Abstract
Background
Studies have reported that people living with HIV have higher burden of subclinical cardiovascular disease, but the data are not adequately synthesized. We performed meta‐analyses of studies of coronary artery calcium and coronary plaque in people living with HIV.
Methods and Results
We performed systematic search in electronic databases, and data were abstracted in standardized forms. Study‐specific estimates were pooled using meta‐analysis. 43 reports representing 27 unique studies and involving 10 867 participants (6699 HIV positive, 4168 HIV negative, mean age 52 years, 86% men, 32% Black) were included. The HIV‐positive participants were younger (mean age 49 versus 57 years) and had lower Framingham Risk Score (mean score 6 versus 18) compared with the HIV‐negative participants. The pooled estimate of percentage with coronary artery calcium >0 was 45% (95% CI, 43%–47%) for HIV‐positive participants, and 52% (50%–53%) for HIV‐negative participants. This difference was no longer significant after adjusting for difference in Framingham Risk Score between the 2 groups. The odds ratio of coronary artery calcium progression for HIV‐positive versus ‐negative participants was 1.64 (95% CI, 0.91–2.37). The pooled estimate for prevalence of noncalcified plaque was 49% (95% CI, 47%–52%) versus 20% (95% CI, 17%–23%) for HIV‐positive versus HIV‐negative participants, respectively. Odds ratio for noncalcified plaque for HIV‐positive versus ‐negative participants was 1.23 (95% CI, 1.08–1.38). There was significant heterogeneity that was only partially explained by available study‐level characteristics.
Conclusions
People living with HIV have higher prevalence of noncalcified coronary plaques and similar prevalence of coronary artery calcium, compared with HIV‐negative individuals. Future studies on coronary artery calcium and plaque progression can further elucidate subclinical atherosclerosis in people living with HIV.
Keywords: calcium score, cardiovascular disease, coronary artery calcium, coronary plaque, human immunodeficiency virus, subclinical atherosclerosis
Subject Categories: Epidemiology, Cardiovascular Disease, Risk Factors
Nonstandard Abbreviations and Acronyms
- FRS
Framingham Risk Score
- PLHIV
people living with HIV
Clinical Perspective
What Is New?
Although several studies on coronary artery calcium and coronary plaque burden in people living with HIV have been published in recent years, the data have not been adequately synthesized, and their relevance to cardiovascular risk stratification in this group is not clear.
This meta‐analysis found that people living with HIV have similar burden of coronary calcium and total coronary plaque and higher burden of noncalcified coronary plaque, compared to HIV‐negative controls.
The findings suggest that a more vulnerable form of subclinical atherosclerosis may develop earlier in people with HIV.
What Are the Clinical Implications?
The findings in this meta‐analysis have implications on cardiovascular disease screening considerations for patients with HIV (especially those with risk factors for cardiovascular disease) at a younger age.
Primary care providers or preventative cardiologists may consider a lower threshold to use coronary calcium scoring to help risk stratify their patients with HIV; coronary computed tomographic angiography can also be considered to identify noncalcified coronary plaque in these patients.
Future randomized clinical trials will help to determine definitively the utility of noninvasive markers such as coronary calcium scoring and computed tomographic angiography in preventing cardiovascular disease in this group.
Patients with HIV infection are living longer because of effective antiretroviral therapy (ART). Consequently, these patients are experiencing an increasing burden of cardiovascular disease (CVD). The focus of care is thus shifting to primary CVD prevention once patients are stable on their ART regimen. 1 In addition to traditional CVD risk factors, people living with HIV (PLHIV) are predisposed to CVD attributable to HIV‐specific factors, including chronic HIV infection, low‐grade inflammation, and cardiometabolic effects of ART. 2 Coronary computed tomography (CT) has increasingly been used to guide treatment strategies in the general population for primary prevention and has also been used for patients with HIV. 3 , 4 Because traditional risk factor calculators may not adequately capture the full extent of the CVD risk in this population, CT imaging markers such as coronary artery calcium (CAC) may be useful to fill in this gap for risk assessment. 5 Prior studies have suggested that patients with HIV have a higher burden of subclinical atherosclerosis, including carotid stenosis and accelerated coronary aging based on CAC score measures. 6 , 7 , 8 Additionally, asymptomatic patients with HIV have been reported to have higher rates of noncalcified plaques in some studies,6 which are considered higher risk for rupture leading to cardiac events. However, studies have not found a consistent association between HIV and CAC burden, and prior reviews have not adequately synthesized the data on CAC and coronary plaque burden in PLHIV. 8 Given inconclusive data from prior studies and reviews, and the rapid expansion of the literature on HIV risk for CAC, we aimed to perform a systematic review and meta‐analysis of all the available evidence on CAC and coronary plaque burden in PLHIV and compare with HIV‐negative individuals to elucidate any differences in subclinical atherosclerotic burden.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Search Strategy
We searched the PubMed and EMBASE databases from inception until September 2020 for English‐language articles on coronary artery calcium (CAC) score and CVD in patients with HIV. PubMed search used the following key search terms related to HIV, CVD, and CAC: Human Immunodeficiency Virus, HIV, AIDS, Acquired Immune Deficiency Syndrome, Cardiovascular Disease, Coronary Artery Disease, Coronary Artery Calcium and Agatston Score. We also scanned reference lists of relevant articles. We used the following inclusion criteria to select studies: (1) observational study (cross‐sectional or longitudinal design); (2) study of patients with HIV (with or without HIV‐negative controls), (3) study on adults >18 years of age, (4) reported data on CAC score (measured using noncontrast cardiac CT) or coronary plaque (measured using coronary CT angiography).
Data Extraction
We extracted prespecified information in duplicate from the publications using standardized forms (performed by C.S., Ah.S., and Am.S.). Relevant study‐level information including study location, study year, design, size, average age of participants (mean or median, whichever was the available summary measure of the age of participants), proportion of men, average values of blood pressure, proportion of smokers, proportion with diabetes mellitus, and average Framingham Risk Score (FRS) values were extracted for HIV‐positive cases and HIV‐negative controls separately and combined, wherever available. The average duration of HIV Infection, proportion on ART, average duration on ART, and average CD4 count were extracted for the HIV‐positive participants. Information on measurement method and results of the following subclinical atherosclerosis measures was recorded for HIV‐positive cases and (where available) HIV‐negative controls: (1) Average CAC Agatston score (mean or median) with measure of dispersion (SD or interquartile range), (2) percentage with CAC >0, (3) percentage with CAC >100, (4) percentage with CAC progression, (5) percentage with coronary plaque, (6) percentage with noncalcified coronary plaque, and (7) percentage with calcified coronary plaque. Information on CAC progression definition for studies reporting these data are as follows: significant increase from baseline (>2.5 on square root scale) with median follow‐up of 19 months 9 ; new CAC with median follow‐up of 5 years 10 ; new CAC >0 or >10‐unit change per year or >10% change per year with follow‐up over 6 years 11 ; new CAC with mean follow‐up of 2.4 years 12 ; new CAC or significant increase from baseline (>2.5 on square root scale) with follow‐up over 2 years 13 ; and new CAC with median follow‐up of 2.2 years (HIV‐positive) and 3.4 years (HIV‐negative) 14 ; CAC percentage increase >15% per year with follow‐up of 0.5 to 3 years 15 ; new CAC or percentage increase >15% per year with median follow‐up of 1.2 years. 16 For studies comparing HIV‐positive and HIV‐negative individuals directly, measures of relative risk (ie, odds ratios) were also extracted for each of the preceding measures, where available. Discrepancies were resolved by consensus, adjudicated by a fourth reviewer (S.E.).
