Abstract
Background
Endomyocardial biopsy (EMB) is part of 2010 Task Force Criteria (TFC) for arrhythmogenic right ventricular cardiomyopathy (ARVC). However, its usage has been curtailed because of its low presumed diagnostic yield, and it is now a poorly used tool. This study aims to analyze the contribution of EMB to the final diagnosis of ARVC.
Methods and Results
We included 104 consecutive patients evaluated for a suspicion of ARVC, who were referred for EMB. Patients with suspected left dominant pattern were excluded from the primary analysis. Subjects were initially stratified according to TFC without considering EMB. After EMB, patients were reclassified accordingly, and the reclassification rate was calculated. EMB yielded a diagnostic finding in 92 patients (85.5%). After including EMB evaluation, 20 (43%) more patients “at risk” received a definite diagnosis of ARVC. Overall, 59 patients received a definite diagnosis of ARVC, 34% only after EMB. EMB appeared to be the better‐performing exam with respect to the final diagnosis (β, 2.2; area uder the curve, 0.73; P<0.05). The reclassification improvement after EMB measured 28%. TFC score increased from 3.5±1.3 to 4.3±1.4 (P<0.001). Notably, active inflammation was present in 6 (10%) patients. Minor complications were reported in only 2% of the cohort. In patients with suspected left‐dominant disease, conventional TFC performed poorly.
Conclusions
Electroanatomic voltage mapping–guided EMB was safe and yielded an optimal diagnostic yield. It allowed upgrading of the diagnosis of nearly one‐third of the patients considered “at risk.” Classical TFC without EMB performed poorly in patients with the left dominant form of ARVC.
Keywords: arrhythmogenic cardiomyopathy, cardiac magnetic resonance, electroanatomic mapping, endomyocardial biopsies, right ventricular arrhythmogenic cardiomyopathy, task force criteria
Subject Categories: Sudden Cardiac Death, Cardiomyopathy, Diagnostic Testing
Nonstandard Abbreviations and Acronyms
- ACM
arrhythmogenic cardiomyopathy
- ALVC
arrhythmogenic left ventricular cardiomyopathy
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- EMB
endomyocardial biopsy
- EVM
electroanatomic voltage mapping
- TFC
Task Force Criteria
Clinical Perspective
What Is New?
Electroanatomical mapping‐guided endomyocardial biopsy (EMB) performed in patients with suspected arrhythmogenic right ventricular cardiomyopathy achieved a diagnostic yield of 86%.
Active inflammation is a not infrequent finding in arrhythmogenic right ventricular cardiomyopathy patients—being found in 10% of our population—with significant implications, especially for sudden death and arrhythmic‐risk stratification.
In patients with suspected arrhythmogenic right ventricular cardiomyopathy and inconclusive results after noninvasive evaluation, EMB allowed upgrading of the diagnosis of nearly one‐third of the patients; our study reinforces the concept that EMB is still a useful, yet underused, tool.
What Are the Clinical Implications?
EMB acquires even greater importance in patients without a genetic diagnosis, in whom the exclusion of phenocopies is essential, and for which noninvasive procedures do not always allow definite results.
Our study strengthens the idea that the relative weight of each individual 2010 Task Force Criteria may not be as equal as currently assumed.
Additionally, conventional Task Force Criteria performed poorly in the diagnosis of inpatients with suspected left‐dominant disease; in this setting, EMB may be of help, although specific criteria are currently lacking.
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an underdiagnosed clinical entity characterized by life‐threatening ventricular arrhythmias and a progressive fibrous of fibro‐fatty replacement of the myocardium. 1 ARVC diagnosis is probably the most challenging in the field of inherited cardiomyopathies because of the absence of a unique diagnostic criterion or test, variable expressivity, and incomplete penetrance. At present, ARVC diagnosis is based on a scoring system known as the 2010 Task Force Criteria (TFC). 2 , 3 Endomyocardial biopsy (EMB) represents 1 of the 6 “pieces” in the puzzle of ARVC diagnosis. However, the role of EMB in the diagnosis of ARVC is still controversial because of its low sensitivity. 4 This is testified by the low number of EMBs being reported in recent ARVC registries and is also supported by current guidelines and societies statements. 5 Yet the early stage of the disease may often go unrecognized by noninvasive evaluation, and EMB also allows to recognize arrhythmogenic cardiomyopathy (ACM) phenocopies (myocarditis, sarcoidosis, or idiopathic dilated cardiomyopathy) apart. 6 , 7 Additionally, growing evidences support the existence of an arrhythmogenic left ventricular cardiomyopathy (ALVC), for which no specific validated diagnostic criteria exists yet. 8 , 9 , 10 For all these reasons, EMB’s role is far from being useless in this setting.
This paper aims to analyze the diagnostic performance of 2010 TFC in a cohort of patients with suspected ARVC. Furthermore, we also aim to assess the diagnostic performance of electroanatomic voltage mapping (EVM) guided EMB and its safety in patients with ARVC.
