Abstract
Background
Racial and ethnic disparities contribute to differences in access and outcomes for patients undergoing heart transplantation. We evaluated contemporary outcomes for heart transplantation stratified by race and ethnicity as well as the new 2018 allocation system.
Methods and Results
Adult heart recipients from 2011 to 2020 were identified in the United Network for Organ Sharing database and stratified into 3 groups: Black, Hispanic, and White. We analyzed recipient and donor characteristics, and outcomes. Among 32 353 patients (25% Black, 9% Hispanic, 66% White), Black and Hispanic patients were younger, more likely to be women and have diabetes mellitus or renal disease (all, P<0.05). Over the study period, the proportion of Black and Hispanic patients listed for transplant increased: 21.7% to 28.2% (P=0.003) and 7.7% to 9.0% (P=0.002), respectively. Compared with White patients, Black patients were less likely to undergo transplantation (adjusted hazard ratio [aHR], 0.87; CI, 0.84–0.90; P<0.001), but had a higher risk of post‐transplant death (aHR, 1.14; CI, 1.04–1.24; P=0.004). There were no differences in transplantation likelihood or post‐transplant mortality between Hispanic and White patients. Following the allocation system change, transplantation rates increased for all groups (P<0.05). However, Black patients still had a lower likelihood of transplantation than White patients (aHR, 0.90; CI, 0.79–0.99; P=0.024).
Conclusions
Although the proportion of Black and Hispanic patients listed for cardiac transplantation have increased, significant disparities remain. Compared with White patients, Black patients were less likely to be transplanted, even with the new allocation system, and had a higher risk of post‐transplantation death.
Keywords: disparities, heart failure, heart transplantation, race and ethnicity
Subject Categories: Heart Failure, Cardiovascular Surgery, Transplantation, Quality and Outcomes, Health Equity
Nonstandard Abbreviations and Acronyms
- aHR
adjusted hazard ratio
- UNOS
United Network for Organ Sharing
Clinical Perspective
What Is New?
Compared with White patients undergoing heart transplantation, Black patients are less likely to be transplanted and more likely to die after transplant.
With the new transplant allocation system, Black, Hispanic, and White patients all had increased likelihood of transplant, however, disparities still persist with Black patients having a lower likelihood of transplantation than White patients.
What Are the Clinical Implications?
Racial and ethnic disparities, particularly for Black patients, continue to exist in heart transplantation despite the new allocation system.
Additional studies are necessary to further understand the causes behind these disparities, including a thorough evaluation of social determinants of health.
Racial and ethnic disparities in cardiac transplantation are well established and have particularly affected Black and Hispanic patients. In cohort analyses of racial and ethnic groups from the previous 3 decades, Black patients were consistently at a higher risk of mortality after cardiac transplantation. 1 , 2 , 3 , 4 Several contributing factors have been hypothesized, including racial and ethnic differences between the donor and recipient, 5 immunologic and genetic mismatch, 6 , 7 access to care, and social determinants of health that disproportionately impact these populations. 8 However, despite some attention from the medical community, elimination of racial and ethnic disparities has been unacceptably slow and inconsistent. 4 , 5 , 8 Given the limited supply of donor hearts and persistent supply‐demand imbalance, it remains crucial to understand disparities among different patient groups to ensure equitable access to cardiac transplantation and optimize outcomes.
In 2018, the United Network for Organ Sharing (UNOS) revised the allocation system from 3 tiers to 6 tiers to expand access to organs for the most medically urgent patients, and reduce disparities as well as regional differences. 9 The older geographic sharing methodology created longer wait times for patients in diverse, highly populated regions, potentially affecting minority recipients more. The new allocation system aimed to remedy these geographic inequities, 9 however, it is unclear how these changes have impacted Black and Hispanic patients. This paper aimed to examine heart transplantation outcomes by racial and ethnic differences over the last decade and assess the impact of the 2018 allocation policy change on access and outcomes.
Methods
Anonymized data and materials are available through request from the Organ Procurement and Transplant Network.
Data Source
This study used the UNOS registry, specifically for heart transplantation. UNOS consists of data from every organ transplant performed in the United States each year. This data are submitted at the time of listing and is updated at transplant and after transplant at 1‐year intervals to account for postoperative outcomes. 10 Since the data are deidentified and publicly available they were deemed exempt by the Yale Institutional Review Board.
Study Population
A retrospective review of the UNOS registry was performed for all heart transplants between January 1, 2011 and May 12, 2020. Multiorgan transplants and patients under the age of 18 years were excluded. Patients were separated into cohorts by self‐reported race and ethnicity: Black, Hispanic, and White. Additionally, there was a supplemental analysis comparing Asian patients with White patients. For our secondary analysis assessing the allocation policy change, patients listed between April 12, 2017 to June 12, 2020 and with >30 days of follow‐up were included. Those with initial listing before October 18, 2018 used the old allocation system while those listed after October 18, 2018 used the new allocation system.
