Abstract
Background
Sodium‐glucose co‐transporter (SGLT) inhibitors reduce cardiovascular outcomes including mortality in several populations; however, their effect on atrial fibrillation/flutter (AF) remains unclear. Our objective was to determine whether SGLT inhibitors reduce AF and whether a history of AF modifies the effect of SGLT inhibitors on the composite of heart failure hospitalization or cardiovascular death.
Methods and Results
We searched MEDLINE, Embase, and CENTRAL to March 2021. Pairs of reviewers identified randomized controlled trials that compared an SGLT inhibitor with placebo or no therapy. We pooled data using RevMan 5.4.1, assessed risk of bias using the Cochrane tool, and determined the overall quality of evidence using Grades of Recommendation, Assessment, Development and Evaluation. Thirty‐one eligible trials reported on AF events (75 279 participants, mean age 62 years, 35.0% women). Moderate quality evidence supported a lower risk of serious AF events with SGLT inhibitors (1.1% versus 1.5%; risk ratio 0.75 [95% CI, 0.66–0.86]; I2=0%). A similar reduction in total AF events was also noted with SGLT inhibitors. Three trials reported on heart failure hospitalization/cardiovascular death stratified by a baseline history of AF (18 832 participants, mean age 66 years, 38.1% women); in patients with a history of AF, SGLT inhibitors resulted in a lower risk in the composite of heart failure hospitalization or cardiovascular death (hazard ratio, 0.70 [95% CI, 0.57–0.85]; I2=0%)—similar to the effect estimate for patients without AF, P value for interaction: 1.00.
Conclusions
SGLT inhibitors may reduce AF events and likely reduce heart failure hospitalization/cardiovascular death to a similar extent in patients with and without AF.
Keywords: atrial fibrillation, atrial flutter, gliflozins, SGLT inhibitors
Subject Categories: Atrial Fibrillation, Meta Analysis
Nonstandard Abbreviation and Acronym
- SGLT
sodium‐glucose co‐transporter
Clinical Perspective
What Is New?
In a pooled analysis of 31 randomized controlled trials (75 279 patients), sodium‐glucose co‐transporter (SGLT) inhibitors were associated with a lower risk of serious and total atrial fibrillation (AF)/flutter events compared with placebo/control.
In a pooled analysis of 3 randomized controlled trials (18 832 patients), SGLT inhibitors reduced the risk of heart failure hospitalization or cardiovascular death to a similar extent in patients with and without AF/flutter at baseline.
What Are the Clinical Implications?
Treatment with SGLT inhibitors may be associated with a lower incidence/recurrence of AF/flutter.
SGLT inhibitors appear to reduce cardiovascular outcomes in patients with and without AF/flutter.
More research is needed to characterize the effect of SGLT inhibitors on AF burden and symptoms.
Atrial fibrillation (AF) affects an estimated 33.5 million adults worldwide and is associated with a significantly increased risk of stroke, heart failure (HF), and overall mortality. 1 Currently, there are limited therapies for primary prevention of AF in at‐risk patients. 2 , 3 , 4 , 5 HF is the most common cause of death in patients with AF. 6 , 7 , 8 AF and HF share many risk factors and have a complex and interdependent pathophysiology; when they occur together, the risk of adverse outcomes significantly increases. 5 , 6 , 7 , 8 , 9 For patients with AF, medical therapies to reduce HF‐related outcomes such as hospitalization and mortality are lacking. 9 Established therapies including beta‐blockers, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers may be less effective at reducing HF hospitalizations and mortality in patients with HF with concomitant AF as compared with those in sinus rhythm. 10 , 11 , 12
Sodium‐glucose co‐transporter (SGLT) inhibitors, originally developed as glucose‐lowering agents, reduce HF hospitalization and cardiovascular death in several populations, including patients with type 2 diabetes mellitus, renal impairment, HF, and cardiovascular disease. 13 , 14 , 15 , 16 , 17 However, whether patients with AF treated with an SGLT inhibitor receive the same risk reductions for HF hospitalization and cardiovascular death as patients without AF has yet to be systematically assessed.
