Abstract
Background
Anticoagulation is indicated for 4 weeks after cardioversion in patients with atrial fibrillation/flutter. We sought to examine whether there is evidence of sex or racial disparity in anticoagulant prescription following cardioversion, and whether postcardioversion anticoagulation affects outcomes.
Methods and Results
We identified a representative sample of Medicare patients who underwent elective electric cardioversion in an outpatient setting from 2015 to 2017. We identified patients who had an anticoagulant prescription for 3 months after the cardioversion date. Multivariable logistic regression was used to assess factors associated with a prescription of an anticoagulant after cardioversion. Cox regression analysis was used to test association of anticoagulation with a composite end point of 90‐day mortality, ischemic stroke, or arterial embolism. The final study cohort included 7860 patients. Overall, 5510 patients (70.1%) received any anticoagulation following cardioversion, while 2350 (29.9%) did not. Patients who did not receive anticoagulation were younger, with a lower burden of most comorbidities. Patients were less likely to receive anticoagulation if they had dementia or atrial flutter, while patients with valvular heart disease, obesity, heart failure, peripheral vascular or coronary disease, or hypertension were more likely to receive anticoagulation. Female sex (adjusted odds ratio, 0.84; 95% CI, 0.75–0.92; P<0.001), Black and Hispanic race (adjusted odds ratio, 0.50; 95% CI, 0.38–0.65; and odds ratio, 0.56; 95% CI, 0.41–0.75, respectively; P<0.001) were independently associated with lower probability of anticoagulant prescription. Postcardioversion anticoagulation was associated with lower risk of the composite end point (adjusted hazard ratio, 0.38; 95% CI, 0.27–0.52; P<0.001).
Conclusions
Racial and sex disparities exist in anticoagulant prescription after outpatient elective cardioversion for atrial fibrillation.
Keywords: anticoagulation, atrial fibrillation, cardioversion, disparities
Subject Categories: Atrial Fibrillation
Clinical Perspective
What Is New?
In patients who undergo elective outpatient direct‐current cardioversion for atrial fibrillation/flutter where postcardioversion anticoagulation is indicated for 4 weeks, Black, Hispanic, and female patients are less likely to receive anticoagulation.
Lack of an anticoagulant prescription following direct‐current cardioversion in patients with atrial fibrillation is associated with higher risk of mortality and ischemic stroke.
What Are the Clinical Implications?
More efforts are needed to eliminate racial and sex disparity in receiving indicated anticoagulation for 4 weeks after elective direct‐current cardioversion in patients with atrial fibrillation.
Atrial fibrillation (AF) is the most prevalent arrhythmia in the United States. 1 Electrical cardioversion is a procedure that is performed with the aim of restoring sinus rhythm. 2 The risk of ischemic stroke, thromboembolism, and mortality is heightened for the first few weeks following cardioversion. Thus, anticoagulation for at least 4 weeks after cardioversion, especially in individuals with CHA2DS2‐VASc score ≥1 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, previous stroke/transient ischemic attack, vascular disease, age 65–74 years, sex category), carries a class I in the current American Heart Association/American College of Cardiology and the European Society of Cardiology guidelines on management of AF. 3 , 4
Sex and racial disparities in the management of AF have been previously demonstrated. Women are less likely to undergo catheter ablation or cardioversion or receive anticoagulation for stroke prophylaxis when indicated compared with men. 5 , 6 , 7 Black patients are less likely to receive appropriate treatment compared with White patients. 8 However, it is unclear if sex or racial disparities in the prescription of oral anticoagulation after cardioversion for AF exist across practices in the United States.
The aims of this study were (1) to examine whether there is evidence of sex or racial disparity in oral anticoagulant prescription after cardioversion and (2) to examine whether anticoagulation following cardioversion is associated with a protective effect against mortality and thromboembolism in a contemporary national cohort from the United States.
Methods
Data used for the study are covered under a data use agreement with the Centers for Medicare and Medicaid Services and are not available for distribution by the authors but may be obtained from the Centers for Medicare and Medicaid Services with an approved data use agreement.
Ethics Approval
The Institutional Review Board at the University of Iowa approved the study with waiver of informed consent.