Statistical Analysis
Only a subset of the studies included both HIV‐positive participants and HIV‐negative controls. To ensure that our conclusions on comparisons by HIV status are valid, we present findings of analyses restricting to studies that recruited both HIV‐positive and negative participants in parallel with results of overall analyses as appropriate. We calculated the weighted mean of study‐level characteristics such as average age, percentage male, percentage Black, percentage smokers, percentage with hypertension, average blood pressure, average CD4 count, and so on, weighted by the appropriate denominators (N). Where appropriate, the P value comparing summary study‐level characteristics between HIV‐positive and HIV‐negative participants was calculated from a linear regression model of each variable upon HIV status, analytically weighted by N for each study (ie, fixed‐effects meta‐regression). Standard errors for the study‐specific prevalence estimates were determined from the point estimate and the sample size (N) assuming a binomial distribution. For studies with data on average CAC score, we included those reporting mean and SD (where available) in meta‐analysis; we used SD and N to estimate the SEM. To obtain an overall summary estimate of the prevalence across studies, we pooled the study‐specific estimates using the inverse‐variance–weighted method under a fixed‐effects model. The fixed‐effects (plural) model does not assume presence of the same underlying effect across the individual studies (unlike fixed [common] effect model) or exchangeability of effect (random‐effects model). 17 , 18 , 19 To minimize the effect of studies with extremely small or extremely large prevalence estimates on the overall estimate, we stabilized the variance of the study‐specific prevalence with the Freeman‐Tukey single arcsine transformation before pooling the study‐specific estimates. 20 Between‐study heterogeneity was assessed using the Cochran's Q and I2 statistics. 21 The I2 statistic estimates the percentage of total variation across studies attributable to true between‐study differences rather than chance. 22 We took I2 value cutoffs of 25%, 50%, and 75% to represent low, medium, and high heterogeneity, respectively. We explored sources of heterogeneity by performing meta‐regression on study‐level characteristics that are known to affect CAC or coronary plaque burden, including average age of participants, percentage male participants, percentage Black participants, and average FRS. To account for difference between the HIV‐positive and HIV‐negative participants in terms of characteristics that influence CAC or coronary plaque burden, we predicted pooled values for CAC and coronary plaque burden adjusted to the mean FRS value across the studies in a meta‐regression model. Additional heterogeneity analyses were performed by subgrouping the studies on the basis of average values of several study‐level variables that are known to affect CAC burden (listed above) into studies with less than the median value and those with greater than the median value for the respective variable. We assessed the presence of publication bias using a funnel plot and the Egger test 23 and by comparing the pooled prevalence between larger and smaller studies. We assessed the robustness of our results by performing an influence analysis in which each individual study was omitted one at a time, and the effect on the pooled estimate was assessed. Methodological quality of included studies was assessed using the Newcastle‐Ottawa Scale. 24 This scale is calculated by assigning points to three aspects of study design, with a maximum total of 10 points: selection of study participants (maximum 5 points), comparability of study groups (maximum 2 points), and ascertainment of the outcome of interest (maximum 3 points). The cut‐offs of 0 to 3, 4 to 7, and 8 to 10 points were arbitrarily used to define high, moderate, and low risk of bias, respectively. P<0.05 was considered statistically significant. We report pooled estimates and 95% CIs. All analyses were performed using Stata software (version 15; StataCorp, College Station, TX). This study is reported according to Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guideline. 25 This study was exempt from institutional review board review because it is a literature‐based, aggregate data meta‐analysis of already published studies and no direct human subjects were involved.
Role of the Funding Source
The funding sources were not involved in the analysis of data or preparation of this manuscript. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
The study selection process is shown in Figure S1. We identified 119 articles for full review on literature search, of which 43 articles were retained. 4 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 Of the retained articles, 22 represented multiple publications from 6 different studies,* yielding 27 unique studies included in the meta‐analyses (Table). The aforementioned 22 articles were retained because they provided complementary data to the information reported in the leading articles.
Table 1.
Baseline Clinical Characteristics by Study
| Study | Place | Study Dates | Study Design | Population Source* | Excluded Prior CAD | N Total | N HIV+ | % Male | % White | % Black | Average Age, y | % DM | % Hypertension | % Smoking | Newcastle‐Ottowa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pereira B (2020) 50 | London, UK | 2009–2019 | Retrospective cross‐sectional study | Clinic associated with medical center | Yes | 739 | 739 | 92.8 | 84.2 | 7.5 | 56 | … | 52.5 | … | 5 |
| Krishnam M (2020) 39 | Irvine, CA | 2010–2015 | Retrospective observational study | Medical center, database | Yes | 143 | 143 | 83.2 | 72.7 | 7 | 46.4 | … | 28.7 | … | 4 |
| Chandra D (2019) 29 | Pittsburgh, PA | Not Listed | Retrospective cross‐sectional study | Local clinics | No | 234 | 177 | 82.1 | … | … | 49.6 | … | 43.5 | … | 9 |
| Senoner T (2019) 53 | Innsbruck, Austria | 2007–2018 | Retrospective cohort study | Medical center | Yes | 138 | 69 | 71 | … | … | 54 | 9 | 37 | 80 | 7 |
| Tarr PE (2018) † , 14 , 57 | Geneva, Switzerland; Zürich, Switzerland | 2013–2016 | Retrospective cross‐sectional Study | Clinical trial, medical centers | Yes | 704 | 428 | 83 | 89 | 7 | 54 | 7 | 43 | 28 | 9 |
| Korada SK (2017) ‡ , 4 , 10 , 38 , 46 , 47 , 52 | Los Angeles, CA; Chicago, IL; Pittsburgh, PA; Columbus, OH; Baltimore, MD; Washington, DC | 2004–2006 | Retrospective cross‐sectional study | Clinical trial | No | 976 | 602 | 100 | 58 | 31 | 54 | 11 | 46 | 28 | 7 |
| Besutti G (2016) § , 9 , 15 , 16 , 28 | Modena, Italy | 2006–2012 | Prospective cross‐sectional study | Clinic associated with medical center | No | 1446 | 1446 | 71 | … | … | 48 | 13 | 38 | 39 | 5 |
| Fitch KV (2016) 36 | Boston, MA | 2006–2012 | Observational study | Local clinics | Yes | 225 | 155 | 60 | 49 | … | 47 | 9 | 22 | 43 | 7 |
| Nadel J (2016) 49 | Sydney, Australia | 2011–2014 | Retrospective observational study | Medical center | No | 97 | 32 | 100 | 81 | … | 60 | 24 | 64 | 18 | 9 |
| Chow D (2015) ‖ | Hawaii, USA; New York, NY; Chicago, IL; Los Angeles, CA; Minneapolis, MN; Winston‐Salem, NC | Not listed | Retrospective cross‐sectional study | Clinical trials | Yes | 2833 | 100 | 100 | 40 | 26 | 59 | 14 | … | 16 | 7 |
| Abd‐Elmoniem KZ (2014) 26 | Bethedsa, MD | 2010–2013 | Prospective cross‐sectional study | NIH Clinical Center | Yes | 46 | 35 | 48 | 37 | 46 | 23 | … | … | … | 7 |
| Longenecker CT (2014) 44 | Cleveland, OH | Not listed | Retrospective cross‐sectional study | Clinical trial | Yes | 147 | 147 | 78 | … | 69 | 46 | … | … | 63 | 5 |
| Baker JV (2014) 13 | Denver, CO; Minneapolis, MN; Providence, RI; St. Louis, MO | 2004–2006 | Prospective cohort study | Clinical trial | No | 436 | 436 | 78 | 59 | 27 | 42 | 9 | … | 41 | 7 |
| Kristoffersen US (2013) 40 | Hvidovre, Denmark | 2008–2010 | Retrospective cross‐sectional study | Clinic associated with medical center | Yes | 210 | 105 | 89 | … | … | 47 | … | … | 37 | 7 |