Methods
Study Population
We included all consecutive patients with a suspicion of ARVC according to 2010 TFC admitted to 2 tertiary referral centers for cardiac arrhythmias (Monzino Cardiology Center, Milan, Italy; and Marche Polytechnic University, Ancona, Italy) between November 2010 and May 2020. Patients with a suspected left‐dominant pattern were excluded from the primary analysis. The study protocol was approved by the Ethical Committee of the Monzino Cardiology Center (R1115/20‐CCM1179) in compliance with institutional standards, national legal requirements, and the Declaration of Helsinki. All patients agreed to participate in the study, providing informed consent. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Collection
Data were retrospectively collected from medical records and included clinical history and diagnostic tests necessary to fulfill the TFC: ECG, Holter recordings, cardiac magnetic resonance (CMR) imaging, echocardiography, genetic testing, and family history. Other clinically relevant diagnostic tests (eg, coronary angiograms, exercise stress tests, and electrophysiology study) were upon discretion of the managing physician. Genetic analysis was always performed by next‐generation sequencing (NGS Illumina NextSeq, with the TruSight Cardio Sequencing Kit). Specifically, we screened patients for pathogenic variants in a prefixed panel of desmosomal (ie, plakophilin‐2 [PKP2], plakoglobin [JUP], desmoglein‐2 [DSG2], desmocollin‐2 [DSC2], and desmoplakin [DSP]) and nondesmosomal genes (TMEM43, RYR2, PLN, SCN5A, and LMNA) that were previously reported to be associated with the disease. 11
Endomyocardial Biopsy
EMB was performed in accordance with international guidelines. 3 , 5 In particular, EMB was required (1) when TFC without EMB were insufficient to achieve a definite diagnosis; and (2) when, although a definite diagnosis of ACM was reached, the possibility of phenocopies was high, in particular in patients without genetic predisposition. Figure 1 depicts the EMB algorithm. A detailed description of EMB is reported in Data S1. 12
Figure 1. Endomyocardial biopsy decisional algorithm.

ACM indicates arrhythmogenic cardiomyopathy; CMR cardiac magnetic resonance; ECHO, echocardiogram; EMB, endomyocardial biopsy; and TFC, Task Force Criteria.
Diagnostic Classification
According to recent guidelines, arrhythmogenic cardiomyopathy is defined as “an arrhythmogenic heart muscle disorder not explained by ischemic, hypertensive or valvular heart disease.” 13 Yet the same terminology is often used referring to either left or biventricular forms of arrhythmogenic cardiomyopathy. Not to be misinterpreted, we specify that when generally referring to both ALVC and ARVC, we will use the term ACM, which does not include infiltrative diseases, channelopathies, noncompaction cardiomyopathy, inflammatory cardiomyopathy, and idiopathic cardiomyopathy.
Two diagnostic classifications of ACM were used. First, patients were classified according to TFC without EMB. Major (2 points) and minor (1 point) criteria were summed and, if the combined score was ≥4, patients were labeled as “definite ACM.” Otherwise, if the combined score was 2 or 3, patients were considered “at risk” for ACM. In particular, patients with a score of 2 were considered with a “possible” diagnosis, while patients with 3 had a “borderline” diagnosis. If the score was <2, the patient was not included in the study.
Second, we reevaluated TFC, taking into account EMB results in each subject. After EMB, patients were reclassified accordingly, and the reclassification rate was calculated.
Statistical Analysis
Data analysis was performed using IBM SPSS Statistics 23. Continuous variables are reported as mean±SD for normally distributed variables, and as median (first to third quartile) for nonnormally distributed variables. Categorical variables are reported as counts and percentage. Comparisons between groups were undertaken with parametric (Student’s t test) or nonparametric tests (Mann‐Whitney U‐test), as appropriate. The comparisons between categorical variables were performed with the χ2 test and the Fisher exact test, as indicated. Using the final diagnosis as a reference, the diagnostic performance of each TFC was evaluated with regard to sensitivity, specificity, and area under the curve. To estimate the relative weights of each different TFC component, logistic regression was used. The diagnostic and classification contribution of EMB was evaluated by assessing the reclassification improvement. Two‐tailed P values <0.05 were considered statistically significant.
Results
Patient Population
A total of 104 patients with suspected ACM were included in our study. Mean age was 43.8±13.9 years, and 70% were men. Patients were referred for ECG abnormalities (15%), family screening (9%), arrhythmias (59%), syncope (12%), and heart failure (5%); see Table 1 and Table S1 for details. Eighty‐five (82%) patients were referred for suspected ARVC, while the remaining 19 (18%) had suspected ALVC. CMR was performed in 102 patients (98%). Sixty‐four patients (62%) underwent genetic testing. A pathognomonic variant was found in 25 patients (39%) in the overall population and in 49% of the patients who reached a definite diagnosis of ARVC. The most common genetic mutations were: PKP2 in 10 patients, DSG2 in 4 patients, and DSP in 6 patients. A detailed report of noninvasive evaluation is displayed in Table S2.
Table 1.
