Abstract
An increasing number of individuals travel to mountainous environments for work and pleasure. However, oxygen availability declines at altitude, and hypoxic environments place unique stressors on the cardiovascular system. These stressors may be exacerbated by exercise at altitude, because exercise increases oxygen demand in an environment that is already relatively oxygen deplete compared with sea‐level conditions. Furthermore, the prevalence of cardiovascular disease, as well as diseases such as hypertension, heart failure, and lung disease, is high among individuals living in the United States. As such, patients who are at risk of or who have established cardiovascular disease may be at an increased risk of adverse events when sojourning to these mountainous locations. However, these risks may be minimized by appropriate pretravel assessments and planning through shared decision‐making between patients and their managing clinicians. This American Heart Association scientific statement provides a concise, yet comprehensive overview of the physiologic responses to exercise in hypoxic locations, as well as important considerations for minimizing the risk of adverse cardiovascular events during mountainous excursions.
Keywords: AHA Scientific Statements, altitude, cardiovascular diseases, exercise, heart failure, hypertension, sudden cardiac death, syncope
More than 100 million people travel to mountainous environments yearly for work or pleasure. Mountain activities frequently involve exercise, which when combined with environmental changes such as temperature, humidity, pressure, and oxygen availability place unique challenges on the cardiovascular system (Figure 1). The high prevalence of cardiovascular disease (≈18 million), hypertension (≈108 million), and heart failure (≈6 million) in the United States, as well as pulmonary hypertension, places many patients at risk of adverse events in these environments. Medical resources are frequently limited, which may compromise outcomes following adverse events. Therefore, it is incumbent on clinicians to understand the unique physiologic challenges associated with exercising in hypoxic environments, and through shared decision‐making processes with patients, to assess the likelihood of adverse events; use necessary strategies, including pretravel assessments; review of travel plans, including location, duration, and type of activities planned; determine evacuation options and identify local medical facilities in the event of emergencies; and adjust medications as indicated to reduce the risk of cardiovascular events from occurring. A staged ascent and acclimatization period may be prudent to reduce the risks of adverse events. The primary objective of this American Heart Association scientific statement is to provide a concise review of the physiology of altitude and hypoxia among individuals with cardiovascular disease, as well as scientific evidence on best practices for managing at‐risk patients who are planning excursions to mountainous locations.
Figure 1. Environmental changes at altitude.
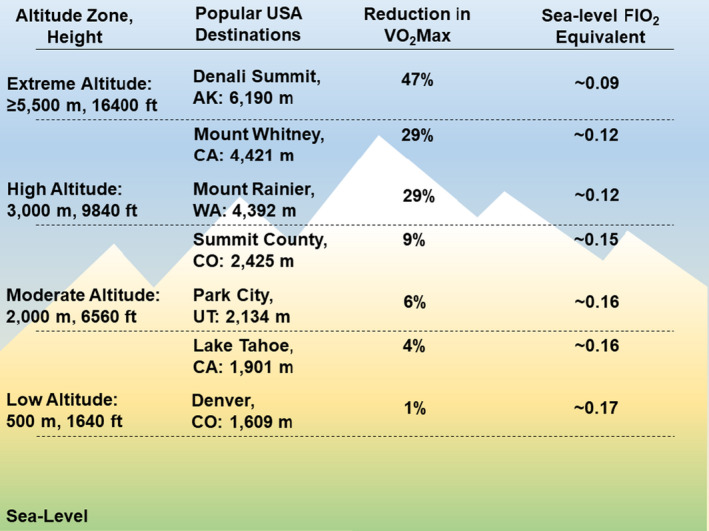
Classification of altitude ranges with associated reductions in the fraction of inspired oxygen (Fio 2) relative to sea level and percent reduction in functional capacity according to maximal oxygen uptake (Vo 2max) as altitude increases. Popular tourist destinations are included for reference.
Physiology of High‐Altitude Exposure
Ascent to altitude is associated with reductions in atmospheric pressure and partial pressure of oxygen. Reductions in alveolar partial pressure of oxygen lower the driving pressure for pulmonary gas exchange, reducing blood oxygen content. 1 As such, hypoxic environments impose unique challenges on the cardiovascular system, particularly during exercise, when there is additional oxygen demand in an environment that is relatively oxygen deprived compared with sea level. The acute response of the cardiovascular system to hypoxia is displayed in Figure 2.
Figure 2. Physiologic response to hypoxia.
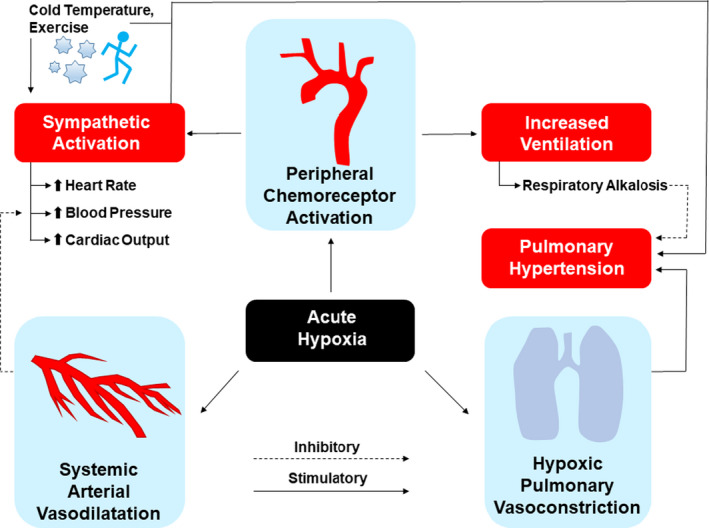
Response to hypoxia at altitude involves systemic arterial vasodilatation, hypoxic pulmonary vasoconstriction, and activation of peripheral chemoreceptors, with downstream effects including sympathetic activation, which increases blood pressure and cardiac output, as well as hyperventilation and pulmonary hypertension. Adapted from Bärtsch et al. 2 © 2007 American Heart Association, Inc.
The cardiovascular response to hypoxia is highly dynamic and evolves from acute (first few hours of exposure) to chronic adaptation as the body adjusts to sustained hypoxia over days to weeks (Figure 3). 3 The acute response is dominated by increased sympathetic tone, which results from hypoxic stimulus of the peripheral chemoreceptors. This activation of the autonomic nervous system increases in proportion to the level of hypoxia and is sustained or increases throughout the duration of altitude exposure. 1 , 2 In response to this surge in sympathetic activity and concomitant vagal withdrawal, heart rate (HR), stroke volume, and cardiac output increase acutely. Blood pressure (BP) initially declines in response to hypoxia‐induced vasodilatation, causing a decline in vascular resistance, but increases as acclimatization evolves to levels that are often higher than sea level for the duration of travel. The time course of this general pattern may be variable among individuals.