The primary objective of this systematic review and meta‐analysis was to explore the association between treatment with an SGLT inhibitor and the occurrence of AF. The secondary objective was to evaluate whether a history of AF modifies the effect of SGLT inhibitors on the composite of HF hospitalization or cardiovascular death.
METHODS
The protocol for this systematic review and meta‐analysis is registered with the International Prospective Register of Systematic Reviews (PROSPERO: CRD42021228865). The data underlying this article are available in the article and in its online supplementary material.
Eligibility Criteria
We included randomized controlled trials (RCTs), irrespective of publication status, date of publication, risk of bias, or language. We included trials assessing SGLT2 inhibitors or dual SGLT1/2 inhibitors. We included trials that enrolled adults regardless of prior AF history or other comorbidities, such as diabetes mellitus, HF, chronic kidney disease (CKD), cardiovascular disease, or multiple cardiovascular risk factors. Outcomes of interest were AF and atrial flutter events and the composite of HF hospitalization or cardiovascular death as defined by study authors. We excluded studies with <100 participants and those with <24 weeks of follow‐up.
Search Methods
We searched MEDLINE, Embase, and Cochrane CENTRAL for keywords related to SGLT inhibitors from inception to March 2021. An academic librarian reviewed the search strategy, which included a validated filter to exclude reports that were not RCTs. 18 We screened the references of eligible papers and consulted experts to identify additional trials.
Selection of Studies
Pairs of reviewers independently screened titles and abstracts for eligibility. Full texts of the potentially eligible studies were retrieved. Pairs of reviewers then independently screened full texts in duplicate and recorded the main reason for exclusion. Disagreements were resolved through discussion.
Data Extraction and Management
Two reviewers independently abstracted data on intervention and outcome, and recorded study and participant characteristics including age, sex, and relevant comorbidities (eg, diabetes mellitus, CKD, HF, and cardiovascular risk). Review authors searched appendices and supplements of published articles and the adverse events reporting section of ClinicalTrials.gov for relevant information. Reviewers compared results and resolved disagreements by discussion with a third party.
Assessment of Risk of Bias
In duplicate, 2 review authors assessed risk of bias. 19 In each trial, reviewers evaluated the following domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, and selective reporting. The results were compared, and disagreements were resolved by discussion. Reviewers assessed performance and detection bias separately. For analysis and presentation purposes, risk of bias was dichotomized as high (or likely high) or low (or likely low). For subgroup analyses, the study‐level risk of bias was assessed for each outcome. If a trial was at risk of selection, performance, detection, or reporting bias for that outcome, it was categorized as high risk of bias.
Effect Estimates
We used risk ratios (RR) to report effect estimates for AF events and hazard ratios (HR) for HF hospitalization/cardiovascular death. We obtained the absolute risk difference for clinical outcomes by applying the RR with 95% CI to the baseline risk in the control group.
We assessed clinical and methodological heterogeneity based on study characteristics. We assessed heterogeneity qualitatively by evaluating overlapping of CIs and quantitatively by using the I2 statistic. 19 Random‐effects models with Mantel‐Haenszel weighting were used because we expected comparisons to show heterogeneity. HR for the composite of HF hospitalization or cardiovascular death were pooled using the generic inverse‐variance method. 19 All analyses followed the intention‐to‐treat principle and were conducted using RevMan 5.4.1 (The Cochrane Collaboration, Denmark) and R software (version 3.6.1; The R Foundation). We considered P<0.05 (2‐sided) to be statistically significant.