Baseline Characteristics Stratified According to the Site of the Disease
|
Overall (n=104) |
Suspected ARVC (n=85) |
Suspected ALVC (n=19) |
|
|---|---|---|---|
| Male sex | 73 (70.2) | 60 (70.6) | 13 (68.4) |
| Age, y | 43.8 (13.9) | 42.6 (13.8) | 49.1 (13.7) |
| Indication | |||
| ECG abnormalities | 16 (15.4) | 11 (12.9) | 5 (26.3) |
| Family screening | 9 (8.7) | 9 (10.6) | 0 (0) |
| Arrhythmias | 61 (58.7) | 52 (61.2) | 9 (47.4) |
| Syncope | 13 (12.5) | 12 (14.1) | 1 (5.3) |
| Heart failure | 5 (4.8) | 1 (1.2) | 4 (21.1) |
| Abnormal ECG | 57 (54.8) | 43 (50.6) | 14 (73.7) |
| Epsilon wave | 4 (3.8) | 4 (44.7) | 0 (0) |
| Negative T wave V1–V3 | 27 (26) | 24 (28.2) | 3 (15.8) |
| Negative T wave V4–V6 | 17 (16.3) | 12 (14.1) | 5 (26.3) |
| Arrhythmias | |||
| PVC >500/24 h | 49 (47.1) | 39 (45.9) | 10 (52.6) |
| NSVT | 34 (32.7) | 27 (31.8) | 7 (36.8) |
| SVT | 25 (24) | 21 (24.7) | 4 (21.1) |
| Endomyocardial biopsy | |||
| Samples number | 3.8 (1.1) | 3.7 (1.1) | 4.3 (1.0) |
| Diagnostic biopsy (%) | 92 (88.5) | 73 (85.9) | 19 (100) |
| Fibrosis at EMB % | 34.1 (9.8–52.0) | 28.3 (9.4–52.5) | 48.8 (26.8–51.8) |
| Residual myocardium (%) | 58.2 (39.5–87.7) | 60.2 (31.7–90.6) | 51.2 (46.5–84.8) |
| Inflammation | 17 (16.3) | 12 (14.1) | 5 (26.3) |
| TFC EMB+ | 4.3 (1.4) | 4.3 (1.5) | 4.1 (1.1) |
| TFC EMB− | 3.4 (1.2) | 3.6 (1.2) | 2.8 (1.1) |
ALVC indicates arrhythmogenic left ventricular cardiomyopathy; ARVC, arrhythmogenic right ventricular cardiomyopathy; EMB, endomyocardial biopsy; NSVT, nonsustained ventricular tachycardia; PVC, premature ventricular complex; SVT, sustained ventricular tachycardia; and TFC, Task Force Criteria.
Continuous variables are shown as Mean ±SD or Median and (interquartile range) (IQR). Discrete variables are presented as number and percentage (%).
Endomyocardial Biopsy
All patients underwent EVM‐guided EMB, which yielded a diagnostic finding in 92 patients (89%). In the remainder of the cohort, the histologic sample was inadequate or not evaluable. A mean of 3.8±1.0 specimens was sampled from each patient. The right ventricle was targeted in 85 (81%) of cases, the left ventricle in 12 (11%), and both ventricles in the remaining 7 (6%). After excluding ALVC patients, 40 (47%) patients presented a histologic pattern diagnostic for ACM. More specifically, 26 (31%) fulfilled a major tissue characterization criterion, while the remaining 14 (16%) fulfilled a minor criterion. Detailed results of the EMB evaluation are reported in Table S3.
Additionally, in 4 patients, EMB excluded ACM, and histologic analyses were suggestive for idiopathic dilated cardiomyopathy. At the end of the diagnostic workout, none of these 4 patients received a definite ACM diagnosis. Notably, only 2 complications were noted (2.2%), both related to vascular access, and both managed conservatively. No cardiac tamponade was observed.
Diagnosis and Task Force Criteria performance
After the initial evaluation, before EMB, 46 (54%) patients were considered at risk (24 [28%] with a possible diagnosis, 22 [26%] with a borderline diagnosis), and 39 (46%) had a definite diagnosis of ACM.
As shown in Figure 2 and Table S4, 20 (43%) patients considered at risk after noninvasive evaluation (12 from the “possible” group and 8 from the “borderline” group) received a definite diagnosis of ACM only after taking EMB into account. In the end, 59 patients received a definite diagnosis of ARVC (34% of these only after EMB). Even in patients who did not reach a definitive diagnosis, 4 were upgraded from possible to borderline. When evaluating the diagnostic performance of each individual TFC component, EMB appeared to be the better performing exam with respect to the final diagnosis of definite ACM (β, 2.2; area under the curve, 0.73; P<0.05 for both) as reported in Figure 3 and Table 2. The reclassification after EMB was of 28%. TFC score increased from 3.5±1.3 per patient to 4.3±1.4 (P<0.001). Table 3 shows how the different components of the TFC contributed to the final diagnosis. As showed by CMR results and confirmed by histologic analysis, 19 patients with suspected ARVC had biventricular involvement (13 with a definite diagnosis and 6 with borderline). A representative example of biventricular ACM is reported in Figure S1. Among the 25 patients with pathogenic mutations, 20 already fulfilled the diagnosis of ARVC, even before performing EMB. Among the 5 without a certain diagnosis, hence genetic carries without phenotypic manifestations, 3 were upgraded from borderline to definite, 1 from possible to borderline, and the last one remained unchanged as possible. Concerning the concordance between EMB and CMR, late gadolinium enhancement was present in 76% of patients with final ARVC definite diagnosis. In addition, the right ventricle was dilated in 69% of the patients, while a reduced ejection fraction of the right ventricle was observed in 46%. Late gadolinium enhancement was present in the absence of dilatation or dysfunction in 2 patients, while an aneurysm or bulging was present in 33%. Overall, all patients with positive EMB had at least a minor criterion by noninvasive imaging evaluation, and 6 patients had a major criterion. Table S5 represents the other positive TFC in patients at risk for ARVC, in whom EMB served as crucial test to reach a definite diagnosis of ARVC.
Figure 2. Reclassifications before and after endomyocardial biopsy.
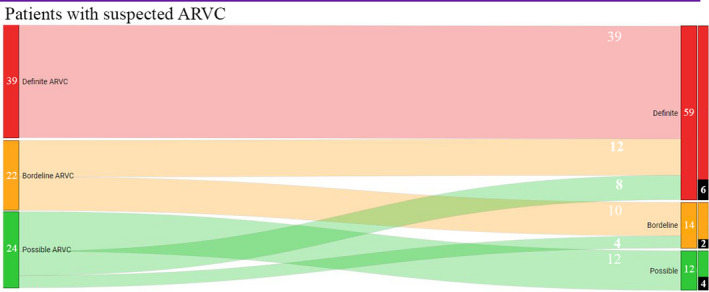
The left column represents diagnostic classification before EMB. On the right we have diagnostic classification after EMB. Marked with black, we highlighted patients whose EMB was positive also for inflammatory infiltrates. ARVC indicates arrhythmogenic right ventricular cardiomyopathy.