Study Cohort and Data Source
Medicare patients from the 5% enhanced sample, who were enrolled in Fee‐for‐Service and Medicare Part D, and underwent elective electric cardioversion in an outpatient setting, for AF or atrial flutter were identified from March 2015 through October 2017, using Current Procedural Terminology codes 92960 and 92961. The Medicare Carrier (Parts B and C) standard analytic files, which includes outpatient encounters, physician billed services, including institutional and outpatient procedures, for a 5% representative rolling cohort of Medicare beneficiaries, was used to extract patients who underwent cardioversion using the above Current Procedural Terminology codes. The Inpatient Medicare Provider Analysis and Review (MedPar) file, which includes all inpatient admissions for Medicare beneficiaries, was used to extract all inpatient admissions. The inpatient admissions and outpatient encounters for 1 year before the cardioversion date were used as a look‐back period to assess patients’ comorbidities burden using billed International Classification of Diseases, Ninth Revision and Tenth Revision (ICD‐9 and ICD‐10) codes. We also calculated a frailty score that was validated in patients with heart failure, pneumonia, myocardial infarction and AF in prior studies. 9 , 10 , 11 The Medicare Beneficiary Summary file was used to extract patients’ demographics, including age, sex, and race; enrollment dates; death dates; residence zip codes; and types of insurance plans for each patient. The race/ethnicity variable “Bene_Race_CD” is reported in inpatient and outpatient encounter claims by the hospital/provider. Finally, prescription medications were extracted from the Pharmacy Drug Event (Part D) file 5% sample, which includes pharmacy claims for Medicare beneficiaries who are enrolled in Part D. Variables included in the Part D file include prescribing physician, filling pharmacy, number of pills and number of days prescribed, and amount paid by plan and patient. We identified patients who filed a claim for an anticoagulant (warfarin, apixaban, rivaroxaban, or dabigatran) 3 months before and for 3 months after the cardioversion date. We also extracted procedures done up to 1 month before the cardioversion and determined patients who underwent transesophageal echocardiogram using Current Procedural Terminology codes (93312, 93314). We excluded patients who were enrolled in Medicare Advantage and patients who were not enrolled in Medicare Part D for at least 3 months before and 3 months after the date of the cardioversion. We also excluded patients who underwent cardioversion in the setting of an inpatient admission, emergency department visit, for ventricular arrhythmias or paroxysmal supraventricular tachycardia. We also extracted the physician specialty, and practice zip code from the cardioversion claim line.
Study Outcomes
Our primary interest was in factors associated with receipt of anticoagulation after cardioversion per guideline recommendations, specifically race and sex disparities. We were also interested in the disparities in patient outcomes and the association of postcardioversion anticoagulation with patient outcomes. We defined a composite outcome representing readmissions with a primary diagnosis of ischemic stroke, transient ischemic attack, or systemic embolism (including acute lower extremity embolic ischemia and splenic and renal infarction), and death within 90 days from the cardioversion date. Study follow‐up was 90 days for the entire cohort, and end of follow‐up was December 2017.
Statistical Analysis
Continuous variables are presented as mean and SD and compared using Student’s t test if normally distributed, or presented as median and interquartile range and compared using the Mann‐Whitney test if nonnormally distributed. Categorical variables are presented as counts and percentages and were compared using the chi‐square test. Multivariable logistic regression was used to assess factors associated with prescription of a postcardioversion anticoagulant. Variables considered for this model included age, sex, race, comorbidities including frailty score, cardioversion indication (AF versus atrial flutter), provider specialty, and practice zip code. A practice was considered to be located in a rural area if it was located in a zip code designated rural by the Federal Office of Rural Health Policy. Variable selection was done in a stepwise approach, with a cutoff P value of 0.2 to enter the model and 0.1 to remain in the model. A second multivariable logistic regression model was constructed to assess factors associated with the composite end point. The exposure of interest in that model was receipt of postcardioversion anticoagulation after adjusting for measured confounders. To assess possible collinearity between different variables, variance inflation factor was calculated for each variable in the model. Both models with candidate variables included were planned a priori. Cox regression model was performed to examine the association of postcardioversion anticoagulation with the composite end point, and Kaplan‐Meier curves for events were generated and compared with log‐rank. In a second model, we analyzed the average treatment effect of postcardioversion anticoagulation on the composite outcome using an augmented inverse probability weighting model with a doubly robust estimator and bootstrapped corrected confidence intervals. 12 In a sensitivity analysis, we assessed the extent to which unmeasured confounders could explain an observed association between postcardioversion anticoagulation and the study primary outcome by calculating the E value for our results. 13 The E value indicates the minimum strength of association that an unmeasured confounder would need to have with both the exposure and outcome to fully explain away a specific treatment‐outcome association. 13 All analysis was performed with SAS version 9.4 (SAS Institute, Cary, NC) and R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population
A total of 19 108 patients underwent a cardioversion for AF or atrial flutter during the study period. We excluded 5928 patients because they were not enrolled in Part D for at least 3 months before and after the cardioversion date, 219 patients because they were enrolled in Medicare Advantage, and 5101 patients who underwent a cardioversion in the setting of an inpatient admission or emergency department visit. The final study cohort included 7860 patients. Table 1 shows baseline characteristics for the study population. Overall, 5510 patients (70.1%) received anticoagulation following cardioversion, while 2350 (29.9%) did not. Patients who did not receive anticoagulation were younger (mean age, 72.9±10 versus 74.8±7.9 years; P<0.001), more likely to be women, of minority race, and with a lower burden of most comorbidities including heart failure, coronary artery disease, lung and kidney disease, anemia, prior bleeding, and prior ischemic stroke. Patients who did not receive anticoagulation had lower frailty scores (median, 2.4 [interquartile range, 0–7.4] versus 3.0 [interquartile range, 0–8.5]; P<0.01) and lower CHA2DS2‐VASc scores (mean, 3.7±1.7 versus 4.2±1.7; P<0.01). Overall, AF was the indication for cardioversion in the majority of patients (89.2%), and 39.0% had a precardioversion transesophageal echocardiogram.
