Abstract
The usual etiologies of giant abdominal cystic masses in infants are mesenteric cyst, enteric duplication cyst, ovarian cyst in females, cystic lymphangioma, however, the presentation of a choledochal cyst in a gigantic form, is unusual. The primary modality for diagnosis of this entity is ultrasound, followed by MRI. The characteristic ultrasound features of a choledochal cyst are a well-defined cystic lesion which may be found to replace any segment of the biliary tree and is distinctly separate from the gallbladder. The associated anomalies are biliary atresia, gallbladder atresia, hepatic fibrosis and those of the pancreatico-biliary ductal system. MRI with MRCP has a conclusive role in confirming the ultrasound diagnosis. Choledochal cysts are currently classified as proposed by Todani et al, into five types. Herein, we report the case study of a 4-month-old male infant afflicted with a gigantic, Type1 Choledochal cyst, complicated by perforation, which was diagnosed by us at the first instance itself, using ultrasound examination and confirmed by MRI. The diagnosis was further confirmed at surgery and histopathology. The recommended treatment of cyst resection accompanied by a hepatico-jejunostomy bypass procedure, was successfully performed in the reported infant.
Keywords: Choledochal cyst, Giant abdominal cyst, Infant, MRI appearances, Ultrasound features
Introduction
Abdominal masses in infants can be classified as solid, cystic or those comprising of mixed contents [1]. The etiology of an abdominal mass in an infant in the order of frequency is likely to be of renal origin in 55% cases, of gastrointestinal tract origin in 15% of cases, of pelvic origin in 15% cases, adrenal masses comprise 10% of cases and a hepato-biliary origin comprises only 5% of all masses [1]. Amongst the hepatobiliary etiology of cystic abdominal masses in children, hepatic mesenchymal hamartoma, hepato-biliary cystadenoma and choledochal cysts have been reported, but are all rare [1,2]. A choledochal cyst is in itself a rare congenital malformation of the biliary tract and gigantic varieties of these are further rare [3], [4], [5], [6], [7], [8], [9], [10]. The more frequently reported gigantic abdominal cysts in children have been found to be due to mesenteric cyst, ovarian cyst in females, enteric duplication cyst and cystic lymphangioma both of intra and retroperitoneal location [1,2].
Ultrasound is the primary imaging modality used to evaluate abdominal masses in children [11]. Herein we present a case of a gigantic abdominal mass in a 4-month-old male infant which was diagnosed by us on the first instance itself by ultrasound examination, to be a giant choledochal cyst complicated by perforation. The ultrasound diagnosis was further substantiated by the characteristic appearances at MRI. The imaging diagnosis was found to be accurate and was confirmed at surgery and at histopathology as well.
Case report
A male infant of 4 months was brought by his parents with complaints of increasing abdominal girth, jaundice and worsening respiratory distress for 1 week. The infant was a child born of non-consanguineous marriage. The birth had occurred at full term, as a vaginal delivery and there was no history of perinatal illness. The infant's mother had undergone antenatal ultrasound examination at an outside centre twice during her pregnancy, which had both been reported as normal. At current presentation, clinical examination revealed a jaundiced child with respiratory distress and respiratory rate of 40 per minute. The abdomen was distended and there was a large palpable mass in the right hypochondrium, which measured approximately 6 × 6 cm in size. The laboratory evaluation revealed Hemoglobin -7.4 g/dl, Hematocrit-24 %, Neutrophils-38% and Lymphocytes-48%; Total bilirubin-7.22 mg/dl, Direct bilirubin-5.01 mg/dl, Indirect bilirubin-2.21 mg/dl, SGOT -171 U/L, SGPT-72 U/L, ALP- 1230 U/L. The clinical differential diagnosis was a hepatic tumor versus choledochal cyst versus mesenteric cyst and the patient was referred for abdominal sonography for further evaluation. Abdominal sonography was performed on a Philips Epiq 7 equipment, using both convex and linear