Abstract
Background
The mechanism by which bystander cardiopulmonary resuscitation (CPR) improves survival following out‐of‐hospital cardiac arrest is unclear. We hypothesized that ventricular fibrillation (VF) waveform measures, as surrogates of myocardial physiology, mediate the relationship between bystander CPR and survival.
Methods and Results
We performed a retrospective cohort study of adult, bystander‐witnessed patients with out‐of‐hospital cardiac arrest with an initial rhythm of VF who were treated by a metropolitan emergency medical services system from 2005 to 2018. Patient, resuscitation, and outcome variables were extracted from emergency medical services and hospital records. A total of 3 VF waveform measures (amplitude spectrum area, peak frequency, and median peak amplitude) were computed from a 3‐second ECG segment before the initial shock. Multivariable logistic regression estimated the association between bystander CPR and survival to hospital discharge adjusted for Utstein elements. Causal mediation analysis quantified the proportion of survival benefit that was mediated by each VF waveform measure. Of 1069 patients, survival to hospital discharge was significantly higher among the 814 patients who received bystander CPR than those who did not (0.52 versus 0.43, respectively; P<0.01). The multivariable‐adjusted odds ratio for bystander CPR and survival was 1.6 (95% CI, 1.2, 2.1), and each VF waveform measure attenuated this association. Depending on the specific waveform measure, the proportion of mediation varied: 53% for amplitude spectrum area, 31% for peak frequency, and 29% for median peak amplitude.
Conclusions
Bystander CPR correlated with more robust initial VF waveform measures, which in turn mediated up to one‐half of the survival benefit associated with bystander CPR. These results provide insight into the biological mechanism of bystander CPR in VF out‐of‐hospital cardiac arrest.
Keywords: bystander CPR, mediation analysis, resuscitation, ventricular fibrillation waveform
Subject Categories: Ventricular Fibrillation, Cardiopulmonary Arrest, Cardiopulmonary Resuscitation and Emergency Cardiac Care, Mechanisms
Nonstandard Abbreviations and Acronyms
- AMSA
amplitude spectrum area
- OHCA
out‐of‐hospital cardiac arrest
- TCPR
telephone‐assisted CPR
Clinical Perspective
What Is New?
Bystander cardiopulmonary resuscitation (CPR), including both unassisted CPR and telephone‐assisted CPR, correlated with more robust initial ventricular fibrillation waveform measures following witnessed, ventricular fibrillation out‐of‐hospital cardiac arrest.
Depending on the specific ventricular fibrillation waveform measure, roughly one‐quarter to one‐half of the survival benefit associated with bystander CPR was mediated by ventricular fibrillation waveform measures.
What Are the Clinical Implications?
Much of the survival benefit conferred by bystander CPR may be related to its effect on the myocardium.
These data may provide novel considerations into how waveform measures might be incorporated to help assess the effectiveness of CPR.
Out‐of‐hospital cardiac arrest (OHCA) is a leading cause of death worldwide. 1 Early cardiopulmonary resuscitation (CPR) initiated by laypeople before arrival of professional rescuers is associated with a greater likelihood of survival and functional recovery. 2 , 3 However, the physiologic mechanisms by which bystander CPR confers a survival advantage are not clear, and a better understanding of these mechanisms could provide insight toward improving CPR performance and effectiveness. Proposed mechanisms include enhanced cerebral blood flow, improved coronary perfusion and myocardial energy stores, reduction of right heart distension, ischemic postconditioning, and some measure of passive ventilation. 4 , 5 , 6 Alternatively, the bystander CPR–survival association may be related to residual confounding, as the provision of bystander CPR is related to prognostic factors such as witnessed status, arrest location, and the perceived viability of the patient. 7 , 8 , 9 , 10
Quantitative measures of the ventricular fibrillation (VF) ECG signal are mathematical functions that characterize ECG features such as frequency, organization, and amplitude. A variety of measures have been designed with the ultimate goal of developing more effective treatment strategies for patients with VF. These measures are associated with dynamic changes in myocardial energy stores (adenosine triphosphate concentrations) and coronary perfusion pressure during the course of resuscitation, and predict clinical outcomes such as responsiveness to shock and functional survival. 11 , 12 , 13 , 14 Quantitative measures of the VF waveform thus potentially reflect physiologic status and provide a means to evaluate how interventions, such as CPR and medications, affect the heart during resuscitation and possibly mediate survival. 15 , 16
To investigate mechanisms by which bystander CPR may improve outcome, we evaluated the association between bystander CPR, VF waveform measures, and survival in patients with a witnessed OHCA and an initial rhythm of VF. We hypothesized that the association between bystander CPR (provided with or without telecommunicator assistance) and outcome is mediated by VF waveform measures independent of Utstein OHCA characteristics.
METHODS
The Institutional Review Board for Human Subjects Research at the University of Washington and the Department of Public Health–Seattle and King County approved this study and determined that it was minimal risk and waived informed consent. The data that support the findings of this study, with the exception of patient ECG data, are available from the corresponding author upon reasonable request.
Study Design, Population, and Setting
We performed a retrospective cohort investigation of adults with nontraumatic, bystander‐witnessed OHCA who presented with an initial rhythm of VF and were treated by a single emergency medical services (EMS) system from 2005 to 2018. The cohort was restricted to bystander‐witnessed arrests because VF waveform measures degrade over time without CPR, and the inclusion of unwitnessed arrests might therefore confound the association between bystander CPR and the initial VF waveform measure value. 17 , 18 Patients were included if there was an available EMS electronic defibrillator recording with ECG and transthoracic impedance signals before the first shock, and this shock was preceded by a pause in CPR of at least 3 seconds. Patients were excluded if (1) a shock was delivered by public access or law enforcement defibrillator before EMS arrival or (2) there was electrical artifact from pacing or other sources visible in the ECG.