Table 1.
Baseline Demographics and Characteristics of the 2 Study Groups
| Variable |
Overall (N=7860) |
Post‐DCCV anticoagulation (N=5510) |
No Post‐DCCV anticoagulation (N=2350) |
P Value |
|---|---|---|---|---|
| Age, y | 74.2±8.6 | 74.8±7.9 | 72.9±10 | <0.001 |
| Male sex | 4488 (57.1) | 3242 (58.8) | 1246 (53.0) | <0.001 |
| White race | 7123 (90.6) | 5077 (92.1) | 2046 (87.1) | <0.001 |
| Black race | 275 (3.5) | 140 (2.5) | 135 (5.7) | |
| Asian race | 94 (1.2) | 58 (1.1) | 36 (1.5) | |
| Hispanic ethnicity | 115 (2.6) | 115 (2.1) | 87 (3.7) | |
| Diabetes mellitus | 2639 (33.6) | 1859 (33.7) | 780 (33.2) | 0.6 |
| Hypertension | 6702 (85.3) | 4869 (88.4) | 1833 (78.0) | <0.001 |
| History of heart failure | 2593 (33.0) | 2057 (37.2) | 536 (22.8) | <0.001 |
| Prior coronary artery disease | 3497 (44.5) | 2647 (48.0) | 850 (36.2) | <0.001 |
| Prior bleeding | 1384 (17.6) | 1013 (18.4) | 371 (15.8) | 0.006 |
| Prior gastrointestinal bleed | 602 (7.7) | 431 (7.8) | 171 (7.3) | 0.4 |
| Prior cerebral bleed | 50 (0.6) | 32 (0.6) | 18 (0.8) | 0.3 |
| Prior ischemic stroke | 1125 (14.3) | 833 (15.1) | 292 (12.4) | 0.002 |
| Prior smoking | 1049 (13.4) | 753 (13.7) | 296 (12.6) | 0.2 |
| Peripheral artery disease | 1832 (23.3) | 1335 (24.2) | 497 (21.2) | 0.003 |
| Liver disease | 403 (5.1) | 287 (5.2) | 116 (4.9) | 0.6 |
| Chronic kidney disease | 1078 (13.7) | 815 (14.8) | 263 (11.2) | <0.001 |
| End‐stage renal disease | 108 (1.4) | 71 (1.3) | 37 (1.6) | 0.3 |
| Prior permanent pacemaker | 826 (10.5) | 647 (11.7) | 179 (7.6) | <0.001 |
| Prior intracardiac defibrillator | 425 (5.4) | 341 (6.2) | 84 (3.6) | <0.001 |
| Valvular heart disease | 3300 (42.0) | 2569 (46.6) | 731 (31.1) | <0.001 |
| Sleep apnea | 1681 (21.4) | 1295 (23.5) | 386 (16.4) | <0.001 |
| Dementia | 319 (4.1) | 196 (3.6) | 123 (5.2) | 0.001 |
| Obesity | 2046 (26.0) | 1529 (27.8) | 517 (22.0) | <0.001 |
| Hypothyroid | 1982 (25.2) | 1417 (25.7) | 565 (24.0) | 0.1 |
| Anemia | 1981 (25.2) | 1442 (26.2) | 539 (22.9) | 0.003 |
| Metastatic tumor | 154 (2.0) | 104 (1.9) | 50 (2.1) | 0.5 |
| Rheumatoid arthritis/connective tissue disease | 603 (7.7) | 419 (7.6) | 184 (7.8) | 0.7 |
| Lung disease | 2260 (28.8) | 1633 (29.6) | 627 (26.7) | 0.008 |
| Depression | 1027 (13.1) | 704 (12.8) | 323 (13.7) | 0.2 |
| Alcohol abuse | 181 (2.4) | 134 (2.4) | 47 (2.0) | 0.2 |
| Frailty score | 2.9 (0–8.2) | 3.0 (0–8.5) | 2.4 (0–7.4) | <0.001 |
| Atrial flutter | 849 (10.8) | 563 (10.2) | 286 (12.2) | 0.01 |
| Precardioversion TEE | 3066 (39.0) | 2458 (44.6) | 608 (25.9) | <0.001 |
