Abstract
Recurrent pericarditis (RP) is a complex inflammatory disorder associated with adverse outcomes and poor quality of life. After the first episode of acute pericarditis, a non‐negligible group of patients will fail to achieve complete remission despite treatment and will be challenged by side effects from the chronic use of medications like corticosteroids. The cause of RP remains unknown in the majority of cases, mainly due to a gap in knowledge of its complex pathophysiology. Over the past 2 decades, the interleukin‐1 (IL‐1) pathway has been uncovered as a key element in the inflammatory cascade, allowing the development of pharmacological targets known as IL‐1 inhibitors. This group of medications has emerged as a treatment option for patients with RP colchicine‐resistance and steroid dependents. Currently, anakinra and rilonacept, have demonstrated beneficial impact in clinical outcomes with a reasonable safety profile in randomized clinical trials. There is still paucity of data regarding the use of canakinumab in the treatment of patients with RP. Although further studies are needed to refine therapeutic protocols and taper of concomitant therapies, IL‐1 inhibitors, continue to consolidate as part of the pharmacological armamentarium to manage this complex condition with potential use as monotherapy. The aim of this review is to highlight the role of IL‐1 pathway in RP and discuss the efficacy, safety, and clinical applicability of IL‐1 inhibitors in the treatment of RP based on current evidence.
Keywords: interleukin‐1 inhibitors, pericarditis, pericardial disease, recurrent pericarditis
Subject Categories: Pericardial Disease
Nonstandard Abbreviations and Acronyms
- AOSD
Adult Onset Still Disease
- CAPS
cryopyrin‐associated periodic syndrome
- DAMPs
damage‐associated molecular patterns
- FDA
Food and Drug Administration
- FMF
Familial Mediterranean Fever
- NRS
numeric rating scale
- PAMPs
pathogen‐associated molecular patterns
- RP
recurrent pericarditis
- TRAPS
tumor necrosis factor alpha‐associated periodic syndrome
Recurrent pericarditis (RP) is associated with significant morbidity and adversely impacts quality of life. 1 Disease burden negatively affects both patients and society with severe symptoms reported in 60% of cases leading to work impairment in half of the patients. 2 RP is defined by flare of symptoms 4 to 6 weeks following the index episode of pericarditis. 3 The annual incidence of pericarditis ranges from 27.7 to 168 cases per 100 000 population/year, with 15% to 30% of these cases reporting recurrent symptoms within 18 months. 4 , 5 , 6 RP is considered a rare condition with an annual incidence in the United States of around 20 000 cases and a prevalence of approximately 37 000 cases. Half of these patients are expected to develop a complication or require a procedure within 2 years of diagnosis. 7 After the first recurrence, half of these patients will continue to have persistent symptoms despite appropriate therapy. 8 The following criteria are established to make the diagnosis of RP: (1) proven first episode of acute pericarditis; (2) recurrence of pericarditis type pain; and (3) association with at least one of the following findings: pericardial friction rub, ECG changes, new or increased pericardial effusion, elevated CRP (C‐reactive protein) , evidence of pericardial inflammation established by an imaging modality (magnetic resonance imaging or computed tomography scan). 3 , 9 , 10
The cause of RP in adults remains unknown in 70% to 90% of cases and is reported as “idiopathic.” The lack of serial investigation during recurrent attacks and a gap in knowledge of its pathophysiology account for this inflated number. 11 , 12 Emerging evidence has demonstrated that auto‐inflammatory pathways, such as the interleukin‐1 (IL‐1), are critical in the disease process. 12 , 13 , 14 Treatment adherence is also a major determinant and premature cessation of therapies from rapid tapering or intolerable side effects are contributors for the perpetuation of inflammation. 7 , 11 , 13 The standard of care in RP includes non‐steroidal NSAIDs, colchicine, and corticosteroids. Due to chronic use‐related side effects from corticosteroids, alternative regimens with azathioprine and human intravenous immunoglobulin have been implemented with variable success, though the level of evidence is limited. 15 , 16
Enhanced understanding of the pathophysiology of this disease and the need for better tolerated therapies, have increased the recognition of the IL‐1 pathway as a promising target therapy. 14 The aim of this review is to highlight the role of IL‐1 pathway in RP and discuss the efficacy, safety, and clinical applicability of the IL‐1 inhibitors in the treatment of RP based on current evidence.
We present a narrative review which focuses on the role of IL‐1 inhibitors in RP. The literature reported in this study corresponds primarily to randomized controlled trials, however, due to scarcity of data, nonrandomized studies, case series and case reports are also discussed.
Pathophysiology of Recurrent Pericarditis and the IL‐1 Pathway
Two main hypotheses have been associated with the pathophysiology of the disease, the auto‐immune and auto‐inflammatory processes. Based on the predominance of one over the other, patients can manifest a non‐inflammatory phenotype (for example, in the setting of underlying autoimmune disease) or an auto‐inflammatory phenotype. 17
Adaptive Immunity or Autoimmune Hypothesis
Adaptive immunity was previously considered the key pathway in the development of RP through several inappropriate response mechanisms as follows: 18
Reactivation of dormant viral particles residing in the pericardium secondary to incomplete viral clearance or steroid‐induced viral replication. 19
Transformation of self‐antigen into foreign antigens promoted by inflammatory tissue as a consequence of cell injury, apoptosis, and oxidative stress. 20
Molecular mimicry between self and foreign antigens that triggers recurrent inflammatory attacks. 21
Production of auto‐antibodies such as anti‐heart, anti‐nuclear, and anti‐intercalated discs found in elevated concentrations in pericardial fluid. 22 , 23
The presence of auto‐antibodies, found in 50% of adults, in addition to clinical findings such as dry eyes, arthralgias, a subacute course and the association with underlying autoimmune disorders are hints towards an autoimmune phenotype of RP. 12 The most common autoimmune conditions associated with RP are systemic lupus erythematosus, systemic sclerosis or Sjogren syndrome and rheumatoid arthritis. 7
Innate Immunity or Autoinflammatory Hypothesis
Similar signal pathways identified in idiopathic RP and auto‐inflammatory conditions such as Tumor necrosis factor Alpha‐associated Periodic Syndrome (TRAPS) and Familial Mediterranean Fever (FMF) have highlighted the role of innate immunity. 24 In these conditions, unprovoked inflammatory response occurred in the absence of T‐cell specific antigens or high titers of autoimmune antibodies. The cytosolic complex of the “inflammasome”, a fundamental component of inflammatory pathways, recognizes pathogens and molecular damage to generate an inflammatory response. 18
Infectious and non‐infectious stimuli acting on the pericardial cells lead to the release of IL‐1α deposits, which alongside with other cytokines/pathogens bind to specific membrane and intracellular receptors in monocytes, further activating the Pathogen‐Associated Molecular Patterns (PAMPs) or Damage‐Associated Molecular Patterns (DAMPs), respectively. 14 , 25 This step is followed by assembly and activation of the 3 components of the Inflammasomes: the adaptor protein ASC, procaspase‐1 enzyme, and a sensor molecule called nucleotide‐binding oligomerization domain–like receptor (NLR). NLR pyrin domain‐containing 3 (NLRP3), the best characterized sensor molecule, stimulates the NF‐KB transcription complex leading to the release of pro IL‐1. Procaspase‐1 of NLRP3 converts the IL‐1ß precursor into its active form. 18 , 26 This proinflammatory cytokine has 2 main isoforms, the previously mentioned IL‐1α that is produced and stored in healthy cells and excreted when damaged, and IL‐1β that is produced by inflammatory leukocytes. IL‐1 β binds to receptors and activates endothelial adhesion molecules facilitating pericardial infiltration with monocytes, neutrophils, and macrophages promoting recurrent pericardial attacks. This inflammatory milieu enhances pericardial inflammation via further activation of NLRP3 amplifying IL‐1 production, self‐perpetuating the inflammatory cycle. Finally, IL‐1β also activates cyclooxygenases, the main target of corticosteroids and non‐steroidal anti‐inflammatory drugs. 18 , 27 , 28 , 29 (Figure 1).