Post‐Hoc Sensitivity Analyses
Considering the rareness of AF events in the underlying data, we performed 2 sensitivity analyses to assess the robustness of the primary results using (1) a 1‐stage individual‐participant‐data logistic regression analysis with Firth's correction for rare events, treating study as a fixed effect in the model, and (2) a fixed‐effects meta‐analysis without homogeneity assumption using multiple estimation methods (Maximum Likelihood Estimator, Woolf's inverse‐variance estimator, and the logarithm of Cochran‐Mantel‐Haenszel statistic). 20 Both sensitivity analyses yield odds ratio (OR), but when events are rare, estimates of OR and RR are nearly identical. 21
Subgroup Analyses
We performed prespecified subgroup analyses to compare the effect of SGLT inhibitors on AF events among different patient populations (diabetes mellitus, HF, CKD, and high cardiovascular risk), SGLT inhibitor medications (empagliflozin, dapagliflozin, canagliflozin, ertugliflozin, and sotagliflozin), and SGLT inhibitor types (dual SGLT1/2 and SGLT2 inhibitors). We performed a simple significance test to investigate differences between subgroups using the methods described in Borenstein et al., involving a standard test for heterogeneity across subgroup results. 19 , 22
Assessment of the Quality of the Evidence
Reviewers used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach to assess the quality of evidence. 23 GRADE appraises the confidence in estimates of effect by considering within‐study risk of bias, directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias. We inspected funnel plots of SEs versus effect estimates for publication bias and small‐study effects.
RESULTS
Screening
The electronic search identified 5224 citations (Figure S1). After reference and full‐text screening, 33 studies met eligibility criteria. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 Table 1 presents details on included studies.
Table 1.
Study Characteristics of Included Randomized Controlled Trials
| Study ID | Study acronym | Trial registration | Sample size | Follow‐up duration | Trial population | Control/placebo and dose | Intervention and dose |
|---|---|---|---|---|---|---|---|
| Bailey, 2010 28 | NCT00528879 | 915 | 102 wk | T2DM | Placebo | Dapagliflozin 2.5 mg, Dapagliflozin 5 mg, Dapagliflozin 10 mg | |
| Barnett, 2014 29 | EMPA‐REG RENAL | NCT01164501 | 741 | 52 wk | T2DM+renal impairment | Placebo | Empagliflozin 10 mg, Empagliflozin 25 mg |
| Bhatt, 2021 55 | SOLOIST‐WHF | NCT03521934 | 1222 | Median: 170 wk | T2DM+worsening HF | Placebo | Sotagliflozin 200/400 mg |
| Bhatt, 2021 56 | SCORED | NCT03315143 | 10 584 | Median: 304 wk | T2DM+cardiovascular risk factors+CKD | Placebo | Sotagliflozin 200/400 mg |
| Bode, 2013 30 | NCT01106651 | 716 | 104 wk | T2DM | Placebo | Canagliflozin 100 mg, Canagliflozin 300 mg | |
| Cannon, 2020 31 | VERTIS‐CV | NCT01986881 | 8246 | Mean: 183 wk | T2DM+CVD | Placebo | Ertugliflozin 5 mg, Ertugliflozin 15 mg |
| Cefalu, 2015 32 | NCT01031680 | 922 | 24 wk | T2DM+CVD+hypertension | Placebo | Dapagliflozin 10 mg | |
| Danne, 2019 33 | inTandem1 | NCT02384941 | 793 | 52 wk | T1DM | Placebo | Sotagliflozin 200 mg, Sotagliflozin 400 mg |
| Ferrannini, 2010 34 | NCT00528372 | 1067 | 102 wk | T2DM | Placebo | Dapagliflozin 2.5 mg, Dapagliflozin 5 mg, Dapagliflozin 10 mg | |