| Precardioversion anticoagulation for at least 3‐4 weeks | 5299 (67.4) | 4876 (88.5) | 423 (18.0) | <0.001 |
| Warfarin | 1647 (21.0) | 1484 (26.9) | 163 (6.9) | |
| Apixaban | 2005 (25.5) | 1867 (33.9) | 138 (5.9) | |
| Rivaroxaban | 1454 (18.5) | 1344 (24.4) | 110 (4.7) | |
| Dabigatran | 410 (5.2) | 387 (7.0) | 23 (1.0) | |
| CHA2DS2‐VASc score | 4.1±1.7 | 4.2±1.7 | 3.7±1.7 | |
| 1 | 403 (5.2) | 200 (3.6) | 203 (8.7) | <0.001 |
| 2 | 1046 (13.3) | 631 (11.5) | 415 (17.7) | |
| 3 | 1599 (20.3) | 1112 (20.2) | 487 (20.7) | |
| 4 | 1881 (23.9) | 1349 (24.5) | 532 (22.6) | |
| 5 | 1438 (18.3) | 1072 (19.5) | 366 (15.6) | |
| 6 | 836 (10.6) | 634 (11.5) | 202 (8.6) | |
| ≥7 | 657 (8.4) | 512 (9.3) | 145 (6.2) | |
| Rural practice zip code | 802 (10.4) | 508 (9.2) | 294 (12.5) | <0.001 |
| Post‐DCCV anticoagulation | ||||
| Warfarin | 1470 (26.7) | NA | NA | |
| Apixaban | 2165 (39.3) | NA | ||
| Rivaroxaban | 1472 (26.7) | NA | ||
| Dabigatran | 403 (7.3) | NA | ||
Results are presented as n (%) and mean±standard deviation.
DCCV indicates direct‐current cardioversion; NA, not applicable; and TEE, transesophageal echocardiography.
Postcardioversion Anticoagulation
In patients who received postcardioversion anticoagulation, apixaban was used in 39.3%, followed by warfarin (26.7%), rivaroxaban (26.7%), and dabigatran (7.3%). Figure 1 shows the logistic regression model for independent factors associated with postcardioversion anticoagulation. Patients were less likely to receive anticoagulation if they were women, Black, or Hispanic or had dementia or atrial flutter, while patients with valvular heart disease, obesity, hypertension, peripheral vascular or coronary artery disease, or systolic or diastolic heart failure, or who underwent precardioversion transesophageal echocardiogram were more likely to receive postcardioversion anticoagulation. Cardioversions performed in rural practices were associated with a lower probability of postcardioversion anticoagulation. Female sex (adjusted odds ratio [OR], 0.84; 95% CI, 0.75–0.92; P<0.001), Black race and Hispanic ethnicity (adjusted OR, 0.50; 95% CI, 0.38–0.65; and OR, 0.56; 95% CI, 0.41–0.75, respectively; P<0.001 for both) were independently associated with lower probability of anticoagulation prescription.
Figure 1. Logistic regression model for factors associated with receipt of anticoagulation following elective electrical cardioversion.
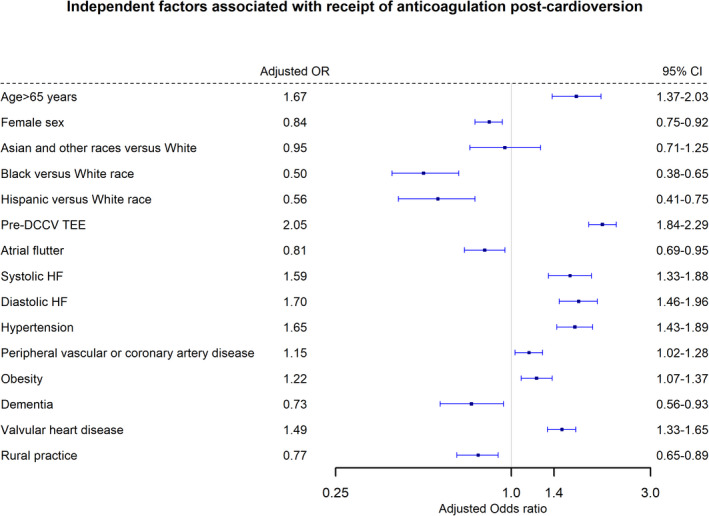
DCCV indicates direct‐current cardioversion; OR, odds ratio; and TEE, transesophageal echocardiography.
