Abstract
Background and purpose
Deep brain stimulation of the thalamus is an effective treatment for multiple neurological disorders. The centromedian and parafascicular nuclei are recently emerging targets for multiple conditions, such as epilepsy and Tourette syndrome; however, their limited visibility on conventional magnetic resonance imaging sequences has been a major obstacle. The goal of this study was to demonstrate the feasibility of a high-resolution and high-contrast targeting sequence for centromedian-parafascicular deep brain stimulation using a recently described magnetic resonance imaging sequence, three-dimensional edge-enhancing gradient echo.
Methods
The three-dimensional edge-enhancing gradient echo sequence was performed on a normal volunteer for a total of six acquisitions. Multi-image co-registration and averaging was performed by first co-registering each of the six scans and then averaging to produce an edge-enhancing gradient echo-multi-image co-registration and averaging scan. The averaging was also performed for two, three, four and five scans to assess the change in the signal-to-noise ratio and identify the ideal balance of image quality and scan time.
Results
The edge-enhancing gradient echo-multi-image co-registration and averaging scan allowed clear boundary delineation of the centromedian and parafascicular nuclei. The signal-to-noise ratio increased as a function of increasing scan number, but the added gain was small beyond four scans for the imaging parameters used in this study.
Conclusions
The recently described three-dimensional edge-enhancing gradient echo sequence provides an easily implementable approach, using widely available magnetic resonance imaging technology without complex post-processing techniques, to delineate centromedian and parafascicular nuclei for deep brain stimulation targeting.
Keywords: Epilepsy, deep brain stimulation, centromedian nucleus, parafascicular nucleus
Introduction
Over the past several decades, there has been an increasing application of neuromodulation to treat various neurological disorders. Recently, there has been increasing interest in the centromedian and parafascicular nuclei complex of the thalamus in numerous conditions, such as Parkinson disease,1,2 epilepsy,3–8 Tourette syndrome,9–13 pain,14–16 and others.
According to the the International Tourette Deep Brain Stimulation Registry and Database, the centromedian/parafascicular region has been reported to be the most commonly targeted brain area for Tourette syndrome worldwide. 9 Retrospective studies of centromedian/parafascicular deep brain stimulation (DBS) have demonstrated beneficial effects on tic frequency and severity which were confirmed by prospective, double-blind randomised trials.10–13 For epilepsy, DBS of the anterior nucleus of the thalamus (ANT) was recently approved by the US Food and Drug Administration for treating refractory epilepsy.17,18 However, patients with temporal onset have better outcomes17,18 from ANT DBS when compared to those with networks that do not involve the limbic circuit, leading to increased interest in other thalamic targets, such as the centromedian nucleus and parafascicular nucleus.19,20 In particular, the centromedian nucleus has been shown as an efficacious target for extra-limbic epilepsy, specifically generalised and frontal epilepsy syndromes. 3
One major barrier to successful surgical therapy has been the limited visibility of these targets on conventional magnetic resonance imaging (MRI) sequences leading to an overreliance on indirect targeting methods with a general consensus that centromedian/parafascicular nuclei are not able to be visualised on conventional MRI. 21 Unfortunately, variations in normal anatomy and acquired shape changes due to atrophy in the setting of epilepsy produce wide variability in the accuracy of such fixed coordinate-based targets, potentially resulting in off-target implants.22,23 Therefore, direct targeting methods with higher accuracy of delineation of intrathalamic nuclei are necessary. The magnetisation-prepared rapid gradient echo (MPRAGE) imaging that applies two inversion times (MP2RAGE) sequence has been proposed as a potential method for centromedian nucleus visualisation, but complex post-processing and edge enhancement was necessary to improve visualisation of the centromedian nucleus. 24
In this proof of concept study, we explore the utility of a recently described three-dimensional (3D) sequence, edge-enhancing gradient echo (3D-EDGE), as an easily implementable method for reliable visualisation and targeting of the centromedian/parafascicular nuclei. 25 3D-EDGE has unique contrast properties that exploit subtle differences in T1 relaxation of myelinated and non-myelinated tissues, making it a potentially valuable approach for detecting histological differences within the thalamic nuclei that are not well detected by existing imaging methods. 25 Improved delineation of targets within the thalamus serves to provide an accurate, patient-specific direct surgical target and may ultimately lead to improved outcomes after DBS.