Figure 1. Inflammasome and activation of Interleukin‐1β.
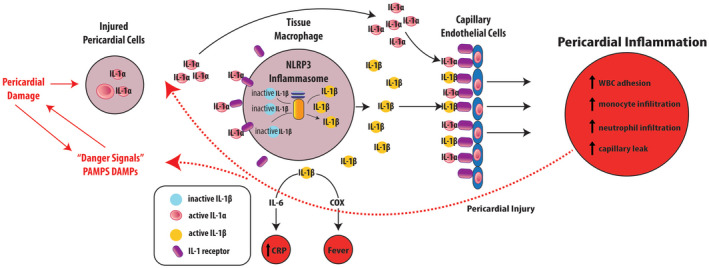
Infectious and sterile noxious stimuli injure the pericardial cells primarily through the activation of pathogen‐associated molecular patterns and damage‐associated molecular patterns, leading to the expression/release of IL‐1α deposits. This promotes along with other triggers, the transcription of the IL‐β precursor and the assembling of the inflammasome in the cytosol of innate immune response cells (ex., tissue macrophage). The inflammasome enzymatic component Procaspase‐1 cleaves the IL‐1ß precursor into its active form. Ultimately, both IL‐1α and IL‐1ß bind to IL‐1 receptors in capillary endothelial cells promoting further pericardial inflammation by various mechanism enhancing pericardial damage which perpetuate the inflammatory cascade of events. COX indicates cyclooxygenase; CRP, C‐reactive protein; DAMPs, damage‐associated molecular patterns; IL, interleukin; NLRP3, nucleotide‐binding oligomerization domain–like receptor pyrin domain‐containing 3; PAMPs, pathogen‐associated molecular patterns; and WBC, white blood cells. Reprinted from Klein et al, 25 with permission. ©2020, Elsevier.
IL‐1 Inhibitors
Anakinra
Anakinra is a recombinant version of the naturally occurring IL‐1 receptor antagonist. It exerts its mechanism of action binding selectively to IL‐1 receptors, blocking the activity of circulating IL‐1α and IL‐1β (Table). It was first approved in 2001 for rheumatoid arthritis and has been studied in different severe inflammatory diseases. 30
Table 1.
Comparison of IL‐1 Antagonists Used in Recurrent Pericarditis in Adults
| Anakinra | Rilonacept | Canakinumab | |
|---|---|---|---|
| Ultrastructure | Recombinant protein | Fc fusion protein | IgG monoclonal antibody |
| IL‐1 α receptor antagonist | + | + | − |
| IL‐1 β receptor antagonist | + | + | + (Binds plasma IL‐ β and prevents interaction with receptor) |
| Half‐life | 4 to 6 h | 7 d | 22 to 26 d |
| Frequency of administration | Daily | Weekly | Every 4 to 8 wks |
| Administration route | Subcutaneous intravenous | Subcutaneous | Subcutaneous |
| Loading dose | − | 320 mg | − |
| Maintenance dose | 2 mg/kg (up to 100 mg) | 160 mg | 4 mg/kg or 150 mg (single dose) |
| Duration of treatment | 6 to 12 months (can extend in patients with recurrences) | 9 months with range of 3 to 14 months (can extend in patients with recurrence) | Up to 2 y |
| Dose adjustment for renal impairment |
CrCl ≥30 mL/min: not required CrCl <30 mL/min or ESRD: consider changing dose to every other day. Not dialyzable (<2.5%) |
Not required |
Not required |
| Dose adjustment for hepatic impairment | Not required | Not required | Not required |
| Adverse events | Injection site reactions, hepatitis, infections | Injection site reactions, infections, neutropenia, hyperlipemia | Injection site reactions, infections, neutropenia |
| Monitoring | CBC, CRP, symptoms/signs of infection, hepatitis B and TB screening at baseline | CBC, CRP, lipid profile, symptoms/signs of infection, hepatitis B and TB screening at baseline | CBC, CRP, symptoms/signs of infections, hepatitis B and TB screening at baseline |
| Main clinical evidence in pericarditis |
|
RHAPSODY Phase III clinical trial and Phase II study. | Case reports & Case Serie |
CBC indicates complete blood count; CrCl, creatinine clearance; CRP, C‐reactive protein; Fc, fragment crystallizable region; IL, interleukin; and TB, tuberculosis.
The cardiovascular efficacy of anakinra was studied in the AIRTRIP (Anakinra Treatment of Recurrent Idiopathic Pericarditis) trial, a randomized‐withdrawal, double‐blinded, placebo‐controlled trial of 21 patients from Italy, with colchicine‐resistant and corticosteroids‐dependent idiopathic RP (at least 3 episodes) and systemic inflammation (elevated CRP>1 mg/dL). The study was conducted in 2 phases. The first, an open‐label phase in which all patients received 2 mg/kg/day of anakinra subcutaneous injection (up to 100 mg) for 2 months. This was followed by the double‐blind withdrawal second phase where patients who responded to anakinra (at least 30% reduction in pericardial pain scale, at least 10% decrease in CRP concentration, and no increase in pericardial effusion on echocardiogram), were randomized to continue treatment or placebo for 6 months. Median follow‐up was 14 months. At the end of the study, the anakinra group had a significantly lower incidence of RP (18.2%, 0.11% per year; 95% CI, 0.03%–0.45%) compared to placebo (90%, 2.06% per year; 95% CI 1.07%–3.97%) (P=0.001) (Figure 2). Time‐to‐flare could not be calculated in patients receiving anakinra, however, in the placebo group the first flare occurred at 72 days after randomization. From 72 days until the end of the study, the percentage of patients free of flare in the anakinra group was significantly higher than placebo (>50% versus 50%, respectively, P<0.001). In patients with recurrences, the time‐to‐flare was significantly longer with anakinra (76.5 versus 28.4 days, respectively, P<0.001). More than half of patients continued colchicine during the second phase (57%). When results were adjusted by colchicine treatment, there were no significant differences in recurrence rate and time‐to‐flare between those treated with colchicine plus anakinra versus anakinra alone. However, the study was not powered to detect differences in monotherapy. In patients with recurrent episodes, the mean time‐to‐flare was significantly shorter in the non‐colchicine group compared to the colchicine group (10 versus 52.7 days, respectively). Corticosteroids treatment was withdrawn from all patients within 6 weeks. 31
Figure 2. Kaplan–Meier analysis of patients with recurrent pericarditis free of relapse in the double‐blind withdrawal phase, from day 0 to day 180 after randomization (intention‐to‐treat analysis).