| Lai S (2013)¶ 12 , 41 , 42 , 43 | Baltimore, MD | 2003–2012 | Retrospective cross‐sectional study | Clinical trial | Yes | 848 | 848 | 63 | 0 | 100 | 46 | 4 | 12 | 83 | 5 |
| Pereyra F (2012) 51 | Boston, MA | Not listed | Prospective cohort study | Local clinics, database | Yes | 152 | 103 | 68 | … | … | 49 | 9 | 23 | 40 | 7 |
| Hsue PY (2012) 37 | San Francisco, CA | Not listed | Prospective cross‐sectional study | Clinical trial | Yes | 311 | 253 | 88 | 62 | 22 | 49 | 5 | 27 | 62 | 7 |
| Duarte H (2012) 33 | Bethesda, MD | Not listed | Prospective cross‐sectional study | NIH Clinical Center | Yes | 52 | 26 | 85 | 48 | 40 | 53 | 25 | 60 | 31 | 7 |
| Fitch K (2012) 35 | Boston, MA | 2006–2010 | Randomized placebo‐controlled trial | Medical center | Yes | 46 | 46 | 76 | 64 | 32 | 47 | … | … | … | 7 |
| d'Ettorre G (2012) 32 | Rome, Italy | Not listed | Retrospective cross‐sectional study | Medical center | No | 55 | 55 | 86 | … | … | 48 | … | … | … | 5 |
| Subramanian S (2012) 55 | Boston, MA | 2009–2011 | Prospective cross‐sectional study | Medical center, database | Yes | 54 | 27 | 93 | … | … | 53 | … | 17 | 15 | 7 |
| Falcone EL (2011) # , 11 , 34 , 45 , 59 | Boston, MA; Providence, RI | 2002–2004 | Retrospective cross‐sectional study | Clinical trial | No | 334 | 334 | 74 | 53 | 34 | 44 | … | … | 50 | 5 |
| Crum‐Cianflone N (2011) 31 | San Diego, CA | 2008–2010 | Retrospective cross‐sectional study | Clinical Trial | No | 223 | 223 | 96 | 49 | 23 | 43 | 6 | 30 | 17 | 5 |
| Monteiro VS (2011) 48 | Recife, Brazil | Not listed | Retrospective cross‐sectional study | Clinics associated with medical centers | Yes | 53 | 53 | 51 | … | … | 43 | 4 | 23 | 19 | 5 |
| Vilela FD (2011) 58 | Rio de Janeiro, Brazil | Not listed | Retrospective cross‐sectional study | Specialized HIV treatment centers | Yes | 40 | 40 | 53 | … | … | 46 | 10 | 55 | 35 | 5 |
| Acevedo M (2002) 27 | Cleveland, OH | Not listed | Prospective cohort study | Local clinics, database | Yes | 85 | 17 | … | … | … | 46 | … | … | … | 7 |
| Talwani R (2002) 56 | Chicago, IL | 1999–2000 | Retrospective cross‐sectional study | Medical center | No | 240 | 60 | 100 | 65 | 27 | 47 | … | … | … | 7 |
| Pooled/combined** | … | … | … | … | … | 10 867 | 6699 | 86 | 51 | 32 | 51 | 10 | 32 | 36 | … |
DM indicates diabetes mellitus.
Some of the studies were based on subjects that were enrolled in existing Clinical Trials, but the design of the reports on coronary artery calcium included in the present meta‐analyses was observational in nature as shown under “Study Design” column.
Tarr PE (2018) contains the same study population as Tarr PE (2020).
Korada SK (2017), contains same study population as Post WS (2014), Monroe AK (2012), Metkus TS (2015), Kingsley LA (2008), and Kingsley LA (2015).
Besutti G (2016) contains same study population as Guaraldi G (2011), Zona S (2012).
Chow C D (2015) contains same study population as Shikuma C (2014).
Lai S (2013) contains same study population as Lai S (2009), Lai S (2005), Lai H (2012); dates of subject enrollment and analysis did not overlap for Lai (2005) and Lai (2013).
Falcone EL (2011) contains same study population as Volpe GE (2013), Mangili A (2007), Falcone EL (2010).
Represents subtotal for N and weighted average for % (weighted by N).
Basic Demographics
A total of 10 867 (6699 HIV‐positive, 4168 HIV‐negative) participants were included in the analyses. Table S1 provides comprehensive details on demographic characteristics of the participants. The mean age of participants ranged from 23 to 60 years across the studies (weighted average, 52 years), the proportion of male participants ranged from 48% to 100% (weighted average, 86%), and the proportion of Black participants ranged from 7% to 100% (weighted average, 32%). The HIV‐positive subgroup was younger (mean±SD age 49±5 versus 57±5 years) and had a lower proportion of male participants (79% versus 96%), compared with the HIV‐negative subgroup. There was higher prevalence of Black participants (37% versus 24%) in the HIV‐positive versus HIV‐negative subgroup. There were 15 studies that included both HIV‐positive cases and HIV‐negative controls allowing direct within study comparison. This subset of studies included a total of 6357 (2189 HIV‐positive, 4168 HIV‐negative) participants, with average age ranging from 23 to 60 years across the studies (weighted average, 55 years), the proportion of male participants ranged from 48% to 100% (weighted average, 93%), and the proportion of Black participants ranged from 7% to 46% (weighted average, 24%). The HIV‐positive subgroup was younger (mean±SD age 51±5 versus 57±5 years) and had a lower proportion of male participants (87% versus 96%) but had similar proportion of Black participants (23% versus 24%) compared with the HIV‐negative subgroup. The majority of studies (18/27) excluded subjects with prior coronary artery disease or percutaneous coronary intervention.
Clinical Characteristics
The weighted mean±SD of clinical characteristics of participants across the studies are detailed in Table S1. In brief, participants with diabetes mellitus comprised 10±4% (HIV positive, 9±4%; HIV negative, 13±3%) across the studies. Participants with hypertension comprised 32±13% (HIV positive, 30±13%; HIV negative, 43±15%). Mean systolic blood pressure across the studies was 123±5 mm Hg for HIV‐positive participants, compared with 125±3 mm Hg for HIV‐negative participants. Smokers comprised 36±20% (HIV positive, 45±18%; HIV negative, 20±14%). The mean FRS for HIV‐positive participants was 6±3 compared with 18±5 (P<0.001) for HIV‐negative participants. The percentage of HIV‐positive participants on ART was 91±12%. Mean duration of ART and mean CD4 count were 6±5 years and 543±89 cells/µL, respectively.
The clinical characteristics were similar when restricting to the 15 studies with both HIV‐positive participants and HIV‐negative controls. The HIV‐positive participants had a higher prevalence of smoking (38±14%; HIV negative, 20±15%), and similar prevalence of diabetes mellitus (9±5% versus 13±3%) and hypertension (38±11% versus 43±13%), compared with HIV‐negative participants. Eight of the studies that included both HIV‐positive and HIV‐negative participants reported FRS scores, which yielded similar findings to the overall analyses (HIV positive, 9±3; HIV negative, 18±5; P=0.002).
CAC Data
The details of CAC measurements, including average CAC score in patients with HIV, in the studies are provided in Table S2. The pooled estimate of percentage with presence of CAC (CAC >0), restricting to 12 studies with HIV‐negative controls, was 45% (43%–47%) for HIV‐positive participants versus 52% (50%–53%) for HIV‐negative participants (Figure 1). The difference was not statistically significant after accounting for a difference in FRS between HIV‐positive and HIV‐negative participants (P=0.23). The predicted prevalence of CAC presence adjusting to FRS value of 8 (the average FRS across 7 studies reporting both FRS and CAC data) was 44% (34%–53%) for HIV‐positive participants and 36% (25%–46%) for HIV‐negative participants. The results were comparable when pooling all the studies with available information on presence of CAC (Figure S2). The combined estimate of the proportion of patients with CAC score >100, restricting to studies that included HIV‐negative controls, was 18% (16%–20%) for HIV‐positive participants and 19% (16%–21%) for HIV‐negative participants (Figure S3). There was significant heterogeneity across the studies for the outcomes assessed (P<0.001; I2>75%). The pooled percentage of CAC progression among HIV‐positive individuals was 13% (11%–16%) among studies that defined CAC progression as development of new CAC only, 13% (9%–17%) among studies that defined CAC progression as significant change in CAC values only, and 21% (18%–24%) among studies that used a combination of both definitions (Figure S4). The odds ratio of plaque progression comparing HIV‐positive versus HIV‐negative participants was 1.64 (95% CI, 0.91–2.37) for the 2 studies that made direct comparisons (Figure S5).