Methods
Image acquisition
The study was approved by the Mayo Clinic Institutional Review Board. The 3D-EDGE sequence was performed on a normal volunteer using a 3 Tesla Siemens Prisma (Siemens Healthineers AG, Erlangen, Germany) with a 64-channel phased-array head and neck coil. The 3D-EDGE utilised a MPRAGE sequence acquired in the sagittal plane with a non-selective inversion pulse with inversion times (TIs) of 442 ms, flip angle of 8°, TR of 2 s, TE of 2.66 ms, isotropic resolution of 1 × 1 × 1 mm, receiver bandwidth of 270 Hz/Px, echo spacing of 6.4 ms, turbo factor of 154, acceleration factor of GRAPPA of 2 and slice partial Fourier of 7/8. The resultant acquisition time was 4.32 minutes. The scan was repeated a total of six times. For image normalisation, a standard MPRAGE and fast grey matter acquisition and T1 inversion recovery (FGATIR) were also acquired, as previously described. 26
Image processing
The six 3D-EDGE acquisitions were co-registered using a linear registration algorithm in Statistical Parametric Mapping v. 12 (https://www.fil.ion.ucl.ac.uk/spm/) with a normalised mutual information objective function and fourth degree B-spline interpolation. The co-registered images were then averaged to generate 3D-EDGE multi-image co-registration and averaging (EDGE-MICRA) images. To assess the effect of incremental image acquisitions, the averaging was performed with two, three, four, five and six total scans.
Image quality
SNR of a single acquisition was compared to each EDGE-MICRA average image (average of two to six scans). SNR was calculated as the mean signal intensity of a region of interest encompassing the thalami divided by the standard deviation of the background signal outside of the head. The plot of SNR versus the number of averages was fit to a polynomial curve in Prism v. 8.1.1 (GraphPad Software, San Diego, CA, USA). The theoretical SNR gain with complex averaging in k-space was also plotted as a reference and calculated as the baseline SNR of a single acquisition multiplied by the square root of the number of acquisitions that were averaged.
Atlas registration
The EDGE-MICRA with six total averaged images was linearly co-registered to the MPRAGE using statistical parametric mapping. The MPRAGE was then normalised to the Montreal Neurological Institute (MNI) template space using the SyN registration approach in advanced normalisation tools (http://stnava.github.io/ANTs/), 27 and the resulting transformation was applied to the EDGE-MICRA. The normalised EDGE-MICRA was resampled to 0.5 × 0.5 × 0.5 mm resolution. An overlay of the Schaltenbrand and Wahren atlas was applied, as derived from the Neuroimaging and Surgical Technologies Lab (http://nist.mni.mcgill.ca) multicontrast PD25 atlas.28–30
Results
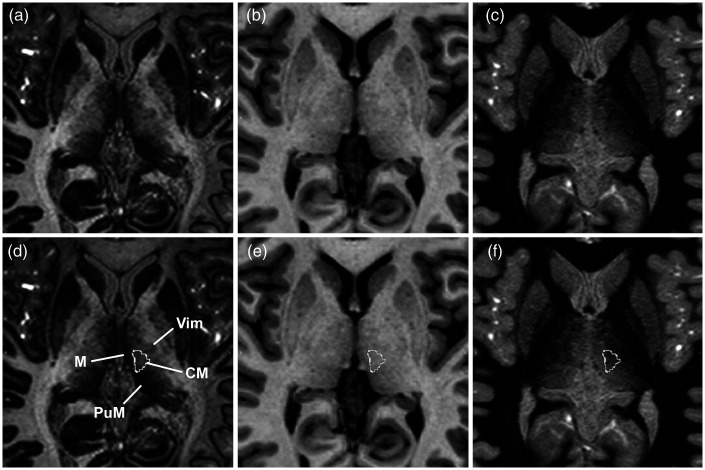
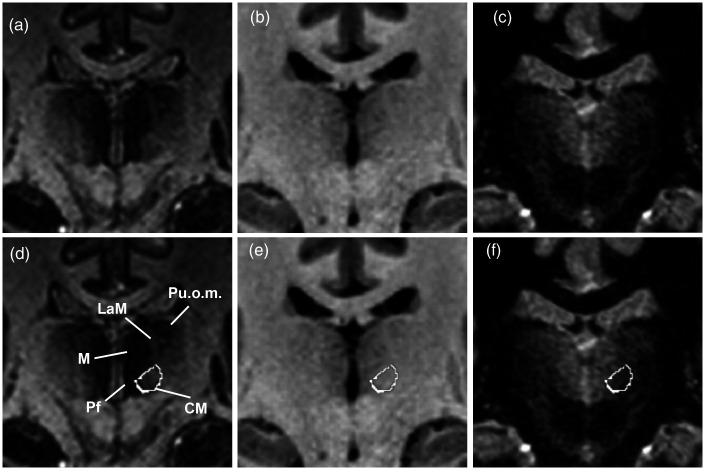
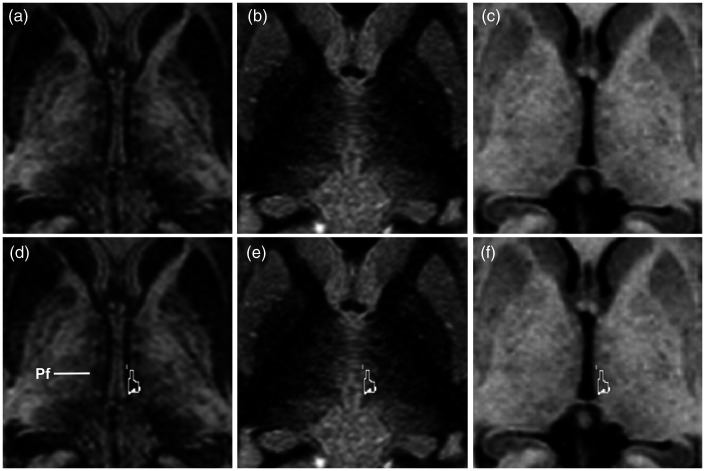
