Abstract
A 15-year-old female with primary amenorrhea, obesity, and insulin resistance, was admitted for further examination due to large pelvic masses found during a transabdominal ultrasound, which was performed while assessing the amenorrhea. The magnetic resonance imaging showed bilateral, multifocal fibrosing tumors, originating from both ovaries. A laparotomy was performed, during which the right ovary masses were enucleated and the left adnexectomy was performed. A histological examination of the tissue material revealed bilateral ovarian fibromas with calcification and ossification. The genetic examination confirmed the diagnosis of Gorlin syndrome. The patient recovered well, and on the first follow-up 1 month after surgery, already experienced her first spontaneous menstrual bleeding.
Keywords: Multiple ovarian fibromas, Gorlin syndrome
Introduction
Adnexal tumors are uncommon in children. Among them, 10%-20% of ovarian tumors are malignant [1], [2], [3]. Ovarian masses are mostly cystic, and solid tumors are very rare. Fibroma is the most common solid ovarian tumor [4], often seen in adults but rarely in children [5]. While radiological investigation, tumor markers and clinical manifestations can point in the right direction, a histological examination following surgery is essential to confirm the exact nature of the mass [1,4]. When an ovarian mass presents in a girl or an adolescent, the highest priority issue is histological pattern and effect on fertility [2,6]. Given this consideration as well as the rarity of malignant diseases, avoiding excessively aggressive surgery is important [2].
This report demonstrates a case of large, bilateral ovarian fibromas in an adolescent, associated with a genetic syndrome called Gorlin syndrome.
Case description
A 15-year-old female was admitted for further investigations due to an ultrasonographically detected abnormal mass in the small pelvis. The patient presented with primary amenorrhea, obesity, and insulin resistance. She had a history of a hydroureteronephrotic transformation in the left kidney, corrected surgically with a ureterocystoneostomy at the age of 1.
Upon admission, the patient was obese, with height of 1.70 m (+1 SD) and weight of 83.5 kg (+3 SD). The patient's body-mass index (BMI) was 28.9 kg/m2, placing the BMI-for-age at the 96th percentile for girls aged 15 years and 3 months. The patient had normal external genitalia; acne, hirsutism or other signs of virilization were not noted. Her secondary sexual characteristics corresponded to the Tanner scale IV.
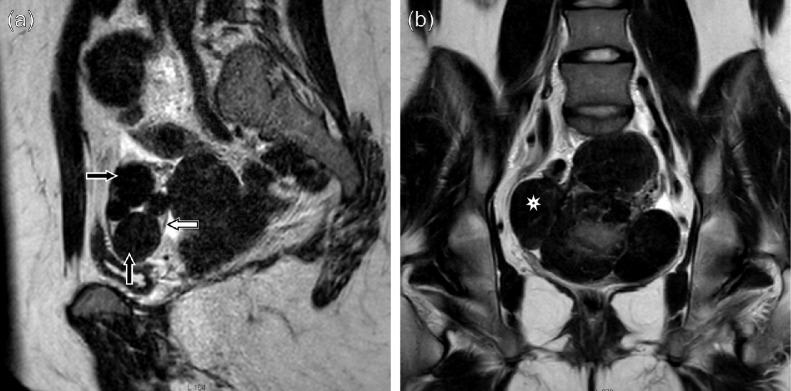
The patient underwent pelvic magnetic resonance imaging (MRI). The MRI revealed 5 masses arising from the right ovary; the size of the largest mass was 3.5 cm × 2.5 cm × 2.1 cm. Normal follicles in the right ovary were not detected on magnetic resonance (MR) images. The left ovary was presumed to be a source of several large fusing masses, forming a conglomerate of size 16 cm × 11 cm × 7.3 cm (Fig. 1). All the lesions were iso- and hypointense to normal muscle in T1-weighted images, and they showed low signal intensity in T2-weighted images. In the T1 gadolinium enhanced series, the tumors showed intensive enhancement with several avascular regions within the largest mass. It was concluded that the pelvic masses were fibrotic in nature, most likely, a bilateral sclerosing multifocal ovarian tumor—a rare stromal tumor presenting with bilateral solid masses [7]. Further abdominal MRI and native chest CT scans excluded tumor dissemination. The patient was referred to skeletal scintigraphy, which detected uptake of the preparation in the pelvic masses; no evidence of bone metastases was found.