Figure 1. Meta‐analysis of prevalence of coronary calcium >0 by HIV status*.
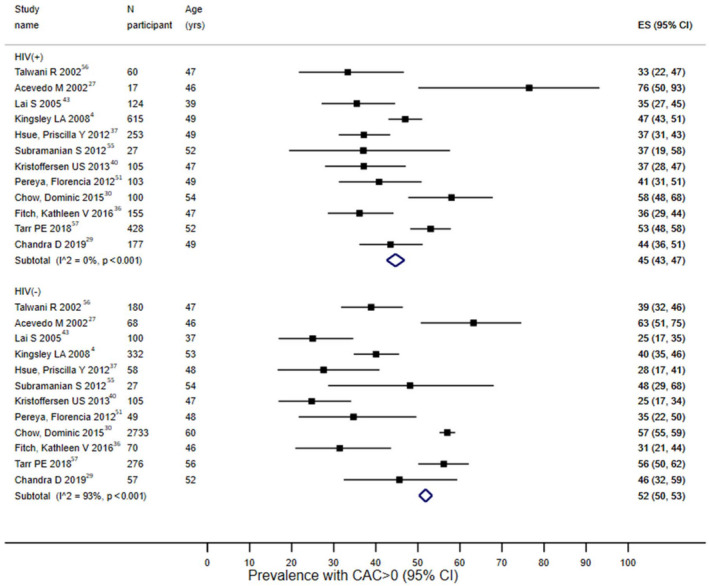
Black boxes represent the prevalence estimates and the horizontal bars about are for the 95% CIs. The blue diamond is for the pooled prevalence estimate and 95% CI. *Analyses restricted to studies that recruited both HIV+ cases and HIV− controls. CAC indicates coronary artery calcium; and ES, prevalence.
Coronary Plaque Data
All the studies reporting coronary plaque data included both HIV‐positive participants and HIV‐negative controls, except for 1 study,41 which was excluded from these analyses. The pooled estimate across the studies for percentage of participants with presence of plaque on coronary CT angiogram was 64% (95% CI, 61%–67%) versus 64% (95% CI, 60%–67%) for the HIV‐positive versus HIV‐negative participants, respectively, with significant heterogeneity across the studies (P<0.001; I2>75%; Figure S6). The predicted prevalence for presence of coronary plaque adjusting to an FRS value of 9 (the average FRS across 6 studies reporting both FRS and coronary plaque data) was 53% (95% CI, 48%–57%) for HIV‐positive participants and 48% (95% CI, 42%–54%) for HIV‐negative participants (P=0.13). The pooled prevalence of participants with calcified plaque was 31% (95% CI, 29%–34%) for those with HIV and 41% (95% CI, 37%–45%) for those without HIV (Figure 2A). By contrast, the corresponding estimate for noncalcified plaque was 49% (95% CI, 47%–52%) for those with HIV and 20% (95% CI, 17%–23%) for those without HIV (Figure 2B). There was significant heterogeneity among the few studies in each subgroup. The pooled odds ratio for presence of plaque (HIV positive versus HIV negative) across 3 studies was 1.09 (95% CI, 1.00–1.17). The corresponding pooled odds ratio estimates for presence of calcified and noncalcified plaque were 0.79 (95% CI, 0.65–0.92) and 1.23 (95% CI, 1.08–1.38), respectively (Figure 3).
Figure 2. Meta‐analysis of prevalence of calcified and non‐calcified plaque prevalence by HIV status*.
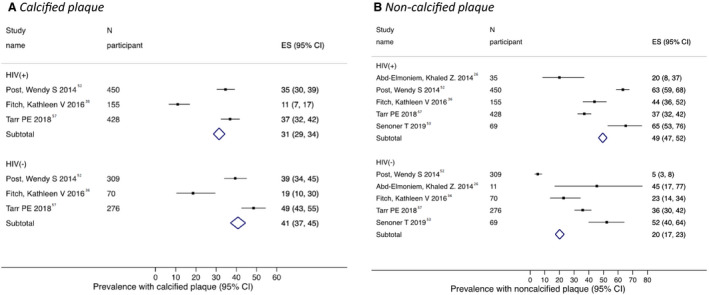
Conventions as per Figure 1. *A, calcified plaque; B, non‐calcified plaque. Analyses restricted to studies that recruited both HIV‐positive cases and HIV‐negative controls.
Figure 3. Meta‐analysis of odds ratio of plaque presence (HIV‐positive vs HIV‐negative) by type of plaque.
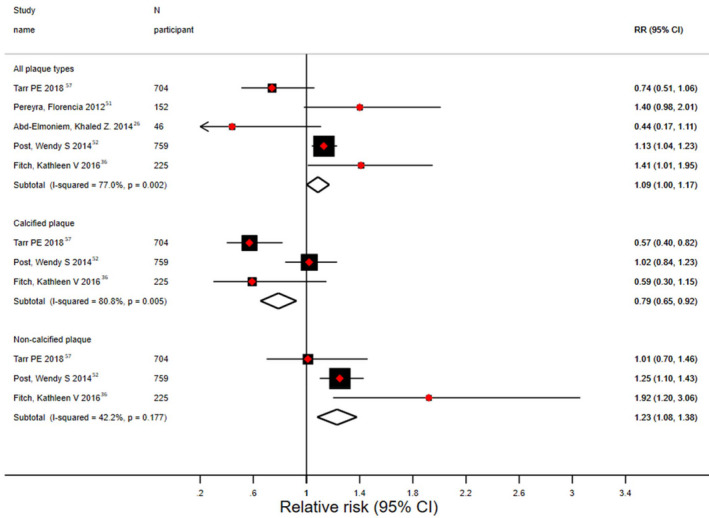
RR indicates relative risk. Red diamonds represent the effect estimates (odds ratios) and the horizontal bars about are for the 95% CIs. The size of the black boxes is proportional to the inverse variance. The black diamond is for the pooled odds ratio estimate and 95% CI—the upper diamond represents random‐effects model estimate and the lower diamond represents fixed‐effect model estimate.
Heterogeneity Assessment
There was evidence of significant heterogeneity across the studies for most of the assessed outcomes, except for odds ratio estimate for noncalcified plaque (where the heterogeneity Q test was not statistically significant and I2 was <50%). Using meta‐regression to assess the source of heterogeneity identified the average age of participants, average FRS, and proportion of male participants were significantly associated with presence of CAC or coronary plaque (direct) across the studies. The proportion of Black participants was significantly associated (inverse) with presence of CAC but not presence of coronary plaque. These identified variables explained between 23% and 81% of between‐study variation in the meta‐regression model (Figures 4 and 5). We also assessed the proportion of participants with hypertension or participants who were smokers, and for HIV‐positive participants only, proportion on ART and average CD4 count in relation to prevalence of CAC >0 in the studies in meta‐regression analyses. Except for proportion of participants with hypertension, the other variables assessed were not significantly associated with presence of CAC across the studies (Figure S7). A substantial amount of heterogeneity still remained across the studies after accounting for these study‐level characteristics. Additional analyses subgrouping the studies on the basis of average values of several variables that affect CAC (including age, percentage male, FRS, percentage with diabetes mellitus) into studies with less than the median value and those with greater than the median value were not able to account for further heterogeneity (Figures S8 and S9).
Figure 4. Meta‐regression of coronary calcium presence study estimates by various study‐level characteristics.