The EDGE-MICRA contrast allowed clear boundary delineation of several relevant thalamic nuclei, including the centromedian and parafascicular nuclei. Axial and coronal videos are shown in Supplementary videos 1 and 2, respectively. Representative images with Schaltenbrand and Wahren atlas overlay are shown in Figure 1. The centromedian nucleus is identified as an area of higher signal intensity compared to its border with the more hypointense mediodorsal nucleus and lateral ventral thalamic nuclei (Figure 1(a) and (d), Figure 2(a) and (d)). On standard MPRAGE (Figure 1(b) and (e), Figure 2(b) and (e)) and FGATIR (Figure 1(c) and (f), Figure 2(c) and (f)), the centromedian nucleus boundaries are not readily discernable. The parafascicular nucleus is located medial and inferior to the centromedian nucleus – inferior to the mediodorsal nucleus – and can be well visualised as a more hypointense structure compared to the more hyperintense lateral boundary (Figure 3(a) and (d)). The boundaries of the parafascicular nucleus are not well delineated on MPRAGE (Figure 3(b) and (e)) or FGATIR (Figure 3(c) and (f)).
Figure 1.
Axial (a) EDGE-MICRA, (b) MPRAGE and (c) FGATIR images at MNI z = 3. Axial (a) EDGE-MICRA image with the centromedian nucleus (CM) outlined from the Schaltenbrand and Wahren atlas. The CM can be visualised as a hyperintense ovoid nucleus lying medial and inferior to the more hypointense mediodorsal nucleus (M), anterior to the more hypointense medial pulvinar (PuM) and medial to the ventral intermediate nucleus (Vim). (e) Axial MPRAGE and (f) FGATIR at the same level shows poor definition of the internal thalamic structure and CM (outline). EDGE-MICRA: three-dimensional edge-enhancing gradient echo multi-image co-registration and averaging; FGATIR: fast grey matter acquisition and T1 inversion recovery; MNI: Montreal Neurological Institute; MPRAGE: magnetisation-prepared rapid gradient echo.
Figure 2.
Coronal (a) EDGE-MICRA, (b) MPRAGE and (c) FGATIR images at MNI y = −20. Coronal (a) EDGE-MICRA image with the centromedian nucleus (CM) outlined from the Schaltenbrand and Wahren atlas. The CM can be visualised as a hyperintense ovoid nucleus lying medial and inferior to the more hypointense mediodorsal nucleus (M) and medial to the parafascicular nucleus (Pf). The lamella medialis (LaM) is also well visualised separating the mediodorsal nucleus from the pulvinar oromedialis (Puom). (e) Coronal MPRAGE and (f) FGATIR at the same level shows poor definition of the internal thalamic structure and CM (outline). EDGE-MICRA: three-dimensional edge-enhancing gradient echo multi-image co-registration and averaging; FGATIR: fast grey matter acquisition and T1 inversion recovery; MNI: Montreal Neurological Institute; MPRAGE: magnetisation-prepared rapid gradient echo.
Figure 3.
Axial (a) EDGE-MICRA, (b) FGATIR and (c) MPRAGE images at MNI z = 0. (d) Axial EDGE-MICRA illustrating the hypointense parafascicular (Pf) thalamic nucleus (outline from the Schaltenbrand and Wahren atlas). Axial (e) FGATIR and (f) MPRAGE show poor definition of the boundaries of Pf (outline). EDGE-MICRA: three-dimensional edge-enhancing gradient echo multi-image co-registration and averaging; FGATIR: fast grey matter acquisition and T1 inversion recovery; MNI: Montreal Neurological Institute; MPRAGE: magnetisation-prepared rapid gradient echo.
