Abstract
Sera from animals infected with Borrelia burgdorferi isolates yield intense immunoblot signals from the B31 ErpA/I/N and ErpB/J/O proteins, which have apparent molecular masses of 19 and 60 kDa, respectively. Since B. burgdorferi proteins with those molecular masses are of immunodiagnostic importance, Lyme disease patient sera were used in studies of B31 lysates and recombinant B31 ErpA/I/N and ErpB/J/O proteins. Immunoblot analyses indicated that only a minority of the patients produced antibodies that recognized the tested B31 Erp proteins. Southern blot analyses of Lyme disease spirochetes cultured from 16 of the patients indicated that all these bacteria contain genes related to the B31 erpA/I/N and erpB/J/O genes, although signal strengths indicated only weak similarities in many cases, suggestive of genetic variability of erp genes among these bacteria. These data indicate that Erp proteins are generally not the 19- and 60-kDa antigens observed on serodiagnostic immunoblots.
Lyme disease is caused by the spirochete Borrelia burgdorferi and other, very closely related, Borrelia genospecies (9). These bacteria are generally difficult to isolate from infected humans, and Lyme disease can be complicated to diagnose clinically due to variability of symptoms between patients. Human infection is frequently, although not always, accompanied by an expanding “bull's-eye” rash, erythema migrans (EM). Lyme disease may or may not also affect various other tissues and organs, resulting in rheumatologic, cardiac and/or neurologic abnormalities (35). In the absence of EM or another characteristic manifestation, serologic testing is often used to assist diagnosis (8, 51, 55). Unfortunately, there is no widely accepted standardized test for Lyme disease, and different laboratories may utilize dissimilar diagnostic procedures and reference materials, possibly yielding conflicting results from different analyses of the same serum sample (7, 8, 17, 24, 55).
The Centers for Disease Control and Prevention (CDC) and other authorities recommend a two-step method for the serodiagnosis of suspected Lyme disease, consisting of a semispecific primary assay (such as enzyme-linked immunosorbent assay or immunodot analysis), followed by a second, more specific immunoblot (Western blot) analysis (4, 6, 8, 13, 50). Immunoblot analyses generally utilize whole-cell lysates of B. burgdorferi, and a number of immunoglobulin G (IgG) and IgM immunoblot bands have been identified as being characteristic of Lyme disease (6, 13, 20, 26, 50). However, the identities have been confirmed for only a small number of these diagnostic antigens (15, 21–23, 40, 53). As a result, it is unclear whether, for example, the 19-kDa IgG immunoblot band observed when using two different reference strains corresponds to the same protein in both bacteria. Cross-reactivity can present an additional problem to serodiagnosis (14, 24, 25, 32); for example, serum antibody binding to the 41-kDa antigen FlaB (flagellin) is suggestive of Lyme disease, but that immunoblot band is not specific for the disorder, since this protein is antigenically similar to flagellar components of other spirochetes (31). As further complications to the use of bacterial lysates for diagnostic immunoblot analyses, it is becoming apparent that the sequences of antigenic proteins often vary considerably between different Lyme disease spirochetes (28, 30, 33, 37, 42, 54) and that protein synthesis can be dramatically influenced by prolonged laboratory cultivation (38) or by variations in culture conditions (10, 27, 39, 46). It is not surprising, then, that serodiagnosis by using whole-cell lysates can be imprecise, and it is clear that tests could be greatly improved through use of purified, recombinant forms of specific, widely conserved antigens.
Within the first 4 weeks of infection, animals experimentally infected with Lyme disease spirochetes consistently produce antibodies directed against borrelial Erp lipoproteins (3, 29, 36, 43, 48, 52). erp genes are located on members of the cp32 plasmid family (a group of closely related 30- to 32-kb circular plasmids, although linear and smaller circular variants have been identified) (2, 11, 12, 45, 47). Individual bacteria can contain several different cp32 plasmids (one clonal culture of isolate B31 carries nine different cp32 plasmids [11]), and so can potentially synthesize a large number of different Erp proteins. All Lyme disease spirochetes that have been examined carry cp32 plasmids and erp genes (2, 3, 5, 11, 12, 19, 29, 34, 41, 47–49, 52, 56), which have also been given various names such as ospE, ospF, p21, pG, elpA, elpB, bbk2.10, bbk2.11, and “upstream homology box genes” (2, 3, 29, 34, 48, 52). Sequence analyses of the known erp genes indicate that, while there may be extensive diversity among these genes, very similar genes can be carried by different bacteria, or even within a single bacterium. For example, the 10 loci of isolate B31 include 17 erp genes, of which erpA, erpI, and erpN encode identical proteins, as do erpB, erpJ, and erpO, and their encoded proteins are designated ErpA/I/N and ErpB/J/O, respectively (11, 12, 43, 44). Additionally, extensive homology is found between the B31 erpA/I/N genes and the B31 erpP gene, and also between the erpB/J/O genes and the erpM, erpQ, and erpX genes of that isolate, so much so that a DNA probe derived from one gene often hybridizes with DNA carrying homologous genes, and antibodies directed against one Erp protein sometimes bind to other Erps (12, 43, 47; our unpublished results).
