Abstract
Background
Infective endocarditis is associated with higher mortality in elderly patients, but the role of surgery in this group has not been fully evaluated. The aim of this study was to assess outcomes of left‐sided infective endocarditis in elderly patients and to determine the influence of surgery on mortality in the elderly.
Methods and Results
A nationwide retrospective study was performed of 2186 patients with left‐sided infective endocarditis recorded in the SRIE (Swedish Registry of Infective Endocarditis), divided into patients aged <65 years (n=864), 65 to 79 years (n=806), and ≥80 years (n=516). Survival analysis was performed using the Swedish National Population Registry, and propensity score matching was applied to assess the effect of surgery on survival among patients of all ages. The rate of surgery decreased with increasing age, from 46% in the <65 group to 6% in the ≥80 group. In‐hospital mortality was 3 times higher in the ≥80 group compared with the <65 group (23% versus 7%) and almost twice that of the 65 to 79 group (12%). In propensity‐matched groups, the mortality rate was significantly lower between the ages of 55 and 82 years in patients who underwent surgery compared with patients who did not undergo surgery. Surgery was also associated with better long‐term survival in matched patients who were ≥75 years (hazard ratio, 0.36; 95% CI, 0.24–0.54 [P<0.001]).
Conclusions
The proportion of elderly patients with infective endocarditis who underwent surgery was low compared with that of younger patients. Surgery was associated with lower mortality irrespective of age. In matched elderly patients, long‐term mortality was higher in patients who did not undergo surgery, suggesting that surgery is underused in elderly patients.
Keywords: elderly, infective endocarditis, outcome, valve surgery
Subject Categories: Cardiovascular Surgery, Infectious Endocarditis, Aging, Risk Factors, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- EuroSCORE
European System for Cardiac Operative Risk Evaluation
- HACEK
Haemophilus species, Aggregatibacter aphrophilus, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae
- IE
infective endocarditis
- SRIE
Swedish Registry of Infective Endocarditis
- TEE
transesophageal echocardiography
Clinical Perspective
What Is New?
The surgery rate in elderly patients with left‐sided infective endocarditis was much lower than in younger patients and surgery was associated with lower mortality among all ages in matched groups of patients who did and did not undergo surgery.
What Are the Clinical Implications?
Surgery seems to be underused in elderly patients with left‐sided infective endocarditis.
Despite improvements in diagnostic and therapeutic strategies for infective endocarditis (IE), neither short‐ nor long‐term mortality (IE) rates have significantly decreased since the 1970s. 1
The incidence of IE in the general population is on the rise. 2 , 3 Epidemiological studies show that this increase is mostly in the elderly population, 4 that it is mainly associated with healthcare procedures, 5 and that age‐related degenerative valve disease is the most common underlying heart condition contributing to the increased incidence of IE in the elderly. 5 , 6 In addition, intracardiac electronic devices such as pacemakers, implantable cardioverter‐defibrillators, and valve protheses implanted surgically or by transcatheter aortic valve replacement are frequently used in this population.
IE in the elderly has a different microbiology than IE in younger patients, a greater propensity for women, and higher mortality. 7 , 8 , 9 Cardiac surgery carries significant risks, and increasing age is one of the most important determinants of poor survival after cardiac surgery. 8 Data on the influence of surgery on survival in elderly populations is scarce, and the few observational studies that have been published have presented data from tertiary centers only, often with a limited number of elderly patients. 10 , 11
The aims of the present study were to: (1) describe clinical and microbiological profiles of elderly patients with left‐sided IE compared with younger patients; (2) assess the short‐ and long‐term outcomes of IE in different age groups; and (3) determine the effect of surgery on mortality in elderly patients with IE using nationwide data from the SRIE (Swedish Registry of Infective Endocarditis).
METHODS
Study Design and Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. We conducted a retrospective cohort study using data in the SRIE on episodes from December 6, 2006, until February 23, 2017. The data were submitted using an internet‐based report. The registry contains information on 4151 episodes of IE in patients aged ≥18 years with suspected or confirmed IE. There are 275 variables in the registry, including demographics, baseline characteristics, mode of acquisition, microbiology, complications, and outcomes. A more detailed description of the SRIE is provided in Data S1. The primary study end points were short‐term and long‐term mortality. The patients were divided into 3 groups according to their age: <65 years (group 1), between 65 and 79 years (group 2), and ≥80 years (group 3). A propensity score–matched analysis was performed comparing patients undergoing surgery with those who had conservative treatment. Our study protocol was approved by the regional ethical review board in Lund (reference number 2017‐1051). Informed consent was waived.
Patient Selection
Patients with left‐sided IE were selected by consecutively employing the following exclusion criteria: (1) “possible IE,” “rejected,” or missing information on the modified Duke criteria (n=1123); (2) right‐sided IE (n=291); (3) IE affecting both sides of the heart (n=212); (4) cardiac implantable electronic device IE (n=183); (5) any nonprimary episode (n=74); and (6) duplicate of episode entry, not a resident in Sweden, or restricted patient identification (n=82). Finally, a total of 2186 patients with left‐sided IE were included in the analysis.
Variables in the SRIE
Between 2006 and 2012, the variables diabetes mellitus, cancer, pacemaker, implantable cardioverter‐defibrillator, chronic intravenous catheter, and end‐stage renal disease (ESRD) were not negated, but only filled if the outcome was positive. We therefore assumed missing entries to be negative. The variable maximal vegetation size was optional and 44% of patients with a vegetation had a reported vegetation size.
Follow‐Up
Follow‐up was defined as the period between start of antimicrobial treatment for IE to the most recent status. In 40 patients, the start date of treatment was missing and was therefore assumed to be the same as the day of admission. Follow‐up was finished in November 2017 using the Swedish National Population Registry, and was 100% complete, comprising 7282 patient‐years with a median follow‐up of 2.8 years (interquartile range, 0.9–5.3 years).