Figure 3. Diagnostic performance of each individual Task Force Criteria (TFC).

Forest plot of the diagnostic odds ratios and 95% CIs. AUC indicates area under the curve; Sn, sensitivity; and Sp, specificity.
Table 2.
Task Force Criteria Components as Predictors of ACM Diagnosis
| B | SE | P value | |
|---|---|---|---|
| I. Imaging | 0.341 | 0.614 | 0.579 |
| II. Biopsy | 2.240 | 0.518* | 0.000* |
| III. Repolarization | 0.305 | 0.434 | 0.482 |
| IV. Depolarization | 1.536 | 1.076 | 0.154 |
| V. Arrhythmias | 1.249 | 0.497 | 0.012* |
| VI. Familiarity | 1.350 | 0.550 | 0.033* |
ACM indicates arrhythmogenic cardiomyopathy; and B, regression coefficient.
Variables with p value less than 0.05
Table 3.
TFC in Suspected ARVC
| TFC | Definite diagnosis (N=59) | Borderline diagnosis (N=14) | Possible diagnosis (N=12) |
|---|---|---|---|
| TFC score (with EMB) | 5.08 (1.1) | 3 (0) | 2 (0) |
| TFC score (without EMB) | 4.03 (1.2) | 2.8 (0.5) | 2 (0) |
| I. Structural | |||
| Major | 22 (38.6) | 4 (26.7) | 1 (7.7) |
| Minor | 31 (52.5) | 11 (73.3) | 9 (75.0) |
| II. Tissue histology: EMB | |||
| Major | 25 (43.9) | 1 (6.7) | 0 (0) |
| Minor | 11 (19.3) | 3 (20) | 0 (0) |
| III. Repolarization | |||
| Major | 19 (33.3) | 1 (6.7) | 1 (7.7) |
| Minor | 7 (11.8) | 6 (40) | 0 (0) |
| IV. Depolarization | |||
| Major | 4 (7.0) | 0 (0) | 0 (0) |
| Minor | 2 (3.5) | 0 (0) | 1 (7.7) |
| V. Arrhythmia | |||
| Major | 13 (22.8) | 1 (6.7) | 0 (0) |
| Minor | 37 (64.9) | 9 (60) | 8 (61.5) |
| VI. Family history | |||
| Major | 19 (32.2) | 0 (0) | 1 (7.7) |
| Minor | 2 (3.4) | 4 (28.6) | 0 (0) |
ARVC indicates arrhythmogenic right ventricular cardiomyopathy; EMB, endomyocardial biopsy; and TFC, Task Force Criteria.
Continuous variables (TFC score) are shown as Mean ± SD. Discrete variables are presented as number and percentage (%).
Patients With Suspected ALVC
Nineteen patients were referred for suspected ALVC. As reported in Table 1 and Table S1, patients with ALVC had nonsignificantly different age compared with patients with ARVC, and the percentage of men was also similar. This subset of patients was more frequently referred for heart failure evaluation (both acute and chronic), and the arrhythmic burden at presentation was nonsignificantly different. Right ventricular function and dimensions, at CMR evaluation, were normal in all patients; conversely, left ventricular late gadolinium enhancement was present in 95% of patients. A pathognomonic genetic variant was present in 3 patients out of the 12 in whom genetic screening was carried out.
If classical TFC without EMB were to be applied in this patient population, only 3 (16%) would have been diagnosed with definite ALVC. The reclassification improvement after EMB was of 68%. TFC score increased from 2.8±1.0 per patient to 3.7±1.3 (P=0.04). Table 4 shows how the different components of the TFC contributed for the final diagnosis. Remarkably, EMB was a significant component (being a major or minor criterion) to the final diagnosis in 86% of definite ALVC.
Table 4.
TFC in Suspected ALVC
| TFC | Definite diagnosis (N=12) | Borderline diagnosis (N=4) | Possible diagnosis (N=3) |
|---|---|---|---|
| TFC score (with EMB) | 4.6 (0.6) | 3.0 (0) | 2.0 (0) |
| TFC score (without EMB) | 3.1 (1.1) | 2.3 (5.6) | 2.0 (0) |
| I. Structural | |||
| Major | 0 (0) | 0 (0) | 0 (0) |
| Minor | 11 (78.6) | 1 (33.3) | 1 (50.0) |
| II. Tissue histology: EMB | |||
| Major | 10 (71.4) | 0 (0) | 0 (0) |
| Minor | 2 (14.3) | 2 (66.7) | 0 (0) |
| III. Repolarization | |||
| Major | 2 (14.3) | 1 (33.3) | 0 (0) |
| Minor | 4 (28.6) | 1 (33.3) | 1 (50.0) |
| IV. Depolarization | |||
| Major | 0 (0) | 0 (0) | 0 (0) |
| Minor | 3 (21.4) | 0 (0) | 0 (0) |
| V. Arrhythmia | |||
| Major | 1 (7.1) | 1 (33.3) | 0 (0) |
| Minor | 10 (71.4) | 0 (0) | 2 (100.0) |
| VI. Family history | |||
| Major | 3 (21.4) | 0 (0) | 0 (0) |
| Minor | 3 (21.4) | 1 (33.3) | 0 (0) |
ALVC indicates arrhythmogenic left ventricular cardiomyopathy; EMB endomyocardial biopsy; and TFC, Task Force Criteria.
