Abstract
Background
The usefulness of right heart catherization (RHC) has long been debated, and thus, we aimed to study the real‐world impact of the use of RHC in cardiogenic shock.
Methods and Results
In the Nationwide Readmissions Database using International Classification of Diseases, Tenth Revision (ICD‐1 0), we identified 236 156 patient hospitalizations with cardiogenic shock between 2016 and 2017. We sought to evaluate the impact of RHC during index hospitalization on management strategies, complications, and outcomes as well as on 30‐day readmission rate. A total 25 840 patients (9.6%) received RHC on index admission. The RHC group had significantly more comorbidities compared with the non‐RHC group. During the index admission, the RHC group had lower death (25.8% versus 39.5%, P<0.001) and stroke rates (3.1% versus 3.4%, P<0.001). Thirty‐day readmission rates (18.7% versus 19.7%, P=0.04) and death on readmission (7.9% versus 9.3%, P=0.03) were also lower in the RHC group. After adjustment, RHC was associated with lower index admission mortality (odds ratio, 0.69; 95% CI, 0.66–0.72), lower stroke rate (odds ratio, 0.81; 95% CI, 0.72–0.90), lower 30‐day readmission (odds ratio, 0.83; 95% CI, 0.78–0.88), and higher left ventricular assist device implantations/orthotopic heart transplants (odds ratio, 6.05; 95% CI, 4.43–8.28) during rehospitalization. Results were not meaningfully different after excluding patients with cardiac arrest.
Conclusions
RHC use in cardiogenic shock is associated with improved outcomes and increased use of downstream advanced heart failure therapies. Further blinded randomized studies are required to confirm our findings.
Keywords: cardiogenic shock, catheterization, outcome, readmission
Subject Categories: Heart Failure, Cardiomyopathy, Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- CS
cardiogenic shock
- MCS
mechanical circulatory support
- NRD
Nationwide Readmissions Database
- OHT
orthotopic heart transplant
- RHC
right heart catheterization
Clinical Perspective
What Is New?
This large real‐world retrospective study of patients with cardiogenic shock demonstrates that right heart catheterization is associated with improved in‐hospital survival and reduced rehospitalizations.
Moreover, though the use of right heart catheterization across the United States is infrequent, the procedure improves downstream use of life‐saving advanced heart failure therapies.
What Are the Clinical Implications?
In patients with cardiogenic shock, hemodynamic profiling using right heart catheterization should be considered to tailor therapies and optimize outcomes.
Additional blinded studies are needed to investigate the clinical implications of hemodynamic‐driven treatment strategies in these patients.
Despite being in use for 50 years, the role of right heart catherization (RHC) continues to be debated, as illustrated by the recent surge despite a previous downward trend. 1 , 2 , 3 The prior decline in use has been attributed to several factors, including no clear benefit in various clinical settings, its inherently invasive nature, increased resource use, and concurrent improvements in noninvasive diagnostic methods in intensive care. 4 , 5 , 6 , 7 A recent report published earlier this year showed a 75% decrease in the use of a pulmonary artery catheter in patients with acute myocardial infarction complicated by cardiogenic shock (CS). 8 In contrast, the American College of Cardiology/American Heart Association guidelines support the use of RHC in patients with heart failure with CS or with respiratory failure requiring mechanical ventilation (class IA), albeit routine use of RHC for management of acute heart failure is not recommended (class III). 9 Importantly, no randomized trial in patients with CS exists.
A recent scientific statement from the American Heart Association for the contemporary management of CS suggests the use of RHC in conjunction with other diagnostic tools in cases of diagnostic uncertainty or in patients with initial treatment unresponsiveness. 10 This recommendation for accurate hemodynamic diagnosis and monitoring is increasingly relevant, with the growing complexity of hospitalized patients, the rising use of temporary mechanical circulatory support (MCS), and the need for swift and early evaluation of candidacy for advanced heart failure therapies. To further define the role of RHC in patients with CS, we conducted an analysis from the Nationwide Readmissions Database (NRD) evaluating outcomes, use of advanced heart failure therapies, and readmission rates.