| Frías, 2016 35 | DURATION‐8 | NCT02229396 | 695 | 104 wk | T2DM | Placebo+exenatide 2 mg | Dapagliflozin 10 mg+exenatide 2 mg |
| Garg, 2017 36 | inTandem3 | NCT02531035 | 1405 | 24 wk | T1DM | Placebo | Sotagliflozin 400 mg |
| Grunberger, 2017 37 | VERTIS RENAL | NCT01986855 | 468 | 52 wk | T2DM+CKD | Placebo | Ertugliflozin 5 mg, Ertugliflozin 15 mg |
| Haering, 2015 38 | EMPA‐REG EXTEND MONO | NCT01289990 | 2705 | 76 wk | T2DM | Placebo | Empagliflozin 10 mg, Empagliflozin 25 mg |
| Heerspink, 2020 39 | DAPA‐CKD | NCT03036150 | 4304 | Median: 125 wk | CKD | Placebo | Dapagliflozin 10 mg |
| Kovacs, 2015 40 | EMPA‐REG PIOTM | NCT01210001 | 499 | 76 wk | T2DM | Placebo | Empagliflozin 10 mg, Empagliflozin 25 mg |
| Mathieu, 2015 42 | NCT01646320 | 320 | 52 wk | T2DM | Placebo | Dapagliflozin 10 mg | |
| McMurray, 2019 25 | DAPA‐HF | NCT03036124 | 4744 | Median: 79 wk | HFrEF | Placebo | Dapagliflozin 10 mg |
| Neal, 2015 26 , 43 | CANVAS | NCT01032629 | 4330 | Mean: 86.5 wk | T2DM+cardiovascular risk factors/history of CVD | Placebo | Canagliflozin 100 mg, Canagliflozin 300 mg |
| Neal, 2017 26 | CANVAS‐R | NCT01989754 | 5813 | Mean: 188.2 wk | T2DM+cardiovascular risk factors/history of CVD | Placebo | Canagliflozin 100 mg, Canagliflozin 300 mg |
| Leiter, 2014 | NCT01042977 | 964 | 104 wk | T2DM+CVD | Placebo | Dapagliflozin 10 mg | |
| Perkovic, 2019 44 | CREDENCE | NCT02065791 | 4401 | Median: 137 wk | T2DM+albuminuric CKD | Placebo | Canagliflozin 100 mg |
| Wilding, 2013 45 | CANTATA‐MSU | NCT01106625 | 469 | 52 wk | T2DM | Placebo | Canagliflozin 100 mg, Canagliflozin 300 mg |
| Packer et al 54 | EMPEROR‐Reduced | NCT03057977 | 3730 | Median: 69 wk | HFrEF | Placebo | Empagliflozin 10 mg |
| Pollock, 2019 46 | DELIGHT | NCT02547935 | 459 | 24 wk | T2DM+albuminuric CKD | Placebo | Dapagliflozin 10 mg |
| Pratley, 2018 47 | VERTIS‐FACTORIAL | NCT02099110 | 1233 | 52 wk | T2DM | Sitagliptin 100 mg | Ertugliflozin 5 mg+Sitagliptin 100 mg, Ertugliflozin 15 mg+Sitagliptin 100 mg |
| Roden, 2013 48 | NCT01177813 | 986 | 24 wk | T2DM | Placebo | Empagliflozin 10 mg, Empagliflozin 25 mg | |
| Rosenstock, 2015 49 | EMPA‐REG BASAL | NCT01011868 | 494 | 78 wk | T2DM | Placebo | Empagliflozin 10 mg, Empagliflozin 25 mg |
| Rosenstock, 2018 50 | VERTIS‐MET | NCT02033889 | 621 | 104 wk | T2DM | Placebo (Glimepiride if glycemic rescue criteria met) | Ertugliflozin 5 mg, Ertugliflozin 15 mg |
| Søfteland, 2017 51 | NCT01734785 | 607 | 24 wk | T2DM | Placebo | Empagliflozin 10 mg, Empagliflozin 25 mg | |
| Wilding, 2012 52 | NCT00673231 | 1240 | 104 wk | T2DM | Placebo | Dapagliflozin 2.5 mg, Dapagliflozin 5 mg, Dapagliflozin 10 mg | |
| Wiviot, 2019 24 | DECLARE‐TIMI 58 | NCT01730534 | 17 190 | Median: 220 wk | T2DM+cardiovascular risk factors/history of CVD | Placebo | Dapagliflozin 10 mg |
| Yale, 2014 53 | DIA3004 | NCT01064414 | 272 | 52 wk | T2DM+renal impairment | Placebo | Canagliflozin 100 mg, Canagliflozin 300 mg |
| Zinman, 2015 27 | EMPA‐REG OUTCOME | NCT01131676 | 7064 | Median: 160 wk | T2DM+high risk of CV events | Placebo | Empagliflozin 10 mg, Empagliflozin 25 mg |
CKD indicates chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; T1DM, type 1 diabetes mellitus; and T2DM, type 2 diabetes mellitus.