Figure 3. Cardiovascular hemodynamic changes that occur in response to acute (minutes to hours) and chronic (days to weeks) exposure to hypoxia compared with sea level.
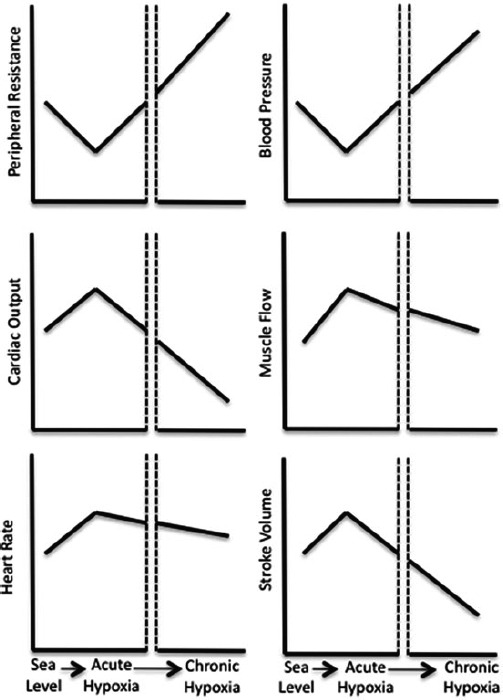
Acutely, heart rate, cardiac output, muscle flow, and stroke volume increase. However, with sustained exposure, all of these variables decrease to levels at or below sea level. Blood pressure and systemic vascular resistance decrease during acute exposure, but may increase progressively with time to levels at or above sea level values. From Baggish et al. 3 Reprinted by permission from Springer. © 2014.
The impact of a hypoxic stimulus may vary between individuals based on medical history, risk factors, and medications. However, there are a few major issues common to all patients with cardiovascular disease that must be emphasized. 1 First, reductions in oxygen availability may exacerbate symptoms. Second, the combination of hypoxia with other environmental stressors (temperature, exercise, dehydration, or injury) may precipitate an acute coronary syndrome (ACS). Third, mountainous locations are in remote environments with limited access to health care. As such, outcomes following an ACS may be worse than sea‐level conditions. Finally, sudden cardiac death (SCD) may occur. For example, the risk of SCD for hiking (1 in 780 000) and downhill skiing (1 in 630 000) at altitude is ≈4‐ and 2‐fold higher than the SCD risk among the general population, respectively, 4 especially on a per‐hour basis when compared with other endurance activities at sea level such as jogging (1 in 3 000 000). 5
Concordant with reductions in partial pressure of oxygen at altitude are reductions in functional capacity. Generally, for healthy individuals, 6 the elderly, 7 and individuals with coronary artery disease (CAD) and heart failure with reduced ejection fraction (HFrEF), 8 maximal oxygen uptake decreases ≈1% for every 100‐m increase in altitude above 1500 m. Thus, younger, healthier individuals, who have higher functional capacity at sea level, will likely tolerate an ≈25% reduction in maximal oxygen uptake at altitudes of 3500 to 4500 m. However, for older individuals or those with cardiovascular disease and heart failure who have a lower functional capacity at sea level, the same ≈25% reduction in work capacity may cause difficulty completing basic tasks such as routine activities of daily living, climbing stairs, or recreational walking. Ventilatory threshold, which represents the intensity of sustainable exercise, occurs at HRs that are comparable to sea level but at lower work intensities. 8 Therefore, pretravel planning should include knowledge of an individual's functional capacity at sea level, duration of anticipated altitude exposure, and activities planned. Development of plans to ensure a safe excursion should be tailored to the patient based on medical history (Figure 4).
Figure 4. Pretravel assessment for patients who are at risk of adverse cardiovascular events in mountainous environments.
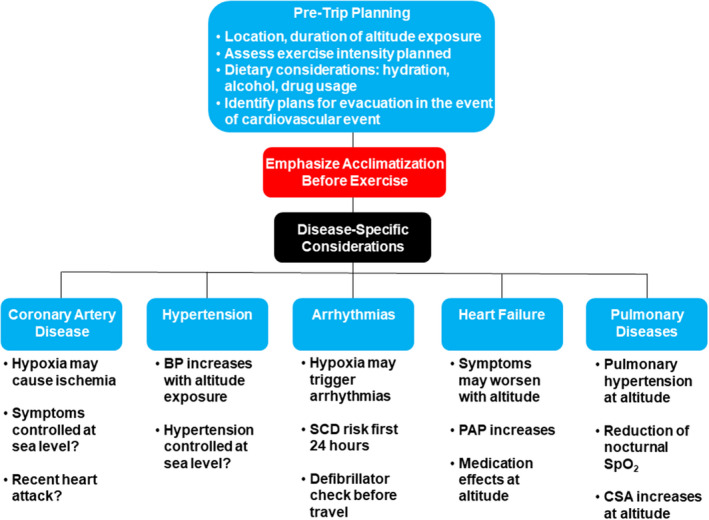
BP indicates blood pressure; CSA, central sleep apnea; PAP, pulmonary arterial pressure; SCD, sudden cardiac death; and Spo 2, systemic oxygen saturation.
Altitude Exposure Among Individuals With CAD
In healthy individuals, altitude exposure causes coronary arterial vasodilatation and increased coronary blood flow. 9 However, atherosclerosis may precipitate paradoxical vasoconstriction of coronary vessels in response to exercise and hypoxia. 1 Furthermore, myocardial oxygen demand increases, even under resting conditions, because of sympathetically mediated increases in HR and BP. Coronary vasoconstriction, combined with altitude‐dependent decreased oxygen carrying capacity and increased myocardial oxygen demand, underlie the increased tendency for ischemia at altitude. Patients with CAD and angina should anticipate that hypoxia will exacerbate anginal symptoms. Increases in cardiac work stimulated by altitude can produce new ischemic symptoms in patients with CAD at sea level. Simulated altitude (eg, the Levy test, which exposes patients to hypoxic conditions [0.10 fraction of inspired oxygen] simulating an altitude of 5486 m) at rest can unmask ischemia and has been used as an electrocardiographic stress test. 10 The Tenth Mountain Division Study found that the double‐product required to induce 1 mm of ST depression at 2500 m was 5% lower than at sea level. 7 Notably, this reduction in ischemic threshold was attenuated after 5 days of acclimatization, 7 which emphasizes the importance of acclimatization before exercise.