Methods
Data Sources
The NRD is part of a family of databases developed for the Healthcare Cost and Utilization Project from the State Inpatient Databases. The NRD is a unique and powerful database designed to support analyses of national readmission rates for all payer types. Readmission rates have been established as an important metric of hospital quality outcomes as defined by the Hospital Readmissions Reduction Program and required as per the Affordable Care Act. 11 It contains data from 27 geographically dispersed states reporting an estimated 36 million discharges nationally. Beginning with the fourth quarter of 2015, the NRD updated its diagnosis and procedure codes using only the International Classification of Diseases, Tenth Revision, Clinical Modification/Procedure Coding System (ICD‐10‐CM/PCS) coding system. Institutional review board approval and informed consent was waived for this study because of the deidentified nature of the database. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We used the NRD from 2016 and 2017 (January to November for each year) to identify first hospitalization for all patients with a diagnosis of CS (using ICD‐10‐CM code R57.0, T8111XA). The ICD‐10‐CM code used for CS has been reported to have a positive predictive value of 93.5% in validation studies. 12 All patients with CS who had a planned admission were excluded. This most probably includes patients previously diagnosed with CS on inotropes or electively admitted for left ventricular assist device (LVAD) implantation or orthotopic heart transplant (OHT). This was deemed necessary to better gauge the effectiveness of RHC/pulmonary artery catheterization in acute CS. Furthermore, all nonelective readmissions within 30 days of discharge were identified. Index admissions that resulted in LVAD implantation or OHT were excluded because patients exhibit different disease courses. If there were ≥2 readmissions after the index admission, only the first one was included for the analysis. Readmission causes and time to readmission were also evaluated. Apart from a hospital performance metric, readmission rates and time to readmission may constitute an additional measure of impact of RHC on management of CS.
To identify patients who underwent RHC, we used ICD‐10‐PCS codes 4A023N6 (Measurement of Cardiac Sampling and Pressure, Right Heart, Percutaneous Approach), 4A023N8 (Measurement of Cardiac Sampling and Pressure, Bilateral, Percutaneous Approach), 4A133B3 (Monitoring of Arterial Pressure, Pulmonary, Percutaneous Approach), 4A1239Z (Monitoring of Cardiac Output, Percutaneous Approach), and 02HP32Z (Insertion of Monitoring Device into Pulmonary Trunk, Percutaneous Approach). We used ICD‐10‐PCS codes 5A02210 (Intra‐aortic Balloon Pump), 5A0221D (Percutaneous Left Ventricular Assist Device), 5A1522F (Extracorporeal Life Support), 4A023N7 (Left Heart Catheterization), Z98.61/Z95.5 (Percutaneous Coronary Intervention), Z95.811 (Left Ventricular Assist Device), and Z94.1 (Orthotopic Heart Transplant) to investigate therapies during index hospitalizations and readmissions. All baseline and outcome information was compared among the patients who did and did not receive RHC during index hospitalization. Two subgroup analyses were also performed: (1) excluding patients with cardiac arrest; (2) excluding patients receing LVAD/OHT on index admission.
Statistical Analysis
In‐hospital outcomes including mortality, acute kidney injury needing hemodialysis, ischemic stroke, and length of stay were compared between the 2 groups. Patient characteristics were presented as mean ±standard deviation for continuous variables or as percentage for categorical variables. Comparisons were performed using the χ 2 test, Student t test, and Wilcoxon rank sum test for categorical and continuous variables with normal and nonnormal distribution, respectively. We performed multivariate logistic regression with inpatient mortality as the dependent variable. We then selected variables with a significance level of P<0.01 and removed variables without clinically meaningful relevance (eg, peptic ulcer disease, depression). The final model included age, sex, insurance status, hospital size and teaching status, Elixhauser Comorbidity Index score, household income, acute myocardial infarction, heart failure, valvular disease, pulmonary circulation disorder, chronic pulmonary disease, renal failure, liver disease, coagulopathy, obesity, fluid/electrolyte disturbances, use of temporary mechanical support (intra‐aortic balloon pump/percutaneous LVAD/ extracorporeal life support and advance heart failure therapies (LVAD/OHT).
We additionally performed 1:1 propensity matching with a caliper width of 0.01 with generation of propensity scores using the psmatch2 command using the Mahalanobis matching method to account for treatment bias. Absolute standardized differences across different variables were <10% (Figure S1). Two‐sided P values <0.05 were considered significant. All analyses were performed using Stata statistical software (version 16; StataCorp, College Station, TX).