Statistical Analysis
Normally distributed continuous variables are presented as mean±SD. Non‐normally distributed continuous variables are expressed as median (interquartile range). Results for categorical data are given as percentages. The cumulative probability of survival was calculated using the Kaplan‐Meier estimator. The log‐rank test was used to compare between‐group differences in survival. The following variables were assessed by univariate Cox regression analysis to determine whether they were associated with long‐term survival: age, sex, diabetes mellitus, ESRD, previous IE, injection drug use, community‐acquired IE, healthcare‐related IE, heart failure, mitral valve location, prosthetic valve IE, vegetations, abscess formation, central nervous system embolism, and pathogen. All variables with a P<0.2 were included in the propensity score–matched analysis and propensity scores were computed by a multivariate logistic regression model. Propensity score matching was conducted using nearest neighbor matching with calipers of 0.2 SD. After propensity score matching, the resulting data set was analyzed using Cox regression. The model allowed for different effects of age for the surgery and nonsurgery groups. This was done in order to estimate whether the effect of age differed between the surgery and the nonsurgery groups. To improve the fit, penalized splines with 4 degrees of freedom were used to model the age variables and the relative mortality rate was plotted. To evaluate the effect of surgery in elderly patients and younger patients separately, the cohort was divided into patients aged <75 years and patients aged ≥75 years. Propensity score matching was performed following the same approach as used to balance the treatment groups in the overall population. Propensity score matching could not be performed for groups 1, 2, and 3 because of the small number of patients who underwent surgery in group 3 (n=30). Survival in the matched groups was estimated with the Kaplan‐Meier method. The long‐term outcome was subsequently determined on the matched data and plotted. Statistical analyses were performed with SPSS statistical software (version 25.0, IBM) and R (The R Foundation, https://www.r‐project.org).
RESULTS
Patient Characteristics
Patients' characteristics are displayed in Table 1. The proportion of women was age dependent, with 48% in group 3 compared with 24% in group 1. Diabetes mellitus was most common in group 2, whereas ESRD and injection drug use decreased with increasing age. The proportion of patients with pacemakers increased with increasing age. The time interval from symptoms to diagnosis was shorter with increasing age, and heart failure was more common in the elderly. However, the incidence of new or major valve insufficiency decreased with increasing age. Abscess formation was 3 times more likely to occur in the <65 group compared with the ≥80 group. In general, any type of embolism was less likely to occur with increasing age. This included central nervous system embolism, which occurred in 19%, 18%, and 13% in groups 1, 2, and 3, respectively (P=0.01).
Table 1.
Patient Characteristics
| Variable | Group 1 | Group 2 | Group 3 |
P Value* All Groups |
P Value † Groups 2 and 3 |
|---|---|---|---|---|---|
| <65 y (n=864) | 65–79 y (n=806) | ≥80 y (n=516) | |||
| Age, y | 50.6±11.8 | 72.1±4.3 | 85.0±3.9 | <0.001 | <0.001 |
| Women | 208 (24) | 249 (31) | 245 (48) | <0.001 | <0.001 |
| Comorbidities | |||||
| Diabetes mellitus (type 1 or type 2) | 106 (13) | 175 (23) | 89 (18) | <0.001 | 0.04 |
| Cancer | 42 (5.0) | 128 (17) | 83 (17) | <0.001 | 0.90 |
| ESRD ‡ , § | 41 (4.9) | 27 (3.5) | 6 (1.2) | 0.002 | 0.01 |
| Risk factors | |||||
| Known heart disease | 181 (21) | 152 (19) | 121 (23) | 0.13 | 0.04 |
| Bicuspid aortic valve | 97 (11) | 10 (1.2) | 2 (0.4) | <0.001 | 0.14 |
| Mitral valve prolapse | 53 (6.1) | 49 (6.1) | 22 (4.3) | 0.29 | 0.15 |
| Congenital heart disease | 24 (2.8) | 9 (1.1) | 1 (0.2) | <0.001 | 0.10 |
| Valve prosthesis | 161 (19) | 215 (27) | 132 (26) | <0.001 | 0.66 |
| Pacemaker | 24 (2.9) | 46 (5.9) | 61 (12) | <0.001 | <0.001 |
| ICD | 13 (1.6) | 15 (2.0) | 2 (0.4) | 0.08 | 0.02 |
| Chronic intravenous catheter | 15 (1.8) | 24 (3.1) | 4 (0.8) | 0.02 | 0.007 |
| Rheumatic heart disease | 4 (0.4) | 7 (0.9) | 3 (0.6) | 0.57 | 0.75 |
| Previous IE | 85 (10) | 31 (3.8) | 22 (4.3) | <0.001 | 0.71 |
| Mode of acquisition | |||||
| IDU | 129 (15) | 5 (0.6) | 0 (0.0) | <0.001 | 0.16 |
| Community acquired | 743 (86) | 675 (84) | 430 (83) | 0.31 | 0.84 |
| Healthcare associated | 93 (11) | 119 (15) | 74 (14) | 0.03 | 0.83 |
| Time from symptoms to diagnosis (days) | 12 (4–30) | 8 (4–25) | 7 (3–19) | <0.001 | 0.049 |
| Clinical features | |||||
| Fever | 683 (79) | 625 (78) | 385 (75) | 0.16 | 0.22 |