Patients with CAD who are asymptomatic at sea level can generally tolerate exercise at moderate altitudes without new ischemia or arrhythmias. 8 In a study of patients who recently underwent revascularization after ACS, there was no evidence of ischemia during exercise after a rapid ascent from 540 to 3454 m. 11 In another study of patients with CAD and HFrEF (left ventricular ejection fraction 39%±6%), no ischemia was induced when patients ascended from 1000 to 2500 m and exercised after 2 days of acclimatization. 8 Several studies have examined patients after revascularization at several time points (6 weeks to 18 months; average, 12±4 months) and altitudes ranging from 2500 to 3454 m. These studies suggest that patients who are >6 months removed from revascularization do not encounter new ischemia, coronary events, or stent complications at altitude. 8 , 11 , 12 , 13 , 14
Patients with established CAD should be counseled to hydrate, adhere to gradual ascent profiles, and temper increases in exercise load to facilitate acclimatization. Guideline‐compliant use of medications should be optimized and continued during sojourns. There are no data to support dose escalation or initiation of new medications before ascent in the absence of sea‐level indications. The interaction between CAD and altitude‐related illnesses such as acute mountain sickness, high‐altitude pulmonary edema, and high‐altitude cerebral edema is unknown. The use of acute hypoxic exercise testing before travel has not been clinically validated, but may be reasonable to consider based on an individual's specific risk profile.
Hypertension and BP Profiles at Altitude
Acute altitude exposure is characterized by an initial reduction in BP that results from hypoxia‐induced systemic vasodilation. However, this initial BP reduction is counterbalanced by chemoreflex‐mediated vasoconstriction, which causes BP to increase over time, especially as oxygen content increases from acclimatization. This increase in BP is proportional to the altitude achieved and remains unchanged throughout the duration of exposure. 1 , 15 The timing and magnitude of these physiological stimuli (immediate hypoxic vasodilation and chemoreflex‐mediated sympathetic activation and vasoconstriction) may vary between individuals, so that the actual integrated effect on measured BP is not entirely predictable. Such a BP increase is more evident when assessed through 24‐hour ambulatory BP monitoring (ABPM) than conventional resting BP measurements, because ABPM demonstrates dynamic responses to environmental factors. 15 In studies incorporating 24‐hour ABPM, exposure of healthy volunteers to progressively high altitudes (up to 5400 m) was associated with an acute (a few hours after exposure), persistent (at least 12 days), and prominent (10–15 mm Hg) increase in systolic and diastolic BP, which was more evident for ABPM values and in older subjects. 15 Of note, the increase in ABPM values at altitude was more significant at night and characterized by a reduction in the nocturnal BP dip that otherwise occurs at sea level, 15 , 16 perhaps enhanced by periodic breathing at altitude.
Knowledge of the effects of different antihypertensive medications on BP at altitude is derived primarily from studies performed on healthy volunteers. Treatment with the angiotensin receptor blocker telmisartan reduced daytime and nighttime BP. 15 However, the benefits were observed up to an altitude of 3400 m and disappeared at higher altitudes (5400 m), likely resulting from concomitant suppression of the renin–angiotensin system that occurs with high‐altitude exposure. 15 Among hypertensive individuals, 24‐hour ABPM found that at an altitude of 3400 m, the combination of telmisartan and the calcium channel blocker nifedipine attenuated altitude‐associated increases in BP. 17 Use of the nonselective β‐blocker/combined α‐blocker carvedilol in healthy subjects resulted in a significant attenuation of the hypertensive response at altitude, but was associated with reduced arterial hemoglobin oxygen saturation and impaired exercise tolerance, 18 perhaps by inhibiting hypoxic pulmonary vasoconstriction and worsening VA/Q mismatch. In contrast, the highly selective β1 adrenergic receptor blocker nebivolol, which also has nitric oxide donating properties, preserved the normal nocturnal BP reduction that occurs at sea level and was associated with a smaller reduction in exercise tolerance at altitude than nonselective β‐blockers. 19 Acetazolamide, a carbonic anhydrase inhibitor, antagonizes the BP rise induced by altitude exposure while also reducing the occurrence of central sleep apnea 16 and preventing acute mountain sickness.
Before travel, clinicians should ensure that BP is controlled at sea level. Medications and dosages should be reviewed, along with travel plans, including duration of travel and ascent profiles. For patients with stable BP and on short excursions (eg, several days), BP monitoring is probably unnecessary. For patients whose BP is difficult to control and who are planning prolonged trips, BP monitoring may be required, and plans developed for BP cutoffs that should trigger patients to rest and avoid strenuous activity, take additional antihypertensive medications, or descend in the event that BP remains uncontrolled. 20 For the most part, the adverse cardiovascular consequences of hypertension occur over much longer time courses than a typical altitude sojourn for a sea‐level native. Acute elevations in BP to high levels are common during resistance exercise 21 and are well tolerated except in patients with severe cardiovascular disease. Although there are no hard data to guide specific cut points, sustained BPs of >180/110 mm Hg should be considered for augmented therapy, especially for those patients with significant underlying cardiovascular disease.
Syncope at High Altitude
Hypoxia‐related syncope is a risk for mountain sojourners, aircraft passengers, and unfit individuals traveling to mountainous locations. 22 Syncope is common, even at moderate altitudes (2700 m), typically occurs <24 hours after ascent, and is likely related to peripheral vasodilatation from acute hypoxic exposure, compounded by cerebral vasoconstriction from hyperventilation‐induced hypocapnia. 23 Among healthy individuals at a simulated altitude of 3660 m, presyncope resulted from ineffective peripheral vasoconstriction refractory to increased sympathetic nerve activity (functional sympatholysis) and insufficient cerebral perfusion because of cerebral vasoconstriction as the result of hypoxic hyperventilation‐induced hypocapnia. 24 These abnormal responses were not reproduced after 14 days of acclimatization, suggesting that normal cardiovascular and cerebrovascular responses to orthostatic stress are preserved with chronic altitude exposure. 25 Postexertional syncope may also occur and is primarily related to hypoxia‐enhanced, exercise‐induced vasodilation. Among healthy men randomized to exercise by repeated bouts of cycling in normoxic versus hypoxic (14.1%) conditions, postexertional BP was significantly lower under hypoxic versus normoxic conditions (84±1 mm Hg versus 87±1 mm Hg). 26 Finally, syncope at altitude may also be associated with meals and alcohol consumption, which may enhance splanchnic or peripheral vasodilation.