Results
Baseline Characteristics
A total of 236 156 patient hospitalizations with a diagnosis of CS were identified between 2016 and 2017, of which only 25 840 (9.6%) underwent RHC. A flow diagram of our study is shown in Figure 1.
Figure 1. Flowdiagram of selection and comparison of right heart catheterization (RHC) andnon‐RHC groups during index admission as well as readmissions.
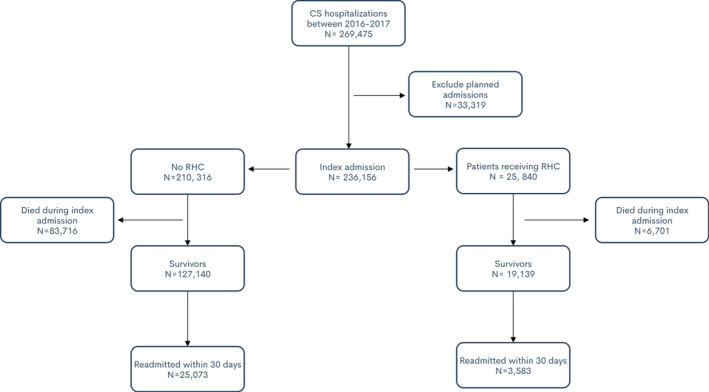
LVAD indicates left ventricular assist device; and OHT, orthotropic heart transplant.
Compared with their counterparts, patients who underwent RHC were more likely to be younger (61.6 years versus 67.3 years, P<0.001) and men (68.3% versus 51.2%, P<0.001). The RHC cohort had a higher morbidity burden including history of congestive heart failure history (92.8% versus 75.1%), arrhythmias (74.7% versus 66.6%), valvular heart disease (38.3% versus 25.8%), peripheral vascular disease (32.2% versus 20.7%, P<0.001), hypertension (75.1% versus 73.7%, P=0.008), complicated diabetes mellitus (29.7% versus 26.0%), acute kidney injury (73% versus 60.4%, P<0.001), chronic renal failure (49.2% versus 38.7%, P<0.001), and obesity (19.6% versus 16.7%, P<0.001). They also had a higher Elixhauser Comorbidity Index score (24.0 versus 20.9, P<0.001). Baseline characteristics are demonstrated in Table 1. Table S1 illustrates baseline and hospital characteristics after excluding patients with cardiac arrest; the comparison between the groups is largely unchanged.
Table 1.
Baseline Hospital and Patient Characteristics
| Demographics | Total, N=236 156 | Non‐RHC, N=210 316 | RHC, N=25 840 | P value |
|---|---|---|---|---|
| Demographics | ||||
| Mean age, y (SD) | 66.6 (14.5) | 67.3 (14.3) | 61.6 (14.4) | <0.001 |
| Women, % | 38.0 | 38.8 | 31.7 | <0.001 |
| Hospital characteristics | ||||
| Hospital size | ||||
| Small, % | 9.4 | 10.1 | 4.2 | <0.001 |
| Medium, % | 23.1 | 24.1 | 14.6 | <0.001 |
| Large, % | 67.4 | 65.7 | 81.1 | <0.001 |
| Hospital case volume (mean) | 347 (493) | 322 (469) | 556 (614) | <0.001 |
| Hospital type | ||||
| Metropolitan nonteaching, % | 18.8 | 20.3 | 6.5 | <0.001 |
| Metropolitan teaching, % | 77.1 | 75.3 | 92.0 | <0.001 |
| Nonmetropolitan hospital, % | 3.9 | 4.2 | 1.3 | <0.001 |
| Elixhauser Comorbidity Index score (mean) | 21.2 (10.1) | 20.9 (10.2) | 24.0 (9.5) | <0.001 |
| Presentation | ||||
| Acute coronary syndrome, % | 44.1 | 45.5 | 32.9 | <0.001 |
| STEMI, % | 13.3 | 13.6 | 10.9 | <0.001 |
| NSTEMI, % | 23.9 | 24.6 | 18.1 | <0.001 |
| Comorbidities | ||||
| Congestive heart failure, % | 75.1 | 73.0 | 92.8 | <0.001 |
| Cardiac arrythmias, % | 67.5 | 66.6 | 74.7 | <0.001 |
| Cardiac arrest, % | 14.2 | 14.9 | 8.1 | <0.001 |
| Valvular heart disease, % | 26.8 | 25.3 | 38.3 | <0.001 |
| Pulmonary circulation disorder, % | 21.5 | 19.3 | 39.5 | <0.001 |
| Chronic pulmonary disease, % | 28.0 | 28.2 | 26.3 | <0.001 |
| Peripheral vascular disease, % | 21.9 | 20.7 | 32.2 | <0.001 |
| Hypertension, % | 73.8 | 73.7 | 75.1 | 0.008 |
| Acute kidney injury, % | 61.8 | 60.4 | 73.0 | <0.001 |
| Chronic Renal failure, % | 39.9 | 38.7 | 49.2 | <0.001 |
| Diabetes mellitus, uncomplicated, % | 15.2 | 15.3 | 14.4 | 0.03 |
| Diabetes mellitus, complicated, % | 26.4 | 26.0 | 29.7 | <0.001 |
| Obesity, % | 17.0 | 16.7 | 19.6 | <0.001 |
| Liver disease, % | 21.4 | 20.9 | 25.2 | <0.001 |