| Vascular phenomena | 274 (32) | 216 (27) | 94 (18) | <0.001 | <0.001 |
| Immunological phenomena | 41 (4.7) | 29 (3.6) | 18 (3.5) | 0.38 | 0.92 |
| Heart failure ‡ | 212 (25) | 227 (28) | 181 (35) | <0.001 | 0.008 |
| Positive blood culture | 647 (75) | 633 (79) | 400 (78) | 0.19 | 0.67 |
| Location | |||||
| Aortic valve | 448 (52) | 445 (55) | 248 (48) | 0.04 | 0.01 |
| Mitral valve | 400 (46) | 366 (45) | 259 (50) | 0.21 | 0.09 |
| Prosthetic valve endocarditis | 93 (12) | 124 (16) | 71 (14) | 0.02 | 0.42 |
| Echocardiographic data | |||||
| Transthoracic echocardiogram | 472 (55) | 440 (55) | 314 (61) | 0.04 | 0.03 |
| Transesophageal echocardiogram | 749 (87) | 701 (87) | 379 (73) | <0.001 | <0.001 |
| Severe aortic insufficiency | 232 (27) | 130 (16) | 46 (8.9) | <0.001 | <0.001 |
| Severe major mitral insufficiency | 194 (23) | 149 (19) | 90 (17) | 0.04 | 0.63 |
| Vegetations § | 693 (80) | 662 (82) | 436 (85) | 0.13 | 0.26 |
| Abscess | 116 (13) | 84 (10) | 22 (4.3) | <0.001 | <0.001 |
| Embolism | |||||
| CNS embolism | 164 (19) | 145 (18) | 66 (13) | 0.01 | 0.01 |
| Meningitis (embolism) | 13 (1.5) | 9 (1.1) | 2 (0.4) | 0.16 | 0.22 |
| Spondylitis (embolism) | 54 (6.3) | 72 (8.9) | 36 (7.0) | 0.10 | 0.21 |
| Other skeletal or joint embolism | 61 (7.1) | 54 (6.7) | 23 (4.5) | 0.13 | 0.09 |
| Skin (embolism) | 90 (10) | 44 (5.5) | 17 (3.3) | <0.001 | 0.07 |
| Coronary embolism | 6 (0.7) | 5 (0.6) | 7 (1.4) | 0.30 | 0.16 |
| Spleen embolism | 40 (4.6) | 27 (3.3) | 5 (1.0) | 0.001 | 0.006 |
| Liver embolism | 11 (1.3) | 8 (1.0) | 2 (0.4) | 0.26 | 0.33 |
| Lung embolism | 25 (2.9) | 11 (1.4) | 8 (1.6) | 0.06 | 0.78 |
| Other embolisms | 60 (6.9) | 48 (6.0) | 18 (3.4) | 0.03 | 0.045 |
Dichotomous variables are expressed as number of patients (percentage within age group). Continuous normally distributed variables are expressed as mean±SD. Non‐normally distributed continuous variables are expressed as median (interquartile range). CNS indicates central nervous system; ESRD, end‐stage renal disease; ICD, implantable cardioverter‐defibrillator; and IDU, injection drug use.
P value, statistical testing among all 3 groups.
P value, statistical testing between groups 2 and 3.
Before or during treatment of infective endocarditis (IE).
Any size visualized by means of echocardiography.
Microbial Pathogenesis of IE
Positive blood culture was recorded in 77% of patients, with no significant difference between age groups, and a causative pathogen was identified in 99% of cases. Table 2 summarizes the microbiological findings. The distribution of pathogens was similar in all groups. Staphylococcus aureus tended to be more common in elderly patients, while the HACEK (Haemophilus species, Aggregatibacter aphrophilus, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae) group was more common in younger patients.
Table 2.
Microbial Pathogenesis of IE
| Pathogen | <65 y (n=864) | 65–79 y (n=806) | ≥80 y (n=516) | P Value* | P Value † |
|---|---|---|---|---|---|
| Staphylococcus aureus ‡ | 268 (31) | 263 (33) | 195 (38) | 0.03 | 0.05 |
| Coagulase negative staphylococci | 35 (4.1) | 43 (5.3) | 30 (5.9) | 0.28 | 0.71 |
| α‐Hemolytic streptococci | 273 (31) | 225 (27) | 148 (29) | 0.23 | 0.76 |
| β‐Hemolytic streptococci | 50 (5.8) | 42 (5.2) | 35 (6.8) | 0.49 | 0.23 |
| Streptococcus pneumoniae | 15 (1.7) | 11 (1.4) | 2 (0.4) | 0.10 | 0.08 |
| Streptococcus bovis | 13 (1.5) | 13 (1.6) | 17 (3.3) | 0.045 | 0.045 |
| Enterococci | 81 (9.4) | 108 (13) | 61 (12) | 0.03 | 0.40 |
| HACEK | 25 (2.9) | 13 (1.6) | 1 (0.2) | 0.001 | 0.01 |
| Other gram‐positive | 47 (5.4) | 51 (6.3) | 15 (2.9) | 0.02 | 0.005 |
| Other gram‐negative | 20 (2.3) | 14 (1.7) | 4 (0.8) | 0.11 | 0.14 |
| Fungi | 5 (0.6) | 5 (0.6) | 2 (0.4) | 0.85 | 0.71 |
| Unknown pathogen | 32 (3.7) | 18 (2.2) | 6 (1.2) | 0.01 | 0.16 |
Variables are expressed as number (percentage) of patients. HACEK indicates Haemophilus species, Aggregatibacter aphrophilus, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae; and IE, infective endocarditis.
Chi‐square test among all 3 groups.
Chi‐square test between groups 2 and 3.
Methicillin‐sensitive S aureus (n=734), methicillin‐resistant S aureus (age <65 years, n=3; age 65–79 years, n=5; age ≥80 years, n=3).
Short‐ and Long‐Term Outcomes
Short‐term outcomes and 1‐ and 5‐year survival are presented in Table 3. Surgery was performed in 46% of cases in group 1, 29% in group 2, and 5.8% in group 3. Short‐term mortality increased with age. The in‐hospital mortality in group 3 was 3 times that of group 1 and twice that of group 2. Long‐term survival was worse in group 2 compared with group 1, and it was the worst in group 3, and there was a significant association between age and long‐term survival (log‐rank, P<0.001). 12 The overall long‐term survival is shown in Figure S1.
Table 3.