Ninety‐Day Outcomes
The composite outcome was higher in patients who did not receive postcardioversion anticoagulation (3.2% versus 1.2%; P<0.001). This was driven mainly by a higher incidence of 90‐day mortality (2.9% versus 0.8%; P<0.001; Table 2). Figure 2 shows the full logistic regression model for the study primary outcome. Anticoagulation following cardioversion was independently associated with a lower risk of the primary outcome (adjusted OR, 0.28; 95% CI, 0.20–0.40; P<0.001). There was no evidence of collinearity in the model (Table S1). On augmented inverse probability weighting, average treatment effect of postcardioversion anticoagulation was −2.7% (95% CI, −1.7% to −3.6%; P<0.01). On Cox regression analysis, postcardioversion anticoagulation was associated with lower risk of the composite end point compared with no anticoagulation (adjusted hazard ratio, 0.38; 95% CI, 0.27–0.52; P<0.001) (Figure 3). On sensitivity analysis, The E value for the adjusted OR of postcardioversion in the full model was 6.6, suggesting that an unobserved confounder associated with the study outcome and with the exposure (postcardioversion anticoagulation) with OR 6.6 and 6.6, respectively, after all covariate adjustment, can explain the observed relationship reported in our study and bring the risk estimate to the null.
Table 2.
Study End Points
|
Post‐DCCV anticoagulation (N=5510) |
No Post‐DCCV anticoagulation (N=2350) |
P Value | |
|---|---|---|---|
| 30‐day mortality | 11 (0.2) | 34 (1.5) | <0.001 |
| 90‐day mortality | 45 (0.8) | 69 (2.9) | <0.001 |
| 90‐day ischemic stroke or TIA | 24 (0.4) | 11 (0.4) | 0.9 |
| Composite end point | 68 (1.2) | 76 (3.2) | <0.001 |
Results are presented as n (%). DCCV, indicates direct current cardioversion; and TIA, transient ischemic attack.
Figure 2. Logistic regression model for factors associated with the composite study end point.
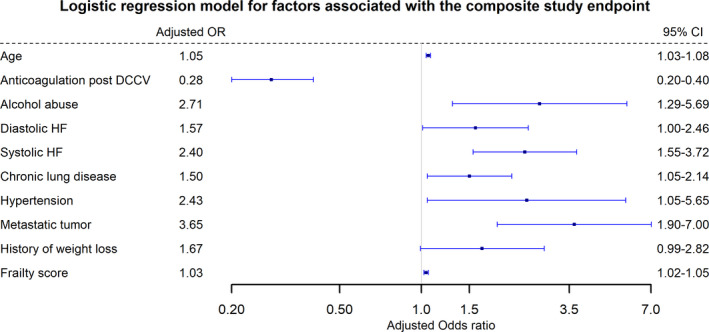
DCCV indicates direct current cardioversion; HF, heart failure; and OR, odds ratio.
Figure 3. Kaplan‐Meier curves for time to composite end point in the 2 study groups, compared by log‐rank test.
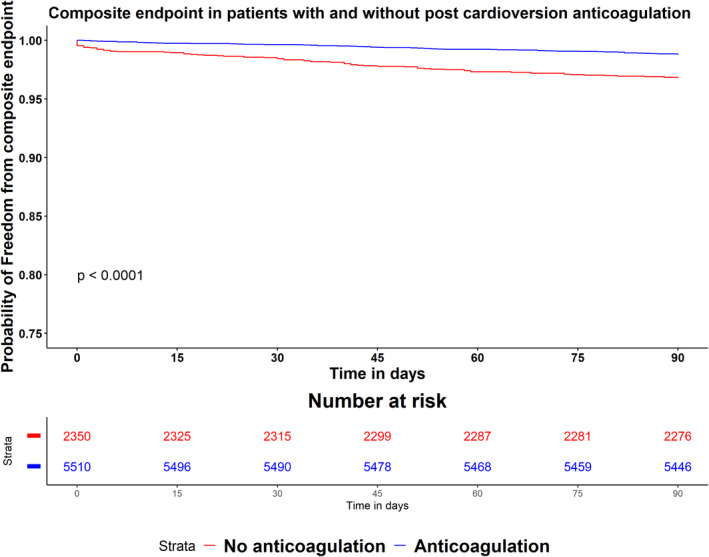
Discussion
In this study, we demonstrate several important findings. First, almost 30% of patients who underwent elective cardioversion in an outpatient setting for AF or atrial flutter did not receive any anticoagulation after cardioversion. Second, patients who identified as women, Black, or Hispanic or those who underwent cardioversion in a rural practice were less likely to receive appropriate anticoagulation after cardioversion. Third, postcardioversion anticoagulation was independently associated with a lower risk of 90‐day mortality and ischemic stroke, with an average treatment effect of −2.7% (95% CI, −1.7% to −3.6%).