The EMS system of the study region serves a population of ≈1.5 million people residing in urban, suburban, and rural settings covering an area of ≈2000 square miles. The EMS system is a 2‐tiered system that is activated by calling 9‐1‐1. Emergency medical dispatchers dispatch care and, if CPR is not already in progress, instruct bystanders to perform chest compressions without rescue breathing. The first tier of the EMS system consists of emergency medical technicians trained in basic life support and equipped with automated external defibrillators. Emergency medical technicians respond to all emergency medical calls and usually arrive first on scene an average of 5 minutes after call receipt. The second tier consists of paramedics trained in advanced life support who arrive on average 8 minutes after call receipt. Paramedics respond to selected, more serious conditions such as OHCA. EMS rescuers are instructed to start or continue CPR, analyze the rhythm, and, if indicated, defibrillate as soon as possible after application of the defibrillator. Resuscitation care is based on the American Heart Association guidelines. 19
Data Collection and Definitions
The EMS system maintains an ongoing registry of all EMS‐treated OHCA organized according to the Utstein guidelines. 20 These registry data include demographic, arrest circumstance, resuscitation process, and clinical outcome data collected from the EMS report, emergency dispatch audio recording, electronic defibrillator recording, and hospital record. Because these data include potentially identifiable information, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author.
Bystander CPR was defined as the provision of chest compressions, with or without ventilations, by a person not responding as part of the organized 9‐1‐1 response EMS system. Bystander CPR was classified into 2 groups: (1) telephone‐assisted CPR (TCPR) if the telecommunicator provided specific instructions regarding compressions to the bystander or (2) unassisted CPR if telecommunicator instructions were not involved with the provision of CPR. EMS response interval was defined as the time of emergency call pickup to arrival time of the first EMS unit. Because the cohort was restricted to witnessed arrests, the EMS response interval was presumed to approximate the time from arrest to EMS arrival. Sustained return of spontaneous circulation (ROSC) was defined as a palpable pulse and measurable blood pressure for 20 consecutive minutes or until emergency department arrival. Neurologically intact survival was defined as survival to hospital discharge with a Cerebral Performance Category of 1 or 2. 20
ECG Data and Calculation of VF Waveform Measures
Investigators, blinded to bystander CPR status and clinical outcomes, used MATLAB (Mathworks, Natick, MA) software to review the ECG and transthoracic impedance signals from the defibrillator recording to extract an artifact‐free, 3‐second ECG segment during a chest compression pause before the initial shock. Defibrillator models included Forerunner 3, Heartstart MRx (Philips Healthcare, Bothell, WA) and Lifepak 12 and Lifepak 15 (Stryker Physio‐Control, Redmond, WA). ECG sampling rates ranged from 125 to 250 Hz across devices; signals at an original sampling rate <250 Hz were resampled to 250 Hz. Each segment was filtered to remove high‐frequency noise and baseline drift using a 1 to 30 Hz fourth‐order Butterworth bandpass filter with forward‐backward implementation.
We calculated 3 VF waveform measures from the 3‐second ECG segments since the degree of mediation potentially varied across measures: amplitude spectrum area (AMSA), peak frequency, and median peak amplitude (Figure 1). 21 , 22 , 23 AMSA was computed from the discrete Fourier transform of the Hanning‐windowed ECG as the sum of each frequency from 3 to 30 Hz multiplied by its magnitude. Peak frequency was defined as the frequency with the greatest power between 1 and 20 Hz on the discrete Fourier transform of the rectangular‐windowed ECG. The median peak amplitude of the ECG was defined as the absolute difference between the median amplitude of the highest 4 peaks and the median amplitude of the lowest 4 valleys.
Figure 1. Examples of 3‐second ECG segments with corresponding ventricular fibrillation waveform measures.
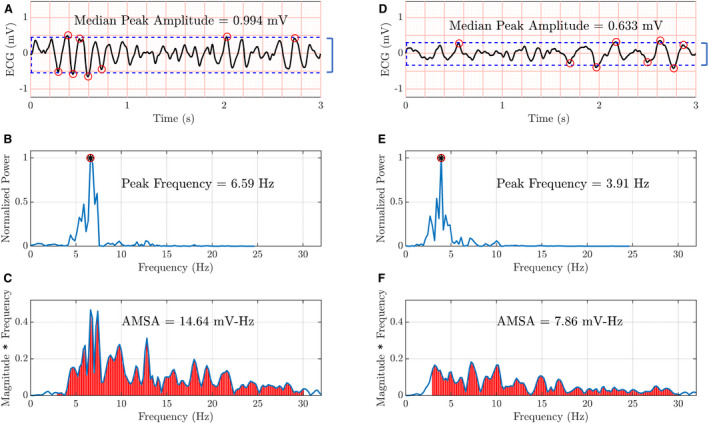
The subplots in the left column (A through C) show the median peak amplitude, peak frequency, and amplitude spectrum area (AMSA), respectively, for the ECG segment in subplot (A). The subplots in the right column (D through F) show the corresponding ventricular fibrillation waveform measures for the ECG segment in subplot (D). The ECG segment on the left has higher, that is, more favorable, ventricular fibrillation waveform measures. Please see text for details of ventricular fibrillation waveform measure calculation.
Statistical Analysis
We used descriptive statistics to summarize demographic, resuscitation process, and clinical outcome variables. The study cohort and excluded cohort were compared using the Wilcoxon rank‐sum test for continuous variables and the chi‐square test for categorical variables. Patients who did and did not receive bystander CPR were compared in a similar manner, as were the bystander CPR subgroups (TCPR and unassisted CPR).
We calculated unadjusted odds ratios for the association between each Utstein element and survival to hospital discharge and computed CIs using the Fisher's exact method for dichotomous variables (bystander CPR, sex, etiology, and location) and logistic regression for continuous variables (age and EMS response time). To investigate the mechanism of the bystander CPR–survival association, we then fit a multivariable logistic regression model with the aforementioned Utstein elements as predictor variables (termed the Utstein model) to calculate the adjusted odds ratio between bystander CPR and this outcome. Each VF measure was then added individually as a predictor variable to the Utstein model; these expanded models were denoted as the mediation models. According to the hypothesized mechanism (Figure 2), bystander CPR may affect survival via the VF waveform measure and via other non‐waveform‐mediated pathways. Attenuation of the regression coefficient for bystander CPR in the mediation model compared with the Utstein model would provide qualitative evidence for mediation. 24
Figure 2. Hypothesized VF waveform‐mediated pathway.