Fig. 1.
MRI images of bilateral fibroma-type masses in both ovaries: (a) several separate masses (arrows) in the right ovary (the border of the ovary is well discernible), (b) massive complex solid tumor that occupies most of the pelvis; given the absence of the left ovary and the similarity of the masses to the formations found in the right ovary, suspicions were raised that the whole conglomerate belonged to the left ovary. This was confirmed by the surgery.
The patient had a normal female karyotype of 46, XX. The sex hormone levels were appropriate for puberty. Also, the thyroid stimulating hormone (TSH) level was in the reference range. Only the prolactin level was insignificantly raised, being 327.9 µU/mL over the reference range of <300 µU/mL. Several tumor markers were tested: levels of Cancer Antigen 125 (CA-125), Carcinoembryonic Antigen (CEA), Alpha Fetoprotein (AFP) and free beta-Chorionic Gonadotropin (beta-HCG) were normal. The Neuron-specific Enolase (NSE) level was faintly elevated (17.4 µg/L), exceeding the reference range of <17.00 µg/L. Additionally, the alkaline phosphatase level (178 U/L) exceeded the reference range of 50-117 U/L. In general, there was no evidence that the tumor was hormonally active.
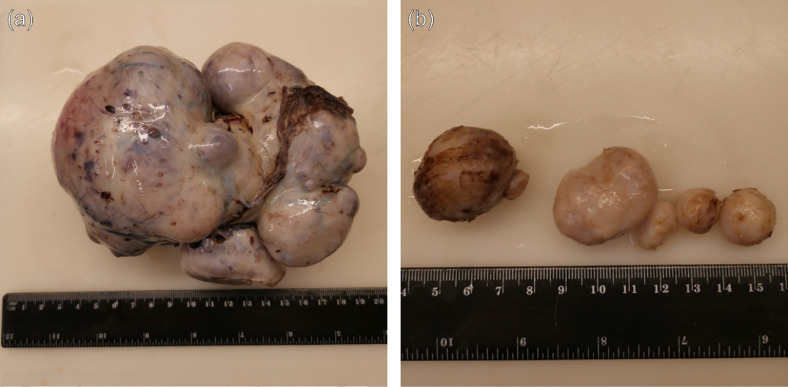
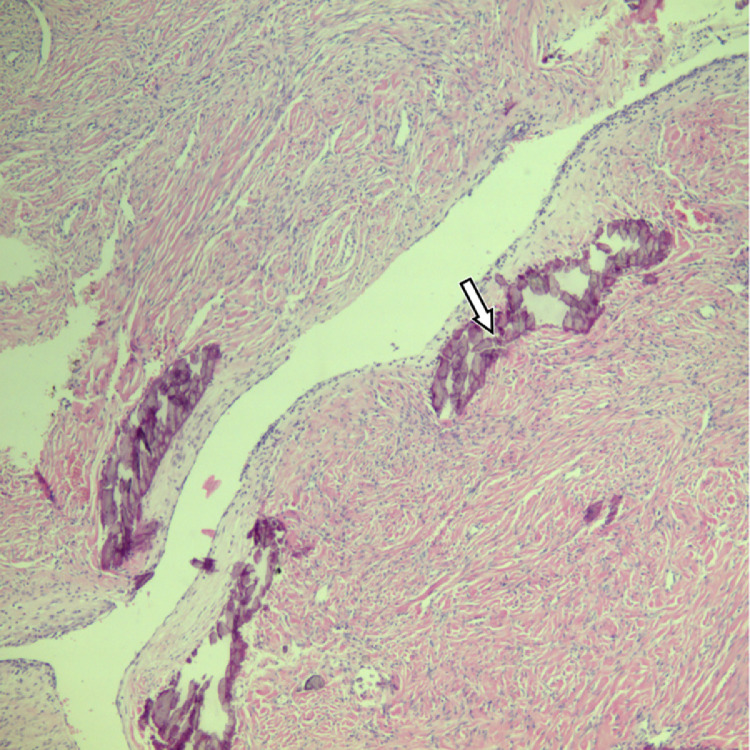
During the laparotomy, normal ovarian tissue on the left side was not visually detected, as it was fully replaced by the tumor tissue consisting of several fused masses, the largest one being around 10 cm in diameter (Fig. 2). This conglomerate had formed adhesions with adjacent structures—the uterus and colon. The left adnexectomy was performed. The right ovary was enlarged, and 5 tumor lesions were palpated ranging from 15 mm till 35 mm in diameter. In order to spare normal ovarian tissue, all the masses from the right ovary were enucleated. Additional biopsy samples were taken from the omentum and peritoneum. The size of the uterus corresponded to the prepubertal age, but with no apparent pathological findings. The histological examination of the surgical sample revealed multiple calcified and ossified ovarian fibromas (Fig. 3), with no evidence of malignancy found in the tissue samples. Due to the relationship of the ovarian fibroma with genetic syndromes [8] including nevoid basal cell carcinoma syndrome (Gorlin syndrome) [5,[9], [10], [11]], the patient was referred to genetic tests which confirmed this syndrome.