| Neurological disorder, other, % | 28.7 | 30.0 | 17.8 | <0.001 |
| Electrolyte disorder, % | 68.8 | 68.3 | 73.1 | <0.001 |
| Deficiency anemia, % | 5.2 | 4.9 | 7.7 | <0.001 |
| Alcohol abuse, % | 6.2 | 6.2 | 6.1 | 0.78 |
| Procedures | ||||
| Left heart catheterization, % | 32.6 | 32.3 | 35.7 | <0.001 |
| Percutaneous coronary intervention, % | 19.7 | 20.1 | 17.2 | <0.001 |
| Intra‐aortic balloon pump, % | 16.3 | 15.1 | 26.5 | <0.001 |
| Percutaneous ventricular assist device, % | 4.8 | 4.2 | 9.4 | <0.001 |
| Extracorporeal life support, % | 2.5 | 2.3 | 4.1 | <0.001 |
| Utilization of Advance heart failure therapies on index admission | ||||
| Left ventricular assist device, % | 1.8 | 0.9 | 8.9 | <0.001 |
| Orthotopic heart transplantation, % | 0.9 | 0.4 | 4.7 | <0.001 |
NSTEMI indicates non–ST‐segment–elevation myocardial infarction; RHC, right heart catheterization; and STEMI, ST‐segment–elevation myocardial infarction.
Invasive therapies during index admission were invariably more common in patients undergoing RHC including temporary MCS (40% versus 21.6% [intra‐aortic balloon pump, 26.5% versus 15.1%; percutaneous left ventricular assist device, 9.4% versus 4.2%; extracorporeal life support, 4.1% versus 2.3%]), and left heart catheterization (35.7% versus 32.3%), albeit percutaneous coronary intervention was less frequent (17.2% versus 20.1%). The utilization of of advance surgical heart failure therapies were significantly higher in the RHC group (LVAD, 8.9% versus 0.9%; and OHT, 4.7% versus 0.4% [Table 1]).
Outcomes During Index Admission
Table 2 illustrates the index admission hospital outcomes. Compared with their counterparts, patients who underwent RHC had a significantly lower mortality rate (25.8% versus 39.5%, P<0.001) and ischemic stroke rate (3.4% versus 3.9%, P=0.018), although these beneficial outcomes were accompanied by a significantly longer length of stay (22.7 days versus 14.3 days, P<0.001). To account for patients who had cardiac arrest before RHC, we performed sensitivity analysis excluding those patients from our cohort. After exclusion, mortality (23.4% versus 35%, P<0.001) and stroke (3.2% versus 3.8%, P=0.04) rates were still lower in the RHC group, with higher hemodialysis rates (3.2% versus 2.5%, P=0.015; Table S2). In a different sensitivity analysis excluding patients not receving LVAD/OHT during index admission, the mortality rate remained lower in patients who underwent RHC (28.7% versus 39.5%, P<0.001; Table S3). On multivariate analysis and after adjusting for baseline differences (Figure 2), the use of RHC was associated with a 31% decrease in mortality rate (odds ratio [OR], 0.69; 95% CI, 0.66–0.72) and 19% reduction in stroke rates (OR, 0.81; 95% CI, 0.72–0.90). Similarly, multivariate analysis after excluding patients with cardiac arrest yielded similar findings for mortality (OR, 0.71; 95% CI, 0.68–0.75) and stroke (OR, 0.8; 95% CI, 0.71–0.9), as shown in Figure 3. Finally, outcomes remained largely consistent when stratified by hospital teaching status (Table S4). Out of 25 486 propensity‐matched hospitalizations (including 98.6% of the RHC cohort), similar trends of lower mortality were observed in patients undergoing RHC (Table S5). The baseline characteristics of matched populations is shown in Table S6.