Unadjusted Short‐Term and Long‐Term Outcomes
| Variable | <65 y (n=864) | 65–79 y (n=806) | ≥80 y (n=516) | P Value* | P Value † |
|---|---|---|---|---|---|
| Surgery for IE | 400 (46) | 231 (29) | 30 (5.8) | <0.001 | <0.001 |
| Length of hospitalization ‡ | 32 (23–43) | 33 (25–45) | 33 (25–42) | 0.18 | 0.16 |
| 30‐d Mortality | 40 (4.7) | 71 (8.9) | 99 (19) | <0.001 | <0.001 |
| 90‐d Mortality | 56 (6.6) | 111 (14) | 158 (31) | <0.001 | <0.001 |
| Overall in‐hospital mortality | 56 (6.5) | 99 (12) | 118 (23) | <0.001 | <0.001 |
| In‐hospital mortality for the surgical group | 16 (4.0) | 28 (12) | 6 (20) | <0.001 | <0.001 |
| In‐hospital mortality for the nonsurgical group | 40 (8.6) | 71 (12) | 112 (23) | <0.001 | <0.001 |
| Long‐term survival, Kaplan‐Meier estimates | |||||
| 1‐y Survival | 89 (87–91) | 77 (74–80) | 55 (51–59) | ||
| Patients at risk, 1 y | 746 | 595 | 274 | ||
| 5‐y Survival | 75 (71–78) | 57 (53–61) | 22 (18–26) | ||
| Patients at risk, 5 y | 323 | 219 | 52 | ||
Dichotomous variables are expressed as number (percentage) of patients. Non‐normally distributed continuous are expressed as median (interquartile range). Estimated 1‐ and 5‐year survival rates are reported as percentage (95% CI). Patients at risk are shown. IE indicates infective endocarditis.
P value, statistical testing among all 3 groups.
P value, statistical testing between groups 2 and 3.
Groups 1 and 2, P=0.17; groups 1 and 3, P=0.88; groups 2 and 3, P=0.16.
Influence of Surgery
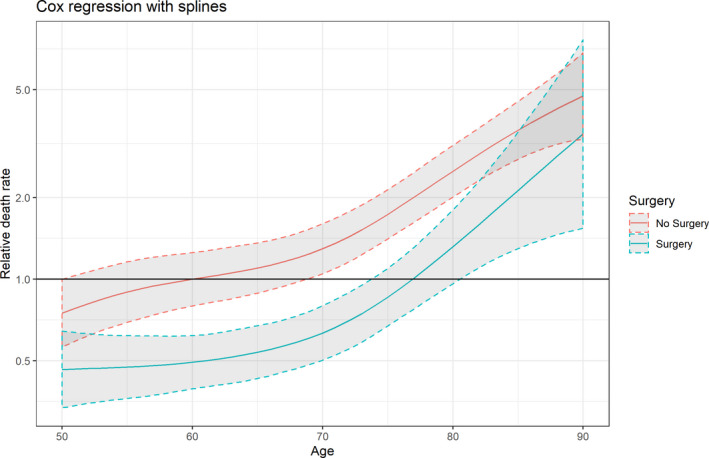
The following variables were used to calculate propensity scores: age, sex, diabetes mellitus, ESRD, previous IE, injection drug use, healthcare‐related IE, heart failure, mitral valve location, prosthetic valve endocarditis, vegetations, and pathogen (Table S1). Propensity score matching resulted in 560 matches (Figure 1). The standardized mean differences are shown in Table 4. The results from Cox regression with splines are shown in Figure 2. The graph shows the relative mortality compared with a 60‐year‐old patient who did not have surgery. Between the ages of 53 years and 82 years, the death rate was significantly lower in the surgery group.
Figure 1. Propensity score matching for (A) all data and (B) unmatched data, with (C) the scatter plot of matched and unmatched patients.
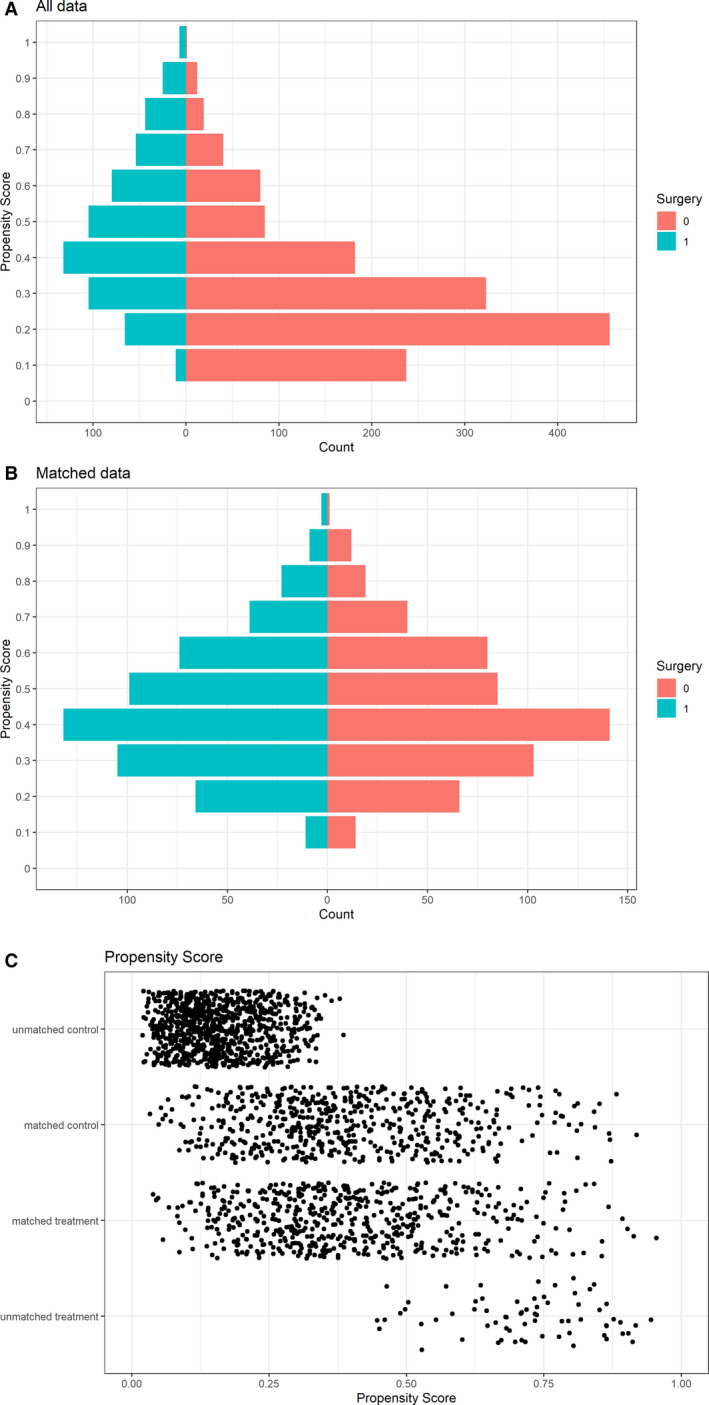
Table 4.