Arrhythmias and SCD at Altitude
Literature on arrhythmias and SCD at altitude is limited, and even less evidence exists in patients with preexisting CAD. Among healthy people exposed to stepwise increases in simulated altitudes up to 8848 m (summit of Mt Everest) over a 40‐day period, increases in resting HR were observed (sea level versus 8848 m: 63±5 bpm versus 89±8 bpm), but there were no significant arrhythmias. 27 During exercise, there were no signs of ischemia and rare episodes of premature ventricular contractions. 27 In another study of healthy individuals, implantable loop recorders monitored HR/rhythm at altitudes >5000 m. 28 All participants reported palpitations during exercise, but there were no signs of ischemia, and palpitations were related to sinus tachycardia. 28 One individual had postexertional atrial flutter with 2:1 conduction. 28
Among patients with established CAD and normal left ventricular systolic function, who had revascularization (coronary artery bypass grafting or percutaneous coronary intervention), exercise at an altitude of up to 3500 m does not appear to provoke arrhythmias that are not present at sea level. 1 , 11 Among patients with CAD and mild to moderately reduced ejection fraction (39%±6%) who are free of arrhythmias at sea level, exercise does not appear to precipitate ischemia or new arrhythmias at up to 2500 m elevation. 8 However, the cardiac demands of exercise at altitude may be greater than anticipated. For example, one study incorporating ambulatory monitoring of downhill skiers found that two‐thirds of individuals had an HR >80% of maximum predicted HR during exercise. 29 The only study evaluating individuals with preexisting arrhythmias but who were otherwise healthy, reported no worsening of arrhythmias up to altitudes of 4500 to 5056 m. 30 However, none of the participants had atrial fibrillation, and it is unknown whether altitude increases the burden of atrial fibrillation. In another study, there were no significant arrhythmias during symptom‐limited exercise at the Jungfraujoch (3454 m) in patients with a history of ACS who were evaluated 6 months after revascularization therapy if left ventricular function was normal and maximum exercise at low altitude was without signs of ischemia. 11
Nevertheless, SCD is the most frequent nontraumatic cause of death at altitude. 4 , 31 , 32 The greatest risk factors for SCD include prior myocardial infarction and traditional risk factors for CAD, especially age and male sex. 32 Additional SCD risks include intense physical exertion before acclimatization and inadequate food/fluid intake. 32 Observational studies have shown that >50% of SCDs at altitude occur on the first day of exposure, and importantly, the risk of SCD is attenuated by 1 night of sleeping above 1000 m, suggesting that even a brief period of acclimatization reduces the SCD risk. 4 A Swiss study followed 276 individuals with implantable cardioverter defibrillators during exposure to altitude, of whom 86 reported stays at above 2000 m, 29 above 2500 m, and were mostly nonacclimatized. Twelve (14% of those exposed above 2000 m) experienced a shock for ventricular arrhythmias, which is similar to the average of 17% during participation in recreational events at sea level. 33
From these data we conclude that among at‐risk patients or those with established but stable CAD, the likelihood of fatal arrhythmias and SCD is low at up to 2500 m, especially after acclimatization. With normal left ventricular function and no arrhythmias at maximal exercise, altitudes of up to 3500 m should be safe. Travel planning should include a few days of acclimatization with reduced or no exercise, particularly if individuals are unaccustomed to the type of activities planned. 29 In this case, the most effective measure of SCD prevention is regular pretravel exercise training and optimizing guideline‐directed medical therapy as indicated. In patients with pacemakers and implantable cardiac defibrillators, normal device function should be assured before traveling and patients made aware of potential mechanical interference with recreational equipment (eg, rucksack belt). Caution is advised in patients showing uncontrolled arrhythmias at sea level, because development of potentially harmful arrhythmias at altitude is unpredictable with additional hypoxia‐related sympathetic activation. The same applies to patients who recently experienced an ACS. A sojourn to altitude should be delayed at least 4 weeks after an uncomplicated ACS and 3 months after a complicated ACS. 34
Heart Failure in High‐Altitude Locations
The physiologic response to hypoxia may be especially problematic for patients with HFrEF. These stressors include sympathetic nerve activation, elevated systemic and pulmonary arterial pressures (PAP), tachycardia, increased lung fluid content, and reductions in stroke volume. 35 Comorbidities, including active CAD, sleep apnea, and pulmonary hypertension, may exacerbate symptoms at altitude, and these conditions need to be stabilized, along with the clinical status of HFrEF, before excursions. A complete blood count should be assessed to ensure that hemoglobin concentration is stable and infusion of intravenous iron considered if anemia is present, 36 especially because iron requirements are markedly increased at altitude. 37
Baseline functional capacity affects the degree of activity that patients with HFrEF will tolerate at altitude. Individuals with a lower maximal oxygen uptake at sea level will have greater difficulty engaging in exercise, compared with those with a higher baseline maximal oxygen uptake. 38 , 39 , 40 Although left and right ventricular systolic function do not appear to be affected by brief exposure to altitude, PAP increases, 39 which may compromise functional capacity, especially in those with preexisting right ventricular dysfunction.
Pharmacologic therapy for patients with HFrEF may also affect exercise tolerance at altitude. Carvedilol, which reduces hyperventilation during exercise at sea level, may limit adaptive hyperventilatory responses needed at high altitude and reduce exercise capacity compared with β1‐selective blockers. 18 , 41 There are no known adverse short‐term effects of renin–angiotensin blocking drugs on altitude exposure, but they may blunt erythropoietin production and full expression of the normal polycythemic response in this population. Diuretics may contribute to volume depletion at altitude. Acetazolamide improves central sleep apnea, 42 prevents pulmonary congestion, counters altitude‐induced reductions in lung diffusion, and blunts onset of acute mountain sickness in HFrEF. However, caution is advised if patients are already on diuretics because of risks of hypokalemia and overdiuresis.