Fig. 2.
Gross pathology of the ovarian fibromas: surgical specimens; (a) the massive complex solid tumor in the left ovary, (b) the diameter of the largest mass is around 10 cm several separate masses in the right ovary.
Fig. 3.
Histological pattern present in the masses: ovarian fibroma with multiple calcification foci (arrow) (hematoxylin-eosin staining, at magnification ×40).
During the follow-up, the gynecological ultrasound showed an adequate blood flow, normal structure, and size of the right ovary, with several small follicles. The size of the uterus was only slightly increased since the surgery, and the patient had started menstruating on the 27th postoperative day. At the 6-month follow-up visit the patient had no complaints, and her uterus had increased in size reaching normal adult range. Her period was regular: lasting 27-29 days with moderate bleeding for 5 days. She had developed a small hemorrhagic cyst in the right ovary, confirming the functionality of the organ. Repeated testing of sex steroids showed values corresponding to normal ovarian function.
Discussion
Ovarian fibromas are generally uncommon, making up only 1%-4.7% of total ovarian tumors [9]. Fibromas occur at all ages being most frequent in late reproductive ages; however, in patients under 30 years of age, fibroids occur in less than 10% of cases [12]. In children, fibroma-type masses are exceptionally rare, and literary data suggest that thecoma-fibroma type tumors represent less than 2% of all pediatric ovarian masses [3,13]. In 90% of cases, ovarian fibromas are unilateral [5]. They are mostly benign, although cases of transformation to malignant disease have been described [4,9,14]. Although ovarian fibromas are mostly seen in peri- and postmenopausal women with the median age of 45 years [4], in young patients sex cord stromal tumors have been occasionally reported (median age 13 years). According to literature, the fibroma-thecoma tumor group is often associated with genetic syndromes [8]. Bilateral fibromas are in turn associated with Gorlin syndrome [3]—nevoid basal cell carcinoma syndrome.
Literature suggests, that in pediatric patients with diagnosed bilateral, calcified ovarian fibromas, Gorlin syndrome must be considered [3,9,15]. Gorlin syndrome is a rare genetic syndrome of multiple basal cell carcinomas related to other abnormalities, such as jaw cysts, skeletal anomalies, medulloblastomas, and ovarian fibromas [15]. Seventy-five percent of calcified bilateral ovarian fibromas are associated with Gorlin syndrome [9]. In turn, it is estimated that fibromas will develop in 2%-25% of females with Gorlin syndrome. In our patient, the tumors were hormonally inactive; however, according to literary data, hormonal activity of ovarian pathology in Gorlin syndrome is possible. Ovarian masses, other than fibroma, i.e., fibrosarcoma and leiomyosarcoma, have also been identified in patients with Gorlin syndrome [15].
In our patient, the expression of this Gorlin syndrome was unusual, in that ovarian fibroids were the only manifestation of the Gorlin syndrome, and the mutation was present in the SUFU gene itself, not in the PTCH gene. PTCH has been identified on chromosome 9q22.3. PTCH, (patched) tumor suppressor gene, modifies the hedgehog signaling pathway, a complex pathway responsible for cell cycle regulation [13]. The SUFU (Sufu negative regulator of hedgehog signaling) gene is located on chromosome 10q24-q25.