Anticoagulation for at least 4 weeks after cardioversion, regardless of thromboembolic risk, carries a class I recommendation in both the American Heart Association/American College of Cardiology and the European Society of Cardiology guidelines for management of AF. 3 , 4 Earlier studies have shown that the risk of ischemic stroke and fatal embolism after cardioversion without anticoagulation can be as high as 5%. 14 , 15 Contemporary studies reported a 2% rate of thromboembolic complications without anticoagulation compared with 0.3% with anticoagulation. 2 Thromboembolic events after cardioversion can happen because of embolization of a preformed atrial thrombus upon restoration of sinus rhythm, or because of de novo thrombus formation after cardioversion from depressed atrial mechanical function, a phenomenon known as atrial stunning. 16 In atrial stunning, recovery of mechanical function of the atrium is delayed compared with the electrical recovery of “P” waves on surface ECG. 17 In our study, the rate of the primary study end point, a composite of ischemic stroke, systemic embolism, and mortality on and off anticoagulation was 1.2% and 3.2%, respectively, comparable with previously reported rates. 2 , 18 , 19 It is unclear why in our study the difference in the composite end point was driven mainly by difference in mortality and not in ischemic stroke. However, it is important to note that in our study, patients who did not receive anticoagulation after cardioversion were younger, had a lower burden of most comorbidities, had lower CHA2DS2‐VASc score, and had lower frailty scores compared with patients who received anticoagulation.
In our study, a history of dementia was significantly associated with a lower likelihood of anticoagulation after cardioversion. However, after adjusting for patient characteristics including frailty, patients who were women, Black, or Hispanic remained less likely to receive anticoagulation. Prior studies have demonstrated sex disparities in AF management. Women were less likely to be prescribed an antiarrhythmic medication or to undergo cardioversion or catheter ablation, compared with men. 5 , 6 , 7 Women also were also less likely to receive chronic anticoagulation for permanent AF when indicated. 20 This is despite our recognition that women have a higher AF‐related risk of fatal or disabling stroke than men. 21 , 22 Several proposed mechanisms to explain the higher risk in women include hormonal factors, menopause, higher platelet activity, and higher thrombotic biomarkers. 23 Similarly, racial disparities in AF management have been shown in the past. Black patients are less likely to receive anticoagulation for AF when indicated compared with White patients after adjusting for clinical differences. 8 Our study extends these findings to show that racial disparities also exist in postcardioversion anticoagulation. It is important to note that Black race was shown to be a risk factor for stroke in AF, and a prior study demonstrated that a modified CHA2DS2‐VASc‐R score, after adding Black race, improved model fit to predict stroke risk. 24 Another important finding in our study is the disparities faced by Hispanic patients in postcardioversion anticoagulation compared with White patients. Hispanic people are one of the largest and fastest‐growing minority populations in the United States. Prior studies have demonstrated that Hispanic patients are less likely to receive left atrial appendage occlusion or catheter ablation for AF. 25 , 26 Hispanic patients are also less likely to receive direct oral anticoagulants and when prescribed warfarin have significantly lower time in therapeutic range. 27 , 28 We expand on these findings by showing that Hispanic patients are less likely to receive indicated anticoagulation after undergoing elective electrical cardioversion for AF.
Another important finding in our study was that patients who underwent cardioversion in rural hospitals were less likely to receive postcardioversion anticoagulation. Almost 25% of the US general population reside in rural areas. Lower quality of healthcare outcomes in rural hospitals has been demonstrated in the past. In one study, adjusted mortality attributable to cardiovascular diseases was significantly higher in rural counties compared with urban counties, and the difference nearly doubled from 1999 to 2017. 29 Another study demonstrated higher in‐hospital mortality in patients admitted with AF in rural hospitals compared with urban settings. 30 Finally, in our study, atrial flutter was independently associated with lower probability of anticoagulation following cardioversion. Risk of embolism after cardioversion for atrial flutter is similar to AF, 31 and the American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend anticoagulation for at least 4 weeks after cardioversion for atrial flutter, in a fashion similar to AF. 3 , 4
Our study has several limitations. First, it included patients from the Medicare population, and thus results may not be generalized to the general population. Second, the risk of residual confounding in an observational study cannot be completely ruled out, although the calculated E value for our risk estimate is significantly high (6.6), which means that any unmeasured confounder should have a significantly high association (6.6 on risk ratio scale) with both the exposure and the outcome of the study after adjustment for measured confounders to bring our results to the null, which is unlikely. Third, we lacked information on echocardiogram and important variables such as ejection fraction, left atrial appendage ligation or occlusion, or concomitant antiplatelet therapy. Fourth, some patients might have been prescribed anticoagulation but did not fill the prescription, so part of the disparity observed could be attributable to these groups (women, Black or Hispanic patients) were less likely to fill their prescription for unclear reasons.