Statistical Analysis
Patient demographics, comorbidities, socioeconomic status, and outcomes were compared between race cohorts using Chi‐square analysis for categorical variables and Mann–Whitney U or Kruskal–Wallis tests for continuous variables. The primary outcomes of interest included transplantation, waitlist mortality, and post‐transplantation survival. Secondary outcomes were ischemic time and travel distance. Unadjusted and adjusted Cox regression was used to predict outcomes of interest including transplantation, waitlist death, and post‐transplant death. Models were adjusted for sex, age, body mass index, insurance payor, work for income, education level, etiology of cardiomyopathy, extracorporeal membrane oxygenation [at listing], intra‐aortic balloon pump [at listing], inotropes, ventilator status, left ventricular assist device, right ventricular assist device, total artificial hearts, diabetes mellitus, end‐stage renal disease, prior cerebrovascular accidents, malignancy, implantable cardioverter‐defibrillator, tobacco use, previous cardiac surgery, and human leukocyte antigen matching. For allocation system analysis, patient demographics, comorbidities, socioeconomic status, and outcomes were compared between allocation systems (within racial and ethnic subgroups) using Chi‐square analysis for categorical variables and Mann–Whitney U tests for continuous variables. Cox regression was used to predict transplantation by allocation system in each cohort and then by race and ethnicity in a cohort of patients just from the new allocation system. The same adjustments were used in this analysis as mentioned above. Changes in racial proportions of listed and transplanted patients over time were analyzed using linear regression. The analysis was performed using SPSS version 26 (IBM, Armonk, NY). Figures were made using GraphPad Prism (GraphPad Software, San Diego, CA).
Results
Baseline Characteristics
In total, we identified 32 353 patients (25% Black, 9% Hispanic, 66% White) during the study period. Baseline patient characteristics stratified by race are shown in Table 1. Compared with Black and Hispanic patients, White patients tended to be older (aged 57 years versus 53 years versus 53 years, respectively, P<0.001), and more likely to have private insurance (P<0.001). In terms of primary etiology of cardiomyopathy, 71.7% of Black patients had a dilated cardiomyopathy, significantly more than White or Hispanic patients (P<0.001). In comparison, White patients were most likely to have an ischemic cardiomyopathy (P<0.001). Significantly more White patients had a history of malignancy, tobacco use, and prior cardiac surgery (P<0.001, all). Hispanic patients had the highest proportion of patients with diabetes mellitus (P<0.001). Donor hearts to Hispanic recipients traveled shorter distances (Black: 100 miles versus Hispanic: 83 miles versus White: 104 miles, P=0.04) (Table S1). Ischemic time, donor age, and donor comorbidities were similar across groups.
Table 1.
Listing Characteristics by Patient Race and Ethnicity
| Variables |
Black (n=7971) |
Hispanic (n=2869) |
White (n=21513) |
P Value |
|---|---|---|---|---|
| Age, y | 53.0 [32.0–60.0] | 53.0 [42.0–61.0] | 57.0 [48.0–64.0] | <0.001 |
| Women | 32.0 | 26.8 | 24.3 | <0.001 |
| BMI | 28.1 [24.4–32.1] | 26.9 [23.7–30.7] | 27.6 [24.2–31.3] | <0.001 |
| Primary payer | <0.001 | |||
| Private | 40.4 | 38.8 | 54.8 | |
| Public | 48.8 | 60.2 | 44.1 | |
| Work for income (at listing) | 7.1 | 7.5 | 11.9 | <0.001 |
| Education level | <0.001 | |||
| Less than high school | 2.4 | 18.2 | 1.7 | |
| High school | 44.4 | 43.5 | 36.8 | |
| Post‐high school | 53.2 | 38.3 | 61.6 | |
| HLA‐matched (≥3 antigens) | 9.6 | 14.2 | 16.4 | <0.001 |
| Cardiac diagnosis | ||||
| Dilated cardiomyopathy | 71.7 | 53.7 | 44.9 | <0.001 |
| Restrictive cardiomyopathy | 3.8 | 1.7 | 2.9 | <0.001 |
| Ischemic cardiomyopathy | 16.9 | 31.2 | 37.6 | <0.001 |
| Congenital cardiomyopathy | 1.3 | 3.7 | 4.2 | <0.001 |
| Hypertrophic cardiomyopathy | 1.1 | 1.7 | 3.0 | <0.001 |
| Valvular cardiomyopathy | 0.9 | 1.7 | 1.4 | <0.001 |
| Cardiac support at time of listing | ||||
| Ventilator | 1.6 | 1.8 | 2.2 | 0.005 |
| Inotropes | 35.4 | 36.1 | 28.4 | <0.001 |
| LVAD | 31.0 | 23.0 | 26.9 | <0.001 |
| RVAD±LVAD or MCS unspecified | 1.6 | 1.2 | 1.6 | 0.310 |
| TAH | 0.5 | 0.5 | 0.5 | 0.996 |
| ECMO | 1.4 | 1.7 | 2.1 | <0.001 |
| IABP | 6.9 | 6.8 | 5.7 | <0.001 |
| Comorbidities | ||||
| Diabetes mellitus | 30.5 | 34.8 | 28.2 | <0.001 |
| Tobacco user | 40.7 | 38.9 | 47.8 | <0.001 |
| Malignancy | 6.8 | 5.1 | 9.8 | <0.001 |
| Prior stroke | 7.4 | 5.3 | 5.9 | <0.001 |
| ESRD | 4.5 | 4.1 | 2.6 | <0.001 |
| AICD | 77.4 | 73.1 | 73.4 | <0.001 |
| Prior cardiac surgery | 34.2 | 34.5 | 43.7 | <0.001 |
| Outcomes | ||||
| Waitlist time (IQR) | 85.0 [25.0–263.0] | 71.0 [20.0–218.0] | 84.0 [23.0–252.0] | <0.001 |
| Old status at listing | <0.001 | |||
| 1A | 25.8 | 27.4 | 23.5 | |
| 1B | 51.7 | 44.0 | 44.1 | |
| 2 | 22.5 | 28.6 | 32.4 | |
| New status at listing | <0.001 | |||
| 1 | 3.0 | 3.4 | 4.7 | |
| 2 | 20.2 | 21.8 | 18.6 | |
| 3 | 13.9 | 13.7 | 10.8 | |
| 4 | 39.6 | 37.2 | 39.8 | |
| 5 | 4.0 | 3.3 | 1.8 | |
| 6 | 19.2 | 20.6 | 24.4 | |
Data are presented as median (interquartile range) for continuous measures, and n (%) for categorical variables.