Table 2.
Index Admission In‐Hospital Outcomes and Therapies
| Outcomes | Total, N=236 156 | Non‐RHC, N=210 316 | RHC, N=25 840 | P value |
|---|---|---|---|---|
| Death, % | 38.0 | 39.5 | 25.8 | <0.001 |
| Stroke, % | 3.9 | 3.9 | 3.4 | 0.018 |
| Need for hemodialysis, % | 3.2 | 2.8 | 3.6 | 0.009 |
| Mechanical ventilation, % | 48.9 | 20.0 | 39.5 | <0.001 |
| Length of stay, d | 15.3 (16.3) | 14.3 (15.1) | 22.7 (20.9) | <0.001 |
RHC indicates right heart catheterization.
Figure 2. Forest plot of the impact of right heart catheterization (RHC) on the cardiovascular outcomes for patients admitted with cardiogenic shock.
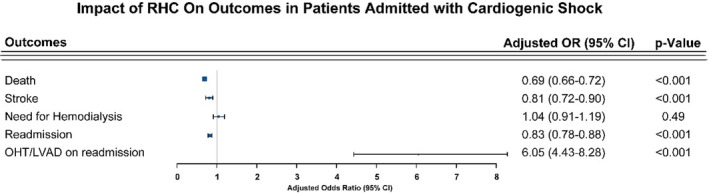
LVAD indicates left ventricular assist device; OHT, orthotropic heart transplant; and OR, odds ratio.
Figure 3. Forest plot of the impact of right heart catheterization (RHC) on the cardiovascular outcomes for patients admitted with cardiogenic shock, excluding patients with cardiac arrest.
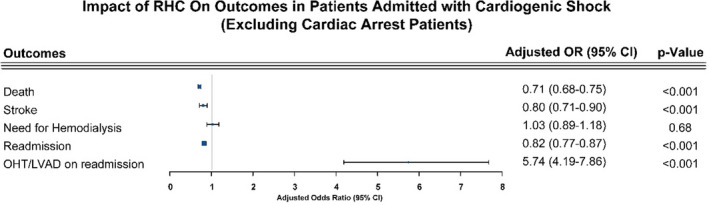
LVAD indicates left ventricular assist device; and OHT, orthotropic heart transplant; and OR, odds ratio.
Readmission Rates, Outcomes, and Causes
Patients with RHC on index admission had a lower rate of hospital readmission (18.7% versus 19.7%, P=0.04) and death rates during readmission (7.9% versus 9.3%, P=0.03). Table 3 shows additional outcomes during readmission. Except for mortality (P=0.06, not shown), on multivariate adjustment, RHC was found to be associated with 30‐day readmission (OR, 0.83; 95% CI, 0.78–0.88) and LVAD/OHT (OR, 6.05; 95% CI, 4.43–8.28) use (Figure 2). Results were not meaningfully affected after exclusion of patients with cardiac arrest as shown in Table S7 and Figure 3. Heart failure exacerbation was the most common cause of readmission (Table S8).
Table 3.
30‐Day Readmission Rate, Death, and Mechanical Circulatory Support Use
| End points | Total, N=146 279 | Non‐RHC, N=127 140 | RHC, N=19 139 | P value |
|---|---|---|---|---|
| 30‐day readmission rate, %* | 19.5 | 19.7 | 18.7 | 0.045 |
| Time to readmission, d, mean (SD)* | 11.6 (8.4) | 11.4 (8.4) | 12.5 (8.3) | <0.001 |
| Death on readmission hospitalization, % | 9.2 | 9.3 | 7.9 | 0.03 |
| Intra‐aortic balloon pump, %* | 1.32 | 1.0% | 2.9% | <0.001 |
| Percutaneous ventricular assist device, %* | 0.44 | 0.40 | 0.68 | 0.11 |
| Extracorporeal life support, %* | 0.38 | 0.27 | 1.0 | 0.004 |
| OHT, %* | 0.30 | 0.14 | 1.27 | <0.001 |
| LVAD, %* | 1.09 | 0.53 | 4.3 | <0.001 |
LVAD indicates left ventricular assist device; OHT, orthotropic heart transplant; and RHC, right heart catheterization.