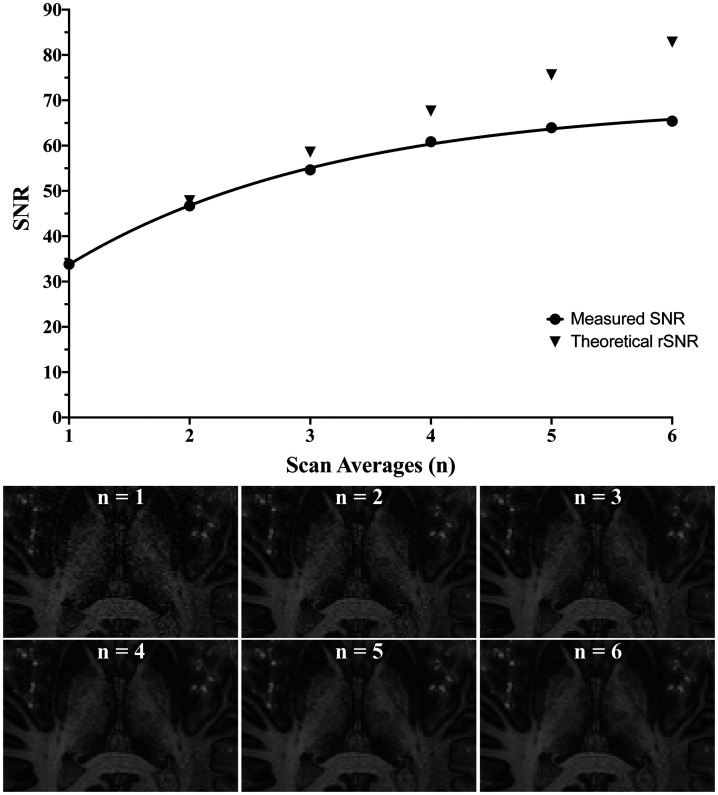
A comparison of differing numbers of averaged images was performed to determine the ideal balance of scan time and satisfactory image quality. As expected, there was an increase in SNR with increasing numbers of acquisitions (SNR(n) = –1.4n2 + 15.7n + 19.9; r2 = 0.998, where n is the number of averages); however, the gain began to plateau after four acquisitions (Figure 4). Most landmarks were apparent at an average of two scans, but with reduced noise and slightly increased visual contrast with higher scan numbers. Visual inspection suggested three averaged acquisitions as an ideal balance between imaging time, SNR and contrast for the utilised scan parameters.
Figure 4.
(Top) Relationship of measured signal-to-noise ratio (SNR; r² = 0.998) and theoretical SNR with increasing number of averaged scans from complex averaging in k-space. (Bottom) Representative images for increasing number of scan averages from a single acquisition (n = 1) to six averaged scans (n = 6).
Discussion
In this study, we describe a novel application of a recently proposed MRI sequence, 3D-EDGE, to delineate the centromedian and parafascicular nuclei for purposes of direct surgical targeting. The unique contrast properties of 3D-EDGE provide a new approach for detecting histological differences in some thalamic nuclei. As the sequence can be obtained using routine magnetic field strengths and base pulse sequences, it is a promising new highly practical approach to DBS targeting within the thalamus.
Middlebrooks et al. recently described the 3D-EDGE sequence for the improved detection of focal cortical dysplasia in epilepsy. 25 The unique contrast of this sequence, however, may have additional applications. The concept of 3D-EDGE is based on the normal variation in longitudinal (T1) relaxation of grey matter and white matter. By selecting an inversion time that is optimised to produce a similar signal intensity in grey matter and white matter, but with opposing polarity, voxels having a variable degree of myelinated and non-myelinated tissue will show different degrees of signal cancelation. The result is effectively a ‘myelin-weighted image’, in which voxels containing a relatively similar volume of myelinated and unmyelinated tissue will show greater signal cancellation. These properties can be exploited to detect subtle variations in myelin content of thalamic nuclei.
While 3D-EDGE is a high contrast sequence, it does suffer from inherently lower SNR compared with conventional MPRAGE. To compensate for the decreased SNR, while maintaining high spatial resolution, a long acquisition time with multiple averages is needed. To achieve satisfactory SNR, we took the approach of averaging multiple acquisitions (MICRA) in the imaging space, rather than increasing the acquisition number in k-space.31,32 While averaging in k-space is a valid alternative approach, the SNR gain with MICRA is close to the theoretical SNR gain with complex averaging in k-space, as shown in Figure 5. As such, we preferred the MICRA approach due to several key advantages. First, by acquiring relatively short individual scans at the penalty of reduced SNR, the effect of motion is minimised compared to a single, long acquisition. The scan is not only less susceptible to motion when shorter, but patient motion during a short scan can also be rectified by excluding problematic individual scans or repeating scans. The MICRA approach also gives greater flexibility more easily to achieve the desired SNR across a variety of platforms, such as lower field strength scanners. Likewise, when high acceleration factors are unachievable (e.g. use of single-channel transmit/receive coil), MICRA can allow the achievement of high spatial resolution and satisfactory SNR without excessively long individual scans that are subject to greater patient motion. Finally, MICRA can be applied irrespective of acceleration techniques used, making it universally applicable to changes in imaging technology.