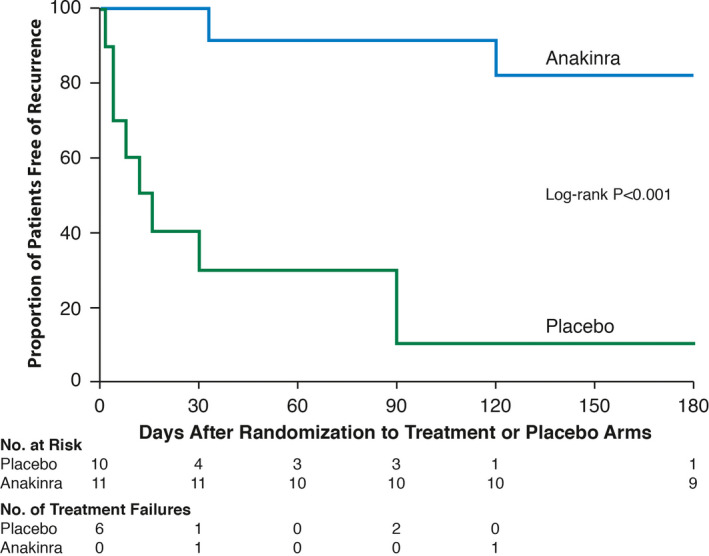
Recurrent pericarditis relapse in the double‐blind withdrawal phase, from day 0 to day 180 after randomization to either anakinra (blue) or placebo (green).
Regarding its safety profile, 95% of patients treated with anakinra developed local reactions in the injection site. In addition, 1 patient developed herpes zoster and 3 patients had elevated transaminases, however, there was no permanent discontinuation of the active drug. One patient developed ischemic optic neuropathy unrelated to the treatment. 31 Its efficacy and safety have been also confirmed in an international registry. The registry included 224 patients with corticosteroids‐dependence and colchicine‐resistant refractory RP (46 ± 14 years old, 63% women, 75% idiopathic, 91% with elevation of CRP, and 88% with pericardial effusion). Refractory pericarditis was defined as the first episode of RP followed by at least 2 recurrences on conventional therapy including NSAIDs, colchicine, and/or corticosteroids. Conventional treatment was maintained, discontinued, or tapered based on clinical evaluation. Patients with hypersensitivity to active substance of anakinra or Escherichia coli‐derived proteins, neutropenia (absolute neutrophil count less than 1.5 × 109/L), active TB and malignancy, were all excluded. Anakinra 100 mg daily subcutaneous injection reduced pericarditis recurrences by 6‐fold (2.33 to 0.39 per patient per year), emergency department admissions by 11‐fold (1.08 to 0.10 per patient per year), and hospitalizations by 7‐fold (0.99 to 0.13 per patient per year) after a median treatment of 6 months. Corticosteroids use was significantly decreased by anakinra (respectively from 80% to 27%; P<0.001). No serious adverse events occurred; adverse events consisted mostly of transient skin reactions (38%) at the injection site. Adverse events led to drug discontinuation in 3% of the patients. A full‐dose treatment duration of more than 3 months followed by a tapering period of more than 3 months were the therapeutic schemes associated with a lower risk of recurrence. 32
These data suggests that anakinra is a promising effective biologic agent in RP. Its short half‐life could be beneficial in clinical scenarios where rapid discontinuation of the medication is needed to allow immunological reconstitution avoiding fatal outcomes in severe infections. Anakinra allows successful withdrawal from steroids reducing its deleterious long‐term side effects. 44 Some of the limitations to its use include high‐cost, long duration of therapy before withdrawal of conventional treatment, and limited evidence on withdrawal protocols. Expert consensus opinion is to gradually taper anakinra based on clinical evidence of remission. Important aspects of management with anakinra are screening for hepatitis B virus and latent tuberculosis before administration, and use is contraindicated in active infection or pre‐existing malignancy. 33
Canakinumab
Canakinumab is IgG1 human monoclonal antibody targeting IL‐1β. It is the IL‐1 inhibitor with the longest half‐life, around 22 to 26 days. Due to its pharmacokinetics, it is administered in adults as a single fixed dose of 150 mg every 4 weeks. Canakinumab is approved for the treatment of various auto‐inflammatory conditions (colchicine resistant FMF, TRAPS, cryopyrin‐associated periodic syndrome [CAPS] amongst others) but has not been used commonly in pericarditis due to its high cost and scarce data mainly limited to case series with mixed results. 34 , 35 , 36 Canakinumab was used in a case series of 3 adult patients with colchicine‐resistant, corticosteroids‐dependent RP, and previous failed response to biologic agents including anakinra. Two patients were diagnosed with adult‐onset Still’s disease (AOSD) and one with seronegative rheumatoid arthritis, all sharing in common evidence of systemic inflammation. In patients with AOSD, the use of canakinumab as monotherapy achieved rapid and long‐term remission (up to 3.5 years) with successful tapering of corticosteroids. In the patient with seronegative rheumatoid arthritis, anakinra was effective by tapering corticosteroids to 5 mg/day. However, anakinra was discontinued due to injection site reaction. After failed trials of other biologics and multiple RP relapses, canakinumab was used and achieved only partial response and was later discontinued due to high cost. 37 AOSD is an autoinflammatory disease characterized by elevation of IL‐1β levels. 38 Similarly, there is evidence that IL‐1β is implicated in the pathogenesis of both seronegative and seropositive RA. 39 Therefore, this explains the response seen in these patients. In a case report of a 6.5‐year‐old boy with colchicine‐resistant and corticosteroids‐dependent idiopathic RP with evidence of systemic inflammation, canakinumab was used as alternative to anakinra after an episode of anaphylactic reaction. The combination of colchicine with canakinumab was successful in achieving remission for 2 years. 36
This suggests that canakinumab can be considered in patients unresponsive to conventional treatment, however, more studies are required to examine its efficacy and safety in adults and children. Of note, canakinumab only targets IL‐1β while anakinra and rilonacept targets both IL‐1α and IL‐1ß which could potentially have clinical implications. Additionally, identification of inflammatory phenotypes is an important aspect of management of RP to target the predominant pathogenic pathway and tailor of therapies for maximal efficacy.
Rilonacept
Rilonacept is a novel IL‐1 inhibitor; it consists of a dimeric fusion protein. It is composed of 3 main components: the ligand binding‐domain of the human IL‐1 receptor (IL‐1RI), IL‐1 receptor accessory protein (IL‐1RAcP), and the Fc portion of the human IgG1. 25 Rilonacept binds to the circulating IL‐1α and IL‐1β and with less affinity to the IL‐1 receptor antagonist acting as an “IL‐1 trap.” This interaction inhibits the engagement of IL‐1β to its receptor, blocking the downstream inflammatory cascade. 29 , 40 , 41 (Figure 3).
Figure 3. Rilonacept mechanism of action.