Continuous variables (TFC score) are shown as Mean ± SD. Discrete variables are presented as number and
percentage (%).
Discussion
In the present study, we evaluated the role of EMB in patients with suspected ACM. The main findings are as follows: (1) EMB, which was always performed under the guidance of EVM, yielded optimal diagnostic performance with a negligible complication rate; and (2) EMB allowed reaching a definite diagnosis of ARVC in 34% of patients considered at risk for ARVC at noninvasive evaluation.
EMB in ACM: The Missing Piece of the Puzzle?
TFC encompass structural, histologic, electrocardiographic, arrhythmic, and familial features, which help the clinician in establishing a diagnosis of ARVC. Since 2010, the role of EMB has progressively declined because of its low sensitivity and inherent risks, especially out of fear of myocardial perforation. This trend was exasperated to the point that some authors stated that “the use of EMB may no longer be justifiable. … ” 14 As for guidelines recommendations, the American Heart Association/American College of Cardiology Foundation/European Society of Cardiology Scientific Statement confers only a class IIB recommendation (level of evidence C) for EMB in patients with suspected ARVC. 5 Indeed, looking at 2 recent large registries describing ARVC, we can note that the percentage of EMB being performed is 7% and 14% for 407 and 140 patients, respectively. 14 , 15 , 16 , 17
However, imaging evaluation is far from being specific for ARVC. Indeed, Bomma et al 18 reported that up to 73% of presumed patients with ARVC were misdiagnosed, based on CMR misinterpretation. Moreover, the agreement between echocardiography and CMR is low, thus reducing the degree of confidence in the results. 15 As for the overall performance of 2010 TFC, this was recently analyzed in a paper by Bosman et al. 14 They found that TFC have both a sensitivity and a specificity of 92%, with 11% false negatives and 14% false positives. In their study, TFC were compared with the opinion of 3 experts. Only 28 of 407 patients underwent EMB and, surprisingly, EMB fulfilled a major criterion for ARVC in just 1 patient. A wider usage of EMB in their study might have reduced the need for expert opinion, thus making the clinical judgment more objective. Additionally, Bosman et al tried to evaluate the relative weight of individual components of TFC. However, they did not include EMB because of the relatively low number of data. We performed a similar analysis, which is reported in Figure 3 and Table 2. Our analysis is limited by selection bias, having included mostly patients with dubious diagnosis. However, in our subset of patients, these results reinforce the concept that EMB, compared with other components of the TFC, appears to be the better performing exam with respect to the final diagnosis of definite ACM.
Additionally, if differentiating patients with ACM from healthy subjects is important, it is equally important to correctly identify patients with sarcoidosis or chronic myocarditis mimicking ACM. Previous studies have demonstrated that noninvasive tests have poor diagnostic yield in this setting. 6 , 7 , 19 , 20 , 21 The main reason is that TFC were assessed relative to healthy individuals, which explains the low specificity when facing other arrhythmogenic diseases. Thus, especially in nonfamiliar forms of ACM, EMB might be the only tool able to adequately differentiate ACM from other phenocopies, as previously reported in the literature. In our paper, we have not specifically addressed this issue, as we just aimed to evaluate the confirmatory role of EMB in the setting of 2010 TFC. Yet it is worth reiterating the fact that 12 patients had histologic signs of active inflammation and no major or minor criteria for ACM, while 4 had a histologic pattern of idiopathic dilated cardiomyopathy. These 16 patients are separately reported in Table S6. In summary, a comprehensive clinical and instrumental evaluation is required to correctly manage these patients, and EMB plays a pivotal role.
Finally, ARVC is a progressive disease. Arrhythmic manifestation and structural abnormalities become more and more pronounced following the natural course of the disease, making the diagnosis certain even without EMB during subsequent follow‐up. One may thus question the utility of EMB, when a close follow‐up may better clarify the diagnosis. However, we believe that the main goal in ARVC management is to anticipate diagnosis and risk stratification at an increasingly earlier stage of the disease, to prevent sudden death attributable to sustained ventricular tachycardia or advanced heart failure.
Old and New Biopsies: What Is the Diagnostic Yield
One of the main reasons leading clinicians to progressively abandon EMB in patients with suspected ARVC is the low presumed sensitivity. 4 If we add the potential risk of serious complications being an invasive procedure, the reason for EMB being progressively abandoned in clinical practice becomes intuitive. It has to be noted that ARVC is a segmental disease, which often spares the septum, which is instead the region most frequently sampled during “old” fluoroscopy‐guided EMB. 4 Obviously, histopathologic findings at EMB may be diagnostic of ARVC if performed in the appropriate position. Hence, the problem is not whether EMB is useful, but whether we are able to correctly identify and sample the diseased tissue.
The first step in this direction was made by Corrado et al, 22 who already demonstrated in 2005 that areas of fibro‐fatty replacement in the right ventricle could be correctly detected by EVM among patients with ARVC. Following this path and adding electrophysiological tools to conventional EMB (ie, intracardiac echo, EVM, steerable catheters and long sheaths, transseptal approach for left ventricular EMB), Casella et al were able to significantly increase the diagnostic yield of EMB in the setting of different structural cardiomyopathies. 6 , 12 , 23 , 24 , 25 , 26 However, a specific analysis of this “new EMB” in a large population of patients with ACM has never been conducted. Our paper demonstrates that in patients with suspected ACM, EMB has optimal diagnostic yield (89%), with a very low complication rate. In particular, in patients with confirmed ACM, EMB satisfied a diagnostic criterion in 52% of the population, and served as fundamental tool for reaching a definite diagnosis in 44%. Notably, the intracardiac complication rate was zero, although the right ventricular free wall was also sampled. This result is largely attributed to the use of intracardiac echocardiography, which enables the operator to biopsy “safe spots” of myocardium, away from thinned aneurismal regions, while readily monitoring for complications.