probes. Abdominal ultrasound revealed a large unilocular cystic lesion in the sub-hepatic location, at porta hepatis, which measured 8.2 × 8.1 × 7 cm in size. The lesion was in the expected location of the common bile duct (CBD) and no other structure resembling a CBD was localized. The cyst showed intense posterior acoustic enhancement. The contents of the cyst were few echogenic debris. The cyst was gigantic and resulted in superior displacement of the liver and a posterior displacement of the right kidney (Fig. 1). The liver appeared normal and the intra hepatic biliary radicals were not dilated. The gall bladder was localized using a high frequency linear probe and was found to be normal. Minimal fluid was seen in the hepatic sub-capsular region (Fig. 1).The pancreas, pancreatic duct and left kidney, bowel and urinary bladder appeared normal. An ultrasound diagnosis of choledochal cyst (Todani Type I), complicated by perforation was arrived at. Subsequently an upper abdomen MRI with MRCP examination was performed under sedation, on a Philips MRI (3 Tesla) equipment. The study revealed that the CBD was replaced by a large unilocular cystic mass located in the sub- hepatic region, in the expected location of the CBD. The cyst measured 8.2 × 7.2 × 8.1 cm in size. The mass was hypointense on T1 and hyperintense on T2W and SPAIR sequences. The cyst showed a fluid-fluid level. Antero-laterally the cyst was extending up till the abdominal wall. On its posterior surface, the cyst was abutting the right kidney and displacing it further posteriorly. Medially the cyst was displacing the pancreatic head to the left side. A minimal amount of fluid was seen in the hepatic sub-capsular region (Fig. 2). However, the pancreas, pancreatic duct and left kidney were normal. The MR appearances corroborated the ultrasound observations and a final imaging diagnosis of Choledochal cyst (Todani Type I) with perforation was arrived at. The pediatric surgery team planned that a surgical bypass be undertaken after stabilizing the infant. The same was achieved with 2 consecutive but uneventful blood transfusions. The surgical procedure planned was a cystectomy followed by a Roux-en-Y-hepatico- jejunostomy bypass. The incision used was a right sub-costal approach which revealed a giant cyst in the location identical to that, delineated by ultrasound and MRI. Additionally, encapsulated fluid was seen in the hepatic sub-capsular region which drained pale yellow contents. However, no site of perforation was identifiable in the cyst. The cyst was aspirated and allowed to collapse in order to facilitate cystectomy. The aspirated fluid was approximately 350 ml and was dark green in color. The decompressed cyst revealed a tubular structure which measured approximately 9 cm in size and it was resected along with the entire extra-hepatic biliary tree (Fig. 3). Reconstruction was performed using a Roux-en-Y limb of jejunum with end-to-end anastomosis of the common hepatic duct with the jejunum. The histo-pathological examination of the specimen was performed using H&E stain, which confirmed a biliary origin of the cyst and also revealed hepatic fibrosis in the adherent hepatic tissue (Fig. 4).The post-operative recovery at hospital was managed under intensive care for the first 72 hours and the infant was stable. However, on the fourth day the parents took the patient away against medical advice and further follow-up was therefore unfortunately not possible.
Fig. 1.
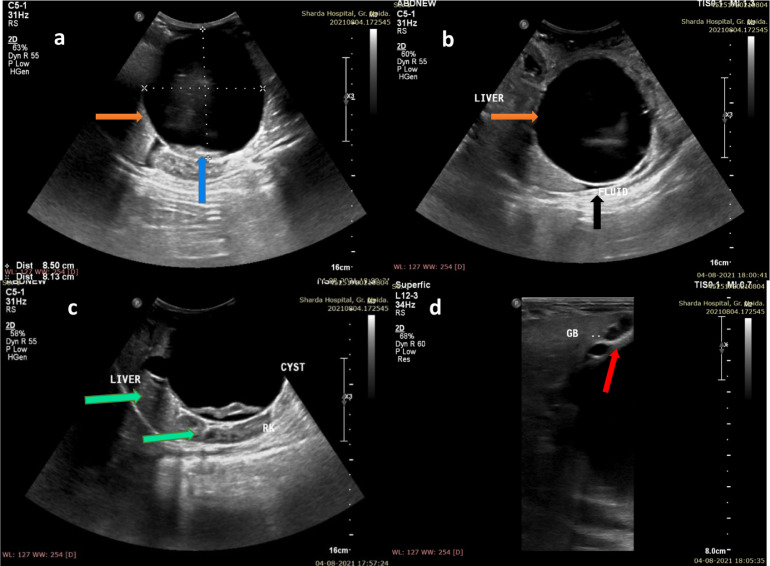
(A,B) Ultrasound of the abdomen in a 4-month male infant, revealed a gigantic cystic lesion in the sub-hepatic location, which measured 8.2 × 7.2 × 8.1 cm in size (orange arrows, A,B). The cyst showed intense posterior acoustic enhancement. The contents of the cyst were a few echogenic debris (blue arrow, A). Minimal fluid was seen in the hepatic sub-capsular region (black arrow, B). The cyst was gigantic and resulted in superior displacement of the liver and a posterior displacement of the right kidney (green arrow, C). The gall bladder was localized using a high frequency linear probe and was found to be normal (red arrow, D) (Color version of figure is available online)
Fig. 2.