The B31 ErpA/I/N and ErpB/J/O proteins are the dominant 19- and 60-kDa antigens of B31 lysates observed when sera from animals infected with that isolate are used in immunoblot analyses (43). The antigenicity and electrophoretic mobilities of these two Erp proteins raise the possibility that they represent the 18- to 20- and 58- to 60-kDa IgG immunoblot bands that are diagnostic for Lyme disease (6, 13, 20, 26, 50). We previously reported that sera from 10 of 10 Lyme disease patients from eastern Long Island, N.Y., contained antibodies that recognized ErpA/I/N, and 8 of the 10 contained antibodies recognizing ErpB/J/O (43). We therefore analyzed sera from additional Lyme disease patients, collected from other geographic locations, to determine whether these B31 Erp proteins are universally recognized by patient antibodies and might be useful components of serodiagnostic tests.
MATERIALS AND METHODS
Bacteria.
B. burgdorferi isolate B31 was originally isolated from an infected tick collected on Shelter Island, N.Y. (near the eastern end of Long Island) (9), and the culture used in this study is infectious to both mice and ticks (39). These bacteria carry plasmids cp32-1, cp32-3, cp32-4, cp32-5, cp32-6, cp32-7, cp32-8, cp32-9, and lp56, and so contain three genes encoding both ErpA/I/N and ErpB/J/O (erpAB on cp32-1, erpIJ on cp32-5, and erpNO on cp32-8) (11, 12). Bacteria were cultivated at 34°C in modified Barbour-Stoener-Kelly medium containing 6% rabbit serum (Sigma, St. Louis, Mo.). After reaching mid-logarithmic phase (approximately 107 bacteria per ml), B31 cultures were harvested by centrifugation, were washed three times with phosphate-buffered saline, were resuspended in distilled water, and were lysed by boiling for 5 min.
Martin Schriefer (CDC, Fort Collins, Colo.) kindly provided B. burgdorferi isolated from 16 Lyme disease patients (described further below): GR 90-2631, MD 91-1458, WI 93-0208, WI 93-1426, WI 91-1222, CA 92-1682, WI 93-0206, WI 93-1414, WI 94-0880, NY 96-1050, NY 96-1055, NY 96-1063, NY 96-1069, NY 96-1078, NY 96-1088, and NY 96-1103. These bacteria have been passaged in culture medium fewer than five times (M. Schriefer, personal communication).
Human and animal sera.
Sera from each of the 16 culture-confirmed Lyme disease patients were obtained from M. Schriefer. Sera were also provided from two additional patients, without corresponding cultured bacteria. The sera were collected at the times after disease onset indicated in Table 1, although all were treated with antibiotics within 2 months of onset (M. Schriefer, personal communication). Eight of the patients were diagnosed in upstate New York (prefix NY), six were diagnosed in Wisconsin (prefix WI), and one each was diagnosed in Michigan, Maryland, California, and Germany (prefixes MI, MD, CA, and GR, respectively). To serve as negative controls, M. Schriefer also provided sera from six healthy individuals who live in areas where Lyme disease is not known to be endemic.
TABLE 1.
Reactivities of human Lyme disease patient sera against B31 whole-cell lysate or recombinant B31 Erp proteins
| Patient designation | Interval of onset to collectiona | Reactivity againstc:
|
|||
|---|---|---|---|---|---|
| ∼19-kDa antigen | rErpA/I/N | ∼60-kDa antigen | rErpB/J/O | ||
| GR 90-2631b | 146 | + | − | + | − |
| MD 91-1458b | 54 | + | − | + | − |
| WI 93-0208b | 263 | − | − | + | − |
| WI 93-1426b | 89 | + | − | + | − |
| WI 91-1222b | 33 | + | − | + | − |
| CA 92-1682b | 141 | + | − | + | − |
| WI 93-0206b | 254 | + | + | + | − |
| WI 93-1414b | 21 | + | − | + | + |
| WI 94-0880b | 7 | + | − | + | − |
| NY 96-1050b | 115 | − | − | + | − |
| NY 96-1055b | 416 | − | − | + | + |
| NY 96-1063b | 10 | + | − | + | − |
| NY 96-1069b | 831 | − | − | + | + |
| NY 96-1078b | 1424 | + | + | + | + |
| NY 96-1088b | 1323 | + | + | + | + |
| NY 96-1103b | 19 | + | − | − | − |
| NY 96-1060 | 25 | − | − | + | − |
| MI 92-1941 | 77 | + | − | + | − |
| NY-1 | ∼30 | − | − | + | − |
| NY-2 | ∼30 | − | − | + | + |
| NY-3 | ∼30 | + | − | + | − |
| NY-4 | ∼30 | − | − | + | − |
| NY-5 | ∼30 | + | − | + | − |
| NY-6 | ∼30 | + | − | + | − |
| NY-7 | ∼30 | + | + | + | + |
| NY-8 | ∼30 | − | − | + | − |
| NY-9 | ∼30 | − | − | − | − |
| NY-10 | ∼30 | + | − | + | − |
| NY-11 | ∼30 | + | − | − | − |
| NY-12 | ∼30 | − | − | + | − |
| NY-13 | ∼30 | + | − | + | − |
| NY-14 | ∼30 | + | − | − | − |
| NY-15 | ∼30 | − | − | + | − |
| NY-16 | ∼30 | + | − | − | − |
| NY-17 | ∼30 | − | − | + | − |
| NY-18 | ∼30 | + | − | + | − |
| NY-19 | ∼30 | − | − | + | + |
| NY-20 | ∼30 | − | − | + | + |
Duration (in days) between the date signs and/or symptoms were first noted and the date a serum sample was collected (M. Schriefer and G. Wormser, personal communications).