Included Studies
Thirty‐one RCTs reported data on AF events. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 These RCTs included a total of 75 279 participants, with a mean age of 62.3±5.0 years and including 35.0% women. In 28 studies, participants were required to have diabetes mellitus (with diabetes mellitus being the key inclusion criterion in 16 studies), 2 trials enrolled patients with HF, 6 enrolled patients with CKD, and 7 enrolled patients at high cardiovascular risk. Twenty‐nine RCTs studied an SGLT2 inhibitor (6 canagliflozin, 11 dapagliflozin, 8 empagliflozin, and 4 ertugliflozin). 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 Two studied the dual SGLT1/2 inhibitor sotagliflozin. 33 , 36 In all trials, data concerning AF events were obtained from adverse‐event reporting. One trial, Dapagliflozin Effect on CardiovascuLAR Events (DECLARE‐TIMI 58), published a dedicated report on AF events. 24 All other trials reported on AF in supplementary appendices and/or on clinical trial databases such as ClinicalTrials.gov (Table S1). None of the studies provided details on the method of ascertainment, including descriptions for rhythm monitoring or other methods for detection of AF. All studies used the Medical Dictionary of Regulatory Affairs (MedDRA) classification system for adverse AF events. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 In this system, serious adverse events are defined as those that resulted in death, were life‐threatening, required inpatient hospitalization or extended a current hospital stay, resulted in an ongoing or significant incapacity, interfered substantially with normal life functions, or put patients in danger or need of medical or surgical intervention to prevent one of the results listed above. Nonserious adverse events are defined by MedDRA as other adverse events that did not meet any of the above criteria. Data were rarely provided regarding the characteristics of AF events (ie, the number of patients with events, whether the events were incident or recurrent, as well as the duration/burden of AF/flutter) or the characteristics of patients with events (including demographics, comorbidities, or medications).
We identified 1 relevant ongoing trial. 57 Empagliflozin and Atrial Fibrillation Treatment (EMPA‐AF; NCT04583813) aims to enroll 400 patients with diabetes mellitus who are overweight and who have HF and AF. Participants will be allocated to empagliflozin or placebo and followed for 24 months with a primary outcome of AF burden. The last publicly posted update on April 2, 2021 listed the trial as not yet recruiting.
Three RCTs reported on HF hospitalization/cardiovascular death in the subgroup of patients with AF; all 3 trials required participants to have diabetes mellitus. 27 , 55 , 56 These RCTs included a total of 18 832 participants, with a mean age of 66.8±2.9 years and 38.1% were women. Among the 3 studies, 1 trial enrolled patients with HF, 1 enrolled patients with CKD, and 1 enrolled patients at high cardiovascular risk. Two of these trials studied the SGLT1/2 inhibitor sotagliflozin and 1 studied the SGLT2 inhibitor empagliflozin. In the 2 sotagliflozin trials, urgent visits for HF were included in the composite outcome in addition to HF hospitalization and cardiovascular death. 55 , 56
Risk of Bias
All 33 included RCTs used a double‐blind design. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 We had no concerns regarding randomization, allocation concealment, performance bias, or incomplete data (Table S2). Because AF was not systematically assessed, we expected that patients with more healthcare encounters, including hospitalizations, would be more likely to have AF captured. SGLT inhibitors have shown a reduction in cardiovascular hospitalizations; therefore, we judged that the included studies were at high risk of detection bias for AF events.