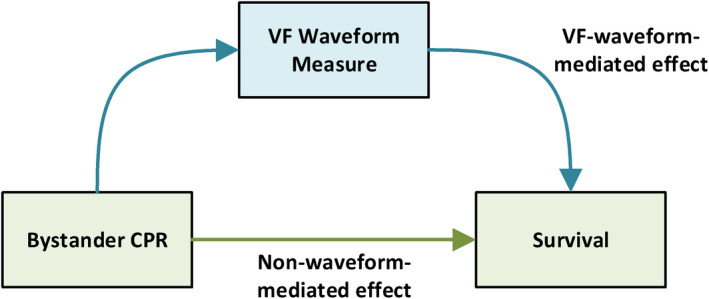
CPR indicates cardiopulmonary resuscitation; and VF, ventricular fibrillation.
To quantify the degree of mediation, we then conducted a causal mediation analysis with each of the VF waveform measures as a potential mediator. 25 Causal mediation analysis is preferred over other types of mediation analysis (such as the difference and product‐of‐coefficients methods) when there are nonlinear relationships or a prevalent binary outcome, which was the case with this data set. 24 , 26 In this approach, the effect of an exposure on an outcome is partitioned into mediated (ie, indirect) and nonmediated (ie, direct) effects. Regression models of the intervention–mediator and mediator–outcome associations are fit, and the regression coefficients are used to simulate values of the mediator and outcome in hypothetical scenarios in which patients do and do not receive the intervention. 26 , 27 The simulated (nonobserved) and observed values are used to calculate mediated and nonmediated effects. The proportion mediated is the mediated effect divided by the sum of the mediated and nonmediated effects.
Following the aforementioned approach, we fit the following 2 regression models: (1) a multivariable linear regression model with the Utstein elements as the predictors and the VF waveform measure as the outcome and (2) the previously described mediation model with survival as the outcome. 25 The waveform‐mediated and the non‐waveform‐mediated effects were estimated from these regression models using the mediatefunction of the R package mediation. 28 The waveform‐mediated effect was defined as the absolute difference in survival that resulted from a change in the VF waveform measure value from the no‐bystander CPR group mean to the bystander CPR group mean. The non‐waveform‐mediated effect was defined as the absolute difference in survival from receiving bystander CPR but without a corresponding change in the VF waveform measure value.
As secondary analyses, we repeated the aforementioned regression and causal mediation analyses first by using neurologically intact survival (Cerebral Performance Category 1 or 2) as the outcome and, second, by analyzing the TCPR and unassisted CPR subgroups separately.
A P value of 0.05 was considered statistically significant, and no adjustment was made for multiple comparisons. The statistical analysis was performed with R version 3.6.2.
RESULTS
From 2005 to 2018, 1953 adults had a bystander‐witnessed, nontraumatic OHCA with a presenting rhythm of VF, 237 of whom were ineligible because of a shock received before EMS arrival. Of the remaining 1716 patients, we were able to extract a 3‐second preshock VF segment from 1072 patients (Figure 3). The 644 excluded patients were older, less likely to present with a cardiac etiology, and less likely to survive to hospital discharge, but did not differ in other characteristics including bystander CPR status (Table S1).
Figure 3. Selection of study population and classification according to bystander CPR status.
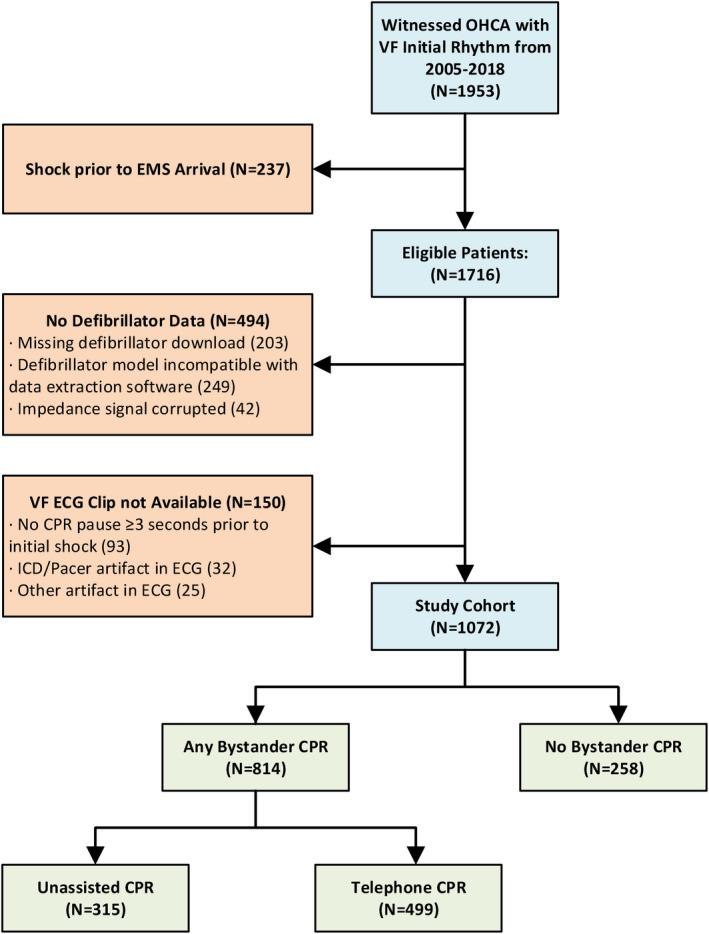
CPR indicates cardiopulmonary resuscitation; EMS, emergency medical services; ICD, implantable cardioverter‐defibrillator; OHCA, out‐of‐hospital cardiac arrest; and VF, ventricular fibrillation.