Bacteria were cultured from these patients, and bacterial DNA was analyzed by Southern blotting (Fig. 2).
Sera were scored for the production of a detectable immunoblot band (+) or the failure to detect a band (−).
An additional 20 sera, selected at random from patients symptomologically diagnosed with Lyme disease in upstate New York, were generously provided by Gary Wormser (New York Medical College, Valhalla). All of these sera were collected approximately 1 month after the probable date of infection (G. Wormser, personal communication) and are designated herein as NY-1 through -20.
Antisera directed against the B31 ErpA/I/N or ErpB/J/O proteins were generated by vaccinating New Zealand White rabbits with one of the respective fusion proteins (described below). Approximately 50 μg of purified protein in complete Freund's adjuvant was injected into each rabbit, followed by booster vaccinations 2 and 4 weeks later with the same dose of protein in incomplete Freund's adjuvant. Rabbits were exsanguinated 2 weeks after the final boost.
Serum was also collected 4 weeks postinfection from a white-footed mouse (Peromyscus leucopus) that had been infected with B31 via tick bite (43).
Immunoblot analyses.
Recombinant B31 ErpA/I/N or ErpB/J/O proteins were purified from Escherichia coli engineered to overexpress polyhistidine-linked fusion proteins (43). Bacteria were lysed either by sonication or suspension in B-PER bacterial protein extraction reagent (Pierce, Rockford, Ill.), and fusion proteins were purified with His-Bind kits (Novagen).
B31 lysates or recombinant Erp proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, were electrotransferred to nitrocellulose membranes, and were blocked with 5% nonfat dried milk in Tris-buffered saline–Tween-20 (20 mM Tris [pH 7.5], 150 mM NaCl, 0.05% [vol/vol] Tween-20). The membranes were incubated with either patient serum, infected mouse serum, or Erp-directed rabbit antiserum by using a Mini-Protean II multiscreen apparatus (Bio-Rad, Hercules, Calif.). Sera were diluted in Tris-buffered saline–Tween 20 at the following ratios: human sera at 1:200, infected mouse serum at 1:250, and ErpA/I/N- and ErpB/J/O-directed rabbit antisera at 1:200 and 1:50, respectively. After incubation with primary antibodies, membranes were incubated with either goat anti-human IgG- or IgM-horseradish peroxidase conjugates (ICN/Cappel, Aurora, Ohio) (for human sera) or protein A-horseradish peroxidase conjugate (Amersham, Piscataway, N.J.) (for mouse or rabbit sera). Bound secondary antibody or protein A conjugates were visualized by enhanced chemiluminescence (Amersham).
DNA analysis.
Each of the 16 B. burgdorferi samples isolated from Lyme disease patients was grown to late logarithmic phase (approximately 108 bacteria per ml) in 100 ml of Barbour-Stoener-Kelly medium, and plasmid DNAs were purified by using plasmid purification kits (Qiagen, Chatsworth, Calif.). Aliquots of each plasmid preparation and, as a control, B31 genomic DNA (47) were digested with EcoRI (New England Biolabs, Beverly, Mass.). Cut DNAs were separated by pulsed-field agarose gel electrophoresis (19) and were transferred (47) to a Biotrans nylon membrane (ICN).
The membrane was alternately hybridized with one of two radiolabeled probes derived from either the B31 erpA or erpB gene. All probes were synthesized from cloned DNAs containing the appropriate sequence by two rounds of PCR, as previously described (19). The erpA-derived probe was amplified from recombinant plasmid pBLS510 by using oligonucleotides E-141 (AGAATAATAGTAATAACTGGG) and E-142 (CTAGTGATATTGCATATTCAG). The erpB-derived probe was amplified from recombinant plasmid pBLS434b by using oligonucleotides E-113 (AGAATTATGCAATTAAAGATTTAG) and E-114 (GATTCTTCTACTTTTTTCACTTTC) (47). Each probe was radiolabeled with [α-32P]dATP (ICN) by random priming (Life Technologies, Gaithersburg, Md.) and was individually incubated overnight with the nylon membrane at 45°C in a solution containing 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% (wt/vol) SDS, and 5 g of nonfat dried milk per liter. The membrane was then washed in a solution of 2× SSC and 0.1% SDS at room temperature, and the hybridized probe was visualized by autoradiography. The membrane was stripped of hybridized probe before reuse by extensive washing with boiling water, and probe removal was confirmed by overnight exposure to X-ray film.
RESULTS
Analyses of B31 extracts.