In conclusion, oral anticoagulation is underused after elective electric cardioversion for AF/atrial flutter, with women and Black and Hispanic patients less likely to receive anticoagulation. Lack of anticoagulation after cardioversion is associated with higher mortality and ischemic stroke within 90 days.
Sources of Funding
Dr Sarrazin is supported by funding from the National Institute on Aging (R01AG055663‐01), and by the Health Services Research and Development Service of the Department of Veterans Affairs.
Disclosures
None.
Supporting information
Table S1
This manuscript w as sent to Carol Ann Remme, MD, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14:195–203. doi: 10.11909/j.issn.1671-5411.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brandes A, Crijns H, Rienstra M, Kirchhof P, Grove EL, Pedersen KB, Van Gelder IC. Cardioversion of atrial fibrillation and atrial flutter revisited: current evidence and practical guidance for a common procedure. Europace. 2020;22:1149–1161. doi: 10.1093/europace/euaa057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 4. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 5. Gleason KT, Dennison Himmelfarb CR, Ford DE, Lehmann H, Samuel L, Jain S, Naccarelli G, Aggarwal V, Nazarian S. Association of sex and atrial fibrillation therapies with patient‐reported outcomes. Heart. 2019;105:1642–1648. doi: 10.1136/heartjnl-2019-314881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kummer BR, Bhave PD, Merkler AE, Gialdini G, Okin PM, Kamel H. Demographic differences in catheter ablation after hospital presentation with symptomatic atrial fibrillation. J Am Heart Assoc. 2015;4:e002097. doi: 10.1161/JAHA.115.002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friberg L, Benson L, Rosenqvist M, Lip GY. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522. doi: 10.1136/bmj.e3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Essien UR, Holmes DN, Jackson LR, Fonarow GC, Mahaffey KW, Reiffel JA, Steinberg BA, Allen LA, Chan PS, Freeman JV, et al. Association of Race/Ethnicity with oral anticoagulant use in patients with atrial fibrillation: findings from the outcomes registry for better informed treatment of atrial fibrillation II. JAMA Cardiol. 2018;3:1174–1182. doi: 10.1001/jamacardio.2018.3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kundi H, Wadhera RK, Strom JB, Valsdottir LR, Shen C, Kazi DS, Yeh RW. Association of frailty with 30‐day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol. 2019;4:1084–1091. doi: 10.1001/jamacardio.2019.3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kundi H, Popma JJ, Reynolds MR, Strom JB, Pinto DS, Valsdottir LR, Shen C, Choi E, Yeh RW. Frailty and related outcomes in patients undergoing transcatheter valve therapies in a nationwide cohort. Eur Heart J. 2019;40:2231–2239. doi: 10.1093/eurheartj/ehz187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, Arora S, Street A, Parker S, Roberts HC, et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775–1782. doi: 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Funk MJ, Westreich D, Wiesen C, Sturmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173:761–767. doi: 10.1093/aje/kwq439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e‐value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 14. Bjerkelund CJ, Orning OM. The efficacy of anticoagulant therapy in preventing embolism related to D.C. electrical conversion of atrial fibrillation. Am J Cardiol. 1969;23:208–216. doi: 10.1016/0002-9149(69)90068-X [DOI] [PubMed] [Google Scholar]
- 15. Arnold AZ, Mick MJ, Mazurek RP, Loop FD, Trohman RG. Role of prophylactic anticoagulation for direct current cardioversion in patients with atrial fibrillation or atrial flutter. J Am Coll Cardiol. 1992;19:851–855. doi: 10.1016/0735-1097(92)90530-Z [DOI] [PubMed] [Google Scholar]
- 16. Fatkin D, Kuchar DL, Thorburn CW, Feneley MP. Transesophageal echocardiography before and during direct current cardioversion of atrial fibrillation: evidence for “atrial stunning” as a mechanism of thromboembolic complications. J Am Coll Cardiol. 1994;23:307–316. doi: 10.1016/0735-1097(94)90412-X [DOI] [PubMed] [Google Scholar]