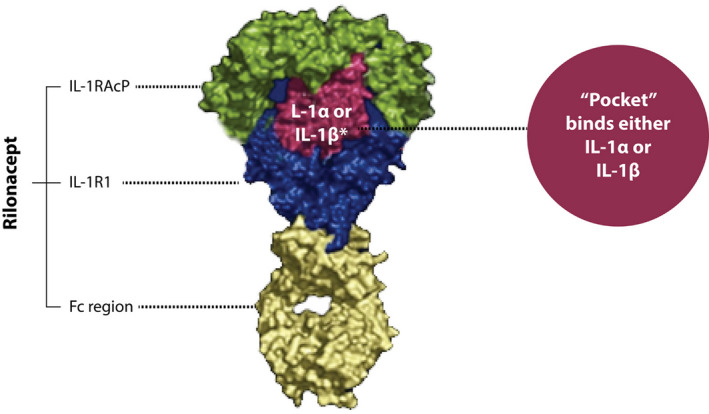
Rilonacept ultrastructure with 3 main components: the ligand binding‐domain of the human IL‐1 receptor, IL‐1 receptor accessory protein, and the Fc portion of the human IgG1. Rilonacept “pocket” binds to IL‐1α and IL‐1ß acting as an IL‐1 trap blocking the interaction with their specific receptor. Fc indicates fragment crystallizable region; IL‐1RAcP, IL‐1 receptor accessory protein; IL‐1RI, IL‐1 receptor; IL, interleukin. Reprinted from Klein et al 25 with permission. ©2020, Elsevier.
In 2008, rilonacept received Food and Drug Administration (FDA) approval for the treatment of the rare genetic condition CAPS. 41 In 2019, the phase II RHAPSODY trial was published. This pilot was an open‐label, single‐arm, 5‐part study that evaluated the efficacy and safety of rilonacept in patients with RP. Eligible candidates included adults (18–75 years) or children (≥6 to <18 years) with at least 2 recurrences, presenting with either active disease (symptoms, CRP>1 mg/dL or delayed Gadolinium enhancement on cardiac magnetic resonance imaging) or without active disease attributable to corticosteroids‐dependence. Based on the clinical presentation and cause, 5 different subgroups (“parts”) were defined. Active treatment consisted of loading dose of 320 mg rilonacept (KPL‐914), administered via subcutaneous injection on day 0, followed by 160 mg SC weekly for 5 more doses. An additional 18‐week treatment‐extension period was optional with the possibility to taper concomitant therapy.
Twenty‐five adults were enrolled from 9 sites in the United States between January 2018 and May 2019 and 23 entered the extended period (mean age 42.8 years, 60% female, majority White, 80% were taking at least 2 anti‐inflammatory medications). For symptomatic patients with elevated CRP>1 mg/dL secondary to post‐pericardiotomy syndrome or idiopathic cause (n=13), pain was reduced by an average of 4 points on the 11‐points pain numeric rating scale (NRS), and CRP normalized in all patients (median time=9 days). For the remaining 3 symptomatic patients with active inflammation on cardiac magnetic resonance imaging (part 2), there was a decrease in NRS score and CRP remained normal (Figure 4). A substantial reduction in recurrences rate was reported with rilonacept with a decreased annualized incidence from 3.9 (SD 3.66) episodes/year prior to study entry to 0.18 (SD 0.62) in patients with idiopathic RP and elevated CRP, and to 0.0 for the remaining study parts. Eleven patients (84.6%) out of 13 who were receiving corticosteroids at baseline were able to discontinue this medication and the remaining 2 reduced the dose without recurrence of symptoms. 42
Figure 4. NRS scores (pain) and CRP levels in symptomatic patients with elevated CRP.
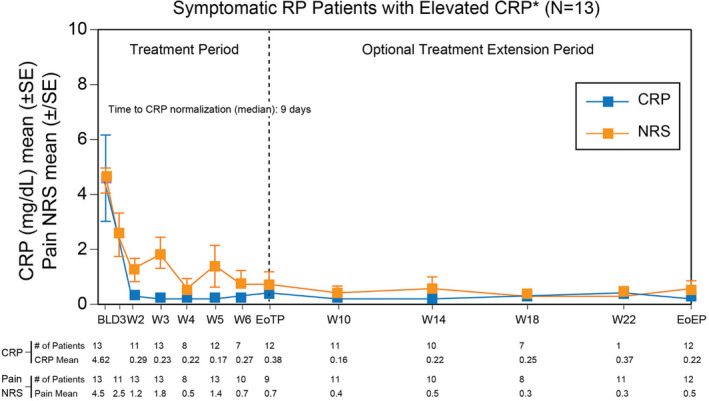
Numerical rating score for pain (NRS) (orange curve) and C‐reactive protein (CRP) levels (blue curve) in patients with active disease and elevated CRP during the treatment period and optional extension treatment period. Normalization of CRP levels was achieved at a median of 9 days. BL indicates baseline; CRP, c‐reactive protein; EoEP, end of extension period; EoTP, end of treatment period; NRS, numerical rating score for pain; SE, standard error; W, week. Adapted from Klein et al. 42
These results were followed by phase III RHAPSODY trial. This study was a double‐blinded, placebo‐controlled, multicenter randomized‐withdrawal trial in patients with symptomatic RP (at least 2 recurrences) and elevated CRP (≥1 mg/dL) colchicine refractory or corticosteroids dependents. Patients were treated in the “run‐in period” with rilonacept for 12 weeks (loading dose 320 mg or 4.4 mg/kg if <18 years of age; followed by weekly 160 or 2.2 mg/kg if <18 years of age). In addition, concomitant anti‐inflammatory therapy was weaned and rilonacept was continued as monotherapy in the last 2 weeks. At the end of this period, patients with clinical response (no symptoms and CRP≤0.5 mg/dL) underwent 1:1 randomization to weekly rilonacept versus placebo. After accrual of a prespecified number of recurrences, the trial was closed, and eligible patients were offered up to 24 months of open‐label rilonacept.
The primary efficacy end point was the development of RP and secondary efficacy end points included assessment of clinical response, time to normalization of the CRP level and the time by which the patients discontinued standard therapy and were receiving rilonacept monotherapy. A total of 86 patients were enrolled, 61 patients completed the run‐in period and underwent randomization before enrollment was stopped (mean age 44.7 years, 57% female, 85% of cases were idiopathic and 15% post–cardiac‐injury). The median duration of rilonacept treatment, including the run‐in period, was 9 months (3–14 months). The rilonacept group had significantly lower recurrences compared to placebo (7% versus 74%; hazard ratio, 0.04; 95% CI 0.01–0.18; P<0.001 by the log‐rank test [Figure 5A]). There was a rapid resolution of pericarditis pain (median of 5 days), normalization of CRP (median time of 7 days), and successful withdrawal of corticosteroids in the treatment group (Figure 5B). Interestingly, the median time from initiation of rilonacept to tapering and discontinuing standard therapy was 7.9 weeks in the run‐in period. There was no re‐introduction of corticosteroids or pericarditis recurrence in the run‐in period. 43 , 44
Figure 5. Rhapsody Phase III trial outcomes.