AICD indicates automated implantable cardioverter defibrillator; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; ESRD, end‐stage renal disease; HLA, human leukocyte antigen; IABP, intra‐aortic balloon pump; LVAD, left ventricular assist device; MCS, mechanical circulatory support; RVAD, right ventricular assist device; and TAH, total artificial heart.
Asian patients were found to be younger than White patients and to have higher rates of diabetes mellitus and lower rates of automated implantable cardioverter defibrillator and prior cardiac surgery (P<0.001, all). Additional data can be found in Table S2.
Transplant Outcomes
In unadjusted analyses, Hispanic patients had shorter median waitlist times (71 months) when compared with Black and White patients (Black: 85 months versus White: 84 months; P<0.001) (Table 1). There were no significant differences in the proportions of waitlist death (Black: 6.3% versus White: 6.7% versus Hispanic: 6.1%; P=0.21). After multivariable adjustment, fewer Black patients were transplanted relative to White patients (adjusted hazard ratio [aHR], 0.87; 95% CI, 0.84–0.90; P<0.001). Black patients had a lower likelihood of waitlist death (aHR, 0.88; CI, 0.78–0.98; P=0.023), but a higher risk of post‐transplant death compared with White patients (aHR, 1.14; CI, 1.04–1.24; P=0.004). There were no statistically significant differences for Hispanic patients compared with White patients (Table 2).
Table 2.
Cox Regression for Outcomes
| Unadjusted hazards ratio | P Value | Adjusted hazards ratio* | P Value | |
|---|---|---|---|---|
| Transplanted | ||||
| White | Reference | Reference | ||
| Black | 0.88 (0.85–0.91) | <0.001 | 0.87 (0.84–0.90) | <0.001 |
| Hispanic | 1.03 (0.98–1.08) | 0.271 | 1.04 (0.99–1.09) | 0.137 |
| Waitlist death | ||||
| White | Reference | Reference | ||
| Black | 0.86 (0.78–0.95) | 0.003 | 0.88 (0.78–0.98) | 0.023 |
| Hispanic | 0.92 (0.79–1.08) | 0.298 | 0.93 (0.78–1.10) | 0.406 |
| Post‐transplant death | ||||
| White | Reference | Reference | ||
| Black | 1.10 (1.01–1.19) | 0.022 | 1.14 (1.04–1.24) | 0.004 |
| Hispanic | 1.04 (0.92–1.17) | 0.553 | 1.00 (0.88–1.14) | 0.996 |
Adjusted for sex, age, BMI, insurance payor, work for income, education level, cardiomyopathy diagnosis, extracorporeal membrane oxygenation, intra‐aortic balloon pump, inotropes, ventilator status, left ventricular assist device, right ventricular assist device, total artificial hearts, diabetes mellitus, end‐stage renal disease, cerebrovascular accidents, malignancy, automated implantable cardioverter defibrillator, tobacco use, prior cardiac surgery, and human leukocyte antigen mismatch (human leukocyte antigen mismatch only included in Post‐Transplant Death Cox Regression).
Asian patients were found to have higher odds of transplantation than White patients after adjusted analysis (aHR, 1.38; CI, 1.28–1.48; P<0.001). There were no differences in waitlist or post‐transplant death (Table S3).
Effects of Allocation System Changes
After the change in allocation system in 2018, significantly more patients were on mechanical support in the form of extracorporeal membrane oxygenation and intra‐aortic balloon pump at the time of listing (both, P<0.001) (Table 3). Median waitlist times decreased in all racial and ethnic groups with the new allocation system (Black: 23 days versus 51 days, P<0.001; Hispanic: 21 days versus 48 days, P<0.001; White: 21 days versus 48 days, P<0.001). Similarly, both median ischemic time (Black: 3.4 hours versus 3.0 hours, P<0.001; Hispanic: 3.4 hours versus 3.1 hours, P=0.001; White: 3.4 hours versus 3.0 hours, P<0.001) and median distance (Black: 247.5 miles versus 84 miles, P<0.001; Hispanic: 184 miles versus 67 miles, P<0.001; White: 231 miles versus 84 miles, P<0.001) increased with the new system.
Table 3.