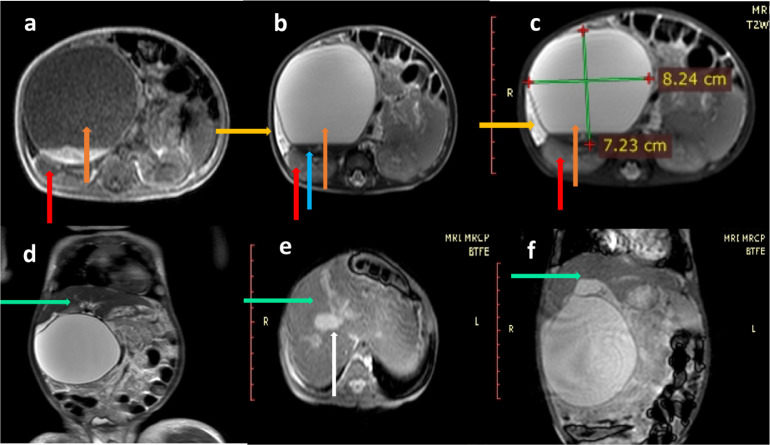
(A,F) MRI of upper abdomen with MRCP in a 4-month infant, T1 W, T2 W and SPAIR sequence in axial planes, revealed a gigantic cystic lesion in the sub-hepatic region (orange arrows), which measured 8.2 × 7.2 × 8.1 cm in size. The cyst was hypo-intense on T1 W, hyper-intense on T2 W and SPAIR sequences (orange arrow, A,C). The cyst also showed a fluid-fluid level (blue arrow, B). The cyst was gigantic and was seen to be indenting the inferior surface of the liver and also causing superior displacement of the organ (D). Antero-laterally the cyst was seen to be extending up till the abdominal wall and posteriorly the cyst was abutting the right kidney and displacing it further posteriorly (red arrows, A,C). Medially the cyst was displacing the pancreatic head to the left side. A minimal amount of fluid was seen in the hepatic sub-capsular region (yellow arrows, A,B). T2 W coronal, BTFE axial and BTFE coronal images showed that the liver was normal (green arrows, D,F). The gall bladder was normal (white arrow, E) (Color version of figure is available online)
Fig. 3.
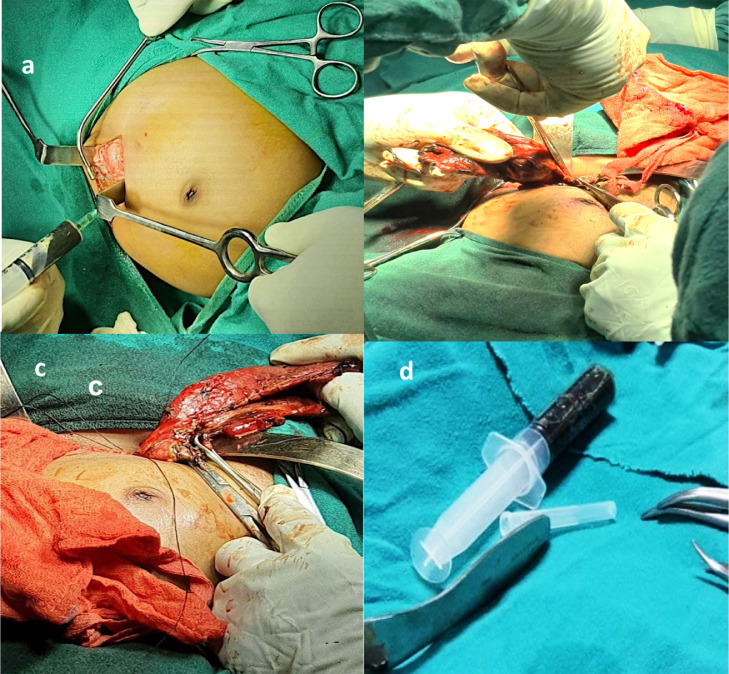
(A,D) The Surgical approach was by a right sub-costal incision (A) and a large cystic lesion was revealed which had to be decompressed before excision (B, C).The aspirate from the choledochal cyst during surgery was a dark green viscous fluid approximately 350 ml, in volume (D).