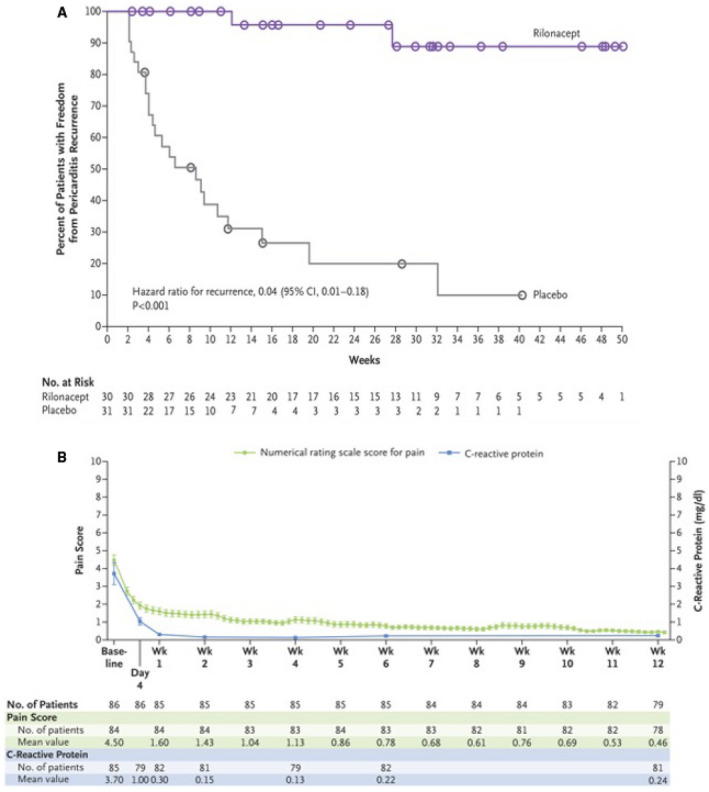
A, Time to the First Adjudicated Pericarditis Recurrence. Curves for the time to the first adjudicated pericarditis recurrence in the randomized‐withdrawal period are shown. Circles indicate the time of data censoring for reasons other than a primary efficacy end‐point event (e.g., a visit at the end of the randomized‐withdrawal period). Overall, 2 patients (7%) in the rilonacept group and 23 (74%) in the placebo group had pericarditis recurrence. The median time to recurrence could not be estimated in the rilonacept group and was 8.6 weeks (95% CI, 4.0 to 11.7) in the placebo group. CI indicates confidence interval; No, number. B, Mean Numerical Rating Scale Scores for Pain and C—reactive protein Levels over the 12‐Week Run‐In Period. Numerical rating scale scores for pain and C‐reactive protein (CRP) levels as assessed by a central laboratory were recorded during the run‐in period, during which all the patients received rilonacept. The mean pain numerical rating scale score and mean CRP level at the baseline visit differ from those recorded for the qualifying pericarditis episode; to allow for the completion of screening procedures, the investigator was permitted to treat each patient with standard‐of‐care medications temporarily during the interval between presentation with the qualifying episode and the baseline visit or trial enrollment. A 3‐day rolling mean was calculated on the basis of non‐missing values over each successive 3‐day interval. In accordance with the protocol, pain was assessed daily with the use of a numerical rating scale (with scores ranging from 0 to 10 and with higher scores indicating greater pain severity). CRP was measured at baseline, on day 4, and at weeks 1, 2, 4, 6, and 12. At week 12, a total of 81 patients had assessments of the CRP level, but 2 of these patients had discontinued treatment before week 12; therefore, only 79 patients were considered to still be participating in the run‐in period. I bars indicate the standard error. No indicates number; Wk, week. Reprinted from Klein et al. 43 with permission. ©2020, Massachusetts Medical Society.
The phase II study demonstrated the safety and tolerability of rilonacept with 92% of the adverse events reported as mild to moderate in severity. The most common side effects included transient injection site reactions (60%), nasopharyngitis (16%), arthralgia (12%), and diarrhea (12%). None of these events led to drug discontinuation and all were treated with conservative measures. No major laboratory abnormalities were reported, however, there was an increase in non‐fasting total cholesterol, low‐density lipoprotein cholesterol, and triglycerides but did not require therapy initiation. 42
The favorable safety profile and tolerability was corroborated in the phase III RHAPSODY study. The most common adverse reactions were injection site reactions (34%), and upper respiratory tract infections (23%). The majority of adverse events were transient, mild to moderate in severity. Four patients had adverse events that warranted drug discontinuation including alopecia, extrinsic allergic alveolitis, systemic allergic reaction and erythema, all reported during the run‐in period. 43 These results suggest rilonacept is an effective and safe therapy for RP. It may be used as a monotherapy and help discontinue standard corticosteroids‐dependent therapy. 43 Some of the limitations to its use include high‐cost, extended half‐life when used in patients at increased risk for infections, as well as the lack of data about long‐term remission and well‐characterized tapering protocol. The results from the planned RESONANCE (Registry of the Natural History of Recurrent Pericarditis in Pediatric and Adult Patients : ClinicalTrials.gov number NCT04687358) are highly anticipated to aid in answering some of these gaps in knowledge. 44
Clinical Application of IL‐1 Inhibitors
The efficacy of IL‐1 inhibitors, specifically, rilonacept and anakinra, has been demonstrated only in patients with RP with at least 2 recurrences secondary to non‐prohibited conditions for IL‐1 inhibitors use (including active infection, tuberculosis, active malignancy, immunocompromised). These patients have failed treatment with conventional therapies (including colchicine[“colchicine resistant”] and corticosteroids), and have evidence of systemic inflammation (fever, elevated CRP ≥1 mg/dL, and/or delayed gadolinium enhancement on cardiac magnetic resonance imaging) or experience recurrence while attempting to withdraw corticosteroids (corticosteroids‐dependent). 31 , 32 , 43 , 44 The European Society of Cardiology 2015 guidelines recommend anakinra as a third line therapy in patients intolerant or non‐responsive to corticosteroids. 3 Although, most recently rilonacept received approval from the (FDA) in March 2021 for its use in RP in adults and children >12 years, no formal American College of Cardiology/American Heart Association guidelines recommendations are available at the moment. 7 , 45 No data is available regarding the role of IL‐1 inhibitors in the treatment of patients with RP without systemic inflammation or after the first recurrence. In essence, additional studies are required to address these latter gaps, and long‐term follow‐up data are required to determine the recurrence rate beyond 8 months for anakinra and 14 months for rilonacept. Better understanding of the pathophysiology of RP, has permitted the identification of phenotypes, allowing the individualization of treatment based on elevated levels of IL1 and inflammatory markers like CRP. Future studies should emphasize the characterization of inflammatory profiles to identify responders to treatment. Figure 6 demonstrates a proposed flowchart for the incorporation of IL‐1 agents in the treatment algorithm for patients with RP.
Figure 6. Proposed flowchart for the introduction of IL‐1 inhibitors in the management of recurrent pericarditis.