Differences based on allocation systems
| Variables | Black | P Value | Hispanic | P Value | White | P Value | |||
|---|---|---|---|---|---|---|---|---|---|
|
Pre‐allocation system changes (n=841) (Apr 1, 2017–Oct 12, 2018) |
Post‐allocation system changes (n=1010) (Oct 12, 2018‒Jun 12, 2020) |
Pre‐allocation system changes (n=340) (Apr 1, 2017–Oct 12, 2018) |
Post‐allocation system changes (n=422) (Oct 12, 2018–Jun 12, 2020) |
Pre‐allocation system changes (n=2458) (Apr 1, 2017–Oct 12, 2018) |
Post‐allocation system changes (n=2503) (Oct 12, 2018–Jun 12, 2020) |
||||
| Age, y | 55.0 [44.0–62.0] | 54.0 [43.0–61.0] | 0.171 | 54.0 [42.0–62.0] | 52.0 [41.8–61.0] | 0.477 | 58.0 [49.0–65.0] | 58.0 [47.0–64.0] | 0.008 |
| Women | 33.9 | 33.7 | 0.919 | 26.6 | 27.5 | 0.506 | |||
| BMI | 27.9 [24.3–31.7] | 27.8 [24.2–32.0] | 0.907 | 26.7 [23.2–29.8] | 26.3 [23.3–30.1] | 0.964 | 27.2 [23.9–31.0] | 27.4 [24.0–31.4] | 0.126 |
| Primary payer | 0.014 | 0.374 | <0.001 | ||||||
| Private | 40.4 | 41.7 | 38.5 | 42.7 | 53.2 | 53.1 | |||
| Public | 59.3 | 56.6 | 60.9 | 56.6 | 46.0 | 44.2 | |||
| Work for income (at listing) | 17.4 | 21.4 | <0.001 | 11.9 | 14.2 | 0.150 | 8.8 | 15.9 | 0.004 |
| Education level | 0.374 | 0.443 | 0.866 | ||||||
| Less than high school | 1.4 | 1.7 | 2.7 | 1.8 | 18.8 | 18.5 | |||
| High school | 35.2 | 33.3 | 44.1 | 44.5 | 44.4 | 42.9 | |||
| Post‐high school | 63.4 | 65.0 | 53.2 | 53.7 | 36.7 | 38.7 | |||
| HLA‐matched (≥3 antigens) | 15.2 | 16.7 | 0.220 | 10.4 | 9.4 | 0.572 | 12.1 | 12.7 | 0.843 |
| Cardiac diagnosis | |||||||||
| Dilated cardiomyopathy | 69.8 | 71.5 | 0.427 | 51.8 | 55.9 | 0.252 | 46.2 | 45.9 | 0.848 |
| Restrictive cardiomyopathy | 6.3 | 6.5 | 0.839 | 2.1 | 2.8 | 0.490 | 3.9 | 4.5 | 0.317 |
| Ischemic cardiomyopathy | 16.1 | 13.5 | 0.117 | 29.7 | 23.9 | 0.073 | 35.2 | 32.7 | 0.071 |
| Congenital cardiomyopathy | 1.0 | 2.2 | 0.037 | 4.7 | 5.5 | 0.643 | 3.9 | 5.1 | 0.047 |
| Hypertrophic cardiomyopathy | 1.9 | 1.3 | 0.288 | 2.4 | 2.4 | 0.988 | 3.5 | 3.6 | 0.854 |
| Valvular | 0.7 | 0.5 | 0.543 | 1.8 | 0.9 | 0.325 | 1.1 | 1.4 | 0.333 |
| Cardiac support at time of listing | |||||||||
| Ventilator | 1.2 | 1.5 | 0.583 | 1.5 | 2.6 | 0.277 | 2.2 | 3.2 | 0.036 |
| Inotropes | 41.6 | 40.6 | 0.656 | 36.8 | 42.4 | 0.113 | 35.2 | 33.1 | 0.117 |
| LVAD | 29.8 | 24.3 | 0.007 | 21.8 | 19.0 | 0.337 | 28.3 | 21.2 | <0.001 |
| RVAD±LVAD or MCS unspecified | 1.0 | 2.2 | 0.037 | 0.9 | 0.5 | 0.488 | 1.3 | 2.0 | 0.041 |
| TAH | 0.6 | 0.5 | 0.771 | 0.0 | 0.5 | 0.204 | 0.3 | 0.4 | 0.489 |
| ECMO | 1.1 | 3.3 | 0.002 | 3.2 | 3.8 | 0.680 | 2.6 | 4.6 | <0.001 |
| IABP | 6.7 | 18.2 | <0.001 | 5.0 | 16.1 | <0.001 | 5.5 | 16.3 | <0.001 |
| Comorbidities | |||||||||
| Diabetes mellitus | 31.3 | 32.0 | 0.744 | 36.2 | 29.6 | 0.055 | 27.8 | 25.6 | 0.083 |
| Tobacco user | 40.9 | 36.5 | 0.054 | 36.5 | 36.8 | 0.921 | 47.2 | 43.1 | 0.004 |
| Malignancy | 7.3 | 8.4 | 0.353 | 4.7 | 2.9 | 0.176 | 10.0 | 11.3 | 0.117 |
| Prior stroke | 8.5 | 8.2 | 0.817 | 5.6 | 5.7 | 0.936 | 6.2 | 6.5 | 0.636 |
| ESRD | 5.6 | 6.0 | 0.680 | 5.6 | 3.6 | 0.176 | 3.1 | 2.9 | 0.717 |
| AICD | 77.6 | 72.2 | 0.008 | 72.5 | 64.3 | 0.016 | 72.9 | 68.4 | 0.001 |
| Prior cardiac surgery | 31.2 | 31.3 | 0.946 | 32.1 | 25.1 | 0.032 | 41.8 | 41.4 | 0.769 |
| Outcomes | |||||||||
| Waitlist time (IQR) | 51.0 [19.0–140.0] | 23.0 [8.0–70.0] | <0.001 | 48.0 [15.0–120.0] | 21.0 [8.0–68.0] | <0.001 | 48.0 [17.0–118.0] | 21.0 [7.0–73.0] | <0.001 |
| Transplant outcomes | |||||||||
| Ischemic time (IQR) | 3.0 [2.3–3.7] | 3.4 [2.9–4.0] | <0.001 | 3.1 [2.2–3.8] | 3.4 [2.7–4.0] | 0.001 | 3.0 [2.3–3.7] | 3.4 [2.8–4.0] | <0.001 |
| Distance traveled (IQR) | 84.0 [12.0–257.8] | 247.5 [94.8–402.5] | <0.001 | 67.0 [15.0–260.0] | 184.0 [50.0–381.0] | <0.001 | 84.0 [13.0–253.0] | 231.0 [87.0–412.0] | <0.001 |
Data are presented as median (interquartile range) for continuous measures, and n (%) for categorical variables.