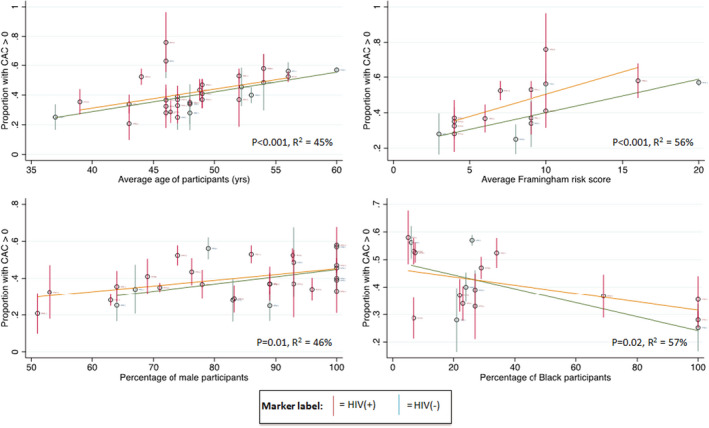
The circles represent prevalence estimates for each study and the vertical bars represent 95% CIs. The red bars represent estimates for HIV‐positive participants, and the green bar represents estimates for HIV‐negative participants. The orange and green transverse lines were fitted using analytical weights of each estimate for HIV‐positive and HIV‐negative participants, respectively. R2 represents the proportion of the between study variance that is explained by the X‐axis variable. CAC, coronary artery calcium.
Figure 5. Meta‐regression of plaque burden study estimates by various study‐level characteristics.

Conventions as per Figure 4.
Study Quality and Risk of Bias Assessment
The Newcastle‐Ottowa scoring system was used to assess the risk of bias among the articles included for analysis. The majority of the studies were of at least moderate quality (see Table and Table S3), indicating mild to moderate risk for bias. Eyeballing of funnel plot and Egger test for bias did not suggest presence of significant publication bias (Figure S10). Influence analyses did not indicate presence of undue effect on the pooled estimate for any single study (data available from authors).
Discussion
In a meta‐analysis of observational studies, which included 6699 HIV positive and 4168 HIV from 27 unique studies reporting on the prevalence of CAC and coronary plaque, we found similar combined prevalence of CAC and total coronary plaque between HIV‐positive and HIV‐negative participants, despite the younger age and overall lower traditional CVD risk factor burden among HIV participants. In addition, there was lower prevalence of calcified plaque and higher prevalence of noncalcified plaque in those with HIV versus HIV‐negative controls. Available data on CAC progression were not conclusive. There was substantial heterogeneity across the studies for most outcomes evaluated, which was partly explained by differences in study‐level characteristics, including the average age of participants, the proportion of male participants, the mean FRS, and the proportion of Black participants.
The lower FRS among those with HIV across the studies reflects the overall lower burden of traditional risk factor profile, including younger age and lower prevalence of hypertension; on the other hand, there were substantially more smokers and fewer men in the HIV group across the studies. Traditional risk factors appeared to contribute to subclinical CVD as demonstrated by the results of the meta‐regression that showed a significant amount of the observed between‐study heterogeneity was explained by some of these factors (eg, age, FRS). The comparable CAC and total plaque prevalence between HIV‐positive and HIV‐negative participants, and the higher prevalence of noncalcified plaque despite a lower overall risk‐factor profile in HIV participants, is consistent with prior reports about the role of nontraditional risk factors, such as low‐grade inflammations associated with HIV infection and adverse effect of ART, that contribute to pathogenesis of CVD in PLHIV. 2 , 60 , 61 The higher occurrence of noncalcified plaque in those with HIV may be partly attributable to the higher proportion of Black participants, 62 who are known to have a higher risk burden of noncalcified plaque, as well as higher inflammatory milieu in those with HIV. 60 Smoking, which was more prevalent among those with HIV, may have likely contributed to the increased prevalence of all plaque types. 63 , 64 However, we did not find significant association between prevalence of smokers and CAC prevalence across the studies, which may be attributable to lack of accurate measurement of smoking (such as current versus former smoker and degree of smoking), and also attributable to inherent limitation in the power of ecological association in meta‐regression. Similarly, we did not find significant association between ART percentage or average CD4 count and CAC prevalence in those with HIV. This may also reflect the limitations of ecological associations in meta‐regression. It may also be attributable to the presumably opposite effects of high ART percentage and higher CD4 count on coronary artery disease, which may partly negate each other. 57 Our findings are consistent with prior literature, including an earlier, more limited review published in 2015, which found that participants with HIV have a higher prevalence of noncalcified plaques, which may be more prone to erosion and rupture. 4 , 5 , 6 , 10 , 29 , 32 , 33 In prior studies assessing actual cardiovascular events in patients with HIV compared with patients without HIV, there seems to be a consensus that patients with HIV are at increased risk of coronary events, in the range of 1.25‐ to 1.75‐fold higher risk. 65 , 66 , 67 , 68 , 69 , 70 The higher prevalence of noncalcified plaque and possibly earlier onset of CAC (as suggested by prior studies and this meta‐analysis), may explain the higher risk of CVD observed in patients with HIV. 70 , 71
Our findings have a number of important potential implications. Although the use of CAC as a risk assessment tool for coronary events is well studied and validated, 3 screening patients with HIV for the presence of CAC may not fully estimate their risk, as they are more likely to have noncalcified plaques 6 that would not be included in CAC scores. Recent emerging data indicate that noncalcified plaques are more vulnerable to rupture than harder, more calcified plaques and thus more likely to lead to myocardial infarction. 72 Furthermore, screening for coronary artery disease using single CAC measurement may not be able to fully capture the risk profile in patients with HIV compared with patients without HIV. There has been prior research on the predictive value of CVD risk score in patients with HIV including FRS, Data Collection on Adverse Effects of Anti‐HIV Drugs, the American College of Cardiology/American Heart Association pooled cohort equations, Systematic Coronary Risk Evaluation high‐risk equation, and Systematic Coronary Risk Evaluation for the Netherlands. Studies comparing these scores, which do not incorporate CAC imaging data, have reported conflicting levels of agreement. 73 , 74 , 75 , 76 , 77 However, most studies included in this systematic review did not report on these risk score measures (aside from FRS); hence, these variables could not be included in subgroup analysis and meta‐regression. Future screening and imaging protocols and risk calculators that take the presence of CAC and nature of coronary plaque into account may have utility to fully capture this increased risk profile in patients with HIV and may need to be considered starting at a younger age for individuals with HIV.
There are several strengths to this review. First, the literature search was comprehensive, yielding the largest data set to date on CAC and coronary plaque in patients with HIV. Second, we used a rigorous process to identify multiple publications of the same study to avoid bias. Of 64 articles originally deemed eligible, 27 unique studies were retained after removing multiple publications. Third, we evaluated multiple subclinical measures of coronary atherosclerosis, including mean CAC, presence of any CAC, high CAC burden (CAC >100), and longitudinal data for CAC progression, as well as various types of coronary plaque—all plaque, noncalcified plaque, and calcified plaque. Finally, we performed extensive subgroup analyses and sensitivity analyses as well as bias assessment, which suggested the robustness of the findings.
The limitations of this meta‐analysis merit consideration. First, there was significant heterogeneity across the studies, leading to wider CIs of the pooled estimates and limiting the generalizability of the findings. We performed heterogeneity analyses and identified factors that explained some of the between‐study differences. Second, the studies included were generally small in size, and nearly half of the studies did not have HIV‐negative controls for comparison. Nonetheless, analyses restricting to studies with both HIV‐positive and HIV‐negative participants yielded comparable results. In addition, we collected detailed information on the characteristics of the HIV‐positive and HIV‐negative participants in each study—including age, sex composition, race, cardiovascular risk factors, and FRS—by pooling these factors for the HIV‐positive and HIV‐negative groups we have attempted to get a sense of the risk factor profile of the comparison groups. Third, data were particularly limited for progression of CAC and nature of plaque in patients with HIV, further reducing the power of the meta‐analysis. Not all articles provided all of our desired measures of subclinical atherosclerosis, further limiting analyses. Fourth, statin usage is known to be associated with increased CAC scores 78 ; however, studies reporting data on statin usage were too few to adjust for this variable in meta‐regression. Fifth, the current study is a literature‐based meta‐analysis, and hence it was not possible to analyze individual data. Finally, while current knowledge suggests that noncalcified plaques are more likely to rupture and cause heart attacks, having actual outcomes data to correlate this with the plaque data in prospective studies would strengthen the relevance of these findings. Further larger studies investigating progression and nature of atherosclerosis are needed.