Figure 5.
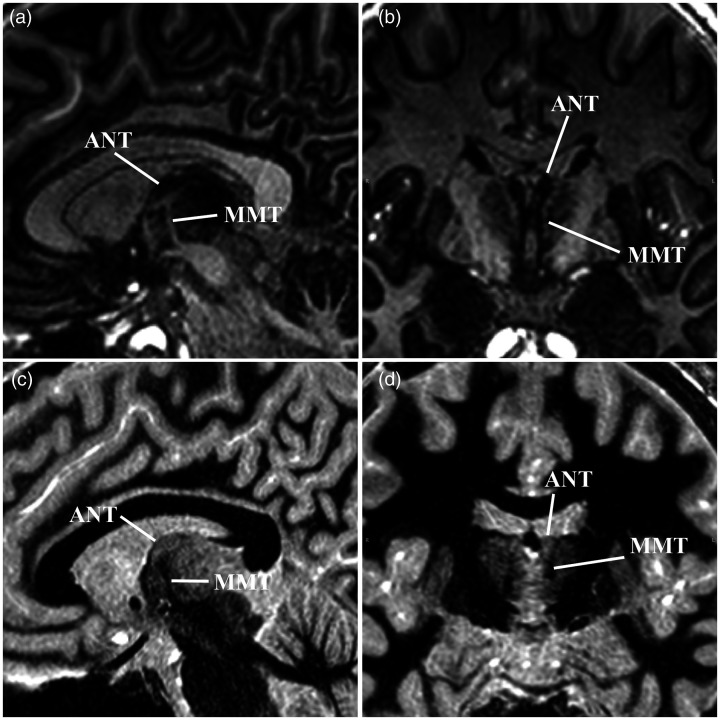
(a) Sagittal EDGE-MICRA images illustrating the hypointense anterior thalamic nucleus (ANT) that lies superior to the termination of the mammillothalamic tract (MMT). (b) Coronal EDGE-MICRA image again showing the ANT as a hypointense structure medial to the more hyperintense internal medullary lamina and MMT. Sagittal (c) and coronal (d) FGATIR images are shown for comparison. EDGE-MICRA: three-dimensional edge-enhancing gradient echo multi-image co-registration and averaging; FGATIR: fast grey matter acquisition and T1 inversion recovery.
DBS of the centromedian and parafascicular nuclei have gained increasing attention in the treatment of many disorders, including Tourette’s syndrome, neuropathic pain and epilepsy, particularly Lennox–Gastaut syndrome. 20 Unfortunately, the results of clinical trials of centromedian/parafascicular DBS have been mixed, which is likely in part due to variation in targeting.3,4,8,33,34 The centromedian nucleus is a spherical structure measuring approximately 1 cm and makes up part of the posterior intralaminar thalamus. The parafascicular nucleus is located medial and inferior to the centromedian nucleus, inferior to the mediodorsal nucleus. 20 To date, few studies have addressed visualisation of the centromedian and parafascicular nuclei. The ability to delineate these structures has largely been elusive with standard structural MRI techniques. Warren et al. recently proposed the use of MPRAGE imaging that applies two inversion times (MP2RAGE) to identify the centromedian and parafascicular complex. 24 Since the contrast of these structures is still minimal on MP2RAGE, additional post-processing was required (application of Sobel edge detection) to achieve adequate boundary visualisation. 24 Li et al. also recently proposed the use of quantitative susceptibility mapping to identify the centromedian nucleus. 35 While promising, the lack of a 3D isotropic acquisition limits the applicability to surgical planning, as well as the requirement of complex post-processing techniques. Due to the lack of the widespread availability of MP2RAGE and quantitative susceptibility mapping, as well as the need for complex post-processing, EDGE-MICRA offers a more easily implementable approach to visualise the centromedian and parafascicular complex using widely available methods.