AICD indicates automated implantable cardioverter defibrillator; BMI, body mass index; ECMO, extracorporeal membrane oxygenation; ESRD, end‐stage renal disease; HLA, human leukocyte antigen; IABP, intra‐aortic balloon pump; IQR, interquartile range; LVAD, left ventricular assist device; MCS, mechanical circulatory support; RVAD, right ventricular assist device; and TAH, total artificial heart.
In adjusted analyses, the chance of transplantation increased for all racial groups (Black: aHR, 1.32; 95% CI, 1.19–1.48; P<0.001 versus Hispanic: aHR, 1.20; 95% CI, 1.01–1.43; P=0.037 versus White: aHR, 1.33; 95% CI, 1.25–1.42; P<0.001) (Table 4). However, compared with White patients, Black patients were still less likely to be transplanted (Black: aHR, 0.90; 95% CI, 0.82–0.99; P=0.024). There was no significant difference for transplantation in Hispanic patients when compared with White patients.
Table 4.
Cox regression for outcomes by allocation system
| Unadjusted hazards ratio | P Value | Adjusted hazards ratio* | P Value | |
|---|---|---|---|---|
| White | ||||
| Transplanted | ||||
| Old allocation system | Reference | Reference | ||
| New allocation system | 1.39 (1.31–1.48) | <0.001 | 1.33 (1.25–1.42) | <0.001 |
| Black | ||||
| Transplanted | ||||
| Old allocation system | Reference | Reference | ||
| New allocation system | 1.41 (1.28–1.57) | <0.001 | 1.32 (1.19–1.48) | <0.001 |
| Hispanic | ||||
| Transplanted | ||||
| Old allocation system | Reference | Reference | ||
| New allocation system | 1.27 (1.09–1.49) | 0.003 | 1.20 (1.01–1.43) | 0.037 |
| New allocation system only | ||||
| Transplanted | ||||
| White | Reference | Reference | ||
| Black | 0.92 (0.85–1.00) | 0.055 | 0.90 (0.82–0.99) | 0.024 |
| Hispanic | 0.94 (0.84–1.05) | 0.283 | 0.89 (0.79–1.01) | 0.076 |
Adjusted for sex, age, body mass index, work for income, education level, insurance payor, cardiomyopathy diagnosis, ECMO, intra‐aortic balloon pump, inotropes, ventilator status, left ventricular assist device, right ventricular assist device, total artificial hearts, diabetes mellitus, ESRD, cerebrovascular accidents, malignancy, automated implantable cardioverter defibrillator, tobacco use, and prior cardiac surgery.
Trends
Over the study period, the proportion of Black and Hispanic patients listed for transplant increased: 21.7% to 28.2% and 7.7% to 9.0%, respectively, (P<0.001) while White patients decreased from 70.6% to 66.5% (P<0.001) (Figure 1). The proportion of Black patients transplanted increased from 20.8 to 27.3% (P=0.003) (Figure 2). White and Hispanic patients both decreased in proportion: 70.7% to 64.3% (P<0.001) and 8.5% to 8.4% (P=0.002), respectively.
Figure 1. Race and ethnicity of patients listed over time: proportion of patients listed for transplantation by race and ethnicity from 2011 to 2020.
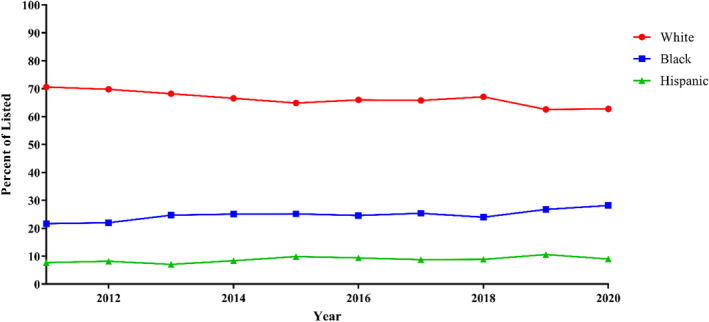
Trends were analyzed with linear regression. Linear regression: P<0.001, all.