In the study cohort, 814 (76%) patients received bystander CPR before EMS arrival. We did not observe statistical differences in the distribution of Utstein characteristics according to bystander CPR status with the exception of longer average EMS response interval (5.3 minutes in the bystander CPR group versus 5.0 minutes in the no‐bystander CPR group) and fewer median number of shocks (3 in the bystander CPR group versus 4 shocks in the no‐bystander CPR group; Table 1). The 3 VF waveform measures before the first shock—AMSA, peak frequency, and median peak amplitude—were significantly higher in recipients of bystander CPR than no‐bystander CPR (P<0.01 for each comparison; Table 1) as were sustained ROSC (77% versus 67%), admission rate to the hospital (75% versus 68%), survival to hospital discharge (52% versus 43%; P<0.01), and neurologically intact survival (48% versus 37%; P<0.01) in the 2 respective groups.
Table 1.
Patient Characteristics, Resuscitation Process Variables, VF Waveform Measures, and Clinical Outcomes According to Bystander CPR Status
| Bystander CPR | P Value | Bystander CPR subgroups | P Value | |||
|---|---|---|---|---|---|---|
| Any | None | Telephone CPR | Unassisted CPR | |||
| Total patients, n | 814 | 258 | 499 | 315 | ||
| Patient characteristics | ||||||
| Female patients, n (%) | 179 (22) | 46 (18) | 0.18 | 112 (22) | 67 (21) | 0.76 |
| Age, y, median (IQR) | 62 (52–72) | 61 (53–74) | 0.47 | 63 (54–72) | 60 (51–71) | 0.013 |
| Cardiac etiology, n (%) | 778 (96) | 245 (95) | 0.81 | 479 (96) | 299 (95) | 0.58 |
| Public location, n (%) | 276 (34) | 94 (36) | 0.50 | 95 (19) | 181 (57) | <0.01 |
| Resuscitation process variables | ||||||
| EMS response time, min, median (IQR) | 5.3 (4.2–6.8) | 5.0 (4.0–6.0) | <0.01 | 5.6 (4.6–7.0) | 5.0 (4.0–6.0) | <0.01 |
| Interval between device power to ECG segment, seconds, median (IQR) | 37 (20–61) | 38 (20–59) | 0.88 | 41 (23–66) | 31 (17–57) | <0.01 |
| Shocks delivered, median (IQR) | 3 (1–6) | 4 (2–7) | <0.01 | 3 (1–6) | 3 (1–5) | 0.50 |
| VF waveform measures | ||||||
| AMSA, median (IQR) | 9.8 (6.6–14) mV‐Hz | 7.5 (4.6–11) mV‐Hz | <0.01 | 10.0 (6.8–14) mV‐Hz | 9.4 (6.1–13) mV‐Hz | 0.15 |
| Peak frequency, median (IQR) | 4.6 (3.2–6.1) Hz | 3.9 (2.9–5.4) Hz | <0.01 | 4.6 (3.2–6.4) Hz | 4.6 (3.2–6.1) Hz | 0.47 |
| Median peak amplitude, median (IQR) | 0.80 (0.55–1.1) mV | 0.67 (0.44–0.94) mV | <0.01 | 0.80 (0.57–1.1) mV | 0.76 (0.54–1.0) mV | 0.18 |
| Clinical outcomes | ||||||
| Sustained ROSC, n (%) | 626 (77) | 173 (67) | <0.01 | 402 (81) | 251 (80) | 0.70 |
| Admit to hospital, n (%) | 612 (75) | 176 (68) | 0.03 | 380 (76) | 232 (74) | 0.47 |
| Survive to hospital discharge, n (%) | 425 (52) | 110 (43) | <0.01 | 265 (53) | 160 (51) | 0.57 |
| Neurologically intact survival, Cerebral Performance Category 1 or 2, n (%)* | 392 (48) | 96 (37) | <0.01 | 244 (49) | 148 (47) | 0.67 |
AMSA indicates amplitude spectrum area; CPR, cardiopulmonary resuscitation; IQR, interquartile range; ROSC, return of spontaneous circulation; and VF, ventricular fibrillation.
Cerebral performance category was missing for 4 patients in the no‐bystander CPR group and 1 patient in the bystander CPR group (unassisted CPR subgroup).
Among patients who received bystander CPR, 499 (61%) required TCPR, and in 315 (39%), CPR was unassisted (Table 1). Patients comprising the unassisted CPR subgroup were on average younger, more likely to arrest in public, and had a shorter average EMS response interval than TCPR recipients. The VF waveform measures were significantly higher in each bystander CPR subgroup than the no‐bystander CPR group (P<0.01 for each comparison). VF waveform measures and all clinical outcomes (sustained ROSC, admission to hospital, survival to hospital discharge, and neurologically intact survival) were not significantly different between the assisted and unassisted CPR subgroups.
Bystander CPR was associated with greater likelihood of survival to hospital discharge compared with no‐bystander CPR in the unadjusted model (odds ratio, 1.5; 95% CI, 1.1–2.0) and in the multivariable adjusted Utstein model (odds ratio, 1.6; 95% CI, 1.2–2.1; Table 2). The addition of each waveform measure attenuated the association between bystander CPR and survival. In the causal mediation analysis, bystander CPR was significantly associated with higher VF waveform measures after adjustment for the other Utstein variables (Table S2). AMSA, peak frequency, and median peak amplitude mediated 53%, 31%, and 29%, respectively, of the association between bystander CPR and survival (Table 3).
Table 2.