Fig. 4.
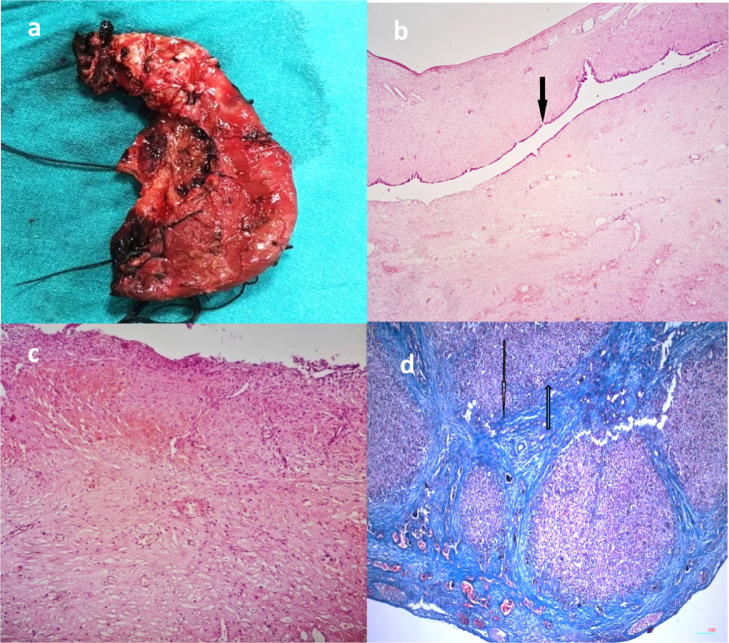
(A,D) Pathology studies of a resected giant abdominal cyst in a 4 month old infant, shows that gross specimen of the resected choledochal cyst (A). Microphotograph of the choledochal cyst wall revealed few foci of chronic inflammation (H&E-200X) (black arrow, (B, C). Micro- photograph of the liver sections showed that early fibrosis was present (Masson Trichrome scanner view) (black arrow, D) (Color version of figure is available online)
Discussion
Choledochal cysts comprise a spectrum of congenital morphological abnormalities of the biliary ductal system, presenting as cystic dilatation of various segments in different combinations [3], [4], [5], [6], [7], [8], [9], [10]. The reported incidence of these anomalies is relatively higher in the Asian population at the rate of 1/1000 births and much rarer in the western population in the rate of 1/100000-1/150000. There is a recognized female preponderance with a male: female ratio of 1:3-4 [4,7].
The earliest reports of this entity are attributed to Vader and Ezlerin 1723 [10]. However, Alonso-Lez and colleagues are credited with first ever classification of the entity in 1959 [5]. The currently followed classification is attributed to Todani et al., which dates back to 1977 [10]. The etiology of the entity has been proposed by Investigators as occurring due to reflux of pancreatic fluid in to the biliary tree, due to an anomalous pancreatico-biliary duct union. It is believed that the pancreatic enzymes cause injury and inflammation to the (common bile) duct walls, leading to their dilatation and cyst formation [5,10]. This particular theory of pancreatic fluid reflux is majorly attributed to Babbit, who had proposed the same vide his publication of 1959 [10].