One Disease, Many Subtypes
ACM is currently thought to represent a much wider spectrum of disease compared with just 10 years ago.
The first is the mixed pattern of ACM with superimposed myocarditis. Bowles et al 27 demonstrated that some cases of ACM are associated with viral genome in the myocardium and inflammatory infiltrates. The actual classification of such patients is still debatable. However, emerging evidences support the notion that this pattern may represent an early stage or a “hot phase” of the disease, associated with ongoing myocyte death and reactive inflammation. 28 These patients are at increased risk for sudden cardiac death attributable to ventricular fibrillation, as compared with the “stable phase,” which is associated with reentrant ventricular arrhythmias. 28 , 29 After EMB, these patients should thus be followed more strictly, and potential preventive tools (eg, implantable cardioverter defibrillator) might be considered, according to clinical judgment. On the other hand, myocarditis (whether infective, toxic, or autoimmune) can mimic ARVC as a disease phenocopy. The sporadic nature of the disease, together with a negative genetic test and clinical follow‐up, besides possible personal history or laboratory test in keeping with external triggers of inflammation, can help in differential diagnosis.
The second subtype is the left‐dominant form of arrhythmogenic cardiomyopathy. 30 No guideline currently reports criteria for ALVC. 9 In our cohort, strictly adhering to the current TFC without considering EMB, only 3 of 19 patients reached a definite ALVC diagnosis, and the differential diagnosis with chronic myocarditis and idiopathic cardiomyopathies was always challenging. EMB is pivotal in this setting, as an appropriate diagnosis poses significant clinical implications on the management of the patient and its relatives. A revised version of the current TFC as well as precise histologic criteria for left dominant forms are urgently needed to better identify and diagnose patients with ALVC.
ARVC and Genetic
ARVC is often a familial disease, and 60% of patients usually carry a causative genetic variant. The high genetic heterogeneity encompasses both desmosomal and nondesmosomal genes. In particular, while ARVC is mainly linked to PKP2 mutations, left ventricular forms are mainly associated with PLN, DSP, DSC2, and DSG2 pathogenic variants. 31 However, the value of genetics in diagnostic criteria is hampered by different limitations, such as the difficult interpretation of variant pathogenicity, the incomplete penetrance, the phenotypic and genetic overlapping with other cardiomyopathies, and the technological limits of the current molecular diagnosis methods.
In particular, the incomplete penetrance and the large variability of clinical manifestations, renders ARVC diagnosis difficult. Indeed, the presence of a putative genetic mutation does not make the patient affected by ARVC. Given the fact that gross structural abnormalities, visible at imaging, are associated with a later stage of the disease, EMB may represent one of the few tools in our hands to adequately identify subclinical ARVC from asymptomatic mutation carriers.
Additionally, some limits of the classical genetic classifications were recently showed by Costa et al, 32 who proved how, according to the new 2015 American College of Medical Genetics and Genomics Criteria, 41.3% of the genetic mutation considerate as putative mutations needed to be reclassified. This led to a downgrade in the diagnosis of 10% of the patients. This knowledge makes the information of papers referring to patients classified before 2015 less accurate, and possibly overemphasizing the effect of the genetic component. Additionally, the genetic panel used in patients with ARVC are under continuous evolution. This is an inherent limitation of all the retrospective studies, being patients tested in 2010, quite different from patients tested in 2019.
Since the identification of a mutation is regarded as a major criterion, it may contribute up to 50% to the diagnosis of ARVC. 3 This is particularly true for left‐dominant forms, for which the other diagnostic criteria are less specific. 9 Therefore, a team of experts is needed for genetic data interpretation, and its weight in the diagnostic criteria is still matter of debate. 3
Limitations
It is not possible to define the number of patients in whom the conventional EMB would have been diagnostic, compared with EVM‐guided EMB. Nonetheless, the overall percentage of positive EMB for ARVC in our study appears higher than the previously reported data with conventional biopsy.
The number of patients referred for arrhythmias represents more than half of patients evaluated in the study. This reflects the fact that the study was performed by the arrhythmology unit of our center. Thus, a selection bias may be present.
It is worth mentioning that the 2010 TFC specify that EMB has to be taken from the right ventricular free wall myocardium. When analyzing TFC score for EMB in suspected ALVC, specimens taken from the left ventricle were considered. In such cases, minor or major criteria were assigned, as if this specimen was taken from the right ventricular free wall. We recognize that this may be a “free” interpretation of the 2010 TFC. Yet we believe that in the absence of standardized criteria for ALVC, this represents the best management strategy in this subset of patients.
Genetic analysis certainly represents a fundamental cornerstone for ARVC diagnosis. We recognize that the low availability of information regarding genetic testing (62% of patients genotyped) is a major limitation of our work. On the other hand, the literature regarding ARVC diagnosis comprises many works that could not provide a full clinical, radiologic, histologic, and genetic evaluations of patients. This is inherent to the retrospective nature of these studies and the different availability provided by different centers. Specifically, EMB evaluation was missing in the vast majority of the cohorts published in recent years or present in <15% of the evaluated patients.
Another potential limitation is the absence of screening for filamin C, which, despite being rarely associated with ARVC, is nowadays usually included in genetic screening panels.