Our patient has SUFU c.246_249del, p.(Asn83Serfs*12). This variant deletes 4 base pairs in exon 2 (of 12 total exons) and generates a frameshift, leading to a premature stop codon at position 12 in a new reading frame. It is predicted to lead to loss of normal protein function, either through protein truncation or nonsense-mediated mRNA decay. Loss of SUFU function is an established disease mechanism. SUFU c.246_249del, p.(Asn83Serfs*12) is classified as likely pathogenic, based on the established association between the gene and the patient's phenotype, the variant's absence in control populations, and variant type (frameshift). Disease caused by SUFU variants is inherited in an autosomal dominant manner. Any offspring of the patient are at 50% risk of inheriting the variant and of being affected. SUFU-related disease may be caused by a de novo variant.
The SUFU gene encodes a component of the Sonic hedgehog (SHH)/Patched signaling pathway. Mutations in genes encoding this pathway are deleterious for normal development, and are associated with cancer predisposing syndromes [16].
Taylor et al. [17] first identified germline variants in SUFU in children with medulloblastoma. Subsequently, Brugiéres et al. [18] identified germline SUFU variants in 8 out of 131 patients (6%) (6 truncating, 1 genomic deletion, 1 missense variant), all under 3 years of age at the time of diagnosis. The reported mutations have been predominantly truncating.
SUFU related nevoid basal cell carcinoma syndrome (NBCCS) is associated with a high risk for medulloblastoma of up to 33% (3/9) and a high meningioma risk post radiation. Facial expressions are likely more subtle in individuals with an SUFU pathogenic variant. Overall, clinical features are milder in individuals with SUFU-related NBCCS, with less basal cell carcinomas, and no jaw cysts reported [19].
When interpreting the radiological picture based on literary sources [7,20], a sclerosing multifocal ovarian tumor was initially suspected. Both types of tumors—sclerosing stromal tumor and thecoma-fibroma, belong to sex cord stromal tumors and consist of fibrous stroma, but are not synonymous [21]. Radiologically, fibroma is described to typically show low signal intensity on T1-weighted MR images, and marked hypointensity on T2-weighted images [3]; the contrast medium enhancement of fibromas is heterogeneous and mild-to moderate [22,23]. The sclerosing stromal tumor also shows hypo- or isointensity, compared to normal muscles in T1-weighted images, and intermediate to low signal intensity in T2-weighted images; however, on postcontrast images, a typical early intensive peripheral enhancement with centripetal progression and hypovascularized central area is present [3,7]. In the case of our patient, the T1 post-contrast image showed intensive contrast enhancement, along with several hypovascularized regions, the largest of which was 5 cm in diameter. It is important to note that, for technical reasons, a postcontrast dynamic series failed and the contrast-enhanced T1 images were obtained 4 minutes after contrast administration, showing intensive enhancement. This could be a reason for misinterpretation, as the contrast enhancement of fibromas in the late postcontrast series could also be significantly intense.
In the case of our patient, the tumor was pure fibroma by histology and consisted only of fibrous tissue. Histologically, a multifocal sclerosing tumor should also have pseudo-lobular pattern in which cellular areas are separated by hypocellular areas consisting of an edematous collagen. Cellular areas should contain a dual population of cells comprising of spindled fibroblastic cells and round to oval to polygonal lipid containing cells (luteinized theca cells). Cellular areas can often reveal a thin-walled capillary network giving hemangiopericytoma-like pattern. Calcification and ossification, as well as the association with Gorlin syndrome, are most common in fibroids [29], however the association of multifocal sclerosing stromal tumors with Gorlin syndrome is also reported [3].
In the case of Gorlin syndrome, fibromas tend to reoccur following tumorectomy, therefore bilateral adnexectomy could be the definitive treatment for such a pathology. However, it would mean a long-term hormonal replacement therapy, inability to produce offspring, as well as possibly increased morbidity in women [24]. Therefore adnexectomy, especially bilateral, is rarely performed in children, and there is very limited information about the follow-up. It is of the utmost importance to choose ovarian sparing techniques whenever possible. In the case that a bilateral adnexectomy is performed, cryopreservation of ovarian tissue could be considered. This could maintain the possibility of conception, whilst at the same time reducing the need for repeated surgery [5,25,26]. In our patient, one ovary was spared giving a chance for fertility in the future. Surprisingly, she started menstruating soon after the surgery. There are several cases reported in literature where women have had Gorlin syndrome with bilateral fibromas, but have experienced normal menstrual cycles and even preserved fertility. However, there are also a few reports about women starting to have irregular menstruations shortly before fibromas were diagnosed. The mechanism regarding the influence of fibromas on the menstrual cycle is unclear, as they are generally not hormonally active [27,28]. Based on the large total volume of the ovarian formations and the resumption of menstruation after their enucleation, it is our opinion that the reason could be the mass effect of the tumors on ovarian tissue.