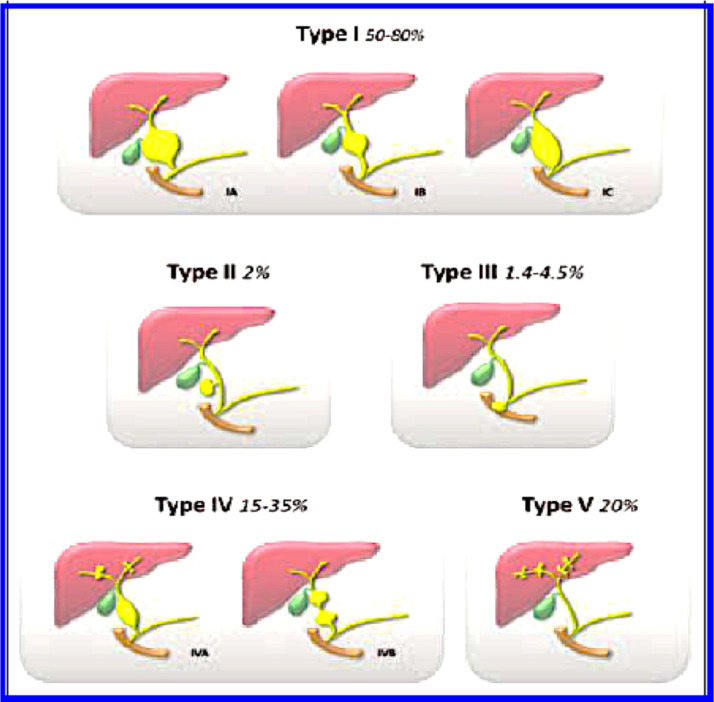
Choledochal cysts were classified by Todani et al. into 5 types. Type I involves the dilatation of the entire common hepatic or of the common bile duct or of a few segments of both and it is the most commonly encountered variety (80%–90% of all choledochal cysts) . Type I choledochal cysts can be further sub-classified into IA, which is a cystic dilatation of the common bile duct as in the presented case. Type IB is a focal segmental dilatation of the common bile duct and type IC is represented by a fusiform dilatation of both, the common hepatic and the common bile duct. Type II is a true diverticulum in any segment of common bile duct. Type III is a choledochocele, located within the duodenal wall at the pancreatico-biliary junction. Type IV choledochal cysts manifests as multiple cysts which can involve both the intrahepatic and extrahepatic biliary tree. Type IV choledochal cysts can be further subdivided into Type IVA and IVB cysts depending on intrahepatic involvement. Type IVB refers to multiple extra hepatic biliary cysts without intrahepatic involvement. Type V choledochal cyst, or Caroli disease, appears as an intrahepatic cystic dilatation without evidence of extra hepatic dilatation (Fig. 5) (Fig. 5, reproduced with permission from reference 12) [12].
Fig. 5.
Reproduced with permission from reference 12 (Santiago I, Loureiro R, Curvo-Semedo L, Marques C, Tardáguila F, Matos C, et al. Congenital cystic lesions of the biliary tree. American Journal of Roentgenoly. 2012;198(4):825-35.) The line diagrams depict the Todani modification of the Alonso-Lej classification and relative percentage of occurrence of each type of choledochal cyst. Type IA, is characterised by a cystic dilatation of the entire extra-hepatic bile duct. Type IB, is typically a focal segmental dilatation of the extra-hepatic bile duct, which is usually located distal to the cystic duct insertion. Type IC, comprises an abnormality comprising a smooth fusiform dilatation of the entire extra-hepatic bile duct. Type II, is a discrete diverticulum of the extra-hepatic bile duct, while Type III, is a dilatation of the intra-duodenal segment of the distal common bile duct. Type IVA, comprises of multiple sites of dilatation of both extra-hepatic and intra-hepatic biliary radicals, while Type IVB, manifests as multiple sites of dilatation only of the extra-hepatic bile duct. Type V, is recognised by multiple sites of saccular or cystic dilatation of only the intra-hepatic biliary tree (synonym: Caroli's disease, or communicating cavernous ectasia)
Majority of choledochal cyst are diagnosed in childhood and 80% are known to be diagnosed within the first decade of life [5,10]. The characteristic presentation of this entity is a unique triad of upper quadrant abdominal pain, palpable mass and jaundice in a young female [5,10]. Known complications of this entity are early development of cholestasis with stone formation, recurrent cholangitis, malignant transformation into cholangiocarcinoma, pancreatitis, portal hypertension, hepato-biliary fibrosis, obstructive jaundice and finally hepatic failure ensues [5,7,10].