There are no published studies on altitude tolerance among patients with heart failure with preserved ejection fraction. However, these patients are likely to have elevated PAP, hypertension, and atrial fibrillation at sea level. HR and BP control are important factors in assessing risks of altitude exposure in these individuals. The role of pretravel testing has not been validated, but echocardiograms, symptom‐limited exercise testing, and ambulatory ECG monitoring have been suggested. 43 In all patients with heart failure, slow ascent (300 m/d sleeping altitude gain when above 2500 m) and stabilization of New York Heart Association functional class at sea level have been emphasized to reduce risks during sojourns. 20
Pulmonary Considerations at Altitude
Although comprehensive reviews of pulmonary disease at altitude are available elsewhere, 44 cardiologists should understand a few key points relevant to patient management. First, although hypoxia causes chemoreceptor‐mediated systemic vasodilatation, in pulmonary circulation, vasoconstriction occurs, increasing PAP and pulmonary vascular resistance (Figure 2). 2 , 44 In healthy individuals, systolic PAP increased to ≈30 mm Hg after brief exposure to hypoxia (fraction of inspired oxygen=0.10). 45 Among patients with treated pulmonary hypertension, resting mean PAP increased from a median (95% CI) of 35 (31–44) mm Hg to 38 (33–46) mm Hg after brief (10 minutes) exposure to simulated altitudes of 2600 m. 46 Thus, patients with preexisting pulmonary hypertension, regardless of cause, should anticipate an increase in PAP at altitude, which may worsen hypoxemia and exercise tolerance. Adults with congenital heart disease may be affected by reduced alveolar‐arterial oxygen gradient and pulmonary hypertension at altitude, with reduced stroke volumes during exercise. 47 The myriad of possible conditions with variable completeness of repair and the limited available data preclude a detailed discussion of these conditions.
In the troposphere (sea level to 11 000 m), the lapse rate, or atmospheric reduction in temperature, is ≈6.5 °C for every 1000 m increase in altitude (or 3.6 °F for every 1000 ft). Colder air may precipitate exercise‐induced asthma and neutrophilic airway inflammation 48 for several reasons. First, the upper airway humidifies and warms inspired air. However, this process may be ineffective/incomplete at altitude because ambient air temperatures are lower than at sea level. Furthermore, resting/exertional breathing rates increase, which reduces transit time of air flowing through the airways. 49 Airways may become inflamed after exposure to colder/drier air, and sensitivity of bronchial smooth muscles may be enhanced.
Symptomatic sleep apnea in the general population has a prevalence of 2% of middle‐aged women and 4% of men. 50 At sea level, apnea‐hypopnea events result from upper airway collapse causing intermittent hypoxia. However, central sleep apnea occurs at altitudes as low as 1600 m, and nocturnal oxygen saturations (Spo 2) may drop substantially. For example, among healthy lowlanders, sleep at 1860 m was associated with a median nocturnal Spo 2 of 96% and apnea‐hypopnea index of 0.1 per hour. 51 However, on the first night of sleep at 4559 m, nocturnal Spo 2 declined to 67%, the apnea‐hypopnea index increased to 60.9 per hour, and there were impairments in subjective assessments of sleep quality. 51 On the third night of sleep at 4559 m, Spo 2 improved to 71%, and sleep quality improved, despite a worsened apnea‐hypopnea index of 86.5 per hour. 51 Lowlanders with untreated obstructive sleep apnea experience exacerbations in sleep‐related breathing disturbances, worsened Spo 2, increased arrhythmia burden, and BP and weight gain at 1680 and 2590 m. 52
Acetazolamide causes metabolic acidosis by increasing renal bicarbonate secretion, which increases ventilation, improving arterial oxygenation and ultimately acclimatization. In a randomized placebo‐controlled, double‐blind crossover trial involving 51 lowlanders with obstructive sleep apnea, the combination of acetazolamide and autoadjusted continuous positive airway pressure increased nocturnal Spo 2 and improved the apnea‐hypopnea index at altitudes of 1690 m and 2590 m compared with the combination of placebo and auto–continuous positive airway pressure. 53 For patients who do not use continuous positive airway pressure routinely, acetazolamide alone is better than no treatment at all, because it improves oxygenation and the apnea‐hypopnea index at altitude. 54 As such, acetazolamide should be considered for patients with obstructive sleep apnea at altitude.
Alcohol consumption is associated with an increased risk of sleep apnea. Alcohol reduces the drive to breathe and predisposes to upper airway collapse by decreasing genioglossal muscle tone and increasing upper airway resistance. Given the predilection for alcohol consumption while vacationing, patients should be counseled on safe practices to minimize alcohol‐associated reductions in arterial oxygenation.
Emergency Preparedness
Although it is difficult to prepare for every possible complication, a few strategies should be considered to reduce risks of cardiac emergencies in mountainous locations. Plans for descent should be established, along with identification of triggers that would prompt such an evacuation. These triggers may include exacerbation of symptoms or arrhythmias occurring at sea level, an ACS event, and unsafe rises in BP above a prespecified cutoff level as determined by the patient's cardiologist. Hospitals closest to the excursion site should be identified. For travelers in remote locations and for those for whom evacuations may be delayed, additional medications, such as aspirin and clopidogrel, antianginal therapies, and additional antihypertensive medications should be packed. Finally, technologies such as smart watches for monitoring HR and arrhythmia may be incorporated to facilitate clinical monitoring until evacuation occurs.
Conclusions
Mountainous environments impose unique stressors on the cardiovascular system. These stressors result primarily from reduced oxygen content and hypoxia‐induced increases in sympathetic activity, which may worsen symptoms of ischemia and heart failure, increase BP, worsen sleep apnea, and precipitate arrhythmias. Patients with cardiovascular disease that is stable at sea level should be able to tolerate excursions at moderate altitudes. However, acclimatization before exercise should be emphasized to reduce risk of adverse events. A thorough pretravel assessment should be completed in all patients to ensure that disease(s) is stable and well controlled under sea‐level conditions. Pretravel exercise testing may be necessary to determine exercise tolerance at altitude. A shared decision‐making process should be incorporated into pretravel assessments and should include a review of medications and counseling on dietary habits. Pretravel consultation with other specialists (eg, pulmonologists) may be necessary. Finally, outcomes following a cardiovascular event in remote environments may be worse than those at sea level because of limited availability or delayed implementation of resources and medical therapies. Patients with cardiovascular disease must accept the risk of an acute cardiovascular event with limited access to advanced medical care, and this risk should be factored into pretravel counseling as appropriate based on an individual's risk profile.