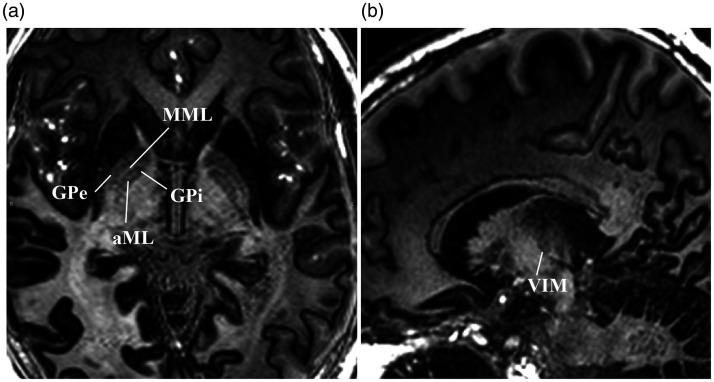
Finally, the focus of the current study was limited to centromedian/parafascicular targeting; however, EDGE-MICRA holds promise for multiple other applications in DBS. DBS of the anterior nucleus of the thalamus (ANT) is the most recently approved DBS indication by the US Food and Drug Administration. The ANT is located in the anterior dorsal thalamus along the medial border with a Y-shaped internal medullary lamina separating it from the adjacent nuclei.36,37 Grewal et al. have previously shown that the location of ANT at the termination of the mammillothalamic tract, as well as the adjacent lamina, can be exploited to visualise ANT by using white matter suppression in the FGATIR sequence. 26 The FGATIR sequence is a fast and easily implementable sequence with superb visualisation of ANT; however, other key nuclei, such as the centromedian and parafascicular, are not well resolved. EDGE-MICRA allows similar to improved visualisation of the mammillothalamic tract and ANT (Figure 5), but with the added advantage of simultaneously visualising other emerging thalamic targets. In addition, the globus pallidus internus, a common target in disorders such as Parkinson’s disease, dystonia and Tourette’s syndrome, is exquisitely delineated by this technique (Figure 6(a)). The lateral boundary with the globus pallidus externus is clearly defined by the medial medullary lamina. In addition, an accessory medullary lamina within the globus pallidus internus can be easily visualised and has not been previously reported with conventional clinical imaging. Thalamic targets for the treatment of tremor, namely the ventral intermedius nucleus, are also well visualised by a hypointense band (Figure 6(b)) that presumably reflects increased myelin content from the traversing dentato–rubro–thalamic tract.
Figure 6.
(a) Axial EDGE-MICRA image showing the globus pallidus externus (GPe) and globus pallidus internus (GPi) separated by the medial medullary lamina (MML). The GPi also contains an accessory medullary lamina (aML). (b) Sagittal EDGE-MICRA images showing the location of the ventral intermedius nucleus (VIM) that can be visualised by an area of slight hypointensity that is presumably related to increased myelin content from the traversing dentate–rubro–thalamic tract. EDGE-MICRA: three-dimensional edge-enhancing gradient echo multi-image co-registration and averaging.
Several limitations are noteworthy. First, the base MPRAGE sequence used for EDGE-MICRA is more widely available than other reported techniques, such as MP2RAGE, application 3D-EDGE contrast has not yet been reported using other variations of 3D inversion-recovery gradient echo schemes, and further validation is needed to determine if it can be reproduced on similar base sequences. Second, although the scan time for EDGE-MICRA is clinically feasible, with acquisition times similar to slightly longer than some MP2RAGE protocols, scan times between 13 and 18 minutes may be required to get the desired SNR. Newly described techniques for image acceleration, such as WaveCAIPI 38 and compressed sensing (CS), 39 have the potential dramatically to reduce imaging times of EDGE-MICRA with minimal SNR penalty. It is worth noting that the EDGE-MICRA technique may be more valuable in situations in which imaging acceleration is not possible (e.g. single-channel transmit/receive coil) and would create excessively long single scan times with MP2RAGE that are susceptible to patient motion.
Conclusion
We have described a novel application of the recently described 3D-EDGE sequence, EDGE-MICRA, as a promising method for direct targeting in DBS. EDGE-MICRA can be easily adapted to widely available scan equipment and a common base sequence (MPRAGE). Further studies are needed to assess the added benefit in targeting accuracy and outcomes with the use of direct targeting using EDGE-MICRA.
Supplementary Material
Conflict of interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: EHM receives unrelated research support from Boston Scientific Corp. and Varian Medical Systems, Inc. The remaining author(s) have no conflicts of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: EHM receives unrelated research support from Boston Scientific Corp. and Varian Medical Systems, Inc., as well as unrelated consultant to Boston Scientific Corp. SSG is a consultant for Medtronic, Inc. and Boston Scientific Corp.
Supplemental material: Supplemental material for this article is available online.