Only unplanned readmissions included.
Discussion
Our study evaluated the association of RHC with mortality and in patients admitted with acute CS using data from a large nationwide registry. We found that an RHC strategy was associated with (1) a significant 31% reduction in mortality in index admission, (2) a 17% reduction in 30‐day readmission, and (3) a 6‐fold increase in use of LVAD/OHT during readmission. Importantly, the above findings did not significantly change after excluding patients with cardiac arrest and/or patients receiving advanced heart failure therapies. We hypothesize that the use of RHC may help to recognize and better characterize the CS patients, leading to increased use of temporary MCS support as well as advanced therapies during the initial encounter. Moreover, among survivors of index hospitalization, the RHC group had more utilization of advanced therapies during readmission.
We found that large academic centers in metropolitan locations more commonly use RHC. This finding has been previously replicated in a study from the National Inpatient Sample showing an increase in RHC use in large academic hospitals after 2005, despite the findings of the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) randomized trial. 13 , 14 These persistent differences in the invasive hemodynamic use among medical centers point to the lack of standardization of care in patients with CS. Whether this is because of differences in resources available among hospitals, variable degrees of familiarity in the use of invasive hemodynamics, or lack of appropriate use, guidelines need to be further elucidated. To that extent, there has lately been considerable effort in the standardization of CS definition and treatment approach by consensus documents endorsed by the American Heart Association and the Society for Cardiovascular Angiography and Intervention. 10 , 15
In addition, patients in the RHC group received more aggressive treatment with temporary MCS that resulted in lower mortality rates, even after adjusting for baseline differences. We believe that these findings should be interpreted in conjunction and are reflective of the higher level of care that patients receive in these centers. This higher use of RHC in large academic and advanced heart failure centers may have led to early, accurate, and appropriate interpretation of hemodynamic data from experienced heart failure specialists and multidisciplinary teams, resulting in a mortality benefit. 13 In addition, RHC likely provided an early objective assessment of the patients’ clinical state. As such, temporary MCS use may have been reflective of the early and objective recognition of severe CS, and our findings have been congruent with the Cardshock Study, an observational, prospective multicenter registry. 16 However, the association of RHC and mortality further underscores the benefits of RHC use in the identification and treatment of CS. Importantly, lower mortality rates were seen in the RHC group despite worse Elixhauser Comorbidity Index scores and baseline comorbidities, implying that RHC was not selectively used in patients who were deemed better candidates and would be expected to have favorable outcomes.
In addition, our findings show that the favorable outcomes of RHC use were extended beyond index admission statistics and led to an overall reduction in 30‐day readmission rate, longer time out of hospital for those who got readmitted, and eventually a 1.4% reduction in readmission mortality for those who received an RHC. Although the ESCAPE trial did not find that RHC‐directed volume optimization was superior with regard to days alive and out of hospital (primary outcome), it did not specifically evaluate patients with CS. 14 However, in a different prospective study by Rossello et al in patients with CS only, the use of RHC was independently associated with a 30‐day (hazard ratio, 0.55; 95% CI, 0.35–0.86; P=0.008) and long‐term mortality benefit (hazard ratio, 0.63; 95% CI, 0.41–0.97; P=0.035). 17 These latter findings support our conclusions, suggesting that RHC‐guided management in CS may result in favorable end points.
Furthermore, we demonstrated that the use of RHC is associated with a >6‐fold increase in LVAD and OHT use during rehospitalization. This finding further substantiates the implications of early and accurate characterization of shock, stratification of patient condition severity, and candidacy for these advanced therapies, all impossible without RHC. Another recent study using National Inpatient Sample data by Hernandez et al found similar associations of increased advanced therapies used in patients with heart failure with CS (LVAD: OR, 3.42; 95% CI, 3.11–3.78; P<0.001 or OHT: 2.0% versus 0.5%; OR, 1.12; 95% CI, 1.06–1.18; P<0.001). 18 The increased numbers of LVAD/OHT on readmission are expected given the prior completed profiling of patients.