Disclosures
Writing Group Disclosures
| Writing group member | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| William K. Cornwell III | University of Colorado | None | NIH, NHLBI K23 (career development award) † | None | None | None | None | None |
| Benjamin D. Levine | University of Texas Southwestern Medical Center, Texas Health Presbyterian Hospital, Dallas Institute for Exercise and Environmental Medicine | None | None | None | None | None | None | None |
| Aaron L. Baggish | Massachusetts General Hospital | None | None | None | None | None | None | None |
| Yadav Kumar Deo Bhatta | NORVIC International Hospital (Nepal) | None | None | None | None | None | None | None |
| Maria Joan Brosnan | St. Vincent's Hospital National Institute of Sports Cardiology (Australia) | None | None | None | None | None | None | None |
| Christoph Dehnert | Medbase, Zurich (Switzerland) | None | None | None | None | None | None | None |
| J. Sawalla Guseh | Massachusetts General Hospital, Harvard Medical School | None | None | None | None | None | None | None |
| Debra Hammer | University of Alberta (Canada) | None | None | None | None | None | None | None |
| Gianfranco Parati | University of Milano‐Bicocca and Istituto Auxologico Italiano San Luca Hospital (Italy) | None | None | None | None | None | None | None |
| Eugene E. Wolfel | University of Colorado–Anschutz Medical Campus | None | None | Medical Education Resources, Inc.* | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be "significant" if (a) the person receives $10 000 or more during any 12‐month period, or 5% or more of the person's gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be "modest" if it is less than "significant" under the preceding definition.
Modest.
Significant.
Reviewer Disclosures
| Reviewer | Employment | Research grant | Other research support | Speakers’ bureau/honoraria | Expert witness | Ownership interest | Consultant/advisory board | Other |
|---|---|---|---|---|---|---|---|---|
| Peter Bartsch | Medizinische Klinik der Universität Heidelberg (Switzerland) | None | None | None | None | None | None | None |
| Peter Hackett | University of Colorado | None | None | None | None | None | None | None |
| Erik R. Swenson | University of Washington | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be "significant" if (a) the person receives $10 000 or more during any 12‐month period, or 5% or more of the person's gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be "modest" if it is less than significant under the preceding definition.
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on April 15, 2021, and the American Heart Association Executive Committee on May 21, 2021. A copy of the document is available at https://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area.
The American Heart Association requests that this document be cited as follows: Cornwell WK 3rd, Baggish AL, Bhatta YKD, Brosnan MJ, Dehnert C, Guseh JS, Hammer D, Levine BD, Parati G, Wolfel EE; on behalf of the American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; and Council on Arteriosclerosis, Thrombosis and Vascular Biology. Clinical implications for exercise at altitude among individuals with cardiovascular disease: a scientific statement from the American Heart Association. J Am Heart Assoc. 2021;10:e023225. DOI: 10.1161/JAHA.121.023225
The expert peer review of American Heart Association–commissioned documents (eg, scientific statements, clinical practice guidelines, systematic reviews) is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit https://professional.heart.org/statements. Select the “Guidelines & Statements” drop‐down menu, then click “Publication Development.”
REFERENCES
- 1. Levine BD. Going high with heart disease: the effect of high altitude exposure in older individuals and patients with coronary artery disease. High Alt Med Biol. 2015;16:89–96. doi: 10.1089/ham.2015.0043 [DOI] [PubMed] [Google Scholar]
- 2. Bärtsch P, Gibbs JS. Effect of altitude on the heart and the lungs. Circulation. 2007;116:2191–2202. doi: 10.1161/CIRCULATIONAHA.106.650796 [DOI] [PubMed] [Google Scholar]
- 3. Baggish AL, Wolfel EE, Levine BD. Cardiovascular system. In: Swenson E, Bärtsch P, eds. High Altitude. Springer; 2014:103–139. doi: 10.1007/978-1-4614-8772-2 [DOI] [Google Scholar]
- 4. Lo MY, Daniels JD, Levine BD, Burtscher M. Sleeping altitude and sudden cardiac death. Am Heart J. 2013;166:71–75. doi: 10.1016/j.ahj.2013.04.003 [DOI] [PubMed] [Google Scholar]
- 5. Thompson PD, Funk EJ, Carleton RA, Sturner WQ. Incidence of death during jogging in Rhode Island from 1975 through 1980. J Am Med Assoc. 1982;247:2535–2538. doi: 10.1001/jama.1982.03320430039028 [DOI] [PubMed] [Google Scholar]
- 6. Fulco CS, Rock PB, Cymerman A. Maximal and submaximal exercise performance at altitude. Aviat Space Environ Med. 1998;69:793–801. [PubMed] [Google Scholar]
- 7. Levine BD, Zuckerman JH, DeFilippi R. Effect of high‐altitude exposure in the elderly. The Tenth Mountain Division Study. Circulation. 1997;96:1224–1232. doi: 10.1161/01.CIR.96.4.1224 [DOI] [PubMed] [Google Scholar]
- 8. Erdmann J, Sun KT, Masar P, Niederhauser H. Effects of exposure to altitude on men with coronary artery disease and impaired left ventricular function. Am J Cardiol. 1998;81:266–270. doi: 10.1016/S0002-9149(97)00901-6 [DOI] [PubMed] [Google Scholar]
- 9. Wyss CA, Koepfli P, Fretz G, Seebauer M, Schirlo C, Kaufmann PA. Influence of altitude exposure on coronary flow reserve. Circulation. 2003;108:1202–1207. doi: 10.1161/01.CIR.0000087432.63671.2E [DOI] [PubMed] [Google Scholar]
- 10. Levy RL, Bruenn HG, Russell N. Electrocardiographic changes caused by induced anoxemia in normal persons and in patients with disease of the coronary arteries. Trans Am Clin Climatol Assoc. 1938;54:71–75. [PMC free article] [PubMed] [Google Scholar]