Adjusted and Unadjusted Odds Ratios for Predicting Clinical Outcomes
| Outcome | Variable | Unadjusted | Utstein model | Mediation models | ||
|---|---|---|---|---|---|---|
| AMSA | Peak frequency | Median peak amplitude | ||||
| Survival to hospital discharge | Utstein elements | |||||
| Bystander CPR | 1.5 (1.1–2.0) | 1.6 (1.2–2.1) | 1.3 (0.92–1.7) | 1.4 (1.0–1.9) | 1.4 (1.0–1.9) | |
| Female patients | 1.4 (1.0–1.9) | 1.4 (1.0–1.9) | 1.3 (0.96–1.9) | 1.2 (0.89–1.7) | 1.4 (0.99–1.9) | |
| Public vs private location | 1.4 (1.0–1.8) | 1.1 (0.80–1.4) | 0.93 (0.70–1.2) | 0.98 (0.74–1.3) | 0.99 (0.75–1.3) | |
| Cardiac | 1.8 (0.94–3.4) | 2.8 (1.5–5.5) | 2.7 (1.4–5.4) | 2.5 (1.3–5.1) | 2.9 (1.5–5.8) | |
| Age/10 | 0.71 (0.65–0.78) | 0.69 (0.63–0.76) | 0.69 (0.62–0.76) | 0.69 (0.63–0.76) | 0.69 (0.62–0.75) | |
| EMS response time | 0.85 (0.79–0.90) | 0.84 (0.78–0.89) | 0.84 (0.79–0.90) | 0.85 (0.80–0.91) | 0.85 (0.79–0.90) | |
| VF waveform measures | ||||||
| AMSA | 2.0 (1.7–2.3) | 1.9 (1.7–2.2) | ||||
| Peak frequency | 2.0 (1.7–2.3) | 1.8 (1.6–2.1) | ||||
| Median peak amplitude | 1.6 (1.4–1.8) | 1.5 (1.3–1.7) | ||||
| Neurologically intact survival (Cerebral Performance Category 1 or 2) | Utstein elements | |||||
| Bystander CPR | 1.5 (1.1–2.1) | 1.7 (1.2–2.3) | 1.3 (0.95–1.8) | 1.5 (1.1–2.0) | 1.5 (1.1–2.0) | |
| Female patients | 1.2 (0.85–1.6) | 1.1 (0.82–1.5) | 1.1 (0.75–1.5) | 0.97 (0.69–1.3) | 1.1 (0.79–1.5) | |
| Public vs private | 1.6 (1.2–2.0) | 1.2 (0.93–1.6) | 1.1 (0.81–1.4) | 1.1 (0.85–1.5) | 1.1 (0.87–1.5) | |
| Cardiac | 1.8 (0.94–3.5) | 2.7 (1.4–5.5) | 2.6 (1.3–5.4) | 2.5 (1.2–5.0) | 2.8 (1.5–5.7) | |
| Age/10 | 0.70 (0.64–0.77) | 0.69 (0.62–0.76) | 0.69 (0.62–0.76) | 0.69 (0.62–0.76) | 0.69 (0.62–0.76) | |
| EMS response time | 0.85 (0.79–0.90) | 0.84 (0.78–0.89) | 0.84 (0.79–0.90) | 0.85 (0.79–0.91) | 0.85 (0.79–0.91) | |
| VF waveform measures | ||||||
| AMSA | 2.1 (1.8–2.4) | 2.0 (1.7–2.3) | ||||
| Peak frequency | 2.1 (1.8–2.4) | 2.0 (1.7–2.3) | ||||
| Mean peak amplitude | 1.6 (1.4–1.8) | 1.5 (1.3–1.7) | ||||
The Utstein model includes bystander CPR, sex, location, etiology, age, and EMS response time as predictor variables. The mediation models include a single VF measure in addition to the Utstein variables. VF waveform measures were log‐transformed and standardized; 95% CIs are in parentheses. AMSA indicates amplitude spectrum area; CPR, cardiopulmonary resuscitation; EMS, emergency medical services; and VF, ventricular fibrillation. Age/10 is the age in years divided by 10.
Table 3.
Results of Causal Mediation Analysis With Different Categorizations of Bystander CPR as the Intervention, Different VF Waveform Measures as Potential Mediators, and Survival to Hospital Discharge and Neurologically Intact Survival as the Outcomes
| Comparison | Outcome | Parameter | Mediator | ||
|---|---|---|---|---|---|
| AMSA | Peak frequency | Mean peak amplitude | |||
| Any bystander CPR vs no CPR | Survival to hospital discharge | VF waveform‐mediated effect | 0.053 | 0.031 | 0.029 |
| Non‐waveform‐mediated effect | 0.047 | 0.071 | 0.072 | ||
| Proportion mediated | 0.53 | 0.31 | 0.29 | ||
| Any bystander CPR vs no CPR | Neurologically intact survival | VF waveform‐mediated effect | 0.054 | 0.030 | 0.028 |
| Non‐waveform‐mediated effect | 0.055 | 0.080 | 0.080 | ||
| Proportion mediated | 0.50 | 0.29 | 0.25 | ||
| Telephone CPR vs no CPR | Survival to hospital discharge | VF waveform‐mediated effect | 0.067 | 0.038 | 0.036 |
| Non‐waveform‐mediated effect | 0.065 | 0.097 | 0.098 | ||
| Proportion mediated | 0.51 | 0.28 | 0.27 | ||
| Unassisted CPR vs no CPR | Survival to hospital discharge | VF waveform‐mediated effect | 0.032 | 0.020 | 0.017 |
| Non‐waveform‐mediated effect | 0.029 | 0.041 | 0.045 | ||
| Proportion mediated | 0.52 | 0.33 | 0.27 | ||
Effect sizes are expressed as absolute differences in survival. AMSA indicates amplitude spectrum area; CPR, cardiopulmonary resuscitation; and VF, ventricular fibrillation.
In the secondary analyses, the regression and mediation analyses results were similar using neurologically intact survival rather than survival to hospital discharge as the outcome (Tables 2 and 3). We observed similar patterns when bystander CPR was stratified into the TCPR and unassisted CPR subgroups (Table 3 and Table S3).
DISCUSSION
In this cohort of bystander‐witnessed OHCA presenting with an initial rhythm of VF, ≈three‐quarters of the 1072 patients received bystander CPR and half survived to hospital discharge. Survival to hospital discharge was about 10% higher in the bystander CPR group than the no‐bystander CPR group (52% versus 43%), as was neurologically intact survival (48% versus 37%), associations that were independent of other Utstein characteristics. Bystander CPR status corresponded to a more robust set of initial VF waveform measures—suggesting more favorable myocardial physiology—that in turn may represent a pathway by which bystander CPR improved outcome.