Standardized Mean Difference for Matched Data
| All Data | Age <75 y | Age ≥75 y | |
|---|---|---|---|
| 560 Pairs | 442 Pairs | 88 Pairs | |
| Risk factor | |||
| Age | 0.0162 | … | |
| Female sex | 0.0405 | −0.0262 | 0.0467 |
| Diabetes mellitus | 0.0055 | −0.0207 | 0.0000 |
| End‐stage renal disease | 0.0298 | 0.0000 | −0.1516 |
| Embolism to the CNS | 0.0042 | −0.0218 | 0.0495 |
| Intravenous drug use | −0.0707 | −0.0456 | … |
| Healthcare‐related | 0.0607 | −0.0321 | 0.1274 |
| Heart failure | 0.0364 | 0.0232 | −0.0679 |
| Mitral valve location | −0.0108 | −0.0183 | −0.1135 |
| Prosthetic valve IE | 0.0255 | −0.0196 | 0.0000 |
| Vegetation | 0.0261 | −0.0455 | 0.1119 |
| Pathogen | 0.0167 | −0.0172 | 0.0100 |
CNS indicates central nervous system; and IE, infective endocarditis
Figure 2. Spline curve showing the effect of age on the relative mortality rates of patients with left‐sided infective endocarditis treated with and without surgery.
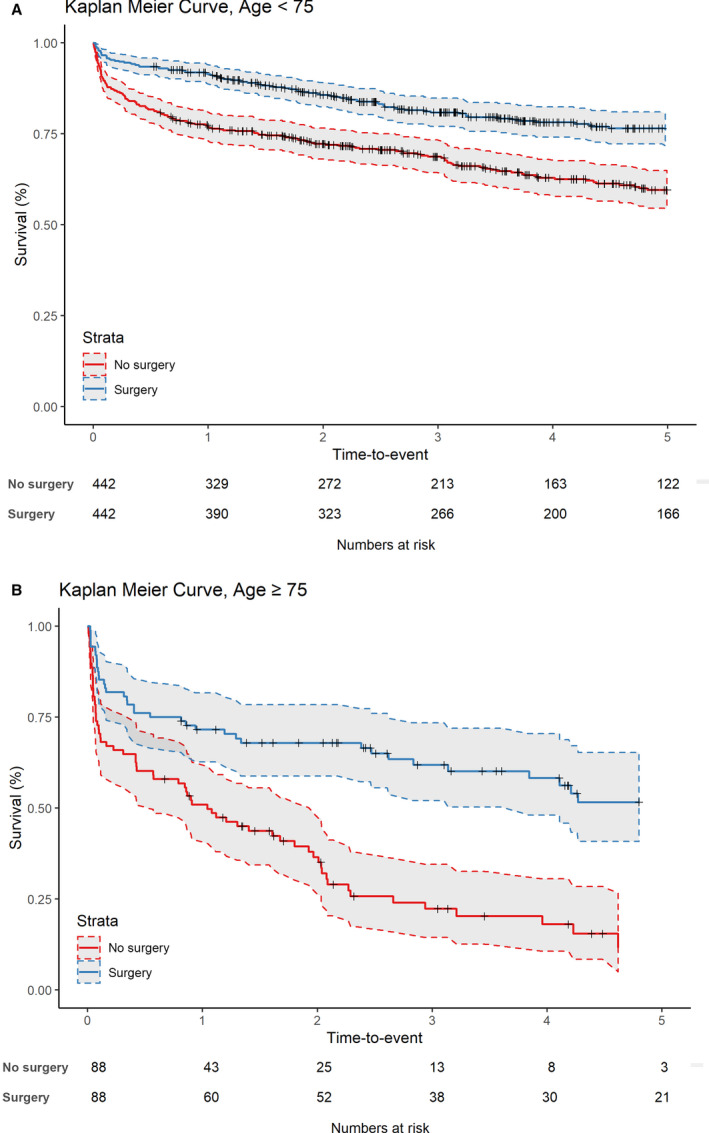
The propensity score matching in patients aged <75 years and those ≥75 years resulted in 442 and 88 matches, respectively (Figures S2 and S3). Standardized mean differences are shown in Table 4. Figure 3 shows the long‐term survival in both matched groups. In the <75 group, 1‐year survival after surgery was 72% compared with 63% without surgery, and in the ≥75 group, 1‐year survival with surgery was 53% compared with 17% without surgery. Cox regression showed that the hazard ratio (HR) for surgery was 0.53 (95% CI, 0.41–0.67; P<0.001) in the <75 group and 0.36 (95% CI, 0.24–0.54; P<0.001) in the ≥75 group.