- 11. Schmid JP, Noveanu M, Gaillet R, Hellige G, Wahl A, Saner H. Safety and exercise tolerance of acute high altitude exposure (3454 m) among patients with coronary artery disease. Heart. 2006;92:921–925. doi: 10.1136/hrt.2005.072520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morgan BJ, Alexander JK, Nicoli SA, Brammell HL. The patient with coronary heart disease at altitude—observations during acute exposure to 3100 meters. J Wilderness Med. 1990;1:147–153. doi: 10.1580/0953-9859-1.3.147 [DOI] [Google Scholar]
- 13. de Vries ST, Komdeur P, Aalbersberg S, van Enst GC, Breeman A, van’t Hof AWJ. Effects of altitude on exercise level and heart rate in patients with coronary artery disease and healthy controls. Neth Heart J. 2010;18:118–121. doi: 10.1007/BF03091749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Messerli‐Burgy N, Meyer K, Steptoe A, Laederach‐Hofmann K. Autonomic and cardiovascular effects of acute high altitude exposure after myocardial infarction and in normal volunteers. Circ J. 2009;73:1485–1491. doi: 10.1253/circj.CJ-09-0004 [DOI] [PubMed] [Google Scholar]
- 15. Parati G, Bilo G, Faini A, Bilo B, Revera M, Giuliano A, Lombardi C, Caldara G, Gregorini F, Styczkiewicz K, et al. Changes in 24 h ambulatory blood pressure and effects of angiotensin II receptor blockade during acute and prolonged high‐altitude exposure: a randomized clinical trial. Eur Heart J. 2014;35:3113–3122. doi: 10.1093/eurheartj/ehu275 [DOI] [PubMed] [Google Scholar]
- 16. Parati G, Revera M, Giuliano A, Faini A, Bilo G, Gregorini F, Lisi E, Salerno S, Lombardi C, Ramos Becerra CG, et al. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur Heart J. 2013;34:759–766. doi: 10.1093/eurheartj/ehs140 [DOI] [PubMed] [Google Scholar]
- 17. Bilo G, Villafuerte FC, Faini A, Anza‐Ramirez C, Revera M, Giuliano A, Caravita S, Gregorini F, Lombardi C, Salvioni E, et al. Ambulatory blood pressure in untreated and treated hypertensive patients at high altitude: the High Altitude Cardiovascular Research‐Andes study. Hypertension. 2015;65:1266–1272. doi: 10.1161/HYPERTENSIONAHA.114.05003 [DOI] [PubMed] [Google Scholar]
- 18. Agostoni P, Contini M, Magini A, Apostolo A, Cattadori G, Bussotti M, Veglia F, Andreini D, Palermo P. Carvedilol reduces exercise‐induced hyperventilation: a benefit in normoxia and a problem with hypoxia. Eur J Heart Fail. 2006;8:729–735. doi: 10.1016/j.ejheart.2006.02.001 [DOI] [PubMed] [Google Scholar]
- 19. Bilo G, Caldara G, Styczkiewicz K, Revera M, Lombardi C, Giglio A, Zambon A, Corrao G, Faini A, Valentini M, et al. Effects of selective and nonselective beta‐blockade on 24‐h ambulatory blood pressure under hypobaric hypoxia at altitude. J Hypertens. 2011;29:380–387. doi: 10.1097/HJH.0b013e3283409014 [DOI] [PubMed] [Google Scholar]
- 20. Parati G, Agostoni P, Basnyat B, Bilo G, Brugger H, Coca A, Festi L, Giardini G, Lironcurti A, Luks AM, et al. Clinical recommendations for high altitude exposure of individuals with pre‐existing cardiovascular conditions: a joint statement by the European Society of Cardiology, the Council on Hypertension of the European Society of Cardiology, the European Society of Hypertension, the International Society of Mountain Medicine, the Italian Society of Hypertension and the Italian Society of Mountain Medicine. Eur Heart J. 2018;39:1546–1554. doi: 10.1093/eurheartj/ehx720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58:785–790. doi: 10.1152/jappl.1985.58.3.785 [DOI] [PubMed] [Google Scholar]
- 22. Perrill CV. High‐altitude syncope: history repeats itself. J Am Med Assoc. 1993;269:587. doi: 10.1001/jama.1993.03500050065015 [DOI] [PubMed] [Google Scholar]
- 23. Nicholas R, O'Meara PD, Calonge N. Is syncope related to moderate altitude exposure. J Am Med Assoc. 1992;268:904–906. doi: 10.1001/jama.1992.03490070086048 [DOI] [PubMed] [Google Scholar]
- 24. Blaber AP, Hartley T, Pretorius PJ. Effect of acute exposure to 3,660m altitude on orthostatic responses and tolerance. J Appl Physiol. 2003;95:591–601. doi: 10.1152/japplphysiol.00749.2002 [DOI] [PubMed] [Google Scholar]
- 25. Thomas KN, Burgess KR, Basnyat R, Lucas SJ, Cotter JD, Fan JL, Peebles KC, Lucas RA, Ainslie PN. Initial orthostatic hypotension at high altitude. High Alt Med Biol. 2010;11:163–167. doi: 10.1089/ham.2009.1056 [DOI] [PubMed] [Google Scholar]
- 26. Horiuchi M, Dobashi S, Kuiuchi M, Endo J, Koyama K, Subudhi AW. Reduction in cerebral oxygenation after prolonged exercise in hypoxia is related to changes in blood pressure. Adv Exp Med Biol. 2016;876:95–100. doi: 10.1007/978-1-4939-3023-4_12 [DOI] [PubMed] [Google Scholar]
- 27. Malconian MK, Rock P, Hultgren H, Donner H, Cymerman A, Groves B, Reeves J, Alexander J, Sutton J, Nitta M, et al. The electrocardiogram at rest and exercise during a simulated ascent of Mt. Everest (Operation Everest II). Am J Cardiol. 1990;65:1475–1480. doi: 10.1016/0002-9149(90)91358-D [DOI] [PubMed] [Google Scholar]
- 28. Woods DR, Allen S, Betts TR, Gardiner D, Montgomery H, Morgan JM, Roberts PR. High altitude arrhythmias. Cardiology. 2008;111:239–246. doi: 10.1159/000127445 [DOI] [PubMed] [Google Scholar]
- 29. Grover RF, Tucker CE, McGroarty SR. The coronary stress of skiing at high altitude. Arch Intern Med. 1990;150:1205–1208. doi: 10.1001/archinte.1990.00390180045007 [DOI] [PubMed] [Google Scholar]
- 30. Wu TY, Ding SQ, Liu JL, Yu MT, Jia JH, Chai ZC, Dai RC, Zhang SL, Li BY, Pan L, et al. Who should not go high: chronic disease and work at altitude during construction of the Qinghai‐Tibet railroad. High Alt Med Biol. 2007;8:88–107. doi: 10.1089/ham.2007.1015 [DOI] [PubMed] [Google Scholar]
- 31. Burtscher M, Pachinger O, Schocke MF, Ulmer H. Risk factor profile for sudden cardiac death during mountain hiking. Int J Sports Med. 2007;28:621–624. doi: 10.1055/s-2007-964850 [DOI] [PubMed] [Google Scholar]