Epidemiologic health services evaluation among human OHCA have demonstrated a beneficial association between bystander CPR and clinical outcomes. 2 Our understanding of the biologic mechanisms by which CPR may affect clinical outcomes, however, has generally relied on animal studies. In swine models of VF arrest, measured characteristics of the VF waveform corresponded to the duration of untreated arrest, the myocardial energy status of the heart, and the likelihood of survival. 17 In a rat model of VF arrest, CPR was able to stabilize and improve myocardial energy status at least transiently. 29 However, translation to human experience has been challenging. In 2 prior studies of 77 and 134 patients, the relationship of EMS CPR with VF waveform measures varied depending on the specific measure, the statistical methodology, and the duration of CPR. 18 , 30 No human study has yet investigated the mechanistic biology of bystander CPR and how it may mediate clinical outcomes.
The lack of such evidence has led some to question bystander CPR as a public health intervention, suggesting that bystander CPR is simply a confounding marker for a well‐trained bystander who can activate 9‐1‐1 more quickly. 7 The current results, however, found that bystander CPR was associated with improved VF waveform measures regardless of the type of bystander CPR. Indeed, the observation that recipients of TCPR experienced a similar benefit as those who received unassisted CPR suggests that bystander CPR is not merely a confounder. Bystanders who require telecommunicator assistance are less likely to comprise a group of well‐trained, quick‐responding bystanders; rather, they likely constitute a more naïve group that is challenged to recognize and respond to cardiac arrest. The current results provide useful evidence that bystander CPR can produce a beneficial biological effect on the myocardium as reflected by waveform measures.
Ventricular fibrillation waveform measures have been associated with myocardial physiological status (adenosine triphosphate stores), suggesting that bystander CPR may work to improve survival through a primary cardiac mechanism. 31 , 32 Although perhaps not surprising, the current results help validate experimental research demonstrating CPR mechanisms related to coronary perfusion and oxygen delivery and their influence on myocardial energetics. 33 , 34 , 35 The VF waveform measures mediated between a quarter to half of the survival benefit, depending on the specific measure. The observed variability in degree of mediation may be related to the measures' inherent abilities to quantify myocardial physiology. AMSA incorporates both amplitude and frequency of the VF waveform, and in a prior study, AMSA was a more accurate predictor of patient outcome than more basic measures of VF frequency alone (such as peak frequency), which in turn were more accurate than measures of VF amplitude alone (such as median peak amplitude). 11
One interpretation of these findings is that none of the selected VF waveform measures optimally reflect the myocardial substrate; it is possible that these waveform measures together mediate a greater proportion of the survival benefit when acting jointly or that alternative waveform measures (such as measures that apply machine learning to improve performance) could serve as more effective mediators. 36 Alternatively, the mechanism by which bystander CPR conveys survival benefit may be distinct from the waveform. 37 Certainly, downstream care by EMS or at the hospital also influences outcome. In addition, bystander CPR may mediate a survival benefit through other mechanisms, most notably cerebral perfusion and oxygenation, that may not be captured by cardiac waveform measures.
The direct relationship between binary (yes/no) bystander CPR and quantitative waveform measures also supports the potential to consider the waveform as a gauge of the quality of bystander CPR. Currently, we are challenged to assess the effectiveness of different approaches to bystander CPR. Although speculative, one approach among patients with VF OHCA might be to evaluate the initial and evolving value of the VF waveform to help gauge the effectiveness of bystander CPR. Such a surrogate measure might be relevant as an efficient means to evaluate the biological comparative effectiveness of different CPR protocols.
The current investigation has limitations. As this study was observational, the association between bystander CPR and survival may still be confounded by unmeasured variables, even though we restricted the cohort to witnessed OHCA, adjusted for the spectrum of Utstein covariates, and evaluated bystander CPR stratified by TCPR and unassisted CPR. Residual confounding would also have impacted the findings of the mediation analysis, which assumed that the intervention–outcome, mediator–outcome, and intervention–outcome associations were unconfounded after adjustment for covariates. Sensitivity analysis was not performed, because the mediation package did not support sensitivity analysis with a logistic regression outcome model. 28 This software also did not offer the ability to perform a multiple mediator analysis that would have investigated the joint effect of all 3 waveform measures.
An additional limitation is that we were not able to measure the quality or quantity of bystander CPR, although future investigations may consider if and how conventional measures of CPR quality (ie, rate, interruptions, and depth) may be related to waveform measures. Finally, there was a period of EMS‐delivered CPR between the bystander care and the sampled ECG segment because EMS rescuers provided at least 30 compressions before rhythm analysis (an “analyze early” EMS approach) under existing treatment protocols. Such a “leveler” of CPR quality before the extracted ECG segment would be expected to bias our results toward the null, suggesting that the current results may underestimate the difference in waveform measures according to bystander CPR status. We should consider these limitations in the context of the study's strengths: an investigation that addressed a gap in our understanding of bystander CPR mechanisms by combining information from a well‐characterized, relatively large VF OHCA cohort with electronic defibrillator ECG data while using advanced methods to evaluate mechanism and mediation.
CONCLUSIONS
Bystander CPR, including both unassisted CPR and TCPR, correlated with more robust initial VF waveform measures, which in turn mediated up to one‐half of the survival benefit associated with bystander CPR. The results provide insight into the biological mechanism of bystander CPR in VF OHCA, suggesting that much of the survival benefit conferred by bystander CPR may be a result of improvement to the myocardium as reflected by more robust waveform measures. This may provide novel considerations for how waveform measures might be incorporated to help assess the effectiveness of CPR. Future investigations might consider approaches to evaluate the quality of CPR as we strive to leverage mechanistic understanding to improve resuscitation outcome.