Conclusion
-
•
When ovarian fibromas are diagnosed in a pediatric patient, genetic surveillance should be performed, since the diagnosis of Gorlin syndrome is suspected.
-
•
When interpreting radiological findings in pelvic formations, attention must be paid to the technical success of the dynamic post-contrast series, as this is what can provide the most important information for a more accurate interpretation of radiological findings.
-
•
Variants in SUFU gene can cause nevoid basal cell carcinoma syndrome without the typical basal cell carcinomas or jaw cysts.
-
•
When treating children and young women with adnexal tumors, ovarian sparing techniques for fertility preservation are important aspects to consider.
Patient consent
The authors have obtained written consent by the patient, stating that the patient agrees to the publication of this paper including the pictures attached to this article.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Mărginean CO, Mărginean C, Chinceşan M, Mărginean MO, Meliţ LE, Săsăran V, et al. Pediatric ovarian tumors, a challenge for pediatrician and gynecologist: three case reports (CARE compliant) Medicine (Baltimore) 2019;98(16):e15242. doi: 10.1097/MD.0000000000015242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eskander RN, Bristow RE. Adnexal masses in pediatric and adolescent females: a review of the literature. Curr Obstet Gynecol Rep. 2012;1(1):25–32. doi: 10.1007/s13669-011-0001-4. [DOI] [Google Scholar]
- 3.Heo SH, Kim JW, Shin SS, Jeong SI, Lim HS, Choi YD, et al. Review of ovarian tumors in children and adolescents: radiologic- pathologic correlation. Radiographics. 2014;34(7):2039–2055. doi: 10.1148/rg.347130144. [DOI] [PubMed] [Google Scholar]
- 4.Leung S.W., Yuen PM. Ovarian fibroma: a review on the clinical characteristics, diagnostic difficulties, and management options of 23 cases. Gynecol Obstet Invest. 2006;62(1):1–6. doi: 10.1159/000091679. [DOI] [PubMed] [Google Scholar]
- 5.Howell CG, Rogers DA, Gable DS, Falls GD. Bilateral ovarian fibromas in children. J Pediatr Surg. 1990;25(6):690–691. doi: 10.1016/0022-3468(90)90366-h. [DOI] [PubMed] [Google Scholar]
- 6.Wallace W.H.B., Anderson R.A., Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6(4):209–218. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]
- 7.Naidu A, Chung B, Simon M, Marshall I. Bilateral sclerosing stromal ovarian tumor in an adolescent. Case Rep Radiol. 2015;2015:1–4. doi: 10.1155/2015/271394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. World Health Organization Classification of Tumours; 2014. WHO classification of tumours of female reproductive organs; pp. 11–86. [Google Scholar]
- 9.Adesina AM, Imaralu JO, Yusuf AO, Ajani MA. Calcified bilateral ovarian fibroma in a 15 year old female: case report and literature review. Int J Med Pharm Case Rep. 2019;12(1):1–7. doi: 10.9734/ijmpcr/2019/v12i130099. [DOI] [Google Scholar]
- 10.Pirschner F, Bastos PM, Contarato GL, Bimbato ACBL, Filho AC. Gorlin syndrome and bilateral ovarian fibroma. Int J Surg Case Rep. 2012;3(9):477–480. doi: 10.1016/j.ijscr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aram S, Moghaddam NA. Bilateral ovarian fibroma associated with Gorlin syndrome. J Res Med Sci. 2009;14(1):57–61. [PMC free article] [PubMed] [Google Scholar]
- 12.Wahal S, Mohindroo S. Bilateral ovarian fibromas in a young patient: a rare occurrence. Chronicles Young Sci. 2014;5(1):69. doi: 10.4103/2229-5186.129342. [DOI] [Google Scholar]
- 13.Ball A, Wenning J, Van Eyk N. Ovarian fibromas in pediatric patients with basal cell nevus (Gorlin) syndrome. J Pediatr Adolesc Gynecol. 2011;24(1):e5–e7. doi: 10.1016/j.jpag.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Heo SH, Kim JW, Shin SS, Jeong SI, Lim HS, Choi YD, et al. Review of ovarian tumors in children and adolescents: radiologic-pathologic correlation. Radiographics. 2014;34(7):2039–2055. doi: 10.1148/rg.347130144. [DOI] [PubMed] [Google Scholar]
- 15.Ball A, Wenning J, Van Eyk N. Ovarian fibromas in pediatric patients with basal cell nevus (Gorlin) syndrome. J Pediatr Adolesc Gynecol. 2011;24(1):e5–e7. doi: 10.1016/j.jpag.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 16.OMIM. SUFU negative regulator of Hedgehog signaling; SUFU. [Last edited 2019 August 23] .[Internet]. [cited 2021 May 11]. Available at: https://www.omim.org/entry/607035?search=sufu&highlight=sufu.