Conclusions
In the present meta‐analysis, we found that participants with HIV had a similar likelihood for having CAC and total coronary plaque as HIV‐negative individuals, with higher prevalence of noncalcified plaque and lower burden of calcified plaque, despite their younger age and lower overall burden of traditional cardiovascular risk factors. Together, these data suggest that PLHIV have higher burden of noncalcified plaques and may develop subclinical atherosclerosis at younger age. Data on CAC progression were more limited. This meta‐analysis was limited by the availability of only few studies for the individual outcomes, significant heterogeneity across studies, aggregate nature of the data, and lack of assessment of clinical CVD events. Thus, conclusions drawn must be interpreted in light of the limitations of the available data. Future large‐scale longitudinal studies with HIV‐negative controls, serial measurement of CAC score and coronary plaque are needed to further assess the characteristics of subclinical atherosclerosis in PLHIV. When possible, ascertainment of incident CVD events in relation to CAC and coronary plaque will help further elucidate the question.
Sources of Funding
This research reported/outlined here was funded by the Department of Veterans Affairs, Veterans Health Administration, and VISN‐1 Career Development award to Dr Erqou. Dr Erqou was also supported by Providence/Boston Center for Aids Research (P30 AI042853), the Rhode Island Foundation, and Lifespan Cardiovascular Institute. This work is partially supported (investigator's time, effort, and publication cost) by the Department of Veterans Affairs Health Service Research and Development Merit Review grant IRP 20‐003 (Dr Wu). The work is also supported (investigator's time and effort) by a Research Project Grant from the National Institutes of Health; the National Heart, Lung, and Blood Institute R01HL139795 (Dr Morrison), an Institutional Development Award from the National Institutes of Health National Institute of General Medical Sciences P20GM103652 (Dr Morrison), and Career Development Award 7IK2BX002527 from the Department of Veterans Affairs Biomedical Laboratory Research and Development Program (Dr Morrison). Drs Yuyun, Morrison, Wu, and Erqou are employees of the Department of Veterans Affair. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Disclosures
None.
Supporting information
Tables S1–S3
Figures S1–S10
Acknowledgments
Author contributions: Dr Soares developed the study concept, extracted data, wrote the first draft of the manuscript. Drs Amjad Samara and Ahamd Samara extracted data and critically revised the manuscript. Drs Yuyun, Echouffo‐Tcheugui, Masri, Morrison, Lin, and Wu provided project guidance and critically revised the manuscript. Dr Erqou conceived the project idea, supervised data extraction, analyzed data, and critically revised the manuscript.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019291
For Sources of Funding and Disclosures, see page 12.
Footnotes
References
- 1. Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So‐Armah K, Freiberg MS, Lloyd‐Jones DM. Patterns of cardiovascular mortality for HIV‐infected adults in the United States: 1999 to 2013. Am J Cardiol. 2016;117:214–220. DOI: 10.1016/j.amjcard.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–147. DOI: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 3. Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. DOI: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4. Kingsley LA, Cuervo‐Rojas J, Munoz A, Palella FJ, Post W, Witt MD, Budoff M, Kuller L. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: multicenter AIDS cohort study. AIDS. 2008;22:1589–1599. DOI: 10.1097/QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raggi P, Corwin C. Heart aging measured with coronary artery calcium scoring and cardiovascular risk assessment algorithms in HIV infected patients. Virulence. 2017;8:539–544. DOI: 10.1080/21505594.2016.1212154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D’Ascenzo F, Cerrato E, Calcagno A, Grossomarra W, Ballocca F, Omedè P, Montefusco A, Veglia S, Barbero U, Gili S, et al. High prevalence at computed coronary tomography of non‐calcified plaques in asymptomatic HIV patients treated with HAART: a meta‐analysis. Atherosclerosis. 2015;240:197–204. DOI: 10.1016/j.atherosclerosis.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 7. Guaraldi G, Zona S, Alexopoulos N, Orlando G, Carli F, Ligabue G, Fiocchi F, Lattanzi A, Rossi R, Modena M, et al. Coronary aging in HIV‐infected patients. Clin Infect Dis. 2009;49:1756–1762. DOI: 10.1086/648080. [DOI] [PubMed] [Google Scholar]
- 8. Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: a systematic review and meta‐analysis of observational studies. Heart. 2009;95:1826–1835. DOI: 10.1136/hrt.2009.177774. [DOI] [PubMed] [Google Scholar]
- 9. Zona S, Raggi P, Bagni P, Orlando G, Carli F, Ligabue G, Scaglioni R, Rossi R, Modena MG, Guaraldi G. Parallel increase of subclinical atherosclerosis and epicardial adipose tissue in patients with HIV. Am Heart J. 2012;163:1024–1030. DOI: 10.1016/j.ahj.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 10. Kingsley LA, Deal J, Jacobson L, Budoff M, Witt M, Palella F, Calhoun B, Post WS. Incidence and progression of coronary artery calcium in HIV‐infected and HIV‐uninfected men. AIDS. 2015;29:2427–2434. DOI: 10.1097/QAD.0000000000000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Volpe GE, Tang AM, Polak JF, Mangili A, Skinner SC, Wanke CA. Progression of carotid intima‐media thickness and coronary artery calcium over 6 years in an HIV‐infected cohort. J Acquir Immune Defic Syndr. 2013;64:51–57. DOI: 10.1097/QAI.0b013e31829ed726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lai H, Detrick B, Fishman EK, Gerstenblith G, Brinker JA, Hollis BW, Bartlett J, Cofrancesco J, Tong W, Tai H, et al. Vitamin D deficiency is associated with the development of subclinical coronary artery disease in African Americans with HIV infection: a preliminary study. J Investig Med. 2012;60:7. DOI: 10.2310/JIM.0b013e318250bf99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baker JV, Hullsiek KH, Singh A, Wilson E, Henry K, Lichtenstein K, Onen N, Kojic E, Patel P, Brooks JT, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS. 2014;28:831–840. DOI: 10.1097/QAD.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tarr PE, Ledergerber B, Calmy A, Doco‐Lecompte T, Schoepf IC, Marzel A, Weber R, Kaufmann PA, Nkoulou R, Buechel RR, et al. Longitudinal progression of subclinical coronary atherosclerosis in Swiss HIV‐positive compared with HIV‐negative persons undergoing coronary calcium score scan and CT angiography. Open Forum Infect Dis. 2020;7:ofaa438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guaraldi G, Scaglioni R, Zona S, Orlando G, Carli F, Ligabue G, Besutti G, Bagni P, Rossi R, Modena MG, et al. Epicardial adipose tissue is an independent marker of cardiovascular risk in HIV‐infected patients. AIDS. 2011;25:1199–1205. DOI: 10.1097/QAD.0b013e3283474b9f. [DOI] [PubMed] [Google Scholar]
- 16. Guaraldi G, Zona S, Orlando G, Carli F, Ligabue G, Fiocchi F, Rossi R, Modena MG, Raggi P. Progression of coronary artery calcium in men affected by human immunodeficiency virus infection. Int J Cardiovasc Imaging. 2012;28:935–941. DOI: 10.1007/s10554-011-9898-y. [DOI] [PubMed] [Google Scholar]
- 17. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. DOI: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–159. DOI: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rice K, Higgins JPT, Lumley T. A re‐evaluation of fixed effect(s) meta‐analysis. J R Stat Soc Ser A Stat Soc. 2017;181:205–227. DOI: 10.1111/rssa.12275. [DOI] [Google Scholar]