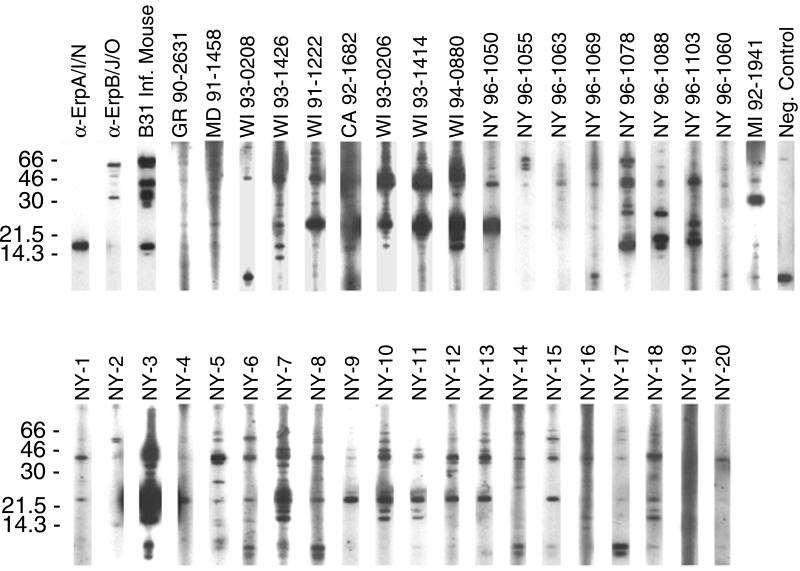
B31 extracts initially cultivated at 23°C then shifted to 34°C produce significantly greater amounts of Erp proteins than do bacteria cultured at a constant 23°C (43, 46). Since the production of Erp proteins by B31 grown at a constant 34°C was unknown, a whole-cell extract was immunoblotted with rabbit polyclonal antisera raised against either recombinant ErpA/I/N or ErpB/J/O, which demonstrated Erp synthesis at this temperature (Fig. 1, first 2 lanes). Immunoblot analyses with the antiserum raised against ErpB/J/O indicated cross-reactivity with additional B31 proteins (Fig. 1) that additional studies demonstrated to be ErpM, ErpQ, and ErpX (our unpublished results). The bacterial lysate was next tested with serum from a mouse that had been infected for 30 days with B31, which yielded strong immunoblot bands corresponding to both ErpA/I/N and ErpB/J/O (Fig. 1, third lane). In contrast to our earlier observations when immunoblotting with B31 that had been shifted from 23 to 34°C (43), the constant 34°C bacteria also synthesized an antigenic protein of approximately 66 kDa (Fig. 1).
FIG. 1.
Immunoblots of B31 whole-cell lysates using polyclonal rabbit antisera raised against recombinant ErpA/I/N or ErpB/J/O (labeled α-ErpA/I/N and α-ErpB/J/O, respectively), serum from a mouse infected with B31 via tick bite (labeled B31 Inf. Mouse), human Lyme disease patient sera (labeled by patient designation), and control serum from a healthy human (CDC serum WY 92-1318, labeled Neg. Control). Enhanced chemiluminescence exposure times were equal for all human serum strips in each panel. Bacteria cultured from the first 16 patients (GR 90-2631 through NY 96-1103) were also analyzed for erpA/I/N- and erpB/J/O-like genes by Southern blotting (Fig. 2). Positions of molecular mass standards (in kilodaltons) are indicated to the left of each panel.
Immunoblot analyses of B31 extract and recombinant Erp proteins with human sera.
The B31 whole-cell extract was next IgG immunoblotted with sera from 38 different human Lyme disease patients (Fig. 1). Even though many of the patients had been infected for comparable lengths of time, the sera did not all produce identical immunoblot banding patterns, nor were all immunoblot signal strengths of comparable intensities. IgM immunoblot analyses with patient sera yielded results similar to those of the IgG immunoblot analyses, with the same or fewer bands being visible (data not shown). Note that immunoblot analyses of some patient sera (e.g., NY 96-1063) yielded results that might be classified as seronegative by CDC criteria despite the fact that B. burgdorferi strains were isolated from those patients, highlighting the need for improved Lyme disease serodiagnostic tests.
Of the 38 patient sera, 23 yielded IgG immunoblots with an approximately 19-kDa band (Fig. 1 and Table 1). Subsequent immunoblotting with recombinant B31 ErpA/I/N protein indicated that only four of the sera contained detectable levels of IgG antibodies which recognized that protein (Table 1). These results indicate that the B31 culture contained at least one additional antigen with an apparent molecular mass of approximately 19 kDa, and close examination of several B31 lysate IgG immunoblots revealed a protein with an electrophoretic mobility slightly slower than that of ErpA/I/N (compare the NY 96-1088 and NY 96-1103 immunoblots in Fig. 1).
A majority of patient sera (33 of 38) contained IgG antibodies that reacted with a B31 protein having an approximate molecular mass of 60 kDa, although there was considerable variability in signal intensity (Fig. 1 and Table 1). However, immunoblot analyses with recombinant B31 ErpB/J/O showed that only 9 of the 38 sera contained detectable levels of IgG antibodies that bound that protein (Table 1). Isolate B31 apparently produces additional proteins with molecular masses of approximately 60 kDa that are recognized by antibodies in some patient sera, possibly the circa 66-kDa antigen that was recognized by the B31-infected mouse, or the previously characterized 60-kDa heat shock protein (25).
None of the control human sera contained antibodies that recognized either recombinant ErpA/I/N or ErpB/J/O (Fig. 1 and data not shown). Some contained antibodies that bound other B. burgdorferi proteins, presumably due to cross-reactivity of antibodies formed in response to unrelated infections.
Southern blot analyses.