Outcomes
AF/Flutter Events
Pooled data from 31 studies (75 279 participants, 951 total events; 2 studies with 0 events in either group; mean follow‐up: 2.6 years) (Figure 1) suggested a significant reduction in the risk of serious AF events in patients who received an SGLT inhibitor (1.1% versus 1.5%; RR 0.75 [95% CI, 0.66–0.86]; I2=0%). 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 Based on the GRADE framework, we judged that this was moderate quality evidence because of a serious risk of bias (Table S3). A post‐hoc sensitivity analysis of all other trials excluding the largest study (DECLARE‐TIMI 58, 21 41.4% of weight, RR 0.74 [95% CI, 0.60–0.90]) yielded consistent results (RR 0.76 [95% CI, 0.64–0.90], I2=0%, P‐for‐interaction=0.80).
Figure 1. Forest plot comparing serious atrial fibrillation or atrial flutter events between patients on SGLT inhibitors vs placebo/control in randomized controlled trials.
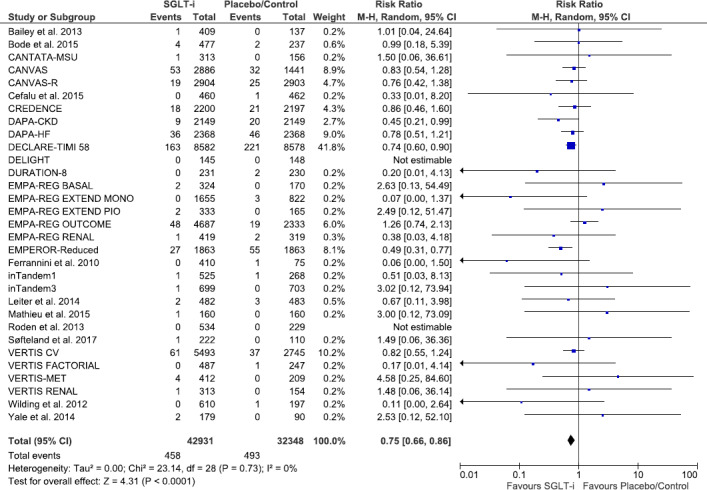
Square markers represent point estimates of RR for individual studies, with square size representing proportional weight given to each study in the meta‐analysis. Horizontal lines indicate 95% CIs. The solid diamond represents the estimated 95% CI for effect size of all meta‐analyzed data. M‐H, Mantel‐Haenszel; RR, relative risk; and SGLT, sodium‐glucose co‐transporter.
In all studies except for DECLARE‐TIMI 58, Dapagliflozin in Patients With Heart Failure and Reduced Ejection Fraction (DAPA‐HF), and Canagliflozin and cardiovascular and renal events in type 2 diabetes (CANVAS), 24 , 25 , 26 AF was only detected by scanning through reports of serious adverse events. These 3 studies also reported other adverse AF events that did not qualify as serious adverse events according to the MedDRA criteria. A post‐hoc meta‐analysis of these 3 studies showed a significant reduction in total (serious and nonserious) AF events (26 223 participants, 1159 events; 4.0% versus 4.9%; RR, 0.83 [95% CI, 0.73–0.94], I2=0%).
Prespecified sensitivity analyses restricting results to either serious (1) AF events only or (2) atrial flutter events only yielded similar effect estimates (Table 2). Subgroup analyses that separated trials according to the type of SGLT inhibitor used (including comparisons of individual agents as well as of dual SGLT1/2 inhibitors versus SGTL2 inhibitors), trial population (history of HF, diabetes mellitus, CKD, or high cardiovascular risk), as well as duration of follow‐up (greater than/equal to versus <2 years of follow‐up) did not result in statistically significant subgroup effects (Table 2, Figure S2). Post‐hoc sensitivity analyses including logistic regression with Firth's correction, treating study as fixed effect as well as fixed effects meta‐analysis without homogeneity assumption using several estimation methods (Maximum Likelihood Estimator, Woolf's inverse‐variance estimator, and the logarithm of Cochran‐Mantel‐Haenszel statistic) demonstrated very similar effect estimates and CIs to random effects meta‐analysis (Table S4).