A choledochal cyst is typically diagnosed using multi-modality imaging, including ultrasound, computed tomography (CT), and magnetic resonance cholangio-pancreatography (MRCP). The role of imaging is to delineate the anatomy of the cyst, its communication with the intrahepatic and extrahepatic biliary tree and to evaluate for any complications or associated anomalies. Ultrasound is the widely used modality, given its low cost and accessibility, and has been shown to be reliable and cost effective, in the paediatric population [11]. Some of the Ultrasound criteria for abnormal intrahepatic duct dilatation or extrahepatic biliary dilatation include a intrahepatic biliary duct and / or common bile duct diameter more than 2 mm in infancy, more than 4 mm in childhood and more than 7 mm after adolescence [13]. Non- obstructive common bile duct dilatation of more than 10 mm in children is also believed to be a diagnostic indicator of a choledochal malformation [7]. Other characteristic ultrasound features are a well-defined cystic lesion at the porta hepatis, which may be replacing any segment of biliary tree and may show communication with the gallbladder. The cystic lesion shows posterior acoustic enhancement [14]. In our patient the Ultrasound features were of a gigantic cyst in the expected location of common bile duct and the cyst was distinctly separate from the gall bladder. In addition loculated fluid in the hepatic sub-capsular region suggested a contained leak from the cyst. MRI with MRCP has currently overtaken CT and has a conclusive role in confirming the ultrasound diagnosis. MRI features in a case of choledochal cyst are a cystic lesion which is hypointense on T1, hyperintense on T2W and SPAIR sequences, which as on ultrasound, replaces any segment of the biliary tree and is distinctly separate from the gallbladder. MRI provides a better evaluation of intrahepatic disease and its complications. MRCP is considered as the best imaging modality in pre-operative diagnosis of biliary tree abnormalities as it delineates the biliary and pancreatic duct structures in detail [3,15]. The case being reported by us qualified on MRI and MRCP as well to be a gigantic Type 1 choledochal cyst with a leak, as explained above.
With the advent of Ultrasound and MRI, the role of Hepato-biliary scintigraphy for choledochal cyst has been rendered almost obsolete. However, on rare occasions it may be used for planning the extent of resection. The characteristic appearance is of a delayed opacification of the bile duct cysts followed by retention of the isotope in the delayed scan [4]. Our case was diagnosed at the first instance on ultrasound and corroborated on MRI, so scintigraphy was not deemed necessary. ERCP was a primary modality used more frequently in adult patients before the availability of cross sectional imaging. However, it remains the primary modality in patients with severe cholangitis due to ductal calculi, who merit endoscopic extraction of the same [4].
The clinical complications of untreated choledochal malformations range from cholestasis with stone formation to recurrent cholangitis, pancreatitis, biliary and hepatic fibrosis and malignant transformation (cholangio-carcinoma). It is known that the Type I choledochal cysts, along with type IV cysts, have the highest risk of malignancy [4,7,16]. Choledochal cyst may also perforate, which can be diagnosed by presence of bile duct edema, bile duct dilatation and ascites combined with the clinical presentation of peritonitis [16]. The patient described by us also had contained leak, as there was loculated fluid in the adjoining hepatic sub- capsular region, which was documented at imaging and confirmed at surgery. However, there were no clinical or imaging features of peritonitis, thereby confirming the leak to be self- contained.
The treatment of choledochal cyst, is an initial control of the complications followed by surgery for the primary cause. Currently the most popular and accepted surgical approach is total excision of the choledochal cyst. It aims to fully excise the cyst and restore biliary enteric drainage into the duodenum via Roux-en-Y hepatico-jejunostomy. The later approach was used in our patient. A laparoscopic approach is also currently being explored as a minimally invasive surgical technique for managing choledochal cysts in children [6, 8,10].
The prognosis of patients with this anomaly is considered good if the patient presents early and surgical correction is timely, although a long term risk of developing cholangiocarcinoma remains [5,17]. Delayed treatment may lead to a series of events, such as the liver cell damage, fibrosis of the biliary channels and liver cirrhosis. Therefore choledochal cysts presenting in adulthood have a worse prognosis than those presenting in childhood [6]. In a patient who survives the surgical treatment despite pre-existing hepatic complications, a long-term surveillance for the likely development of malignancy is essential [5,17].
Conclusion
Choledochal cysts are rare anomalies of the biliary tree and are typically known to present in young adult females. Presentation in childhood, that too in the early infancy period is very infrequent and its presentation as a gigantic cyst with perforation, in infancy is further rare. The role of radiology and that of an astute Radiologist, are both paramount in achieving an early and accurate diagnosis and salvaging this otherwise clinically catastrophic situation. The characteristic imaging features have been highlighted in our report, which we believe will increase awareness of this entity by Radiologists and Clinicians alike.
Patient Consent
The authors affirm that patient consent has been obtained from patient party (in the regional language) for publication of the case reports on grounds of maintaining anonymity.
Footnotes
Funding: The authors affirm no funding was received for this research.
The Departments of Radiology, Pediatric Surgery and Pathology, School Of Medical Sciences and Research, Sharda Hospital, Sharda University, Greater Noida, 201306, UP, India and Department of Radiology Icahn School of Medicine, at Mount Sinai West, New York, USA.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2021.10.051.