- 17.Taylor MD, Liu L, Raffel C, Hui C-c, Mainprize TG, Zhang X, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31(3):306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 18.Brugières L, Pierron G, Chompret A, Paillerets BB-d, Rocco FD, Varlet P, et al. Incomplete penetrance of the predisposition to medulloblastoma associated with germ-line SUFU mutations. J Med Genet. 2010;47(2):142–144. doi: 10.1136/jmg.2009.067751. [DOI] [PubMed] [Google Scholar]
- 19.Evans DG, Oudit D, Smith MJ, Rutkowski D, Allan E, Newman WG, et al. First evidence of genotype-phenotype correlations in Gorlin syndrome. J Med Genet. 2017;54(8):530–536. doi: 10.1136/jmedgenet-2017-104669. [DOI] [PubMed] [Google Scholar]
- 20.Chang YW, Hong SS, Jeen YM, Kim MK, Suh ES. Bilateral sclerosing stromal tumor of the ovary in a premenarchal girl. Pediatr Radiol. 2009;39(7):731–734. doi: 10.1007/s00247-009-1190-0. [DOI] [PubMed] [Google Scholar]
- 21.Al Harbi R, McNeish IA, El-Bahrawy M. Ovarian sex cord-stromal tumors: an update on clinical features, molecular changes, and management. Int J Gynecol Cancer. 2021;31(2):161–168. doi: 10.1136/ijgc-2020-002018. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Wang J, Chen X, Wang Y, Wang Z, Li D. Computed tomography and magnetic resonance imaging features of ovarian fibrothecoma. Oncol Lett. 2017;14(1):1172–1178. doi: 10.3892/ol.2017.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinagare AB, Meylaerts LJ, Laury AR, Mortele KJ. MRI features of ovarian fibroma and fibrothecoma with histopathologic correlation. Am J Roentgenol. 2012;198(3):296–303. doi: 10.2214/AJR.11.7221. [DOI] [PubMed] [Google Scholar]
- 24.Sarrel PM, Sullivan SD, Nelson LM. Hormone replacement therapy in young women with surgical primary ovarian insufficiency. Fertil Steril. 2016;106(7):1580–1587. doi: 10.1016/j.fertnstert.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michaeli J, Weintraub M, Gross E, Ginosar Y, Ravitsky V, Eizenman E, et al. Fertility preservation in girls. Obstet Gynecol Int. 2012;2012:1–10. doi: 10.1155/2012/139193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98(3):720–725. doi: 10.1016/j.fertnstert.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Ono M, Nishijima S, Amagata T, Ikeda T, Yakubo K, Akatsuka S, et al. Two consecutive births after ovarian preservation in a Gorlin syndrome patient. J Obstet Gynaecol (Lahore) 2015;35(1):96–97. doi: 10.3109/01443615.2014.937682. [DOI] [PubMed] [Google Scholar]
- 28.Morse CB, McLaren JF, Roy D, Siegelman ES, Livolsi VA, Gracia CR. Ovarian preservation in a young patient with Gorlin syndrome and multiple bilateral ovarian masses. Fertil Steril. 2011;96(1):e47–e50. doi: 10.1016/j.fertnstert.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 29.Scalia AC, Farulla A, Fiocchi F, Alboni C, Torricelli P, et al. Imaging features of uterine and ovarian fibromatosis in Nevoid Basal Cell Carcinoma Syndrome. J Radiol Case Rep. 2018;12(9):21–30. doi: 10.3941/jrcr.v12i9.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]