Figure 3. Propensity score matching for long‐term survival of in (A) patients aged <75 years and (B) patients ≥75 years.
DISCUSSION
The present study presents nationwide data on left‐sided IE in different age groups, including >500 patients aged ≥80 years. The results are likely generalizable, as the study included data from patients at secondary and tertiary care centers in an entire nation. We found that the rate of surgical treatment decreased dramatically with increasing age, despite an increase in heart failure and a higher proportion of patients with S aureus IE. In matched‐groups analysis, patients who underwent surgery had a significantly lower death rate among a wide age range. In addition, after matching elderly patients ≥75 years who underwent surgery with those who did not, those who had surgery showed significantly better survival.
Left‐sided IE in the elderly is associated with poor prognosis. Our results show that mortality increases significantly with older age. In‐hospital mortality in the ≥80 group was 3 times that of the <65 group. These findings are similar to previous results showing increased in‐hospital mortality with increasing age, 2 , 5 , 7 , 8 , 9 , 11 , 13 , 14 , 15 although the cutoffs differ among studies.
Although in‐hospital mortality increases with advanced age, López‐Wolf et al, 9 Oliver et al, 7 and Gatti et al 16 have all found that older age is not an independent predictor of in‐hospital mortality. However, several other studies have shown that advanced age is independently associated with in‐hospital mortality. 5 , 11 , 17 In‐hospital mortality may be explained to a greater extent by the severity of IE and by comorbidities than by age. Long‐term survival is also worse with older age, and in the present study only 22% of patients aged ≥80 years survived beyond 5 years. This is confirmed by studies that have shown that age is an independent predictor of long‐term mortality. 7 , 15
In the current study, <6% of cases of IE in the ≥80 group were treated with surgery, while surgery was performed in almost half of the cases in the <65 group. Other authors have also found that elderly patients undergo surgery less frequently than younger patients, 5 , 7 , 8 , 9 , 15 but most have reported higher numbers of patients who undergo surgery than the current study. Durante‐Mangoni et al 5 reported that 37% of patients >70 years underwent surgery for IE, and Arminanzas et al 15 and Di Salvo et al 13 reported that 21% and 41% of patients aged ≥80 years underwent surgery. The reason for this discrepancy is unclear, but it could be that secondary care centers that report patients to the SRIE are not referring the oldest patients for tertiary care. There is some justification for deferring surgery in the elderly, whether because there is a lower incidence of valve impairment in this group, or because the relatively high comorbidity among these patients makes the surgical risk unacceptable. 8 , 9 Nevertheless, Oliver et al 7 posited that the European Society of Cardiology guidelines have been poorly implemented among older patients, in that a significant proportion of patients who had an indication for surgery were did not undergo surgery. They concluded that surgery was underused in patients aged ≥80 years because patients who underwent surgery in the oldest group had a much better 1‐year survival than those who did not undergo surgery. Furthermore, Arminanzas et al 15 have shown that the absolute difference between the proportion of patients with surgical indication and the proportion of patients actually undergoing surgery increases with age. Whereas 52% of octogenarian patients in that study had an indication for surgery, only 21% ultimately had surgery. In contrast, 68% of patients who were <65 years had a surgical indication, and 53% had surgery. 15
Advanced age is strongly associated with short‐term mortality following any type of cardiac surgery and is included in all risk models for mortality after cardiac surgery. 18 This is also true of surgery for IE, where short‐term mortality is higher among elderly patients who undergo either urgent or elective surgery. 8 In our study, the in‐hospital mortality among patients aged ≥80 years who underwent surgery was high and was similar to the in‐hospital mortality of patients who did not undergo surgery. After propensity score matching of surgical and nonsurgical cases, we were able to show that long‐term mortality expressed as the relative mortality rate was significantly lower among a wide age range, from 54 to 82 years, in patients who underwent surgery compared with comparable patients who did not undergo surgery. We could further show that a 60‐year‐old patient who did not undergo surgery had the same mortality risk as a 77‐year‐old patient who did undergo surgery. Although the margin of error was too wide to demonstrate a statistically significant difference, the death rate was lower in patients who underwent surgery up to 90 years of age compared with those who did not undergo surgery. The propensity score matching was good, and no variables had a standardized mean difference of >0.1. When we applied propensity score matching analysis after splitting the patients into <75 and ≥75 age groups, we found that the benefit of surgery was significant in both sets of matched groups, and the HR of surgery was lower in the ≥75 group than in the younger group, indicating a greater effect of surgery in this group.
The rate of S aureus IE increased with age. S aureus IE accounted for 37% of cases in the ≥80 group, and was significantly more common in this group than in the patients who were aged 65 to 79 years. This was the case even though conditions that are often associated with S aureus bacteremia, such as diabetes mellitus, ESRD, and cancer, were all less common in the older group. Both López‐Wolf et al 7 , 9 and Oliver et al, 7 , 9 to the contrary, have reported lower rates of S aureus in older patients, but a higher incidence of enterococci and Streptococcus bovis. Our analysis showed no significant difference in the frequency of enterococci among age groups. This also contrasts with the reports from López et al and Arminanzas et al, which showed an increased incidence of enterococci among elderly patients. 9 , 15 S bovis was most common in elderly patients in both studies. This is to be expected, as this pathogen is associated with gastrointestinal lesions that are more common in the elderly. 19
Elderly patients were more likely to have transthoracic echocardiography, and the use of transesophageal echocardiography (TEE) decreased with increasing age, despite the fact that the sensitivity of TEE in detecting vegetative lesions is superior to that of transthoracic echocardiography, also in the elderly. 9 , 20 This suggests that elderly patients are treated differently than younger patients, which is concerning, because the proportion of IE cases where TEE is the only evidence of IE was found to increase with age. 5 Wider application of TEE could therefore lead to an important diagnostic gain in older patients. However, cooperation of elderly patients for TEE may be difficult because of cognitive disorder or agitation caused by IE.