Table 2.
Pooled Risk Ratio of Atrial Fibrillation and Atrial Flutter Events
| Event rate (SGLT inhibitor) | Event rate (placebo) | Pooled risk ratio (random effects model) | Test for interaction | |||
|---|---|---|---|---|---|---|
| Atrial fibrillation events only | 29 RCTs | n=72 955 | 0.92% | 1.31% | RR, 0.75 (95% CI, 0.65–0.87), I2, 0% | |
| Atrial flutter events only | 13 RCTs | n=57 264 | 0.23% | 0.32% | RR, 0.74 (95% CI, 0.53– 1.03), I2, 1% | |
| Trial population | ||||||
| DM | 16 RCTs | n=11 916 | 0.23% | 0.27% | RR, 0.74 (95% CI, 0.35– 1.55), I2: 0% | P=0.75 |
| Renal disease | 6 RCTs | n=10 462 | 0.57% | 0.85% | RR, 0.69 (95% CI, 0.43– 1.09), I2, 0% | |
| CVD/risk factors | 7 RCTs | n=44 439 | 1.36% | 1.78% | RR, 0.79 (95% CI, 0.68– 0.92), I2, 0% | |
| HFrEF | 2 RCTs | n=8462 | 1.52% | 1.94% | RR, 0.62 (95% CI, 0.51– 1.21), I2: 53% | |
| Duration of follow‐up | ||||||
| ≥2 y | 13 RCTs | n=51 519 | 1.13% | 1.56% | RR: 0.77 (95% CI: 0.66, 0.90), I2: 0% | P=0.52 |
| <2 y | 18 RCTs | n=23 760 | 0.93% | 1.43% | RR: 0.70 (95% CI– 0.55, 0.90), I2: 0% | |
| Medication | Canagliflozin (n=15 983) vs dapagliflozin (n=30 993) vs empagliflozin (n=16 048) vs ertugliflozin (n=10 060) vs sotagliflozin (n=2195) | P=0.88 | ||||
| SGLT2 inhibitor vs dual SGLT1/2 inhibitor | Canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin (n=73 084) vs sotagliflozin (n=2195) | P=0.73 | ||||
CVD indicates cardiovascular disease; DM, diabetes mellitus; HFrEF, heart failure with reduced ejection fraction; RCTs, randomized controlled trials; RR, relative risk; and SGLT, sodium‐glucose co‐transporter.
HF Hospitalization or Cardiovascular Death
Three trials reported on HF hospitalization/cardiovascular death stratified by a history of AF at the time of enrollment (18 832 participants, 2060 events, mean follow‐up: 4.6 years) (Figure 2). 27 , 55 , 56 Among patients with diabetes mellitus and a history of AF, SGLT inhibitors compared with placebo resulted in a lower risk of HF hospitalization or cardiovascular death (HR, 0.70 [95% CI, 0.57–0.85]; I2=0%). This was similar to what was observed in patients without AF (HR, 0.70 [95% CI, 0.61–0.79]; I2=0%; P‐for‐interaction: 1.00). Based on the GRADE framework, we judged that this was high‐quality evidence.
Figure 2. Forest plot demonstrating composite of heart failure hospitalization/urgent visit or cardiovascular death between patients on SGLT inhibitors vs placebo/control in randomized controlled trials stratified by presence or absence of atrial fibrillation/flutter at baseline.