The use of RHC in patients who are sicker and have significant baseline comorbidities comes as no surprise. Patients with preexisting conditions, such as heart failure, arrhythmias, valvular abnormalities, and renal failure, frequently represent a diagnostic challenge in the acute setting of undifferentiated shock. In these instances, and given the residual limitations of noninvasive hemodynamic methods, RHC provides clinicians with timely and accurate diagnostic results and reliable hemodynamics that affect a patient’s outcomes and length of stay.
Interestingly, patients who presented with acute coronary syndrome, including ST‐segment–elevation myocardial infarction, were less likely to receive RHC. A previous Medicare beneficiaries’ study found that RHC use in myocardial infarction has been steadily declining since 1999, 19 as prior studies suggested increased mortality in this population. 20 , 21 , 22 Nonetheless, even after adjustment for acute myocardial infarction, our study suggests RHC is associated with survival benefit, contrary to prior studies.
Based upon our results, patients in the RHC group appear to have higher rates of acute kidney injury and hemodialysis during index hospitalization. Although the nature of the data set precludes understanding the underlying mechanisms, a few plausible explanations exist. First, the higher rates of acute kidney injury could just represent the severity of CS and cardiorenal syndrome in this group. Second, acute kidney injury may have been the byproduct of increased use of temporary MCS or occur as a result of contrast‐induced nephrotoxicity. Thus, renal dysfunction may trigger further hemodynamic evaluation using RHC. Thus after multivariate adjustment, RHC does not change the need for hemodialysis in CS patients.
Limitations
The findings of our study must be interpreted within the context of their limitations. First, the use of an administrative data set for this study makes it prone to errors of coding of diagnosis and procedures. Second, the data set also lacks the severity of CS, hemodynamic data, echocardiographic variables, as well as the temporality of interventions that are important clinical parameters in these patients and could affect interpretation of our conclusions. Moreover, the non‐RHC group may include patients who could have expired before RHC was performed, which was partially addressed by excluding patients with cardiac arrest. Third, given the observational nature of the study, direct causality cannot be inferred toward the reported outcomes, although the findings of our analysis provide solid evidence for the strong association of the benefit of RHC in patients with CS. Fourth, the NRD database does not capture deaths or other end points that occurred out of the hospital and thus may underestimate overall risk during follow‐up. However, we believe that despite these limitations, the NRD captures relevant clinical data to provide scientific rigor and validity that make our findings relevant and meaningful.
Conclusions
Our findings provide insights into the impact of RHC in patients with CS. Our findings merit consideration of RHC in patients with CS to improve mortality, either through recovery or the use of advanced heart failure therapies. Further blinded randomized studies are required to confirm our findings.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S8
Figure S1
For Sources of Funding and Disclosures, see page 8.
References
- 1. Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States, 1993–2004. JAMA. 2007;298:423–429. DOI: 10.1001/jama.298.4.423. [DOI] [PubMed] [Google Scholar]
- 2. Pandey A, Khera R, Kumar N, Golwala H, Girotra S, Fonarow GC. Use of pulmonary artery catheterization in US patients with heart failure, 2001–2012. JAMA Intern Med. 2016;176:129. DOI: 10.1001/jamainternmed.2015.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swan HJ, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow‐directed balloon‐tipped catheter. N Engl J Med. 1970;283:447–451. DOI: 10.1056/NEJM197008272830902. [DOI] [PubMed] [Google Scholar]
- 4. Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, Laporta DP, Viner S, Passerini L, Devitt H, et al. A randomized, controlled trial of the use of pulmonary‐artery catheters in high‐risk surgical patients. N Engl J Med. 2003;348:5–14. DOI: 10.1056/NEJMoa021108. [DOI] [PubMed] [Google Scholar]