- 20. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta‐analysis of prevalence. J Epidemiol Community Health. 2013;67:974–978. DOI: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. DOI: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emerging Risk Factors C , Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. DOI: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. DOI: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lo CK, Mertz D, Loeb M. Newcastle‐Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. DOI: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. DOI: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abd‐Elmoniem KZ, Unsal AB, Eshera S, Matta JR, Muldoon N, McAreavey D, Purdy JB, Hazra R, Hadigan C, Gharib AM. Increased coronary vessel wall thickness in HIV‐infected young adults. Clin Infect Dis. 2014;59:1779–1786. DOI: 10.1093/cid/ciu672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Acevedo M, Sprecher D, Calabrese L, Pearce G, Coyner D, Halliburton S, White R, Sykora E, Kondos G, Hoff J. Pilot study of coronary atherosclerotic risk and plaque burden in HIV patients: “a call for cardiovascular prevention.” Atherosclerosis. 2002;163:6. DOI: 10.1016/S0021-9150(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 28. Besutti G, Raggi P, Zona S, Scaglioni R, Santoro A, Orlando G, Ligabue G, Leipsic J, Sin DD, Man S, et al. Independent association of subclinical coronary artery disease and emphysema in HIV‐infected patients. HIV Med. 2016;17:178–187. DOI: 10.1111/hiv.12289. [DOI] [PubMed] [Google Scholar]
- 29. Chandra D, Gupta A, Fitzpatrick M, Haberlen SA, Neupane M, Leader JK, Kingsley LA, Kleerup E, Budoff MJ, Witt M, et al. Lung function, coronary artery disease, and mortality in HIV. Ann Am Thorac Soc. 2019;16:687–697. DOI: 10.1513/AnnalsATS.201807-460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chow D, Young R, Valcour N, Kronmal RA, Lum CJ, Parikh NI, Tracy RP, Budoff M, Shikuma CM. HIV and coronary artery calcium score: comparison of the Hawaii aging with HIV cardiovascular study and Multi‐Ethnic Study of Atherosclerosis (MESA) cohorts. HIV Clin Trials. 2015;16:130–138. DOI: 10.1179/1528433614Z.0000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crum‐Cianflone N, Krause D, Wessman D, Medina S, Stepenosky J, Brandt C, Boswell G. Fatty liver disease is associated with underlying cardiovascular disease in HIV‐infected persons(*). HIV Med. 2011;12:463–471. DOI: 10.1111/j.1468-1293.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. d’Ettorre G, Francone M, Mancone M, Ceccarelli G, Ascarelli A, Vullo F, Baroncelli S, Galluzzo MC, Catalano C, Strano S, et al. Significant coronary stenosis detected by coronary computed angiography in asymptomatic HIV infected subjects. J Infect. 2012;64:82–88. DOI: 10.1016/j.jinf.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 33. Duarte H, Matta JR, Muldoon N, Masur H, Hadigan C, Gharib AM. Non‐calcified coronary plaque volume inversely related to CD4(+) T‐cell count in HIV infection. Antivir Ther. 2012;17:763–767. DOI: 10.3851/IMP1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Falcone EL, Mangili A, Tang AM, Jones CY, Woods MN, Polak JF, Wanke CA. Micronutrient concentrations and subclinical atherosclerosis in adults with HIV. Am J Clin Nutr. 2010;91:1213–1219. DOI: 10.3945/ajcn.2009.28816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fitch K, Abbara S, Lee H, Stavrou E, Sacks R, Michel T, Hemphill L, Torriani M, Grinspoon S. Effects of lifestyle modification and metformin on atherosclerotic indices among HIV‐infected patients with the metabolic syndrome. AIDS. 2012;26:587–597. DOI: 10.1097/QAD.0b013e32834f33cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fitch KV, DeFilippi C, Christenson R, Srinivasa S, Lee H, Lo J, Lu MT, Wong K, Petrow E, Sanchez L, et al. Subclinical myocyte injury, fibrosis and strain in relationship to coronary plaque in asymptomatic HIV‐infected individuals. AIDS. 2016;30:2205–2214. DOI: 10.1097/QAD.0000000000001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsue PY, Ordovas K, Lee T, Reddy G, Gotway M, Schnell A, Ho JE, Selby V, Madden E, Martin JN, et al. Carotid intima‐media thickness among human immunodeficiency virus‐infected patients without coronary calcium. Am J Cardiol. 2012;109:742–747. DOI: 10.1016/j.amjcard.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Korada SKC, Zhao DI, Tibuakuu M, Brown TT, Jacobson LP, Guallar E, Bolan RK, Palella FJ, Margolick JB, Martinson JJ, et al. Frailty and subclinical coronary atherosclerosis: the multicenter AIDS cohort study (MACS). Atherosclerosis. 2017;266:240–247. DOI: 10.1016/j.atherosclerosis.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krishnam M, Chae EJ, Hernandez‐Rangel E, Karangiah E, Gupta G, Budoff M. Utility of routine non‐gated CT chest in detection of subclinical atherosclerotic calcifications of coronary arteries in hospitalised HIV patients. Br J Radiol. 2020;93:20190462. DOI: 10.1259/bjr.20190462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kristoffersen US, Lebech AM, Wiinberg N, Petersen CL, Hasbak P, Gutte H, Jensen GB, Hag AM, Ripa RS, Kjaer A. Silent ischemic heart disease and pericardial fat volume in HIV‐infected patients: a case‐control myocardial perfusion scintigraphy study. PLoS One. 2013;8:e72066. DOI: 10.1371/journal.pone.0072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lai S, Bartlett J, Lai H, Moore R, Cofrancesco JJ, Pannu H, Tong W, Meng W, Sun H, Fishman E. Long‐term combination antiretroviral therapy is associated with the risk of coronary plaques in African Americans with HIV infection. AIDS Patient Care STDS. 2009;23:10. DOI: 10.1089/apc.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lai S, Fishman EK, Gerstenblith G, Brinker J, Tai H, Chen S, Li J, Tong W, Detrick B, Lai H. Vitamin D deficiency is associated with coronary artery calcification in cardiovascularly asymptomatic African Americans with HIV infection. Vasc Health Risk Manag. 2013;9:493–500. DOI: 10.2147/VHRM.S48388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lai S, Lima J, Lai H, Vlahov D, Celentano D, Tong W, Bartlett J, Margolick J, Fishman E. Human immunodeficiency virus 1 infection, cocaine, and coronary calcification. Arch Intern Med. 2005;165:6. DOI: 10.1001/archinte.165.6.690. [DOI] [PubMed] [Google Scholar]
- 44. Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, Lederman MM, Storer N, Labbato DE, McComsey GA. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014;28:969–977. DOI: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mangili A, Jacobson DL, Gerrior J, Polak JF, Gorbach SL, Wanke CA. Metabolic syndrome and subclinical atherosclerosis in patients infected with HIV. Clin Infect Dis. 2007;44:1368–1374. DOI: 10.1086/516616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Metkus TS, Brown T, Budoff M, Kingsley L, Palella FJ Jr, Witt MD, Li X, George RT, Jacobson LP, Post WS. HIV infection is associated with an increased prevalence of coronary noncalcified plaque among participants with a coronary artery calcium score of zero: multicenter AIDS cohort study (MACS). HIV Med. 2015;16:635–639. DOI: 10.1111/hiv.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Monroe AK, Dobs AS, Xu X, Palella FJ, Kingsley LA, Post WS, Witt MD, Brown TT. Low free testosterone in HIV‐infected men is not associated with subclinical cardiovascular disease. HIV Med. 2012;13:358–366. DOI: 10.1111/j.1468-1293.2011.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Monteiro V, Lacerda H, Uellendahl M, Chang T, Maria de Albuquerque V, Zirpoli J, Ximenes R, Militão de Albuquerque M, Filho D, Filho D. Calcium score in the evaluation of atherosclerosis in patients with HIV/AIDS. Arq Bras Cardiol. 2011;97:7. [DOI] [PubMed] [Google Scholar]
- 49. Nadel J, O'Dwyer E, Emmanuel S, Huang J, Cheruvu S, Sammel N, Brew B, Otton J, Holloway CJ. High‐risk coronary plaque, invasive coronary procedures, and cardiac events among HIV‐positive individuals and matched controls. J Cardiovasc Comput Tomogr. 2016;10:391–397. DOI: 10.1016/j.jcct.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 50. Pereira B, Mazzitelli M, Milinkovic A, Moyle G, Ranasinghe S, Mandalia S, Pozniak A, Asboe D, Nelson M, Al‐Hussaini A, et al. Use of coronary artery calcium scoring to improve cardiovascular risk stratification and guide decisions to start statin therapy in people living with HIV. J Acquir Immune Defic Syndr. 2020;85:98–105. DOI: 10.1097/QAI.0000000000002400. [DOI] [PubMed] [Google Scholar]