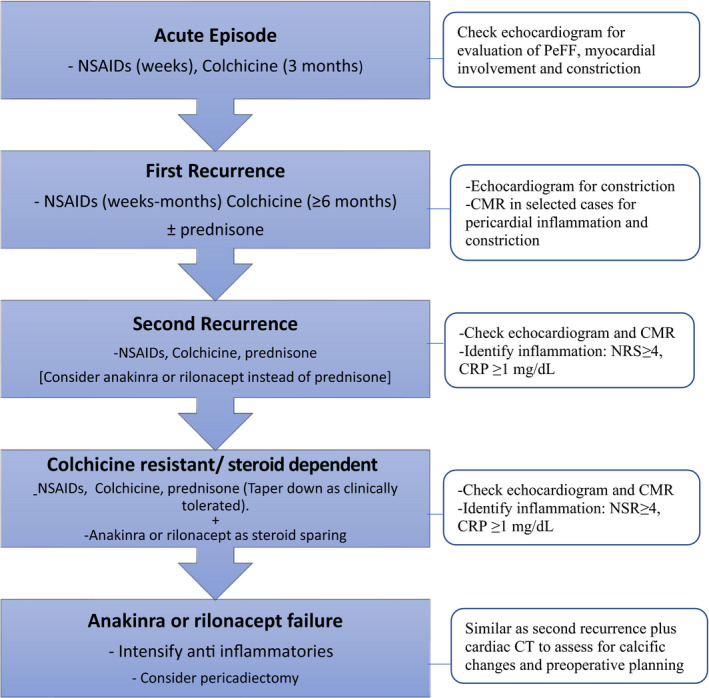
CMR indicates cardiac magnetic resonance; CRP, C‐reactive protein; CT, computed tomography; NRS, numerical rating scale; and PeFF, pericardial effusion.
IL‐1 Inhibitors in Special Populations
Pregnancy
There is limited data regarding the safety of anakinra during pregnancy and lactation. 35 In humans, case series of women with various types of auto‐inflammatory conditions utilizing anakinra during pregnancy were reported with favorable outcomes. 46 , 47 The current expert consensus is that anakinra can be used before and after pregnancy. 48 From the largest series of 23 pregnant women exposed to anakinra with 21 reported healthy infants, there were 2 miscarriages and 1 case of congenital malformation. Fourteen infants were breast fed without complications or developmental delay after a median follow up of 18 months. 47 It is also reassuring that the natural equivalent of anakinra IL‐1 receptor antagonist is present in human milk posing no risk to infants. 48
The safety data of rilonacept and canakinumab in pregnancy and lactation are even more scarce. The case series from Youngstein and colleagues reported the use of canakinumab in 8 pregnant women resulting in 7 live births. Four infants were breast fed with no reported serious infections or developmental abnormalities at a mean follow‐up of 2.2 years. 47
Regarding rilonacept, only animal studies have shown a possible harmful effect on embryo‐fetal development, however, no human studies exist to explore this toxic effect. Therefore, rilonacept is classified as category C and should be used when benefits to the patient outweighs risk of fetal toxicity. Otherwise, it should be discontinued before pregnancy. It is also unknown whether rilonacept is secreted during lactation. 45
Children
Albeit there is limited data about the use of anakinra in pediatric patients, available results in children favor its safety and efficacy. In a multicenter retrospective study of 12 children (age at commencement of anakinra 6–16 years) with colchicine‐resistant and corticosteroids‐dependent RP, anakinra 1 to 2 mg/kg/day reduced 95% of flares compared to pre‐treatment and allowed rapid withdrawal of concomitant treatment including corticosteroids. 49 Based on data in adults and limited evidence in children, it is reasonable to use anakinra in pediatrics and perhaps at early stages for long‐term control of recurrences. Future randomized control trials in this group of patients will help in building on the available evidence on RP management in this population. 35
Although no pediatric patients were enrolled in RHAPSODY PHASE II, a total of 7 patients between 12 and 17 years of age received rilonacept in the run‐in period of the RHAPSODY phase III trial. Subsequently, 1 patient was randomized to rilonacept and 2 to placebo during the randomized‐withdrawal period without safety concerns based on this variable. 42 , 43 FDA approval was granted in RP including children >12 years, in concordance with its previous authorization for use in children of this age with auto‐inflammatory syndromes. 43 , 45
COVID‐19
Although these medications could hypothetically mitigate the immune response elicited by the mRNA COVID‐19 vaccines, patients with pericarditis treated with immunosuppressive therapy were not included in the vaccines trials and this remains only speculative. 50 , 51 Furthermore, COVID‐19 vaccines can be administered in patients on treatment with these drugs. Anti‐IL1 agents appear to be well tolerated in the context of COVID‐19 infection and it seems to be safe to continue treatment if necessary, for symptoms control. 52 At the moment, no data is available regarding the use of this group of medications concomitantly with other viral vectors or live attenuated virus based COVID‐19 vaccines.
Drug Interactions
There are no studies that investigated drug interactions with IL‐1 inhibitors. Elevated IL‐1 levels in RP may inhibit the production of CYP450 enzymes. Therefore, it is hypothesized that IL‐1 inhibitors may restore the production of CYP450 enzymes requiring further evaluation of drug concentrations and dose adjustments of medications metabolized by this enzyme. The concomitant use of IL‐1 inhibitors with a TNF‐alpha blocking agent is not recommended due to the presumed potentiation of immunosuppression and risk for serious infections. 45
Conclusions
Improved understanding of the pathophysiology of RP has shifted the paradigm for the management of this debilitating disease. Identification of targets along the IL‐1 pathway has permitted the therapeutic use of 3 IL‐1 inhibitors. Clinical data have highlighted the efficacy of mainly 2 drugs, rilonacept and anakinra, while there is still paucity of data for canakinumab. These drugs demonstrated substantial effect ameliorating clinical symptoms, decreasing inflammatory markers and the incidence of recurrences with a favorable safety profile. Differences in the pharmacokinetics of these medications, allows physicians to individualize treatment based on the patients immunosuppresive risk, preventing infection‐related complications. Further studies are needed to expand the knowledge of interactions with other medications, safety and efficacy in special populations such as in pregnancy, and to delineate the appropriate length of therapy and taper protocols.