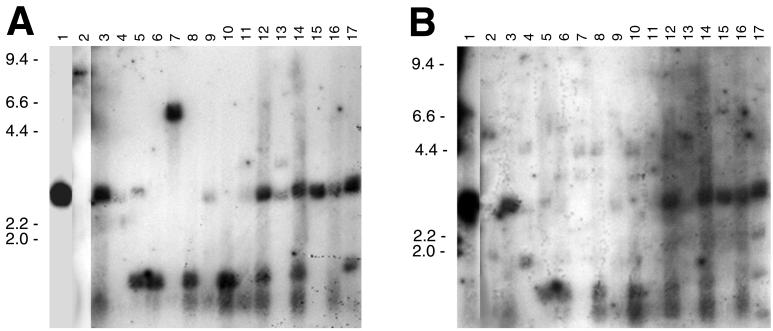
As discussed above, all previously examined Lyme disease spirochetes contain erp loci. However, the lack of immunoblot reactivity against the recombinant B31 ErpA/I/N and ErpB/J/O proteins by most patient sera raised the possibility that the bacteria infecting these patients lack genes similar to those of B31. To test this hypothesis, Southern blot analyses of DNA from the human Borrelia isolates were performed by using probes derived from the B31 erpA and erpB genes.
A number of different Lyme disease borreliae are known to contain genes that are greater than 80% identical in nucleotide sequence to the B31 erpA, erpI, and erpN genes (2, 11, 12, 29, 34, 43, 44, 47–49). Since many of the identified erpA/I/N-like genes contain an EcoRI site near their 5′ ends, a probe that excludes that sequence was used. Southern blotting with this B31 erpA-derived probe indicated that all of the human patient isolates contain DNA sequences similar to the B31 erpA/I/N genes (Fig. 2A). Although approximately equal amounts of total DNAs were used in the blotting, signal intensities varied between isolates, suggesting that the bacteria carry genes with varying degrees of similarity to those of B31, and/or that some bacteria also carry multiple B31 erpA/I/N-like genes with overlapping EcoRI digestion patterns. Multiple bands were observed for several isolates, indicating that these bacteria may carry multiple homologous genes. Alternatively, since none of the human isolates have been cloned in the laboratory, it is possible that the cultures contain mixtures of unrelated bacteria. There was no direct correlation between erpA Southern blot signal strength from a bacterial isolate and production of ErpA/I/N-binding IgG antibodies by humans infected with those bacteria (Table 1 and Fig. 2A).
FIG. 2.
Southern blots of B31 and human isolate DNAs digested with EcoRI and hybridized with probes derived from B31 erpA (A) and erpB (B). DNA from B31 (lane 1) and bacteria cultured from patients GR 90-2631 (2), MD 91-1458 (3), WI 93-0208 (4), WI 93-1426 (5), WI 91-1222 (6), CA 92-1682 (7), WI 93-0206 (8), WI 93-1414 (9), WI 94-0880 (10), NY 96-1050 (11), NY 96-1055 (12), NY 96-1063 (13), NY 96-1069 (14), NY 96-1078 (15), NY 96-1088 (16), and NY 96-1103 (17). Positions of DNA size standards (in kilobases) are indicated to the left of each panel.
Genes similar to B31 erpB/J/O have been identified from only three B. burgdorferi isolates (2, 11, 12, 19, 43, 47, 48), but there are few published reports of deliberate attempts to characterize such genes. At low Southern blot stringency, all cultured bacteria were found to contain DNA that hybridized with the erpB probe, albeit such signals were barely detectable in many cases (Fig. 2B). All bacteria isolated from patients whose sera contained ErpB/J/O-binding IgG antibodies contained DNA that gave strong Southern blot signals, although the converse was not necessarily true (Table 1 and Fig. 2B).
Blotting of B31 DNA with the erpA- and erpB-derived probes revealed single hybridization signals corresponding to an approximately 3-kb EcoRI fragment (Figs. 2A and B, lanes 1), as would be expected from restriction maps of the plasmids carrying the B31 erpAB, erpIJ, and erpNO genes and of the plasmid carrying the similar B31 erpPQ locus (11, 12, 45). Many of the human isolates exhibited digestion profiles similar to B31, suggestive of widespread cp32 plasmid sequence conservation. Other restriction patterns were observed, however. For example, an approximately 1.5-kb EcoRI fragment from several isolates was detected with the erpA probe, suggesting other patterns of sequence conservation in the plasmids of those bacteria. Larger erpA-hybridizing EcoRI fragments were also observed, with sizes of approximately 9 and 6.6 kb in the bacteria cultured from patients GR 90-2631 and CA 92-1682, respectively (Fig. 2A, lanes 2 and 7), and an approximately 3.5-kb fragment from both NY 96-1050 and NY 96-1063 (Fig. 2A, lanes 11 and 13).
DISCUSSION
Within 4 weeks of infection with B. burgdorferi isolate B31, laboratory animals produce antibodies that recognize all members of the Erp protein family (43; our unpublished results). Additionally, immunoblot analyses with sera from such infected animals yield relatively intense signals from B31 proteins with apparent molecular masses of 19 and 60 kDa, which were previously demonstrated to be ErpA/I/N and ErpB/J/O, respectively (43). Other researchers have indicated that immunoblot antigens with these approximate sizes are of diagnostic importance when testing human Lyme disease patient sera. In the present study, we observed that while a majority of sampled Lyme disease patient sera contained antibodies that bound B31 proteins of approximately 19 and 60 kDa, in only a small percentage of cases were these antigens Erp proteins. Similar findings were also reported by Nguyen et al. (36) and Wallich et al. (52), who found that only minor proportions of patient sera contained antibodies that recognized other recombinant Erp proteins.