- 32. Burtscher M. Risk and protective factors for sudden cardiac death during leisure activities in the mountains: an update. Heart Lung Circ. 2017;26:757–762. doi: 10.1016/j.hlc.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 33. Kobza R, Duru F, Erne P. Leisure‐time activities of patients with ICDs: findings of a survey with respect to sports activity, high altitude stays, and driving paterns. Pacing Clin Electrophysiol. 2008;31:845–849. doi: 10.1111/j.1540-8159.2008.01098.x [DOI] [PubMed] [Google Scholar]
- 34. Dehnert C, Bärtsch P. Can patients with coronary heart disease go to high altitude? High Alt Med Biol. 2010;11:183–188. doi: 10.1089/ham.2010.1024 [DOI] [PubMed] [Google Scholar]
- 35. Agostoni P. Considerations on safety and treatment of patients with chronic heart failure at high atltitude. High Alt Med Biol. 2013;14:96–100. doi: 10.1089/ham.2012.1117 [DOI] [PubMed] [Google Scholar]
- 36. Ponikowski P, Kirwan B‐A, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double‐blind, randomised, controlled trial. Lancet. 2020;396:1895–1904. doi: 10.1016/S0140-6736(20)32339-4 [DOI] [PubMed] [Google Scholar]
- 37. Okazaki K, Stray‐Gundersen J, Chapman RF, Levine BD. Iron insufficiency diminishes the erythropoietic response to moderate altitude exposure. J Appl Physiol (1985). 2019;127:1569–1578. doi: 10.1152/japplphysiol.00115.2018 [DOI] [PubMed] [Google Scholar]
- 38. Agostoni P, Cattadori G, Guazzi M, Bussotti M, Conca C, Lomanto M, Marenzi G, Guazzi M. Effects of simulated altitude‐induced hypoxia on exercise capacity in patients with chronic heart failure. Am J Med. 2000;109:450–455. doi: 10.1016/S0002-9343(00)00532-5 [DOI] [PubMed] [Google Scholar]
- 39. Schmid JP, Nobel D, Brugger N, Novak J, Palau P, Trepp A, Wilhelm M, Saner H. Short‐term high altitude exposure at 3454 m is well tolerated in patients with stable heart failure. Eur J Heart Fail. 2015;17:182–186. doi: 10.1002/ejhf.227 [DOI] [PubMed] [Google Scholar]
- 40. Vona M, Mazzuero G, Lupi A, Vettorato C, Bosso P, Cohen‐Solal A. Effects of altitude on effort tolerance in non‐acclimatized patients with ischemic left ventricular dysfunction. Eur J Cardiovasc Prev Rehabil. 2006;13:617–624. doi: 10.1097/01.hjr.0000220583.27140.9b [DOI] [PubMed] [Google Scholar]
- 41. Valentini M, Revera M, Bilo G, Caldara G, Savia G, Styczkiewicz K, Parati S, Gregorini F, Faini A, Branzi G, et al. Effects of beta‐blockade on exercise performance at high altitude: a randomized, placebo‐controlled trial comparing the efficacy of nebivolol versus carvedilol in healthy subjects. Cardiovasc Ther. 2012;30:240–248. doi: 10.1111/j.1755-5922.2011.00261.x [DOI] [PubMed] [Google Scholar]
- 42. Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double‐blind, prospective study. Am J Respir Crit Care Med. 2006;173:234–237. doi: 10.1164/rccm.200507-1035OC [DOI] [PubMed] [Google Scholar]
- 43. Donegani E, Hillebrandt D, Windsor J, Gieseler U, Rodway G, Schoffl V, Kupper T. Pre‐existing cardiovascular conditions and high altitude travel. Consensus statement of the Medical Commission of the Union Internationale des Associations d'Alpinisme (UIAA MedCom) Travel Medicine and Infectious Disease. Travel Med Infect Dis. 2014;12:237–252. doi: 10.1016/j.tmaid.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 44. Luks AM, Swenson ER. Travel to high altitude with pre‐existing lung disease. Eur Respir J. 2007;29:770–792. doi: 10.1183/09031936.00052606 [DOI] [PubMed] [Google Scholar]
- 45. Ghofrani HA, Reichenberger F, Kohstall MG, Mrosek EH, Seeger T, Olschewski H, Seeger W, Grimminger F. Sildenafil increased exercise capacity during hypoxia at low altitudes and at mount everest base camp. A randomized, double‐blind, placebo‐controlled crossover trial. Ann Intern Med. 2004;141:169–177. doi: 10.7326/0003-4819-141-3-200408030-00005 [DOI] [PubMed] [Google Scholar]
- 46. Groth A, Saxer S, Bader PR, Lichtblau M, Furian M, Schneider SR, Schwarz EI, Bloch KE, Ulrich S. Acute hemodynamic changes by breathing hypoxic and hyperoxic gas mixtures in pulmonary arterial and chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2018;270:262–267. doi: 10.1016/j.ijcard.2018.05.127 [DOI] [PubMed] [Google Scholar]
- 47. Garcia JA, McMinn SB, Zuckerman JH, Fixler DE, Levine BD. The role of the right ventricle during hypobaric hypoxic exercise: insights from patietns after the Fontan operation. Med Sci Sports Exerc. 1993;31:269–276. doi: 10.1097/00005768-199902000-00011 [DOI] [PubMed] [Google Scholar]
- 48. Seys SF, Daenen M, Dilissen E, Van Thienen R, Bullens DM, Hespel P, Dupont LJ. Effects of high altitude and cold air exposure on airway inflammation in patients with asthma. Thorax. 2013;68:906–913. doi: 10.1136/thoraxjnl-2013-203280 [DOI] [PubMed] [Google Scholar]
- 49. Sheel AW, MacNutt MJ, Querido JS. The pulmonary system during exercise in hypoxia and the cold. Exp Physiol. 2010;95:422–430. doi: 10.1113/expphysiol.2009.047571 [DOI] [PubMed] [Google Scholar]
- 50. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. doi: 10.1513/pats.200709-155MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nussbaumer‐Ochsner Y, Ursprung J, Siebenmann C, Maggiorini M, Bloch KE. Effect of short‐term acclimatization to high altitude on sleep and nocturnal breathing. Sleep. 2012;35:419–423. doi: 10.5665/sleep.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nussbaumer‐Ochsner Y, Schuepfer N, Ulrich S, Bloch KE. Exacerbation of sleep apnoea by frequent central events in patients with the obstructive sleep apnoea syndrome at altitude: a randomized trial. Thorax. 2010;65:429–435. doi: 10.1136/thx.2009.125849 [DOI] [PubMed] [Google Scholar]
- 53. Latshang TD, Nussbaumer‐Ochsner Y, Henn RM, Ulrich S, Lo Cascio CM, Ledergerber B, Kohler M, Bloch KE. Effect of acetazolamide and AutoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude. J Am Med Assoc. 2012;308:2390–2398. doi: 10.1001/jama.2012.94847 [DOI] [PubMed] [Google Scholar]
- 54. Bloch KE, Latshang TD, Ulrich S. Patients with obstructive sleep apnea at altitude. High Alt Med Biol. 2015;16:110–116. doi: 10.1089/ham.2015.0016 [DOI] [PubMed] [Google Scholar]