Disclosures
Dr Massimo Imazio discloses that he serves as scientific advisory board for Kiniksa and SOBI. Dr Allan Klein discloses that he received a research grant from Kiniksa and serves as scientific advisory board for Kiniksa, Sobi, and Pfizer. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Cacoub P, Marques C. Acute recurrent pericarditis: from pathophysiology towards new treatment strategy. Heart. 2020;106:1046–1051. doi: 10.1136/heartjnl-2019-316481. [DOI] [PubMed] [Google Scholar]
- 2. LeWinter M, Kontzias A, Lin D, Cella D, DerSarkissian M, Zhou M, Duh MS, Lim‐Watson M, Magestro M. Burden of recurrent pericarditis on health‐related quality of life. Am J Cardiol. 2021;141:113–119. doi: 10.1016/j.amjcard.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 3. Adler Y, Charron P, Imazio M, Badano L, Barón‐Esquivias G, Bogaert J, Brucato A, Gueret P, Klingel K, Lionis C, et al. ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2015;2015:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Imazio M, Cecchi E, Demichelis B, Chinaglia A, Ierna S, Demarie D, Ghisio A, Pomari F, Belli R, Trinchero R. Myopericarditis versus viral or idiopathic acute pericarditis. Heart. 2008;94:498–501. doi: 10.1136/hrt.2006.104067. [DOI] [PubMed] [Google Scholar]
- 5. Sogaard KK, Farkas DK, Ehrenstein V, Bhaskaran K, Botker HE, Sorensen HT. Pericarditis as a marker of occult cancer and a prognostic factor for cancer mortality. Circulation. 2017;136:996–1006. doi: 10.1161/CIRCULATIONAHA.116.024041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Imazio M, Brucato A, Cemin R, Ferrua S, Maggiolini S, Beqaraj F, Demarie D, Forno D, Ferro S, Maestroni S, et al. A randomized trial of colchicine for acute pericarditis. N Engl J Med. 2013;369:1522–1528. doi: 10.1056/NEJMoa1208536. [DOI] [PubMed] [Google Scholar]
- 7. Klein A, Cremer P, Kontzias A, Furqan M, Tubman R, Roy M, Lim‐Watson M, Magestro M. US Database Study of Clinical Burden and Unmet Need in Recurrent Pericarditis. J Am Heart Assoc. 2021. Aug 3;10(15):e018950. doi: 10.1161/JAHA.120.018950. Epub 2021 Jul 21. PMID: 34284595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imazio M, Brucato A, Cemin R, Brucato A, Ferrua S, Belli R, Maestroni S, Trinchero R, Spodick DH, Adler Y. Colchicine for recurrent pericarditis (CORP): a randomized trial. Ann Intern Med. 2011;155:409–410. doi: 10.7326/0003-4819-155-7-201110040-00359 [DOI] [PubMed] [Google Scholar]
- 9. Imazio M, Belli R, Brucato A, Cemin R, Ferrua S, Beqaraj F, Demarie D, Ferro S, Forno D, Maestroni S, et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP‐2): a multicentre, double‐blind, placebo‐controlled, randomised trial. Lancet. 2014;383:2232–2237. doi: 10.1016/S0140-6736(13)62709-9. [DOI] [PubMed] [Google Scholar]
- 10. Lazaros G, Vasileiou P, Koutsianas C, Antonatou K, Stefanadis C, Pectasides D, Vassilopoulos D. Anakinra for the management of resistant idiopathic recurrent pericarditis. Initial experience in 10 adult cases. Ann Rheum Dis. 2014;73:2215–2217. doi: 10.1136/annrheumdis-2014-205990 [DOI] [PubMed] [Google Scholar]
- 11. Dauphin C, Merlin E, Chalard A, Trésorier R, Lusson J‐R, Soubrier M. Recurrent pericarditis: current challenges and future prospects. Res Rep Clin Cardiol. 2016;7:99–108. [Google Scholar]
- 12. Brucato A, Valenti A, Maisch B. Acute and recurrent pericarditis: still idiopathic? J Am Coll Cardiol. 2017;69:2775. doi: 10.1016/j.jacc.2017.02.072 [DOI] [PubMed] [Google Scholar]
- 13. Imazio M, Brucato A, Derosa FG, Lestuzzi C, Bombana E, Scipione F, Leuzzi S, Cecchi E, Trinchero R, Adler Y. Aetiological diagnosis in acute and recurrent pericarditis: when and how. J Cardiovasc Med (Hagerstown). 2009;10:217–230. doi: 10.2459/JCM.0b013e328322f9b1. [DOI] [PubMed] [Google Scholar]
- 14. Brucato A, Emmi G, Cantarini L, Di Lenarda A, Gattorno M, Lopalco G, Marcolongo R, Imazio M, Martini A, Prisco D. Management of idiopathic recurrent pericarditis in adults and in children: a role for il‐1 receptor antagonism. Intern Emerg Med. 2018;13:475–489. doi: 10.1007/s11739-018-1842-x. [DOI] [PubMed] [Google Scholar]
- 15. Vianello F, Cinetto F, Cavraro M, Battisti A, Castelli M, Imbergamo S, Marcolongo R. Azathioprine in isolated recurrent pericarditis: a single centre experience. Int J Cardiol. 2011;147:477–478. doi: 10.1016/j.ijcard.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 16. Imazio M, Lazaros G, Picardi E, Vasileiou P, Carraro M, Tousoulis D, Belli R, Gaita F. Intravenous human immunoglobulins for refractory recurrent pericarditis: a systematic review of all published cases. J Cardiovasc Med (Hagerstown). 2016;17:263–269. doi: 10.2459/JCM.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 17. Maestroni S, Di Corato PR, Cumetti D, Chiara DBLC, Ghidoni S, Prisacaru L, Cantarini L, Imazio M, Penco S, Pedrotti P, et al. Recurrent pericarditis: autoimmune or autoinflammatory? Autoimmun Rev. 2012;12:60–65. doi: 10.1016/j.autrev.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 18. Cremer PC, Kumar A, Kontzias A, Tan CD, Rodriguez ER, Imazio M, Klein AL. Complicated pericarditis: understanding risk factors and pathophysiology to inform imaging and treatment. J Am Coll Cardiol. 2016;68:2311–2328. doi: 10.1016/j.jacc.2016.07.785. [DOI] [PubMed] [Google Scholar]
- 19. Artom G, Koren‐Morag N, Spodick DH, Brucato A, Guindo J, Bayes‐de‐Luna A, Brambilla G, Finkelstein Y, Granel B, Bayes‐Genis A, et al. Pretreatment with corticosteroids attenuates the efficacy of colchicine in preventing recurrent pericarditis: a multi‐centre all‐case analysis. Eur Heart J. 2005;26:723–727. doi: 10.1093/eurheartj/ehi197. [DOI] [PubMed] [Google Scholar]
- 20. Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, Bassetto F, Doria A. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol. 2010;87:385–395. doi: 10.1189/jlb.0709517. [DOI] [PubMed] [Google Scholar]
- 21. Cusick MF, Libbey JE, Fujinami RS. Molecular mimicry as a mechanism of autoimmune disease. Clin Rev Allergy Immunol. 2012;42:102–111. doi: 10.1007/s12016-011-8294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Imazio M, Brucato A, Doria A, Brambilla G, Ghirardello A, Romito A, Natale G, Palmieri G, Trinchero R, Adler Y. Antinuclear antibodies in recurrent idiopathic pericarditis: prevalence and clinical significance. Int J Cardiol. 2009;136:289–293. doi: 10.1016/j.ijcard.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 23. Caforio ALP, Brucato A, Doria A, Brambilla G, Angelini A, Ghirardello A, Bottaro S, Tona F, Betterle C, Daliento L, et al. Anti‐heart and anti‐intercalated disk autoantibodies: evidence for autoimmunity in idiopathic recurrent acute pericarditis. Heart. 2010;96:779–784. doi: 10.1136/hrt.2009.187138. [DOI] [PubMed] [Google Scholar]