- 5. Teboul J‐L, Saugel B, Cecconi M, De Backer D, Hofer CK, Monnet X, Perel A, Pinsky MR, Reuter DA, Rhodes A, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016;42:1350–1359. DOI: 10.1007/s00134-016-4375-7. [DOI] [PubMed] [Google Scholar]
- 6. Richard C, Warszawski J, Anguel N, Deye N, Combes A, Barnoud D, Boulain T, Lefort Y, Fartoukh M, Baud F, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2003;290:2713–2720. [DOI] [PubMed] [Google Scholar]
- 7. Shah MR, Hasselblad V, Stevenson LW, Binanay C, O’Connor CM, Sopko G, Califf RM. Impact of the pulmonary artery catheter in critically Ill patients. JAMA. 2005;294:1664. DOI: 10.1001/jama.294.13.1664. [DOI] [PubMed] [Google Scholar]
- 8. Vallabhajosyula S, Shankar A, Patlolla SH, Prasad A, Bell MR, Jentzer JC, Arora S, Vallabhajosyula S, Gersh BJ, Jaffe AS, et al. Pulmonary artery catheter use in acute myocardial infarction‐cardiogenic shock. ESC Heart Fail. 2020;7:1234–1245. DOI: 10.1002/ehf2.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. ACCF/AHA guideline for the management of heart failure. Circulation. 2013;128:e240–e327. DOI: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 10. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. DOI: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 11. Zuckerman RB, Sheingold SH, Orav EJ, Ruhter J, Epstein AM. Readmissions, observation, and the hospital readmissions reduction program. N Engl J Med. 2016;374:1543–1551. DOI: 10.1056/NEJMsa1513024. [DOI] [PubMed] [Google Scholar]
- 12. Lauridsen MD, Gammelager H, Schmidt M, Nielsen H, Christiansen CF. Positive predictive value of International Classification of Diseases, 10th revision, diagnosis codes for cardiogenic, hypovolemic, and septic shock in the Danish National Patient Registry. BMC Med Res Methodol. 2015;15:23. DOI: 10.1186/s12874-015-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khera R, Pandey A, Kumar N, Singh R, Bano S, Golwala H, Kumbhani DJ, Girotra S, Fonarow GC. Variation in hospital use and outcomes associated with pulmonary artery catheterization in heart failure in the United States. Circulation. 2016;9:e003226. DOI: 10.1161/CIRCHEARTFAILURE.116.003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. [DOI] [PubMed] [Google Scholar]
- 15. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. DOI: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 16. Sionis A, Rivas‐Lasarte M, Mebazaa A, Tarvasmäki T, Sans‐Roselló J, Tolppanen H, Varpula M, Jurkko R, Banaszewski M, Silva‐Cardoso J, et al. Current use and impact on 30‐day mortality of pulmonary artery catheter in cardiogenic shock patients: results from the cardshock study. J Intensive Care Med. 2020;35:1426–1433. DOI: 10.1177/0885066619828959. [DOI] [PubMed] [Google Scholar]
- 17. Rossello X, Vila M, Rivas‐Lasarte M, Ferrero‐Gregori A, Sans‐Roselló J, Duran‐Cambra A, Sionis A. Impact of pulmonary artery catheter use on short‐ and long‐term mortality in patients with cardiogenic shock. Cardiology. 2017;136:61–69. DOI: 10.1159/000448110. [DOI] [PubMed] [Google Scholar]
- 18. Hernandez GA, Lemor A, Blumer V, Rueda CA, Zalawadiya S, Stevenson LW, Lindenfeld J. Trends in utilization and outcomes of pulmonary artery catheterization in heart failure with and without cardiogenic shock. J Card Fail. 2019;25:364–371. DOI: 10.1016/j.cardfail.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 19. Ikuta K, Wang Y, Robinson A, Ahmad T, Krumholz HM, Desai NR. National trends in use and outcomes of pulmonary artery catheters among medicare beneficiaries, 1999–2013. JAMA Cardiol. 2017;2:908. DOI: 10.1001/jamacardio.2017.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zion MM, Balkin J, Rosenmann D, Goldbourt U, Reicher‐Reiss H, Kaplinsky E, Behar S. Use of pulmonary artery catheters in patients with acute myocardial infarction. Chest. 1990;98:1331–1335. DOI: 10.1378/chest.98.6.1331. [DOI] [PubMed] [Google Scholar]
- 21. Cohen MG, Kelly RV, Kong DF, Menon V, Shah M, Ferreira J, Pieper KS, Criger D, Poggio R, Ohman EM, et al. Pulmonary artery catheterization in acute coronary syndromes: insights from the GUSTO IIb and GUSTO III trials. Am J Med. 2005;118:482–488. DOI: 10.1016/j.amjmed.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 22. Gore JM, Goldberg RJ, Spodick DH, Alpert JS, Dalen JE. A community‐wide assessment of the use of pulmonary artery catheters in patients with acute myocardial infarction. Chest. 1987;92:721–727. DOI: 10.1378/chest.92.4.721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8
Figure S1