One final limitation is the absence of follow‐up, which may have possible implications on the classification of some patients. However, we believe that in this cohort, what is important is not only to reach a diagnosis, but also to reach it as early as possible, to adopt all possible preventive measures.
Conclusions
This study confirms the high diagnostic efficacy and safety of EVM‐guided EMB in patients with ARVC. It reinforces the concept that EMB is still a useful (yet unfortunately underused) tool, allowing upgrading of the diagnostic status of one‐third of patients with a suspect ARVC. EMB acquires an even greater importance in patients without a genetic diagnosis, in whom the exclusion of phenocopies is essential, and for which noninvasive procedures do not allow definite results. Our study reinforces the idea that the relative weight of each individual TFC may not be as equal as currently assumed. In patients with suspected left‐dominant disease, conventional TFC performed poorly. EMB may be of help, although specific criteria are currently lacking.
Sources of Funding
None.
Disclosures
Dr Russo has received consulting fees/honoraria from Biosense Webster. Dr Di Biase is a consultant for Biosense Webster, Boston Scientific, Stereotaxis, Rhythm Management, and Abbott; and has received speaking honoraria from Medtronic, Pfizer, Bristol Meyers, and Biotronik. Dr Natale is a consultant for Biosense Webster, Abbott, and Janssen; and has received speaking honoraria from Boston Scientific, Biosense Webster, Abbott, Biotronik, and Medtronic. Dr Tondo receives consulting fees/honoraria from Abbott, Medtronic, Boston Scientific, and Biosense Webster; and serves as a member of EU Medtronic Advisory Board and Boston Scientific Advisory Board. The remaining authors have no disclosures to report.
Supporting information
Data S1
Table S1–S6
Figure S1
M. Casella and M. Bergonti contributed equally.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376:61–72. DOI: 10.1056/NEJMra1509267. [DOI] [PubMed] [Google Scholar]
- 2. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MGPJ, Daubert JP, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. DOI: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16:e301–e372. DOI: 10.1016/j.hrthm.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 4. Angelini A, Basso C, Nava A, Thiene G. Endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy. Am Heart J. 1996;132:203–206. [DOI] [PubMed] [Google Scholar]
- 5. Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of. Circulation. 2007;28:3076–3093. DOI: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 6. Pieroni M, Dello Russo A, Marzo F, Pelargonio G, Casella M, Bellocci F, Crea F. High prevalence of myocarditis mimicking arrhythmogenic right ventricular cardiomyopathy differential diagnosis by electroanatomic mapping‐guided endomyocardial biopsy. J Am Coll Cardiol. 2009;53:681–689. DOI: 10.1016/j.jacc.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 7. Ott P, Marcus FI, Sobonya RE, Morady F, Knight BP, Fuenzalida CE. Cardiac sarcoidosis masquerading as right ventricular dysplasia. Pacing Clin Electrophysiol. 2003;26:1498–1503. DOI: 10.1046/j.1460-9592.2003.t01-1-00217.x. [DOI] [PubMed] [Google Scholar]
- 8. Casella M, Gasperetti A, Gaetano F, Busana M, Sommariva E, Catto V, Sicuso R, Rizzo S, Conte E, Mushtaq S, et al. Long‐term follow‐up analysis of a highly characterized arrhythmogenic cardiomyopathy cohort with classical and non‐classical phenotypes‐a real‐world assessment of a novel prediction model: does the subtype really matter. Europace. 2020;22:797–805. DOI: 10.1093/europace/euz352. [DOI] [PubMed] [Google Scholar]
- 9. Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari MD, Migliore F, Pilichou K, Rampazzo A, Rigato I, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol. 2020;319:106–114. DOI: 10.1016/j.ijcard.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 10. Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, Basso C, Bauce B, Brunckhorst C, Bucciarelli‐Ducci C, et al. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J. 2019;41:1414–1429. DOI: 10.1093/eurheartj/ehz669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier‐Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. DOI: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Casella M, Dello Russo A, Bergonti M, Catto V, Conte E, Sommariva E, Gasperetti A, Vettor G, Tundo F, Sicuso R, et al. Diagnostic yield of electroanatomic voltage mapping in guiding endomyocardial biopsies. Circulation. 2020;142:1249–1260. DOI: 10.1161/CIRCULATIONAHA.120.046900. [DOI] [PubMed] [Google Scholar]
- 13. Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 2011;8:1308–1339. DOI: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 14. Bosman LP, Cadrin‐Tourigny J, Bourfiss M, Aliyari Ghasabeh M, Sharma A, Tichnell C, Roudijk RW, Murray B, Tandri H, Khairy P, et al. Diagnosing arrhythmogenic right ventricular cardiomyopathy by 2010 Task Force Criteria: clinical performance and simplified practical implementation. Europace. 2020;22:787–796. DOI: 10.1093/europace/euaa039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borgquist R, Haugaa KH, Gilljam T, Bundgaard H, Hansen J, Eschen O, Jensen HK, Holst AG, Edvardsen T, Svendsen JH, et al. The diagnostic performance of imaging methods in ARVC using the 2010 Task Force criteria. Eur Heart J Cardiovasc Imaging. 2014;15:1219–1225. DOI: 10.1093/ehjci/jeu109. [DOI] [PubMed] [Google Scholar]