Figure 2. Race and ethnicity of patients transplanted over time: proportions of patients transplanted by race and ethnicity from 2011 to 2020.
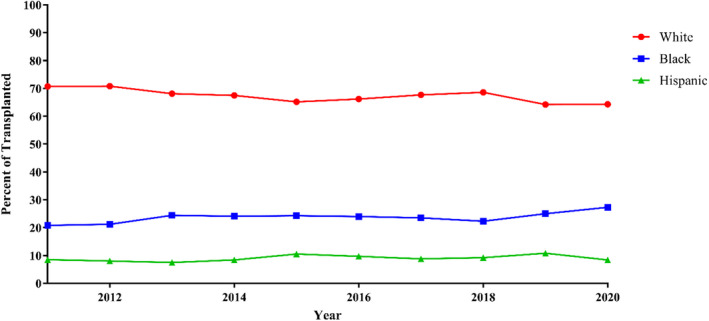
Trends were analyzed with linear regression. Linear regression: Black: P=0.003, Hispanic: P=0.002, White: P<0.001.
Discussion
In this analysis from the UNOS database, we assessed for racial and ethnic differences in waitlist disparities and post‐heart transplantation outcomes over the last decade and in relationship to the recent allocation policy change. We found several important findings of note. First, over the 10‐year study period, the proportion of both Black and Hispanic patients listed for heart transplantations have increased. However, despite these encouraging findings, Black patients were significantly less likely than White patients to be transplanted. Second, compared with White patients, Black individuals were more likely to die post‐transplantation. Finally, we also describe the first report from the 2018 allocation system change on racial and ethnic disparities. Importantly, all groups have experienced decreased waitlist times and an increased chance of transplantation. However, compared with White patients, racial disparities remain as Black patients continued to have a lower likelihood of transplantation after the allocation change.
Over the study period, we found the proportion of Black and Hispanic patients transplanted has increased from 21.7% to 28.2% and 7.7% to 9.0%, respectively. In a previous study of trends, Liu et al. 1 demonstrated that the proportion of racial and ethnic minorities increased from 1987 to 2009, and by 2005 to 2009 accounted for nearly one third of all transplants. Our findings add to this previous study by further delineating trends by Black and Hispanic race and ethnicity. Although these trends are improving, our data suggest that access to heart transplantation remains unequal. One previously postulated explanation for longer waitlist times and lower rates of transplantation, specifically for Black patients, includes immunologic factors, such as higher panel reactive antibody values. 5 , 11 Used during the screening process for heart transplantation, panel reactive antibody values identify patients with antibodies to known human leukocyte antigens, and can lower the chance of a compatible transplant. 5 Another potential reason for these disparities in access may be clinician bias. 12 Several qualitative studies have noted that racial minorities are less likely referred for transplantation and more likely to be referred for mechanical circulatory support than clinically similar White patients. 13 Findings from these qualitative studies are supported by recent UNOS analyses showing racial minorities are more likely referred for mechanical circulatory support, which may explain in part the disparities we note in transplantation. 14
Despite some improving trends in transplantation equity, unacceptable racial disparities remain pervasive in outcomes. In 2 previous studies, Liu et al. and Kilic et al. demonstrated that Black cardiac transplantation recipients were significantly more likely to experience post‐transplant mortality than White and Hispanic patients. 1 , 4 In our contemporary analysis, these disparities persisted as Black patients had a higher likelihood of post‐transplant mortality after adjustment when compared with White patients. There are a number of mechanisms that may contribute to worse outcomes in Black patients. Several studies have linked socioeconomic factors such as living in lower‐income neighborhoods as independent risk factors for poorer outcomes in both heart failure and transplant outcomes. 15 , 16 These results are likely not only because of lack of access to care, but also because of the physical and social environment in which these patients reside. 17 , 18 Other studies have suggested that poorer outcomes may be partially because of factors such as human leukocyte antigen mismatch, which is more common in Black individuals, and associated with higher rates of post‐transplant rejection. 5 , 19 Finally, several studies have linked differential gene expression profiles as well as response to specific immunosuppressive regimens. 6 , 7 Future studies are needed to delineate the impact of each of these factors on worse outcomes for Black patients undergoing heart transplantation.
Following the 2018 allocation system change, we found that access improved for Black and Hispanic patients relative to prior years. The old system was criticized for the overuse of exception statuses for status 1A and for a geographic sharing scheme that was not equitable or consistent with the final rule. 20 , 21 This geographic sharing scheme created a waitlist time that was considerably higher, especially for patients in diverse, highly populated areas of the country. 9 As a result, the allocation system changes included a 6‐tier system and aimed to increase the distance at which hearts could be retrieved for donations, thereby aiming to alleviate possible sources of inequity in heart distribution. 9 While the changes improved waitlist times for all races, we found disparities still persist with Black patients having a lower chance of transplantation compared with White patients. These findings highlight the continued need for further allocation interventions to end inequity.