All Lyme disease spirochetes, including those studied in this work, have been found to contain erp genes, yet a majority of the tested humans did not produce antibodies that recognized the examined B31 Erp proteins. There are several possible explanations for the lack of antibodies in the patient sera that recognized B31 recombinant Erp proteins, any or all of which could have contributed to our results. First, the Southern blotting data reported herein, in combination with previously reported sequencing results (2, 11, 29, 34, 44, 47–49), suggest that there are often differences among the erp gene sequences of different Lyme disease bacteria. This diversity of erp gene sequences is due, at least in part, to intergenic recombination among these genes (44). Second, the intense response of B31-infected animals to ErpA/I/N and ErpB/J/O may be due to the presence of three genes each for these proteins, possibly resulting in protein levels that are greater than those for proteins encoded by single erp genes. If triplication of erpA/I/N- and erpB/J/O-like genes is rare among Lyme disease borreliae, then this may have contributed to the general lack of antibodies in patient sera that bound B31 ErpA/I/N and ErpB/J/O. Third, it has been reported that some B. burgdorferi do not express all their erp loci during the initial stages of mammalian infection (1, 16). It is possible that some of the sampled Lyme disease patients were infected with bacteria carrying genes that encode proteins quite similar to B31 ErpA/I/N and ErpB/J/O, but which were not synthesized during the times prior to antibiotic treatment and serum acquisition. Fourth, differences of infection duration, degree of bacterial dissemination, or immunocompetence or other variations among the patients assayed could all have contributed to the results of our studies.
The lack of antibodies in the patient sera recognizing the B31 ErpA/I/N or ErpB/J/O proteins does not appear to be related to the general paucity of B. burgdorferi-directed antibodies. As examples, sera from patients WI 94-0880 and NY-3 both yielded strong immunoblots, with readily detectable signals from both 19- and 60-kDa B31 proteins, yet neither contained antibodies that bound either B31 Erp protein. While it is possible that the recombinant Erp proteins may have different conformations than the native proteins, this is not likely to be a significant reason for the lack of antibody binding when using the recombinant proteins, since serum from the mouse infected with B31 contained significant levels of antibodies that bound the recombinant Erps. Those results suggest that if the tested humans were infected with bacteria that synthesized proteins similar to B31 ErpA/I/N and ErpB/J/O, antibodies directed against such proteins would have been detected in our testing.
The present findings stand in contrast to our earlier study of Lyme disease patients from eastern Long Island, N.Y., which found that a majority of the sera contained antibodies recognizing recombinant B31 ErpA/I/N and ErpB/J/O proteins (43). Since B31 was isolated from Shelter Island, located between the forks of eastern Long Island, the results from our studies may reflect variations between Lyme disease-causing bacteria, with those near Shelter Island being more similar to B31 than spirochetes found elsewhere. It has been argued that Lyme disease borreliae are essentially clonal (18, 54), which predicts localized similarities, and further analyses of erp genes from additional B. burgdorferi isolated on eastern Long Island and elsewhere would address this hypothesis.
In conclusion, our studies indicated that while animals infected with B. burgdorferi isolate B31 produced significant levels of antibodies directed against the ErpA/I/N and ErpB/J/O proteins, sampled infected humans rarely produced antibodies that recognized either B31 protein. These data indicate that recombinant B31 ErpA/I/N and ErpB/J/O are not broadly applicable for serodiagnosis of human Lyme disease.
ACKNOWLEDGMENTS
This work was supported by grants AI44254 from the National Institutes of Health and 949 from the University of Kentucky Chandler Medical Center Research Fund. Jennifer Miller is the recipient of a Kentucky Opportunity Fellowship for Academic Excellence.
We thank Martin Schriefer, Katie Davis, and Gary Wormser for their gifts of human sera; Tom Schwan for providing mouse serum; Ralph Larson for assistance in producing rabbit antisera; Darrin Akins for frank discussions of unpublished data; and Patti Rosa, Tom Schwan, Tim Kowalik, Jim Bono, Steve Porcella, Kit Tilly, and Abdallah Elias for their helpful advice, assistance in producing recombinant Erp proteins, and in carrying out bacterial cultivation.