Sources of Funding
This work was supported in part by a grant from the Laerdal Foundation to Ms Bessen and a grant from the Washington Research Foundation to Dr Coult. Dr Hsu and Dr Kwok are supported by a grant from the American Heart Association Strategically Focused Research Network on Arrhythmias and Sudden Cardiac Death. The granting organizations had no role in the study design, conduct, analysis, interpretation, or manuscript preparation.
Disclosures
Philips Healthcare, Inc., has provided funding for research related to defibrillator algorithms to Dr Kwok through the University of Washington. Dr Kudenchuk reports grants from National Institutes of Health/National Institute of Neurological Disorders and Stroke outside of the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Acknowledgments
Author contributions: Ms Bessen, Dr Coult, and Ms Blackwood were responsible for data acquisition. Drs Kwok and Coult computed the ECG waveform measures. Ms Blackwood and Dr Kwok performed the statistical analysis. All authors contributed substantially to the study design, interpretation of the results, and the drafting of the manuscript. All authors approved the submitted and final version.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.020825
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:E139–E596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2. Sasson C, Rogers MAM, Dahl J, Kellermann AL. Predictors of survival from out‐of‐hospital cardiac arrest: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2009;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576 [DOI] [PubMed] [Google Scholar]
- 3. Hasselqvist‐Ax I, Riva G, Herlitz J, Rosenqvist M, Hollenberg J, Nordberg P, Ringh M, Jonsson M, Axelsson C, Lindqvist J, et al. Early cardiopulmonary resuscitation in out‐of‐hospital cardiac arrest. N Engl J Med. 2015;372:2307–2315. doi: 10.1056/NEJMoa1405796 [DOI] [PubMed] [Google Scholar]
- 4. Kern KB, Garewal HS, Sanders AB, Janas W, Nelson J, Sloan D, Tacker WA, Ewy GA. Depletion of myocardial adenosine triphosphate during prolonged untreated ventricular fibrillation: effect on defibrillation success. Resuscitation. 1990;20:221–229. doi: 10.1016/0300-9572(90)90005-Y [DOI] [PubMed] [Google Scholar]
- 5. Bobrow BJ, Ewy GA, Clark L, Chikani V, Berg RA, Sanders AB, Vadeboncoeur TF, Hilwig RW, Kern KB. Passive oxygen insufflation is superior to bag‐valve‐mask ventilation for witnessed ventricular fibrillation out‐of‐hospital cardiac arrest. Ann Emerg Med. 2009;54:656–662. doi: 10.1016/j.annemergmed.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 6. Rea TD, Cook AJ, Hallstrom A. CPR during ischemia and reperfusion: a model for survival benefits. Resuscitation. 2008;77:6–9. doi: 10.1016/j.resuscitation.2007.10.010 [DOI] [PubMed] [Google Scholar]
- 7. Bardy GH. A critic’s assessment of our approach to cardiac arrest. N Engl J Med. 2011;364:374–375. doi: 10.1056/NEJMe1012554 [DOI] [PubMed] [Google Scholar]
- 8. Blewer AL, Ibrahim SA, Leary M, Dutwin D, McNally B, Anderson ML, Morrison LJ, Aufderheide TP, Daya M, Idris AH, et al. Cardiopulmonary resuscitation training disparities in the United States. J Am Heart Assoc. 2017;6:e006124. doi: 10.1161/JAHA.117.006124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sasson C, Magid DJ, Chan P, Root ED, McNally BF, Kellermann AL, Haukoos JS; CARES Surveillance Group . Association of neighborhood characteristics with bystander‐initiated CPR. N Engl J Med. 2012;367:1607–1615. doi: 10.1056/NEJMoa1110700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Starks MA, Schmicker RH, Peterson ED, May S, Buick JE, Kudenchuk PJ, Drennan IR, Herren H, Jasti J, Sayre M, et al. Association of neighborhood demographics with out‐of‐hospital cardiac arrest treatment and outcomes: where you live may matter. JAMA Cardiol. 2017;2:1110. doi: 10.1001/jamacardio.2017.2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coult J, Blackwood J, Sherman L, Rea T, Kudenchuk P, Kwok H. Ventricular fibrillation waveform analysis during chest compressions to predict survival from cardiac arrest. Circ Arrhythm Electrophysiol. 2019;12:1–10. doi: 10.1161/CIRCEP.118.006924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Callaway CW, Menegazzi JJ. Waveform analysis of ventricular fibrillation to predict defibrillation. Curr Opin Crit Care. 2005;11:192–199. doi: 10.1097/01.ccx.0000161725.71211.42 [DOI] [PubMed] [Google Scholar]
- 13. Salcido DD, Menegazzi JJ, Suffoletto BP, Logue ES, Sherman LD. Association of intramyocardial high energy phosphate concentrations with quantitative measures of the ventricular fibrillation electrocardiogram waveform. Resuscitation. 2009;80:946–950. doi: 10.1016/j.resuscitation.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 14. Reynolds JC, Salcido DD, Menegazzi JJ. Correlation between coronary perfusion pressure and quantitative ECG waveform measures during resuscitation of prolonged ventricular fibrillation. Resuscitation. 2012;83:1497–1502. doi: 10.1016/j.resuscitation.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schoene P, Coult J, Murphy L, Fahrenbruch C, Blackwood J, Kudenchuk P, Sherman L, Rea T. Course of quantitative ventricular fibrillation waveform measure and outcome following out‐of‐hospital cardiac arrest. Heart Rhythm. 2014;11:230–236. doi: 10.1016/j.hrthm.2013.10.049 [DOI] [PubMed] [Google Scholar]