Strengths and Limitations
The major strength of this study is that it includes data from a nationwide registry on IE, with patients treated at secondary and tertiary centers. The large number of elderly patients allowed us to compare the effects of surgery from young to advanced age. The limitation of this study is that it is retrospective in nature, albeit on a prospective database, with potential biases. It is not mandatory to report all IE episodes to the SRIE, which may lead to underreporting, especially of elderly patients and patients who are not treated by infectious disease specialists. The SRIE only recently introduced a category for patients with an indication for surgery who do not undergo surgery. Our analyses were therefore performed on all eligible patients with left‐sided IE, including those who did not have an indication for surgery. Matching patients who underwent surgery to patients who did not with propensity scores may have limitations in that there may be factors that influence whether patients undergo surgery or not that are not apparent to the authors. We were not able to include estimated surgical risk in our propensity score models. The SRIE does not include European System for Cardiac Operative Risk Evaluation (EuroSCORE) or any other surgical risk estimation. Although many of the variables that are used to calculate EuroSCORE (such as age, female sex, and diabetes mellitus) were used in calculating the propensity scores, several important EuroSCORE variables are not included in the SRIE, which prevented us from calculating the EuroSCORE.
Conclusions
The current study adds valuable information on the influence of surgery in elderly patients. We have shown that surgery is associated with a lower death rate among all ages in matched groups of patients who did and did not undergo surgery, with a significantly lower death rate between the ages of 54 and 82 years. When patients aged ≥75 years who underwent surgery were matched with patients who did not undergo surgery, there was significantly better survival associated with surgery. When the low proportion of elderly patients who undergo surgery is taken into consideration, we conclude that surgery is underused in the elderly.
Sources of Funding
This work was supported by the ALF (Avtalet om Läkarutbildning och Forskning) agreement, grant to Ragnarsson.
Disclosures
None.
Supporting information
(J Am Heart Assoc. 2021;10:e020221. DOI: 10.1161/JAHA.120.020221.)
See Editorial by Ghanta and Pettersson
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Sigurdur Ragnarsson, Email: sigurdur.ragnarsson@med.lu.se.
Magnus Rasmussen, Email: magnus.rasmussen@med.lu.se.
REFERENCES
- 1. Fedeli U, Schievano E, Buonfrate D, Pellizzer G, Spolaore P. Increasing incidence and mortality of infective endocarditis: a population‐based study through a record‐linkage system. BMC Infect Dis. 2011;11:48. DOI: 10.1186/1471-2334-11-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoen B, Alla F, Selton‐Suty C, Béguinot I, Bouvet A, Briançon S, Casalta JP, Danchin N, Delahaye F, Etienne J, et al. Changing profile of infective endocarditis: results of a 1‐year survey in France. JAMA. 2002;288:75–81. DOI: 10.1001/jama.288.1.75 [DOI] [PubMed] [Google Scholar]
- 3. Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000–13: a secular trend, interrupted time‐series analysis. Lancet. 2015;385:1219–1228. DOI: 10.1016/S0140-6736(14)62007-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Slipczuk L, Codolosa JN, Davila CD, Romero‐Corral A, Yun J, Pressman GS, Figueredo VM. Infective endocarditis epidemiology over five decades: a systematic review. PLoS One. 2013;8:e82665. DOI: 10.1371/journal.pone.0082665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Durante‐Mangoni E, Bradley S, Selton‐Suty C, Tripodi MF, Barsic B, Bouza E, Cabell CH, Ramos AI, Fowler V Jr, Hoen B, et al. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med. 2008;168:2095–2103. DOI: 10.1001/archinte.168.19.2095 [DOI] [PubMed] [Google Scholar]
- 6. Benito N, Miró JM, de Lazzari E, Cabell CH, del Río A, Altclas J, Commerford P, Delahaye F, Dragulescu S, Giamarellou H, et al. Health care‐associated native valve endocarditis: importance of non‐nosocomial acquisition. Ann Intern Med. 2009;150:586–594. DOI: 10.7326/0003-4819-150-9-200905050-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oliver L, Lavoute C, Giorgi R, Salaun E, Hubert S, Casalta J‐P, Gouriet F, Renard S, Saby L, Avierinos J‐F, et al. Infective endocarditis in octogenarians. Heart. 2017;103:1602–1609. DOI: 10.1136/heartjnl-2016-310853 [DOI] [PubMed] [Google Scholar]
- 8. López J, Revilla A, Vilacosta I, Sevilla T, Villacorta E, Sarriá C, Pozo E, Rollán MJ, Gómez I, Mota P, et al. Age‐dependent profile of left‐sided infective endocarditis: a 3‐center experience. Circulation. 2010;121:892–897. DOI: 10.1161/CIRCULATIONAHA.109.877365 [DOI] [PubMed] [Google Scholar]
- 9. López‐Wolf D, Vilacosta I, San Román JA, Fernández C, Sarriá C, López J, Revilla A, Manchado R. [Infective endocarditis in octogenarian patients]. Rev Esp Cardiol. 2011;64:329–333. DOI: 10.1016/j.recesp.2010.05.005 [DOI] [PubMed] [Google Scholar]