REFERENCES
- 1.Akins D R, Bourell K W, Caimano M J, Norgard M V, Radolf J D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Investig. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins D R, Caimano M J, Yang X, Cerna F, Norgard M V, Radolf J D. Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect Immun. 1999;67:1526–1532. doi: 10.1128/iai.67.3.1526-1532.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akins D R, Porcella S F, Popova T G, Shevchenko D, Baker S I, Li M, Norgard M V, Radolf J D. Evidence for in vivo but not in vitro expression of a Borrelia burgdorferi outer surface protein F (OspF) homologue. Mol Microbiol. 1995;18:507–520. doi: 10.1111/j.1365-2958.1995.mmi_18030507.x. [DOI] [PubMed] [Google Scholar]
- 4.American College of Physicians. Guidelines for laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1106–1108. doi: 10.7326/0003-4819-127-12-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 5.Amouriaux P, Assous M, Margarita D, Baranton G, Saint Girons I. Polymerase chain reaction with the 30-kb circular plasmid of Borrelia burgdorferi B31 as a target for detection of the Lyme borreliosis agents in cerebrospinal fluid. Res Microbiol. 1993;144:211–219. doi: 10.1016/0923-2508(93)90046-5. [DOI] [PubMed] [Google Scholar]
- 6.Association of State and Territorial Public Health Laboratory Directors. Second National Conference on Serologic Diagnosis of Lyme Disease. Washington, D.C.: Association of Public Health Laboratories; 1995. Recommendations; pp. 1–5. [Google Scholar]
- 7.Bakken L L, Callister S M, Wand P J, Schell R F. Interlaboratory comparison of test results for detection of Lyme disease by 516 participants in the Wisconsin State Laboratory of Hygiene/College of American Pathologists proficiency testing program. J Clin Microbiol. 1997;35:537–543. doi: 10.1128/jcm.35.3.537-543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown S L, Hansen S L, Langone J J. Role of serology in the diagnosis of Lyme disease. JAMA. 1999;282:62–66. doi: 10.1001/jama.282.1.62. [DOI] [PubMed] [Google Scholar]
- 9.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 10.Carroll J A, Garon C F, Schwan T G. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67:3181–3187. doi: 10.1128/iai.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casjens S, Palmer N, van Vugt R, Huang W M, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson R J, Haft D, Hickey E, Gwinn M, White O, Fraser C. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 12.Casjens S, van Vugt R, Tilly K, Rosa P A, Stevenson B. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J Bacteriol. 1997;179:217–227. doi: 10.1128/jb.179.1.217-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. Morbid Mortal Weekly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 14.Coleman J L, Benach J L. Characterization of antigenic determinants of Borrelia burgdorferi shared by other bacteria. J Infect Dis. 1992;165:658–666. doi: 10.1093/infdis/165.4.658. [DOI] [PubMed] [Google Scholar]
- 15.Coleman J L, Benach J L. Identification and characterization of an endoflagellar antigen of Borrelia burgdorferi. J Clin Investig. 1989;84:322–330. doi: 10.1172/JCI114157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das S, Barthold S W, Stocker Giles S, Montgomery R R, Telford S R, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Investig. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davidson M M, Ling C L, Chisholm S M, Wiseman A D, Joss A W L, Ho-Yen D O. Evidence-based diagnosis of Lyme disease. Eur J Clin Microbiol Infect Dis. 1999;18:484–489. doi: 10.1007/s100960050328. [DOI] [PubMed] [Google Scholar]
- 18.Dykhuizen D E, Polin D S, Dunn J, Wilske B, Preac-Mursic V, Dattwyler R J, Luft B J. Borrelia burgdorferi is clonal: implications for taxonomy and vaccine development. Proc Natl Acad Sci USA. 1993;90:10163–10167. doi: 10.1073/pnas.90.21.10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Hage N, Lieto L D, Stevenson B. Stability of erp loci during Borrelia burgdorferi infection: recombination is not required for chronic infection of immunocompetent mice. Infect Immun. 1999;67:3146–3150. doi: 10.1128/iai.67.6.3146-3150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engstrom S M, Shoop E, Johnson R C. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol. 1995;33:419–427. doi: 10.1128/jcm.33.2.419-427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs R, Jauris S, Lottspeich F, Preac-Mursic V, Wilske B, Soutschek E. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22kDa protein (pC) in Escherichia coli. Mol Microbiol. 1992;6:503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 22.Gilmore R D, Kappel K J, Johnson B J B. Molecular characterization of a 35-kilodalton protein of Borrelia burgdorferi, an antigen of diagnostic importance in early Lyme disease. J Clin Microbiol. 1997;35:86–91. doi: 10.1128/jcm.35.1.86-91.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmore R D, Murphee R L, James A M, Sullivan S A, Johnson B J B. The Borrelia burgdorferi 37-kilodalton immunoblot band (P37) used in serodiagnosis of early Lyme disease is the flaA gene product. J Clin Microbiol. 1999;37:548–552. doi: 10.1128/jcm.37.3.548-552.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goossens H A T, van den Bogaard A E, Nohlmans M K E. Evaluation of fifteen commercially available serological tests for diagnosis of Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 1999;18:551–560. doi: 10.1007/s100960050347. [DOI] [PubMed] [Google Scholar]
- 25.Hansen K, Bangsborg J M, Fjordvang H, Pedersen N S, Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988;56:2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauser U, Lehnert G, Wilske B. Validity of interpretation criteria for standardized Western blots (immunoblots) for serodiagnosis of Lyme borreliosis based on sera collected throughout Europe. J Clin Microbiol. 1999;37:2241–2247. doi: 10.1128/jcm.37.7.2241-2247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Indest K J, Ramamoorthy R, Sole M, Gilmore R D, Johnson B J B, Philipp M T. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jauris-Heipke S, Roßle B, Wanner G, Habermann C, Rössler D, Fingerle V, Lehnert G, Lobentanzer R, Pradel I, Hillenbrand B, Schulte-Spechtel U, Wilske B. Osp17, a novel immunodominant outer surface protein of Borrelia afzelii: recombinant expression in Escherichia coli and its use as a diagnostic antigen for serodiagnosis of Lyme borreliosis. Med Microbiol Immunol. 1999;187:213–219. doi: 10.1007/s004300050095. [DOI] [PubMed] [Google Scholar]