The solutions to address these disparities are necessary but not necessarily straightforward. Several of the mechanisms for disparities may ultimately stem from structural racism in the treatment of cardiovascular disease, which was recently highlighted in an American Heart Association Scientific Statement. 22 Structural racism encompasses the societal practices that drive inequities in quality of housing and neighborhood environments, economic advancement, and education opportunities among others. Tangible examples include differential usage of guideline‐directed medical therapy, poor inclusion of minorities in clinical trials, and a greater proportion of minorities that are uninsured and underinsured. 23 Given the multifactorial causes of disparities, Nayak et al. suggest multifactorial solutions aimed at increased minority enrollment in clinical trials, expanding academic‐community partnership, and increased diversification of the healthcare workforce to name a few solutions. 23 To fully eradicate these disparities in cardiac transplantation, it will be necessary to address societal policies that lead to disparate cardiovascular disease outcomes.
Limitations
This study has several notable limitations in addition to being retrospective. First, differences between races about key social determinants of health, including access to care, social support systems, and income could not be assessed because of limitations of the UNOS database. Second, while we found that the percentage of Black and Hispanic patients listed and receiving cardiac transplantation has increased, these improvements do not necessary reflect the proportion of patients living with advanced heart failure. Our analysis of the UNOS database includes only patients accepted for listing by a transplant center. Third, while UNOS does capture other racial categories such as Asian and Pacific Islander, we a priori decided to focus on the 3 previously defined patient populations but have included a comparison of Asian and White patients as a supplemental analysis. In addition, we recognize that race is often an imprecise term, and that we have not included individuals who identified as having a mixed racial background. Fourth, we acknowledge that the allocation system policy has only been in effect for <2 years and that the heart transplantation community practices are continuing to evolve under the new system. In particular, the impact of the COVID‐19 pandemic on heart transplantation from 2020 remains incompletely understood, and likely resulted in atypical patterns of care. 24
Conclusions
Over the last decade, Black patients were both significantly less likely to undergo cardiac transplantation than White patients and were significantly more likely to experience post‐transplant mortality after adjustment. Overall trends suggest that racial disparities in listing and transplant have narrowed, but significant work is still needed. In particular, the new allocation system has shown improved rates of transplantation and decreased waitlist times for each race and ethnicity. However, Black patients were still less likely than White patients to undergo transplantation. Overall, these findings suggest that the new allocation system may be narrowing previously noted racial disparities in cardiac transplantation, but additional investigation is required to better understand and address continued disparities.
Sources of Funding
Dr Miller reports funding through the Yale National Clinician Scholars Program and by Clinical and Translational Science Award Grant Number TL1 TR001864 from the National Center for Advancing Translational Science, a component of the National Institutes of Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of National Institutes of Health.
Disclosures
Dr Desai works under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures used for public reporting and pay for performance programs. He reports research grants and consulting for Amgen, Astra Zeneca, Boehringer Ingelheim, Cytokinetics, Medicines Company, Relypsa, Novartis, and SCPharmaceuticals. Dr Ahmad is a consultant for Amgen, Cytokinetics, Relypsa, and Novartis. The other authors have no disclosures.
Supporting information
Table S1‐S3
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.021067.
For Sources of Funding and Disclosures, see page 11.
See Editorial by Lewsey and Breathett.
References
- 1. Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent racial disparities in survival after heart transplantation. Circulation. 2011;123:1642–1649. DOI: 10.1161/CIRCULATIONAHA.110.976811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh TP, Almond C, Givertz MM, Piercey G, Gauvreau K. Improved survival in heart transplant recipients in the United States: racial differences in era effect. Circ Heart Fail. 2011;4:153–160. DOI: 10.1161/CIRCHEARTFAILURE.110.957829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen JG, Weiss ES, Arnaoutakis GJ, Russell SD, Baumgartner WA, Conte JV, Shah AS. The impact of race on survival after heart transplantation: an analysis of more than 20,000 patients. Ann Thorac Surg. 2010;89:1956–1964; discussion 1963–1964. DOI: 10.1016/j.athoracsur.2010.02.093. [DOI] [PubMed] [Google Scholar]
- 4. Kilic A, Higgins RS, Whitson BA, Kilic A. Racial disparities in outcomes of adult heart transplantation. Circulation. 2015;131:882–889. DOI: 10.1161/CIRCULATIONAHA.114.011676. [DOI] [PubMed] [Google Scholar]