- 10. Wu Z, Chen Y, Xiao T, Niu T, Shi Q, Xiao Y. The clinical features and prognosis of infective endocarditis in the elderly from 2007 to 2016 in a tertiary hospital in China. BMC Infect Dis. 2019;19:937. DOI: 10.1186/s12879-019-4546-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramírez‐Duque N, García‐Cabrera E, Ivanova‐Georgieva R, Noureddine M, Lomas JM, Hidalgo‐Tenorio C, Plata A, Gálvez‐Acebal J, Ruíz‐Morales J, de la Torre‐Lima J, et al. Surgical treatment for infective endocarditis in elderly patients. J Infect. 2011;63:131–138. DOI: 10.1016/j.jinf.2011.05.021 [DOI] [PubMed] [Google Scholar]
- 12. Delahaye F, Goulet V, Lacassin F, Ecochard R, Selton‐Suty C, Hoen B, Etienne J, Briançon S, Leport C. Characteristics of infective endocarditis in France in 1991: a 1‐year survey. Eur Heart J. 1995;16:394–401. DOI: 10.1093/oxfordjournals.eurheartj.a060923 [DOI] [PubMed] [Google Scholar]
- 13. Di Salvo G, Thuny F, Rosenberg V, Pergola V, Belliard O, Derumeaux G, Cohen A, Iarussi D, Giorgi R, Casalta JP, et al. Endocarditis in the elderly: clinical, echocardiographic, and prognostic features. Eur Heart J. 2003;24:1576–1583. DOI: 10.1016/S0195-668X(03)00309-9 [DOI] [PubMed] [Google Scholar]
- 14. Forestier E, Fraisse T, Roubaud‐Baudron C, Selton‐Suty C, Pagani L. Managing infective endocarditis in the elderly: new issues for an old disease. Clin Interv Aging. 2016;11:1199–1206. DOI: 10.2147/CIA.S101902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Armiñanzas C, Fariñas‐Alvarez C, Zarauza J, Muñoz P, González Ramallo V, Martínez Sellés M, Miró Meda JM, Pericás JM, Goenaga MÁ, Ojeda Burgos G, et al. Role of age and comorbidities in mortality of patients with infective endocarditis. Eur J Intern Med. 2019;64:63–71. DOI: 10.1016/j.ejim.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 16. Gatti G, Perrotti A, Obadia J‐F, Duval X, Iung B, Alla F, Chirouze C, Selton‐Suty C, Hoen B, Sinagra G, et al. Simple scoring system to predict in‐hospital mortality after surgery for infective endocarditis. J Am Heart Assoc. 2017;6:e004806. DOI: 10.1161/JAHA.116.004806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Selton‐Suty C, Célard M, Le Moing V, Doco‐Lecompte T, Chirouze C, Iung B, Strady C, Revest M, Vandenesch F, Bouvet A, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1‐year population‐based survey. Clin Infect Dis. 2012;54:1230–1239. DOI: 10.1093/cid/cis199 [DOI] [PubMed] [Google Scholar]
- 18. Geissler HJ, Hölzl P, Marohl S, Kuhn‐Régnier F, Mehlhorn U, Südkamp M, de Vivie ER. Risk stratification in heart surgery: comparison of six score systems. Eur J Cardiothorac Surg. 2000;17:400–406. DOI: 10.1016/S1010-7940(00)00385-7 [DOI] [PubMed] [Google Scholar]
- 19. Ballet M, Gevigney G, Gare JP, Delahaye F, Etienne J, Delahaye JP. Infective endocarditis due to Streptococcus bovis. A report of 53 cases. Eur Heart J. 1995;16:1975–1980. DOI: 10.1093/oxfordjournals.eurheartj.a060856 [DOI] [PubMed] [Google Scholar]
- 20. Werner GS, Schulz R, Fuchs JB, Andreas S, Prange H, Ruschewski W, Kreuzer H. Infective endocarditis in the elderly in the era of transesophageal echocardiography: clinical features and prognosis compared with younger patients. Am J Med. 1996;100:90–97. DOI: 10.1016/S0002-9343(96)90017-0 [DOI] [PubMed] [Google Scholar]
- 21. Bjursten H, Rasmussen M, Nozohoor S, Götberg M, Olaison L, Rück A, Ragnarsson S. Infective endocarditis after transcatheter aortic valve implantation: a nationwide study. Eur Heart J. 2019;40:3263–3269. DOI: 10.1093/eurheartj/ehz588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clarelin A, Rasmussen M, Olaison L, Ragnarsson S. Comparing right‐ and left sided injection‐drug related infective endocarditis. Sci Rep. 2021;11:1177. DOI: 10.1038/s41598-020-80869-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aldman MH, Rasmussen M, Olaison L, Påhlman LI. Endocarditis due to Staphylococcus lugdunensis‐a retrospective national registry‐based study. Eur J Clin Microbiol Infect Dis. 2021;40:1103–1106. DOI: 10.1007/s10096-020-04134-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vähäsarja N, Lund B, Ternhag A, Götrick B, Olaison L, Hultin M, Krüger Weiner C, Naimi‐Akbar A. Incidence of infective endocarditis caused by viridans group streptococci in Sweden—effect of cessation of antibiotic prophylaxis in dentistry for risk individuals. J Oral Microbiol. 2020;12:1768342. DOI: 10.1080/20002297.2020.1768342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Vlasselaer A, Rasmussen M, Nilsson J, Olaison L, Ragnarsson S. Native aortic versus mitral valve infective endocarditis: a nationwide registry study. Open Heart. 2019;6:e000926. DOI: 10.1136/openhrt-2018-000926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bläckberg A, Nilson B, Özenci V, Olaison L, Rasmussen M. Infective endocarditis due to Streptococcus dysgalactiae: clinical presentation and microbiological features. Eur J Clin Microbiol Infect Dis. 2018;37:2261–2272. DOI: 10.1007/s10096-018-3367-7 [DOI] [PubMed] [Google Scholar]
- 27. Lindell F, Söderquist B, Sundman K, Olaison L, Källman J. Prosthetic valve endocarditis caused by Propionibacterium species: a national registry‐based study of 51 Swedish cases. Eur J Clin Microbiol Infect Dis. 2018;37:765–771. DOI: 10.1007/s10096-017-3172-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nilson B, Olaison L, Rasmussen M. Clinical presentation of infective endocarditis caused by different groups of non‐beta haemolytic streptococci. Eur J Clin Microbiol Infect Dis. 2016;35:215–218. DOI: 10.1007/s10096-015-2532-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