- 29.Lam T T, Nguyen T-P K, Montgomery R R, Kantor F S, Fikrig E, Flavell R A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994;62:290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livey I, Gibbs C P, Schuster R, Dorner F. Evidence for lateral transfer and recombination in OspC variation in Lyme disease Borrelia. Mol Microbiol. 1995;18:257–269. doi: 10.1111/j.1365-2958.1995.mmi_18020257.x. [DOI] [PubMed] [Google Scholar]
- 31.Luft B J, Dunn J J, Dattwyler R J, Gorgone G, Gorevic P D, Schubach W H. Cross-reactive antigenic domains of the flagellin protein of Borrelia burgdorferi. Res Microbiol. 1993;144:251–257. doi: 10.1016/0923-2508(93)90009-q. [DOI] [PubMed] [Google Scholar]
- 32.Magnarelli L A, Anderson J F, Johnson R C. Cross reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987;156:183–187. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- 33.Marconi R T, Samuels D S, Landry R K, Garon C F. Analysis of the distribution and molecular heterogeneity of the ospD gene among the Lyme disease spirochetes: evidence for lateral gene exchange. J Bacteriol. 1994;176:4572–4582. doi: 10.1128/jb.176.15.4572-4582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marconi R T, Sung S Y, Norton Hughes C A, Carlyon J A. Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J Bacteriol. 1996;178:5615–5626. doi: 10.1128/jb.178.19.5615-5626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nadelman R B, Wormser G P. Lyme borreliosis. Lancet. 1998;352:557–565. doi: 10.1016/S0140-6736(98)01146-5. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen T-P K, Lam T T, Barthold S W, Telford S R, Flavell R A, Fikrig E. Partial destruction of Borrelia burgdorferi within ticks that engorged on OspE- or OspF-immunized mice. Infect Immun. 1994;62:2079–2084. doi: 10.1128/iai.62.5.2079-2084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts W C, Mullikin B A, Lathigra R, Hanson M S. Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect Immun. 1998;66:5275–5285. doi: 10.1128/iai.66.11.5275-5285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwan T G, Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987;156:852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- 39.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson W J, Cieplak W, Schrumpf M E, Barbour A G, Schwan T G. Nucleotide sequence and analysis of the gene in Borrelia burgdorferi encoding the immunogenic P39 antigen. FEMS Microbiol Lett. 1994;119:381–388. doi: 10.1111/j.1574-6968.1994.tb06917.x. [DOI] [PubMed] [Google Scholar]
- 41.Simpson W J, Garon C F, Schwan T G. Borrelia burgdorferi contains repeated DNA sequences that are species specific and plasmid associated. Infect Immun. 1990;58:847–853. doi: 10.1128/iai.58.4.847-853.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson B, Barthold S W. Expression and sequence of outer surface protein C among North American isolates of Borrelia burgdorferi. FEMS Microbiol Lett. 1994;124:367–372. doi: 10.1111/j.1574-6968.1994.tb07310.x. [DOI] [PubMed] [Google Scholar]
- 43.Stevenson B, Bono J L, Schwan T G, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevenson B, Casjens S, Rosa P. Evidence of past recombination events among the genes encoding the Erp antigens of Borrelia burgdorferi. Microbiology. 1998;144:1869–1879. doi: 10.1099/00221287-144-7-1869. [DOI] [PubMed] [Google Scholar]
- 45.Stevenson B, Casjens S, van Vugt R, Porcella S F, Tilly K, Bono J L, Rosa P. Characterization of cp18, a naturally truncated member of the cp32 family of Borrelia burgdorferi plasmids. J Bacteriol. 1997;179:4285–4291. doi: 10.1128/jb.179.13.4285-4291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevenson B, Tilly K, Rosa P A. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J Bacteriol. 1996;178:3508–3516. doi: 10.1128/jb.178.12.3508-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sung S Y, Lavoie C P, Carlyon J A, Marconi R T. Genetic divergence and evolutionary instability in ospE-related members of the upstream homology box gene family in Borrelia burgdorferi sensu lato complex isolates. Infect Immun. 1998;66:4656–4668. doi: 10.1128/iai.66.10.4656-4668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trevejo R T, Krause P J, Sikand V K, Schreifer M E, Ryan R, Lepore T, Porter W, Dennis D T. Evaluation of two-test serodiagnostic method for early Lyme disease in clinical practice. J Infect Dis. 1999;179:931–938. doi: 10.1086/314663. [DOI] [PubMed] [Google Scholar]
- 51.Tugwell P, Dennis D T, Weinstein A, Wells G, Shea B, Nichol G, Hayward R, Lightfoot R, Baker P, Steere A C. Laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1109–1123. doi: 10.7326/0003-4819-127-12-199712150-00011. [DOI] [PubMed] [Google Scholar]
- 52.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed only in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallich R, Moter S E, Simon M M, Ebnet K, Heiberger A, Kramer M D. The Borrelia burgdorferi flagellum-associated 41-kilodalton antigen (flagellin): molecular cloning, expression, and amplification of the gene. Infect Immun. 1990;58:1711–1719. doi: 10.1128/iai.58.6.1711-1719.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang I-N, Dykhuizen D E, Qiu W, Dunn J J, Bosler E M, Luft B J. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wormser G P, Aguero-Rosenfeld M E, Nadelman R B. Lyme disease serology: problems and opportunities. JAMA. 1999;282:79–80. doi: 10.1001/jama.282.1.79. [DOI] [PubMed] [Google Scholar]
- 56.Zückert W R, Meyer J. Circular and linear plasmids of Lyme disease spirochetes have extensive homology: characterization of a repeated DNA element. J Bacteriol. 1996;178:2287–2298. doi: 10.1128/jb.178.8.2287-2298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]