ORCID iD
Lela Okromelidze https://orcid.org/0000-0002-1296-0522
References
- 1.Stefani A, Peppe A, Pierantozzi M, et al. Multi-target strategy for Parkinsonian patients: the role of deep brain stimulation in the centromedian–parafascicularis complex. Brain Res Bull 2009; 78: 113–118. DOI: 10.1016/j.brainresbull.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 2.Peppe A, Gasbarra A, Stefani A, et al. Deep brain stimulation of CM/PF of thalamus could be the new elective target for tremor in advanced Parkinson’s disease? Parkinsonism Relat Disord 2008; 14: 501–504. DOI: 10.1016/j.parkreldis.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 3.Valentin A, Garcia Navarrete E, Chelvarajah R, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 2013; 54: 1823–1833. DOI: 10.1111/epi.12352 [DOI] [PubMed] [Google Scholar]
- 4.Fisher RS, Uematsu S, Krauss GL, et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia 1992; 33: 841–851. DOI: 10.1111/j.1528-1157.1992.tb02192.x [DOI] [PubMed] [Google Scholar]
- 5.Velasco F, Velasco AL, Velasco M, et al. Deep brain stimulation for treatment of the epilepsies: the centromedian thalamic target. In: Sakas DE, Simpson BA. (eds) Operative Neuromodulation: Vol. 2: Neural Networks Surgery. Vienna: Springer Vienna, 2007, pp. 337–342. [DOI] [PubMed] [Google Scholar]
- 6.Klinger N, Mittal S. Deep brain stimulation for seizure control in drug-resistant epilepsy. Neurosurg Focus FOC 2018; 45: E4. DOI: 10.3171/2018.4.Focus1872 [DOI] [PubMed] [Google Scholar]
- 7.Zhou JJ, Chen T, Farber SH, et al. Open-loop deep brain stimulation for the treatment of epilepsy: a systematic review of clinical outcomes over the past decade (2008–present). Neurosurg Focus FOC 2018; 45: E5. DOI: 10.3171/2018.5.Focus18161 [DOI] [PubMed] [Google Scholar]
- 8.Son BC, Shon YM, Choi JG, et al. Clinical outcome of patients with deep brain stimulation of the centromedian thalamic nucleus for refractory epilepsy and location of the active contacts. Stereotact Funct Neurosurg 2016; 94: 187–197. DOI: 10.1159/000446611 [DOI] [PubMed] [Google Scholar]
- 9.International Tourette Deep Brain Stimulation Database and Registry. https://tourettedeepbrainstimulationregistry.ese.ufhealth.org 2021. (accessed 8 January 2021).
- 10.Houeto JL, Karachi C, Mallet L, et al. Tourette’s syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry 2005; 76: 992–995. DOI: 10.1136/jnnp.2004.043273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visser-Vandewalle V, Temel Y, Boon P, et al. Chronic bilateral thalamic stimulation: a new therapeutic approach in intractable Tourette syndrome. J Neurosurg 2003; 99: 1094. DOI: 10.3171/jns.2003.99.6.1094 [DOI] [PubMed] [Google Scholar]
- 12.Ackermans L, Temel Y, Cath D, et al. Deep brain stimulation in Tourette's syndrome: two targets? Movement Disord 2006; 21: 709–713. DOI: 10.1002/mds.20816 [DOI] [PubMed] [Google Scholar]
- 13.Kim JP, Min H-K, Knight EJ, et al. Centromedian-parafascicular deep brain stimulation induces differential functional inhibition of the motor, associative, and limbic circuits in large animals. Biol Psychiatry 2013; 74: 917–926. DOI: 10.1016/j.biopsych.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krauss JK, Pohle T, Weigel R, et al. Deep brain stimulation of the centre median-parafascicular complex in patients with movement disorders. J Neurol Neurosurg Psychiatry 2002; 72: 546–548. DOI: 10.1136/jnnp.72.4.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ray CD, Burton CV. Deep Brain Stimulation for Severe, Chronic Pain. Vienna: Springer Vienna, 1980, pp. 289–293. [DOI] [PubMed]
- 16.Mundinger F, Salomão JF. Deep Brain Stimulation in Mesencephalic Lemniscus Medialis for Chronic Pain. Vienna: Springer Vienna, 1980, pp. 245–258. [DOI] [PubMed]
- 17.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010; 51: 899–908. DOI: 10.1111/j.1528-1167.2010.02536.x [DOI] [PubMed] [Google Scholar]
- 18.Salanova V, Witt T, Worth R, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015; 84: 1017–1025. DOI: 10.1212/WNL.0000000000001334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipescu C, Lagarde S, Lambert I, et al. The effect of medial pulvinar stimulation on temporal lobe seizures. Epilepsia 2019; 60: e25–e30. DOI: 10.1111/epi.14677 [DOI] [PubMed] [Google Scholar]
- 20.Ilyas A, Pizarro D, Romeo AK, et al. The centromedian nucleus: anatomy, physiology, and clinical implications. J Clin Neurosci 2019; 63: 1–7. DOI: 10.1016/j.jocn.2019.01.050 [DOI] [PubMed] [Google Scholar]