- 16. Affatato R, Li Y, Ristagno G. See through ECG technology during cardiopulmonary resuscitation to analyze rhythm and predict defibrillation outcome. Curr Opin Crit Care. 2016;22:199–205. doi: 10.1097/MCC.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 17. Salcido DD, Kim Y‐M, Sherman LD, Housler G, Teng X, Logue ES, Menegazzi JJ. Quantitative waveform measures of the electrocardiogram as continuous physiologic feedback during resuscitation with cardiopulmonary bypass. Resuscitation. 2012;83:505–510. doi: 10.1016/j.resuscitation.2011.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eftestøl T, Wik L, Sunde K, Steen PA. Effects of cardiopulmonary resuscitation on predictors of ventricular fibrillation defibrillation success during out‐of‐hospital cardiac arrest. Circulation. 2004;110:10–15. doi: 10.1161/01.CIR.0000133323.15565.75 [DOI] [PubMed] [Google Scholar]
- 19. Kleinman ME, Brennan EE, Goldberger ZD, Swor RA, Terry M, Bobrow BJ, Gazmuri RJ, Travers AH, Rea T. Part 5: adult basic life support and cardiopulmonary resuscitation quality 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015;132:S414–S435. doi: 10.1161/CIR.0000000000000259 [DOI] [PubMed] [Google Scholar]
- 20. Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, Bossaert LL, Brett SJ, Chamberlain D, de Caen AR, et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein resuscitation registry templates for out‐of‐hospital cardiac arrest. Circulation. 2015;132:1286–1300. doi: 10.1161/CIR.0000000000000144 [DOI] [PubMed] [Google Scholar]
- 21. Eftestøl T, Sunde K, Ole Aase S, Husøy JH, Steen PA. Predicting outcome of defibrillation by spectral characterization and nonparametric classification of ventricular fibrillation in patients with out‐of‐hospital cardiac arrest. Circulation. 2000;102:1523–1529. doi: 10.1161/01.CIR.102.13.1523 [DOI] [PubMed] [Google Scholar]
- 22. Marn‐Pernat A, Weil MH, Tang W, Pernat A, Bisera J. Optimizing timing of ventricular defibrillation. Crit Care Med. 2001;29:2360–2365. doi: 10.1097/00003246-200112000-00019 [DOI] [PubMed] [Google Scholar]
- 23. Weaver WD, Cobb LA, Dennis D, Ray R, Hallstrom AP, Copass MK. Amplitude of ventricular fibrillation waveform and outcome after cardiac arrest. Ann Intern Med. 1985;102:53–55. doi: 10.7326/0003-4819-102-1-53 [DOI] [PubMed] [Google Scholar]
- 24. VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. doi: 10.1146/annurev-publhealth-032315-021402 [DOI] [PubMed] [Google Scholar]
- 25. Imai K, Keele L, Tingley D, Yamamoto T. Unpacking the black box of causality: learning about causal mechanisms from experimental and observational studies. Am Polit Sci Rev. 2011;105:765–789. doi: 10.1017/S0003055411000414 [DOI] [Google Scholar]
- 26. Lee H, Herbert RD, McAuley JH. Mediation analysis. JAMA. 2019;321:697–698. doi: 10.1001/jama.2018.21973 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Z, Zheng C, Kim C, Van Poucke S, Lin S, Lan P. Causal mediation analysis in the context of clinical research. Ann Transl Med. 2016;4:425. doi: 10.21037/atm.2016.11.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw. 2014;59:1–38.26917999 [Google Scholar]
- 29. Choi HJ, Nguyen T, Park KS, Cha KC, Kim H, Lee KH, Hwang SO. Effect of cardiopulmonary resuscitation on restoration of myocardial ATP in prolonged ventricular fibrillation. Resuscitation. 2013;84:108–113. doi: 10.1016/j.resuscitation.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 30. Gundersen K, Kvaløy J, Kramer‐Johansen J, Steen P, Eftestøl T. Development of the probability of return of spontaneous circulation in intervals without chest compressions during out‐of‐hospital cardiac arrest: an observational study. BMC Med. 2009;7:6. doi: 10.1186/1741-7015-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neumar RW, Browns CG, Robitaille P‐ML, Altschul RA. Myocardial high energy phosphate metabolism during ventricular fibrillation with total circulatory arrest. Resuscitation. 1990;19:199–226. doi: 10.1016/0300-9572(90)90103-L [DOI] [PubMed] [Google Scholar]
- 32. Neumar RW, Brown CG, Van Ligten P, Hoekstra J, Altschuld RA, Baker P. Estimation of myocardial ischemic injury during ventricular fibrillation with total circulatory arrest using high‐energy phosphates and lactate as metabolic markers. Ann Emerg Med. 1991;20:222–229. doi: 10.1016/S0196-0644(05)80927-8 [DOI] [PubMed] [Google Scholar]
- 33. Cobb LA, Fahrenbruch CE, Walsh TR, Copass MK, Olsufka M, Breskin M, Hallstrom AP. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out‐of‐hospital ventricular fibrillation. JAMA. 1999;281:1182–1188. doi: 10.1001/jama.281.13.1182 [DOI] [PubMed] [Google Scholar]
- 34. Berg RA, Hilwig RW, Kern KB, Ewy GA. Precountershock cardiopulmonary resuscitation improves ventricular fibrillation median frequency and myocardial readiness for successful defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Ann Emerg Med. 2002;40:563–570. doi: 10.1067/mem.2002.129866 [DOI] [PubMed] [Google Scholar]
- 35. Yeh ST, Lee H‐L, Aune SE, Chen C‐L, Chen Y‐R, Angelos MG. Preservation of mitochondrial function with cardiopulmonary resuscitation in prolonged cardiac arrest in rats. J Mol Cell Cardiol. 2009;47:789–797. doi: 10.1016/j.yjmcc.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coult J, Rea TD, Blackwood J, Kudenchuk PJ, Liu C, Kwok H. A method to predict ventricular fibrillation shock outcome during chest compressions. Comput Biol Med. 2021;129:104–136. doi: 10.1016/j.compbiomed.2020.104136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hall M, Phelps R, Fahrenbruch C, Sherman L, Blackwood J, Rea TD. Myocardial substrate in secondary ventricular fibrillation: insights from quantitative waveform measures. Prehosp Emerg Care. 2011;15:388–392. doi: 10.3109/10903127.2011.561407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