- 51. Pereyra F, Lo J, Triant VA, Wei J, Buzon MJ, Fitch KV, Hwang J, Campbell JH, Burdo TH, Williams KC, et al. Increased coronary atherosclerosis and immune activation in HIV‐1 elite controllers. AIDS. 2012;26:2409–2412. DOI: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Post WS, Budoff M, Kingsley L, Palella FJ Jr, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458–467. DOI: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Senoner T, Barbieri F, Adukauskaite A, Sarcletti M, Plank F, Beyer C, Dichtl W, Feuchtner GM. Coronary atherosclerosis characteristics in HIV‐infected patients on long‐term antiretroviral therapy: insights from coronary computed tomography‐angiography. AIDS. 2019;33:1853–1862. DOI: 10.1097/QAD.0000000000002297. [DOI] [PubMed] [Google Scholar]
- 54. Shikuma CM, Barbour JD, Ndhlovu LC, Keating SM, Norris PJ, Budoff M, Parikh N, Seto T, Gangcuangco LM, Ogata‐Arakaki D, et al. Plasma monocyte chemoattractant protein‐1 and tumor necrosis factor‐alpha levels predict the presence of coronary artery calcium in HIV‐infected individuals independent of traditional cardiovascular risk factors. AIDS Res Hum Retroviruses. 2014;30:142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. DOI: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Talwani R, Falusi O, Mendes de Leon C, Nerad J, Rich S, Proia L, Sha B, Smith K, Kessler H. Electron beam computed tomography for assessment of coronary artery disease in HIV‐infected men receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;30:5. DOI: 10.1097/00042560-200206010-00008. [DOI] [PubMed] [Google Scholar]
- 57. Tarr PE, Ledergerber B, Calmy A, Doco‐Lecompte T, Marzel A, Weber R, Kaufmann PA, Nkoulou R, Buechel RR, Kovari H, et al. Subclinical coronary artery disease in Swiss HIV‐positive and HIV‐negative persons. Eur Heart J. 2018;39:2147–2154. DOI: 10.1093/eurheartj/ehy163. [DOI] [PubMed] [Google Scholar]
- 58. Vilela FD, de Lorenzo AR , Tura BR, Ferraiuoli GI, Hadlich M, de Lima Barros MV , Ribeiro Lima AB, Meirelles V. Risk of coronary artery disease in individuals infected with human immunodeficiency virus. Braz J Infect Dis. 2011;15:521–527. DOI: 10.1016/S1413-8670(11)70245-8. [DOI] [PubMed] [Google Scholar]
- 59. Falcone EL, Mangili A, Skinner S, Alam A, Polak JF, Wanke CA. Framingham Risk Score and early markers of atherosclerosis in a cohort of adults infected with HIV. Antivir Ther. 2011;16:1–8. DOI: 10.3851/IMP1682. [DOI] [PubMed] [Google Scholar]
- 60. Kuller LH, Tracy R, Belloso W, Wit SD, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. DOI: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV‐infected adults. J Infect Dis. 2012;205(suppl 3):S375–S382. DOI: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nance JW Jr, Bamberg F, Schoepf UJ, Kang DK, Barraza JM Jr, Abro JA, Bastarrika G, Headden GF, Costello P, Thilo C. Coronary atherosclerosis in African American and white patients with acute chest pain: characterization with coronary CT angiography. Radiology. 2011;260:373–380. DOI: 10.1148/radiol.11110158. [DOI] [PubMed] [Google Scholar]
- 63. Hou ZH, Lu B, Li ZN, An YQ, Gao Y, Yin WH. Coronary atherosclerotic plaque volume quantified by computed tomographic angiography in smokers compared to nonsmokers. Acad Radiol. 2019;26:1581–1588. DOI: 10.1016/j.acra.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 64. Cheezum MK, Kim A, Bittencourt MS, Kassop D, Nissen A, Thomas DM, Nguyen B, Glynn RJ, Shah NR, Villines TC. Association of tobacco use and cessation with coronary atherosclerosis. Atherosclerosis. 2017;257:201–207. DOI: 10.1016/j.atherosclerosis.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Freiberg MS, Chang C‐C, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. DOI: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Friis‐Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El‐Sadr WM, Reiss P, Thiebaut R, Morfeldt L, De Wit S, Pradier C, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. [DOI] [PubMed] [Google Scholar]
- 67. Lang S, Mary‐Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Bingham A, Costagliola D; French Hospital Database on H‐AC . Increased risk of myocardial infarction in HIV‐infected patients in France, relative to the general population. AIDS. 2010;24:1228–1230. DOI: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 68. Mary‐Krause M, Cotte L, Simon A, Partisani M, Costagliola D; Clinical Epidemiology Group from the French Hospital Database . Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV‐infected men. AIDS. 2003;17:2479–2486. DOI: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 69. Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, Gerstoft J. Ischemic heart disease in HIV‐infected and HIV‐uninfected individuals: a population‐based cohort study. Clin Infect Dis. 2007;44:1625–1631. DOI: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 70. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. DOI: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eyawo O, Brockman G, Goldsmith CH, Hull MW, Lear SA, Bennett M, Guillemi S, Franco‐Villalobos C, Adam A, Mills EJ, et al. Risk of myocardial infarction among people living with HIV: an updated systematic review and meta‐analysis. BMJ Open. 2019;9:e025874. DOI: 10.1136/bmjopen-2018-025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid‐term follow‐up. J Am Coll Cardiol. 2015;66:337–346. DOI: 10.1016/j.jacc.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 73. Dhillon S, Sabin CA, Alagaratnam J, Bagkeris E, Post FA, Boffito M, Anderson J, Vera J, Williams I, Johnson M, et al. Level of agreement between frequently used cardiovascular risk calculators in people living with HIV. HIV Med. 2019;20:347–352. DOI: 10.1111/hiv.12731. [DOI] [PubMed] [Google Scholar]
- 74. Friis‐Møller N, Ryom L, Smith C, Weber R, Reiss P, Dabis F, De Wit S, Monforte AD, Kirk O, Fontas E, et al. An updated prediction model of the global risk of cardiovascular disease in HIV‐positive persons: the data‐collection on adverse effects of anti‐HIV drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23:214–223. DOI: 10.1177/2047487315579291. [DOI] [PubMed] [Google Scholar]
- 75. Friis‐Møller N, Thiébaut R, Reiss P, Weber R, D'Arminio Monforte A, De Wit S, El‐Sadr W, Fontas E, Worm S, Kirk O, et al. Predicting the risk of cardiovascular disease in HIV‐infected patients: the data collection on adverse effects of anti‐HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. DOI: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 76. Krikke M, Hoogeveen RC, Hoepelman AI, Visseren FL, Arends JE. Cardiovascular risk prediction in HIV‐infected patients: comparing the Framingham, Atherosclerotic Cardiovascular Disease Risk Score (ASCVD), systematic coronary risk evaluation for the Netherlands (SCORE‐NL) and data collection on adverse events of anti‐HIV drugs (D:A:D) risk prediction models. HIV Med. 2016;17:289–297. DOI: 10.1111/hiv.12300. [DOI] [PubMed] [Google Scholar]
- 77. Thompson‐Paul AM, Lichtenstein KA, Armon C, Palella FJ Jr, Skarbinski J, Chmiel JS, Hart R, Wei SC, Loustalot F, Brooks JT, et al. Cardiovascular disease risk prediction in the HIV outpatient study. Clin Infect Dis. 2016;63:1508–1516. DOI: 10.1093/cid/ciw615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Osei AD, Mirbolouk M, Berman D, Budoff MJ, Miedema MD, Rozanski A, Rumberger JA, Shaw L, Al Rifai M, Dzaye O, et al. Prognostic value of coronary artery calcium score, area, and density among individuals on statin therapy vs. non‐users: the Coronary Artery Calcium Consortium. Atherosclerosis. 2021;316:79–83. DOI: 10.1016/j.atherosclerosis.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S10