- 16. Cadrin‐Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, Bourfiss M, Fortier A, Lie ØH, Saguner AM, et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2019;40:1850–1858. DOI: 10.1093/eurheartj/ehz103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aquaro GD, De Luca A, Cappelletto C, Raimondi F, Bianco F, Botto N, Lesizza P, Grigoratos C, Minati M, Dell’Omodarme M, et al. Prognostic value of magnetic resonance phenotype in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2020;75:2753–2765. DOI: 10.1016/j.jacc.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 18. Bomma C, Rutberg J, Tandri H, Nasir K, Roguin A, Tichnell C, Rodriguez R, James C, Kasper E, Spevak P, et al. Misdiagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol. 2004;15:300–306. DOI: 10.1046/j.1540-8167.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- 19. Yared K, Johri AM, Soni AV, Johnson M, Alkasab T, Cury RC, Hung J, Mamuya W. Cardiac sarcoidosis imitating arrhythmogenic right ventricular dysplasia. Circulation. 2008;118:e113–e115. DOI: 10.1161/CIRCULATIONAHA.107.755215. [DOI] [PubMed] [Google Scholar]
- 20. Rastegar N, Burt JR, Corona‐Villalobos CP, Te Riele AS, James CA, Murray B, Calkins H, Tandri H, Bluemke DA, Zimmerman SL, et al. Cardiac MR findings and potential diagnostic pitfalls in patients evaluated for arrhythmogenic right ventricular cardiomyopathy. Radiographics. 2014;34:1553–1570. DOI: 10.1148/rg.346140194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gasperetti A, Rossi VA, Chiodini A, Casella M, Costa S, Akdis D, Büchel R, Deliniere A, Pruvot E, Gruner C, et al. Differentiating hereditary arrhythmogenic right ventricular cardiomyopathy from cardiac sarcoidosis fulfilling 2010 ARVC Task Force Criteria. Heart Rhythm. 2020;18:231–238. DOI: 10.1016/j.hrthm.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 22. Corrado D, Basso C, Leoni L, Tokajuk B, Bauce B, Frigo G, Tarantini G, Napodano M, Turrini P, Ramondo A, et al. Three‐dimensional electroanatomic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2005;111:3042–3050. DOI: 10.1161/CIRCULATIONAHA.104.486977. [DOI] [PubMed] [Google Scholar]
- 23. Casella M, Dello Russo A, Vettor G, Lumia G, Catto V, Sommariva E, Ribatti V, Biagioli V, Tundo F, Carbucicchio C, et al. Electroanatomical mapping systems and intracardiac echo integration for guided endomyocardial biopsy. Expert Rev Med Devices. 2017;14:609–619. DOI: 10.1080/17434440.2017.1351875. [DOI] [PubMed] [Google Scholar]
- 24. Casella M, Pizzamiglio F, Dello Russo A, Carbucicchio C, Al‐Mohani G, Russo E, Notarstefano P, Pieroni M, D’Amati G, Sommariva E, et al. Feasibility of combined unipolar and bipolar voltage maps to improve sensitivity of endomyocardial biopsy. Circ Arrhythm Electrophysiol. 2015;8:625–632. DOI: 10.1161/CIRCEP.114.002216. [DOI] [PubMed] [Google Scholar]
- 25. Santangeli P, Hamilton‐craig C, Russo AD, Pieroni M, Casella M, Pelargonio G, Biase LD, Smaldone C, Bartoletti S, Narducci ML, et al. Imaging of scar in patients with ventricular arrhythmias of right ventricular origin: cardiac magnetic resonance versus electroanatomic mapping. J Cardiovasc Electrophysiol. 2011;22:1359–1366. DOI: 10.1111/j.1540-8167.2011.02127.x. [DOI] [PubMed] [Google Scholar]
- 26. Avella A, d'AMATI G, Pappalardo A, Re F, Silenzi PF, Laurenzi F, De girolamo P, Pelargonio G, Russo AD, Baratta P, et al. Diagnostic value of endomyocardial biopsy guided by electroanatomic voltage mapping in arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Electrophysiol. 2008;19:1127–1134. DOI: 10.1111/j.1540-8167.2008.01228.x. [DOI] [PubMed] [Google Scholar]
- 27. Bowles NE, Ni J, Marcus F, Towbin JA. The detection of cardiotropic viruses in the myocardium of patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2002;39:892–895. DOI: 10.1016/S0735-1097(02)01688-1. [DOI] [PubMed] [Google Scholar]
- 28. Pilichou K, Thiene G, Bauce B, Rigato I, Lazzarini E, Migliore F, Perazzolo Marra M, Rizzo S, Zorzi A, Daliento L, et al. Arrhythmogenic cardiomyopathy. Orphanet J Rare Dis. 2016;11:33. DOI: 10.1186/s13023-016-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Basso C, Corrado D, Bauce B, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2012;5:1233–1246. DOI: 10.1161/CIRCEP.111.962035. [DOI] [PubMed] [Google Scholar]
- 30. Sen‐Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ. Left‐dominant arrhythmogenic cardiomyopathy: an under‐recognized clinical entity. J Am Coll Cardiol. 2008;52:2175–2187. DOI: 10.1016/j.jacc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 31. Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JDH, Murray B, te Riele ASJM, van den Berg MP, Bikker H, et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy‐associated mutation carriers. Eur Heart J. 2015;36:847–855. DOI: 10.1093/eurheartj/ehu509. [DOI] [PubMed] [Google Scholar]
- 32. Costa S, Gasperetti A, Akdis D, Suna G, Medeiros Domingo A, Brunckhorst C, Duru F, Saguner AM. Impact of genetic reclassification on ARVC diagnosis based on the 2010 task force criteria. Eur Heart J. 2020;41. DOI: 10.1093/ehjci/ehaa946.2104. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1–S6
Figure S1