- 21.Cukiert A, Lehtimaki K. Deep brain stimulation targeting in refractory epilepsy. Epilepsia 2017; 58 (Suppl. 1): 80–84. DOI: 10.1111/epi.13686 [DOI] [PubMed] [Google Scholar]
- 22.Grewal SS, Middlebrooks EH, Okromelidze L, et al. Variability between direct and indirect targeting of the anterior nucleus of the thalamus. World Neurosurg 2020; 139: e70–e77. DOI: 10.1016/j.wneu.2020.03.107 [DOI] [PubMed] [Google Scholar]
- 23.Wu C, D’Haese PF, Pallavaram S, et al. Variations in thalamic anatomy affect targeting in deep brain stimulation for epilepsy. Stereotact Funct Neurosurg 2016; 94: 387–396. DOI: 10.1159/000449009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren AEL, Dalic LJ, Thevathasan W, et al. Targeting the centromedian thalamic nucleus for deep brain stimulation. J Neurol Neurosurg Psychiatry 2020; 91: 339–349. DOI: 10.1136/jnnp-2019-322030 [DOI] [PubMed] [Google Scholar]
- 25.Middlebrooks EH, Lin C, Westerhold E, et al. Improved detection of focal cortical dysplasia using a novel 3D imaging sequence: edge-enhancing gradient echo (3D-EDGE) MRI. Neuroimage Clin 2020; 28: 102449. DOI: 10.1016/j.nicl.2020.102449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grewal SS, Middlebrooks EH, Kaufmann TJ, et al. Fast gray matter acquisition T1 inversion recovery MRI to delineate the mammillothalamic tract for preoperative direct targeting of the anterior nucleus of the thalamus for deep brain stimulation in epilepsy. Neurosurg Focus 2018; 45: E6. DOI: 10.3171/2018.4.FOCUS18147 [DOI] [PubMed] [Google Scholar]
- 27.Avants BB, Epstein CL, Grossman M, et al. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008; 12: 26–41. DOI: 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao Y, Beriault S, Pike GB, et al. Multicontrast multiecho FLASH MRI for targeting the subthalamic nucleus. Magn Reson Imaging 2012; 30: 627–640. DOI: 10.1016/j.mri.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 29.Xiao Y, Fonov V, Beriault S, et al. Multi-contrast unbiased MRI atlas of a Parkinson’s disease population. Int J Comput Assist Radiol Surg 2015; 10: 329–341. DOI: 10.1007/s11548-014-1068-y [DOI] [PubMed] [Google Scholar]
- 30.Xiao Y, Fonov V, Chakravarty MM, et al. A dataset of multi-contrast population-averaged brain MRI atlases of a Parkinsons disease cohort. Data Brief 2017; 12: 370–379. DOI: 10.1016/j.dib.2017.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Middlebrooks EH, Ver Hoef L, Szaflarski JP. Neuroimaging in epilepsy. Curr Neurol Neurosci Rep 2017; 17: 32. DOI: 10.1007/s11910-017-0746-x [DOI] [PubMed] [Google Scholar]
- 32.Fleming Beattie J, Martin RC, Kana RK, et al. Hippocampal dentation: structural variation and its association with episodic memory in healthy adults. Neuropsychologia 2017; 101: 65–75. DOI: 10.1016/j.neuropsychologia.2017.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velasco AL, Velasco F, Jimenez F, et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox–Gastaut syndrome. Epilepsia 2006; 47: 1203–1212. DOI: 10.1111/j.1528-1167.2006.00593.x [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Lim SC, Yang DW, et al. Thalamo-cortical network underlying deep brain stimulation of centromedian thalamic nuclei in intractable epilepsy: a multimodal imaging analysis. Neuropsychiatr Dis Treatment 2017; 13: 2607–2619. DOI: 10.2147/NDT.S148617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Li Y, Gutierrez L, et al. Imaging the centromedian thalamic nucleus using quantitative susceptibility mapping. Front Hum Neurosci 2019; 13: 447. DOI: 10.3389/fnhum.2019.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira TA, Jr, Middlebrooks EH, Tzu WH, et al. Postmortem dissections of the papez circuit and nonmotor targets for functional neurosurgery. World Neurosurg 2020; 144: e866–e875. DOI: 10.1016/j.wneu.2020.09.088 [DOI] [PubMed] [Google Scholar]
- 37.Bouwens van der Vlis TAM, Schijns O, Schaper F, et al. Deep brain stimulation of the anterior nucleus of the thalamus for drug-resistant epilepsy. Neurosurg Rev 2019; 42: 287–296. DOI: 10.1007/s10143-017-0941-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polak D, Setsompop K, Cauley SF, et al. Wave-CAIPI for highly accelerated MP-RAGE imaging. Magn Reson Med 2018; 79: 401–406. DOI: 10.1002/mrm.26649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pawar K, Chen ZL, Zhang JX, et al. Application of compressed sensing using chirp encoded 3D GRE and MPRAGE sequences. Int J Imag Syst Tech 2020; 30: 592–604. DOI: 10.1002/ima.22401 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.