- 17. Khan IA. Atrial stunning: basics and clinical considerations. Int J Cardiol. 2003;92:113–128. doi: 10.1016/S0167-5273(03)00107-4 [DOI] [PubMed] [Google Scholar]
- 18. Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma C‐S, Le Heuzey J‐Y, Talajic M, Scanavacca M, Vardas PE, Kirchhof P, et al. Rivaroxaban vs. vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J. 2014;35:3346–3355. doi: 10.1093/eurheartj/ehu367 [DOI] [PubMed] [Google Scholar]
- 19. Dentali F, Botto GL, Gianni M, Ambrosino P, Di Minno MN. Efficacy and safety of direct oral anticoagulants in patients undergoing cardioversion for atrial fibrillation: a systematic review and meta‐analysis of the literature. Int J Cardiol. 2015;185:72–77. doi: 10.1016/j.ijcard.2015.03.096 [DOI] [PubMed] [Google Scholar]
- 20. Thompson LE, Maddox TM, Lei L, Grunwald GK, Bradley SM, Peterson PN, Masoudi FA, Turchin A, Song Y, Doros G, et al. Sex differences in the use of oral anticoagulants for atrial fibrillation: a report from the national Cardiovascular Data Registry (NCDR((R))) PINNACLE registry. J Am Heart Assoc. 2017;6:e005801. doi: 10.1161/JAHA.117.005801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 22. Volgman AS, Manankil MF, Mookherjee D, Trohman RG. Women with atrial fibrillation: greater risk, less attention. Gend Med. 2009;6:419–432. doi: 10.1016/j.genm.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 23. Cove CL, Albert CM, Andreotti F, Badimon L, Van Gelder IC, Hylek EM. Female sex as an independent risk factor for stroke in atrial fibrillation: possible mechanisms. Thromb Haemost. 2014;111:385–391. doi: 10.1160/TH13-04-0347 [DOI] [PubMed] [Google Scholar]
- 24. Kabra R, Girotra S, Vaughan SM. Refining stroke prediction in atrial fibrillation patients by addition of African‐American ethnicity to CHA2DS2‐VASc score. J Am Coll Cardiol. 2016;68:461–470. doi: 10.1016/j.jacc.2016.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sparrow R, Sanjoy S, Choi Y‐H, Elgendy IY, Jneid H, Villablanca PA, Holmes DR, Pershad A, Alraies C, Sposato LA, et al. Racial, ethnic and socioeconomic disparities in patients undergoing left atrial appendage closure. Heart. 2021. [epub ahead of print]. doi: 10.1136/heartjnl-2020-318650 [DOI] [PubMed] [Google Scholar]
- 26. Eberly LA, Garg L, Yang L, Markman TM, Nathan AS, Eneanya ND, Dixit S, Marchlinski FE, Groeneveld PW, Frankel DS. Racial/ethnic and socioeconomic disparities in management of incident paroxysmal atrial fibrillation. JAMA Netw Open. 2021;4:e210247. doi: 10.1001/jamanetworkopen.2021.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tedla YG, Schwartz SM, Silberman P, Greenland P, Passman RS. Racial disparity in the prescription of anticoagulants and risk of stroke and bleeding in atrial fibrillation patients. J Stroke Cerebrovasc Dis. 2020;29:104718. doi: 10.1016/j.jstrokecerebrovasdis.2020.104718 [DOI] [PubMed] [Google Scholar]
- 28. Golwala H, Jackson LR, Simon DN, Piccini JP, Gersh B, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas L, et al. Racial/ethnic differences in atrial fibrillation symptoms, treatment patterns, and outcomes: insights from outcomes registry for better informed treatment for atrial fibrillation registry. Am Heart J. 2016;174:29–36. doi: 10.1016/j.ahj.2015.10.028 [DOI] [PubMed] [Google Scholar]
- 29. Cross SH, Mehra MR, Bhatt DL, Nasir K, O'Donnell CJ, Califf RM, Warraich HJ. Rural‐urban differences in cardiovascular mortality in the US, 1999–2017. JAMA. 2020;323:1852–1854. doi: 10.1001/jama.2020.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Neal WT, Sandesara PB, Kelli HM, Venkatesh S, Soliman EZ. Urban‐rural differences in mortality for atrial fibrillation hospitalizations in the United States. Heart Rhythm. 2018;15:175–179. doi: 10.1016/j.hrthm.2017.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gallagher MM, Hennessy BJ, Edvardsson N, Hart CM, Shannon MS, Obel OA, Al‐Saady NM, Camm AJ. Embolic complications of direct current cardioversion of atrial arrhythmias: association with low intensity of anticoagulation at the time of cardioversion. J Am Coll Cardiol. 2002;40:926–933. doi: 10.1016/S0735-1097(02)02052-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