- 5. Morris AA, Kransdorf EP, Coleman BL, Colvin M. Racial and ethnic disparities in outcomes after heart transplantation: a systematic review of contributing factors and future directions to close the outcomes gap. J Heart Lung Transplant. 2016;35:953–961. DOI: 10.1016/j.healun.2016.01.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khush KK, Pham MX, Teuteberg JJ, Kfoury AG, Deng MC, Kao A, Anderson AS, Cotts WG, Ewald GA, Baran DA, et al. Gene expression profiling to study racial differences after heart transplantation. J Heart Lung Transplant. 2015;34:970–977. DOI: 10.1016/j.healun.2015.01.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moayedi Y, Fan C‐P, Miller RJH, Tremblay‐Gravel M, Posada JGD, Manlhiot C, Hiller D, Yee J, Woodward R, McCaughan JA, et al. Gene expression profiling and racial disparities in outcomes after heart transplantation. J Heart Lung Transplant. 2019;38:820–829. DOI: 10.1016/j.healun.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 8. Young BA. Health disparities in advanced heart failure treatment: the intersection of race and sex. JAMA Netw Open. 2020;3:e2011034. DOI: 10.1001/jamanetworkopen.2020.11034. [DOI] [PubMed] [Google Scholar]
- 9. Taylor LJ, Fiedler AG. Balancing supply and demand: review of the 2018 donor heart allocation policy. J Card Surg. 2020;35:1583–1588. DOI: 10.1111/jocs.14609. [DOI] [PubMed] [Google Scholar]
- 10. Goldstein BA, Thomas L, Zaroff JG, Nguyen J, Menza R, Khush KK. Assessment of heart transplant waitlist time and pre‐ and post‐transplant failure: a mixed methods approach. Epidemiology. 2016;27:469–476. DOI: 10.1097/EDE.0000000000000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris AA, Cole RT, Veledar E, Bellam N, Laskar SR, Smith AL, Gebel HM, Bray RA, Butler J. Influence of race/ethnic differences in pre‐transplantation panel reactive antibody on outcomes in heart transplant recipients. J Am Coll Cardiol. 2013;62:2308–2315. DOI: 10.1016/j.jacc.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 12. Breathett K, Yee E, Pool N, Hebdon M, Crist JD, Knapp S, Larsen A, Solola S, Luy L, Herrera‐Theut K, et al. Does race influence decision making for advanced heart failure therapies? J Am Heart Assoc. 2019;8:e013592. DOI: 10.1161/JAHA.119.013592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Breathett K, Yee E, Pool N, Hebdon M, Crist JD, Yee RH, Knapp SM, Solola S, Luy L, Herrera‐Theut K, et al. Association of gender and race with allocation of advanced heart failure therapies. JAMA Netw Open. 2020;3:e2011044. DOI: 10.1001/jamanetworkopen.2020.11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Breathett K, Allen LA, Helmkamp L, Colborn K, Daugherty SL, Blair IV, Jones J, Khazanie P, Mazimba S, McEwen M, et al. Temporal trends in contemporary use of ventricular assist devices by race and ethnicity. Circ Heart Fail. 2018;11:e005008. DOI: 10.1161/CIRCHEARTFAILURE.118.005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bikdeli B, Wayda B, Bao H, Ross JS, Xu X, Chaudhry SI, Spertus JA, Bernheim SM, Lindenauer PK, Krumholz HM. Place of residence and outcomes of patients with heart failure: analysis from the telemonitoring to improve heart failure outcomes trial. Circ Cardiovasc Qual Outcomes. 2014;7:749–756. DOI: 10.1161/CIRCOUTCOMES.113.000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wayda B, Clemons A, Givens RC, Takeda K, Takayama H, Latif F, Restaino S, Naka Y, Farr MA, Colombo PC, et al. Socioeconomic disparities in adherence and outcomes after heart transplant: a UNOS (United Network for Organ Sharing) registry analysis. Circ Heart Fail. 2018;11:e004173. DOI: 10.1161/CIRCHEARTFAILURE.117.004173. [DOI] [PubMed] [Google Scholar]
- 17. Evans JD, Kaptoge S, Caleyachetty R, Di Angelantonio E, Lewis C, Parameshwar KJ, Pettit SJ. Socioeconomic deprivation and survival after heart transplantation in England: an analysis of the United Kingdom Transplant Registry. Circ Cardiovasc Qual Outcomes. 2016;9:695–703. DOI: 10.1161/CIRCOUTCOMES.116.002652. [DOI] [PubMed] [Google Scholar]
- 18. DePasquale EC, Kobashigawa JA. Socioeconomic disparities in heart transplantation: a universal fix? Circ Cardiovasc Qual Outcomes. 2016;9:693–694. DOI: 10.1161/CIRCOUTCOMES.116.003210. [DOI] [PubMed] [Google Scholar]
- 19. Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long‐term survival following cardiac transplantation. Transplant Proc. 1997;29:1460–1463. DOI: 10.1016/S0041-1345(96)00567-2. [DOI] [PubMed] [Google Scholar]
- 20. Snyder JJ, Salkowski N, Wey A, Pyke J, Israni AK, Kasiske BL. Organ distribution without geographic boundaries: a possible framework for organ allocation. Am J Transplant. 2018;18:2635–2640. DOI: 10.1111/ajt.15115. [DOI] [PubMed] [Google Scholar]
- 21. Goff RR, Uccellini K, Lindblad K, Hall S, Davies R, Farr M, Silvestry S, Rogers JG. A change of heart: preliminary results of the US 2018 adult heart allocation revision. Am J Transplant. 2020;20:2781–2790. DOI: 10.1111/ajt.16010. [DOI] [PubMed] [Google Scholar]
- 22. Churchwell K, Elkind MSV, Benjamin RM, Carson AP, Chang EK, Lawrence W, Mills A, Odom TM, Rodriguez CJ, Rodriguez F, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142:e454–e468. DOI: 10.1161/CIR.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 23. Nayak A, Hicks AJ, Morris AA. Understanding the complexity of heart failure risk and treatment in black patients. Circ Heart Fail. 2020;13:e007264. DOI: 10.1161/CIRCHEARTFAILURE.120.007264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeFilippis EM, Farr MA, Givertz MM. Challenges in heart transplantation in the era of COVID‐19. Circulation. 2020;141:2048–2051. DOI: 10.1161/CIRCULATIONAHA.120.047096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3