- 24. Park H, Bourla AB, Kastner DL, Colbert RA, Siegel RM. Lighting the fires within: the cell biology of autoinflammatory diseases. Nat Rev Immunol. 2012;12:570–580. doi: 10.1038/nri3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klein AL, Imazio M, Brucato A, Cremer P, LeWinter M, Abbate A, Lin D, Martini A, Beutler A, Chang S, et al. Rationale for and design of a pivotal phase 3 trial to assess efficacy and safety of rilonacept, an interleukin‐1alpha and interleukin‐1beta trap, in patients with recurrent pericarditis. Am Heart J. 2020;228:81–90. doi: 10.1016/j.ahj.2020.07.004 [DOI] [PubMed] [Google Scholar]
- 26. Petrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 27. March CJ, Mosley B, Larsen A, Cerretti DP, Braedt G, Price V, Gillis S, Henney CS, Kronheim SR, Grabstein K, et al. Cloning, sequence and expression of two distinct human interleukin‐1 complementary DNAs. Nature. 1985;315:641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- 28. Gery I, Gershon R, Waksman BH. Potentiation of the t‐lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972;136:128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dinarello CA, Simon A, Van der Meer JW. Treating inflammation by blocking interleukin‐1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Tassell BW, Varma A, Salloum FN, Das A, Seropian IM, Toldo S, Smithson L, Hoke NN, Chau VQ, Robati R, et al. Interleukin‐1 trap attenuates cardiac remodeling after experimental acute myocardial infarction in mice. J Cardiovasc Pharmacol. 2010;55:117–122. doi: 10.1097/FJC.0b013e3181c87e53. [DOI] [PubMed] [Google Scholar]
- 31. Brucato A, Imazio M, Gattorno M, Lazaros G, Maestroni S, Carraro M, Finetti M, Cumetti D, Carobbio A, Ruperto N, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the airtrip randomized clinical trial. JAMA. 2016;316:1906–1912. doi: 10.1001/jama.2016.15826. [DOI] [PubMed] [Google Scholar]
- 32. Imazio M, Andreis A, De Ferrari GM, Cremer PC, Mardigyan V, Maestroni S, Luis SA, Lopalco G, Emmi G, Lotan D, et al. Anakinra for corticosteroid‐dependent and colchicine‐resistant pericarditis: the IRAP (International Registry of Anakinra for Pericarditis) study. Eur J Prev Cardiol. 2020;27:956–964. doi: 10.1177/2047487319879534. [DOI] [PubMed] [Google Scholar]
- 33. Lazaros G, Imazio M, Brucato A, Vassilopoulos D, Vasileiou P, Gattorno M, Tousoulis D, Martini A. Anakinra: an emerging option for refractory idiopathic recurrent pericarditis: a systematic review of published evidence. J Cardiovasc Med (Hagerstown). 2016;17:256–262. doi: 10.2459/JCM.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 34. Cremer PC, Kumar A, Kontzias A, Tan CD, Rodriguez ER, Imazio M, Klein AL. Complicated pericarditis: understanding risk factors and pathophysiology to inform imaging and treatment. J Am Coll Cardiol. 2016;68:2311–2328. doi: 10.1016/j.jacc.2016.07.785. [DOI] [PubMed] [Google Scholar]
- 35. Tombetti E, Mule A, Tamanini S, Matteucci L, Negro E, Brucato A, Carnovale C. Novel pharmacotherapies for recurrent pericarditis: current options in 2020. Curr Cardiol Rep. 2020;22:59. doi: 10.1007/s11886-020-01308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Epcacan S, Sahin S, Kasapcopur O. Anaphylactic reaction to anakinra in a child with steroid‐dependent idiopathic recurrent pericarditis and successful management with canakinumab. Cardiol Young. 2019;29:549–551. doi: 10.1017/S1047951119000672. [DOI] [PubMed] [Google Scholar]
- 37. Kougkas N, Fanouriakis A, Papalopoulos I, Bertsias G, Avgoustidis N, Repa A, Sidiropoulos P. Canakinumab for recurrent rheumatic disease associated‐pericarditis: a case series with long‐term follow‐up. Rheumatology (Oxford). 2018;57:1494–1495. doi: 10.1093/rheumatology/key077. [DOI] [PubMed] [Google Scholar]
- 38. Kastner DL, Aksentijevich I, Goldbach‐Mansky R. Autoinflammatory disease reloaded: a clinical perspective. Cell. 2010;140:784–790. doi: 10.1016/j.cell.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist‐deficient mice. J Exp Med. 2000;191:313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bettiol A, Lopalco G, Emmi G, Cantarini L, Urban ML, Vitale A, Denora N, Lopalco A, Cutrignelli A, Lopedota A, et al. Unveiling the efficacy, safety, and tolerability of anti‐interleukin‐1 treatment in monogenic and multifactorial autoinflammatory diseases. Int J Mol Sci. 2019;20:1898. doi: 10.3390/ijms20081898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Van Tassell BW, Toldo S, Mezzaroma E, Abbate A. Targeting interleukin‐1 in heart disease. Circulation. 2013;128:1910–1923. doi: 10.1161/CIRCULATIONAHA.113.003199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klein AL, Lin D, Cremer PC, Nasir S, Luis SA, Abbate A, Ertel A, LeWinter M, Beutler A, Fang F, et al. Efficacy and safety of rilonacept for recurrent pericarditis: results from a phase II clinical trial. Heart. 2020;107:488–496. doi: 10.1136/heartjnl-2020-317928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Klein AL, Imazio M, Cremer P, Brucato A, Abbate A, Fang F, Insalaco A, LeWinter M, Lewis BS, Lin D, et al. Phase 3 trial of interleukin‐1 trap rilonacept in recurrent pericarditis. N Engl J Med. 2021;384:31–41. doi: 10.1056/NEJMoa2027892. [DOI] [PubMed] [Google Scholar]
- 44. Klein AL, Imazio M, Paolini JF. Phase 3 trial of interleukin‐1 trap rilonacept in recurrent pericarditis. N Engl J Med. 2021;384: 15:1474–1476. 10.1056/nejmc2101978. [DOI] [PubMed] [Google Scholar]
- 45. ARCALYST . Package insert. Kiniksa Pharmaceuticals (UK) L.
- 46. Ilgen U, Kucuksahin O. Anakinra use during pregnancy: report of a case with familial mediterranean fever and infertility. Eur J Rheumatol. 2017;4:66–67. doi: 10.5152/eurjrheum.2017.16075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Youngstein T, Hoffmann P, Gül A, Lane T, Williams R, Rowczenio DM, Ozdogan H, Ugurlu S, Ryan J, Harty L, et al. International multi‐centre study of pregnancy outcomes with interleukin‐1 inhibitors. Rheumatology (Oxford). 2017;56:2102–2108. doi: 10.1093/rheumatology/kex305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Götestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer‐Betz R, Elefant E, Chambers C, da Silva J, Nelson‐Piercy C, Cetin I, Costedoat‐Chalumeau N, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795–810. doi: 10.1136/annrheumdis-2015-208840. [DOI] [PubMed] [Google Scholar]
- 49. Finetti M, Insalaco A, Cantarini L, Meini A, Breda L, Alessio M, D'Alessandro M, Picco P, Martini A, Gattorno M. Long‐term efficacy of interleukin‐1 receptor antagonist (anakinra) in corticosteroid‐dependent and colchicine‐resistant recurrent pericarditis. J Pediatr. 2014;164:1425–1431.e1. doi: 10.1016/j.jpeds.2014.01.065. [DOI] [PubMed] [Google Scholar]
- 50. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162B2 mRNA Covid‐19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Imazio M, Brucato A, Lazaros G, Andreis A, Scarsi M, Klein A, De Ferrari GM, Adler Y. Anti‐inflammatory therapies for pericardial diseases in the COVID‐19 pandemic: safety and potentiality. J Cardiovasc Med (Hagerstown). 2020;21:625–629. doi: 10.2459/JCM.0000000000001059. [DOI] [PubMed] [Google Scholar]