Appendix. Supplementary materials
References
- 1.Light DE, Pianki FR, Ey EH. Abdominal Mass in an Infant. J Am Osteopath Coll Radiol. 2015 [Google Scholar]
- 2.Ferrero L, Guanà R, Carbonaro G, Cortese MG, Lonati L, Teruzzi E, et al. Cystic intra-abdominal masses in children. Pediatr Rep. 2017;9(3):7284. doi: 10.4081/pr.2017.7284. PMID: 29081933; PMCID: PMC5643949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon JH. Magnetic resonance cholangiopancreatography diagnosis of choledochal cyst involving the cystic duct: report of three cases. Brit J Radiol. 2011;84(997):e18–e22. doi: 10.1259/bjr/77844300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar M, Rajagopalan S. Choledochal cyst. Med JArmed Forces India. 2012;68(3):296–298. doi: 10.1016/j.mjafi.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soares KC, Arnaoutakis DJ, Kamel I, Rastegar N, Anders R, Maithel S, et al. Choledochal cysts: presentation, clinical differentiation, and management. J Am Coll Surg. 2014;219(6):1167–1180. doi: 10.1016/j.jamcollsurg.2014.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moslim MA, Takahashi H, Seifarth FG, Walsh RM, Morrisstiff G. Choledochal cyst disease in a western center: a 30-year experience. J Gastrointest Surg. 2016;20(8):1453–1463. doi: 10.1007/s11605-016-3181-4. [DOI] [PubMed] [Google Scholar]
- 7.Banks JS, Saigal G, D'Alonzo JM, Bastos MD, Nguyen NV. Choledochal malformations: surgical implications of radiologic findings. Am J Roentgenol. 2018;210(4):748–760. doi: 10.2214/ajr.17.18402. [DOI] [PubMed] [Google Scholar]
- 8.Ndoye NA, Wellé IB, Cissé L, Guèye D, Diouf C, Mbaye PA, et al. Choledochal cyst in children in dakar: diagnostic and therapeutic aspects. Afr J Paediatr Surg. 2021;18:168–170. doi: 10.4103/ajps.AJPS_4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhua AK, Bhatnagar V, Kandasamy D, Mitra A, Ranjan A, Varshney A. Giant choledochal cyst in infancy-a rare entity. Tropical gastroenterology. 2015;36(4):283–285. doi: 10.7869/tg.311. [DOI] [PubMed] [Google Scholar]
- 10.Suleiman JM, Msuya D, Philemon R, Sadiq A, Amsi P, Lodhia J. A giant choledochal cyst: a case reported from tanzania. Int J Surg Case Rep. 2021;81 doi: 10.1016/j.ijscr.2021.105829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papaioannou G, McHugh K. Investigation of an abdominal mass in childhood. Br Inst Radiol. 2004;16(2):114–123. doi: 10.1259/imaging/11980186. [DOI] [Google Scholar]
- 12.Santiago I, Loureiro R, Curvo-Semedo L, Marques C, Tardáguila F, Matos C, et al. Congenital cystic lesions of the biliary tree. Am J Roentgenol. 2012;198(4):825–835. doi: 10.2214/AJR.11.7294. [DOI] [PubMed] [Google Scholar]
- 13.Lee HC. Ultrasonography of choledochal cysts in children. J Med Ultrasound. 2007;15(3):191–196. [Google Scholar]
- 14.Kim OH, Chung HJ, Choi BG. Imaging of the choledochal cyst. Radiographics. 1995;15(1):69–88. doi: 10.1148/radiographics.15.1.7899614. [DOI] [PubMed] [Google Scholar]
- 15.Lee HK, Park SJ, Yi BH, Lee AL, Moon JH, Chang YW. Imaging features of adult choledochal cysts: a pictorial review. Korean J Radiol. 2009;10(1):71–80. doi: 10.3348/kjr.2009.10.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Tang Y, Wang Z, Wang Q, Wang D. Clinical value of ultrasound in diagnosing pediatric choledochal cyst perforation. Am J Roentgenol. 2015;204:630–635. doi: 10.2214/AJR.14.12935. [DOI] [PubMed] [Google Scholar]
- 17.Ono S, Fumino S, Shimadera S, Iwai N. Long-term outcomes after hepaticojejunostomy for choledochal cyst: a 10- to 27-year follow-up. J Pediatr Surg. 2010;45(2):376–378. doi: 10.1016/j.jpedsurg.2009.10.078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.