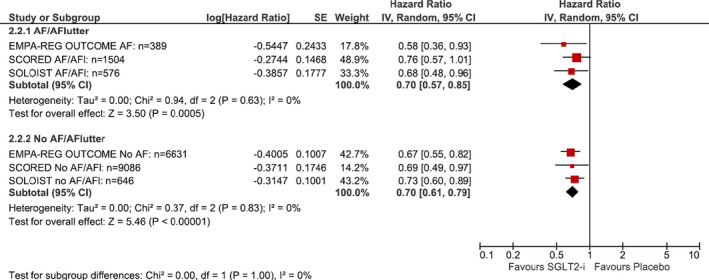
Square markers represent point estimate of HR for individual studies, with square size representing proportional weight given to each study in the meta‐analysis. Horizontal lines indicate 95% CIs. The solid diamonds represent the estimated 95% CI for effect size of all meta‐analyzed data. AF indicates atrial fibrillation; AFl, atrial flutter; EMPA‐REG OUTCOME, Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes; HR, hazard ratio; SGLT, sodium‐glucose co‐transporter; SOLOIST, Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure; and SCORED, Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease.
Publication Bias
The funnel plot for AF events was symmetrical, suggesting against the presence of publication bias (Figure S3). Publication bias was not assessed for the composite outcome of HF hospitalization or cardiovascular death given the small number of available studies.
DISCUSSION
To our knowledge, this is the largest and most comprehensive systematic review to address the role of SGLT inhibitors in reducing the incidence and recurrence of AF with twice as many total AF events as any prior meta‐analysis. 58 , 59 This is also the first pooled report on the impact of SGLT inhibitors on HF hospitalization and cardiovascular death in patients who are known to have AF.
In this systematic review and meta‐analysis of RCTs, SGLT inhibitors resulted in a 25% (95% CI, 14%–34%) relative risk reduction in serious AF events across 31 RCTs and a similar relative reduction in total AF events, suggesting that SGLT inhibitors may reduce the incidence and recurrence of AF. Consistent effects were observed across subgroups of trials in patients with high cardiovascular risk, HF, or CKD and across comparisons of individual agents. These trials were limited in how they captured and defined AF events. Appropriately designed RCTs with standardized monitoring and adjudication of incident AF and rigorous capture of AF‐related healthcare utilization are needed to confirm whether SGLT inhibitors reduce new‐onset AF and other patient‐important AF events. Nonetheless, the consistent reduction in AF events highlights the promise of SGLT inhibitors both in primary prevention for high‐risk patients and as therapy to reduce progression in those with AF. Retrospective analyses of large, population‐wide pharmacovigilance databases have also suggested that patients prescribed SGLT inhibitors have a lower rate of AF compared with patients treated with other glucose‐lowering therapies. 60 , 61 There are multiple possible mechanisms through which SGLT inhibitors may reduce AF such as through reduction in body weight, blood pressure, and volume. 62 , 63 Mechanistic studies and animal models indicate that SGLT inhibitors may reduce atrial fibrosis and adverse remodeling, in addition to improving cellular metabolism and bioenergetics such as ion handling and mitochondrial function. 64 , 65 , 66 Reductions in HF could also reduce AF, and vice versa. 62 , 63
This meta‐analysis also suggests that patients with diabetes mellitus with and without AF benefit from the same risk reduction in HF hospitalization and cardiovascular death from SGLT inhibitors. The pooled estimate from 3 trials showed a 30% reduction in the hazard of HF hospitalization or cardiovascular death, without statistical heterogeneity. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes (EMPA‐REG OUTCOME) was the only trial to report a dedicated post‐hoc analysis assessing the treatment effect of SGLT inhibitors on the subgroup of patients with AF. 27 HF hospitalization or cardiovascular death were 1.85 to 3.43 times more frequent in patients with than in those without AF, suggesting a much higher baseline risk in these patients. 27 Because patients with and without AF benefited from a similar relative reduction in these outcomes, these findings suggest that patients with AF could benefit from a greater absolute risk reduction from SGLT inhibitors when compared with patients without AF. Whether SGLT inhibitors improve outcomes of patients with AF who would not have been eligible for these trials, in particular those without diabetes mellitus, is uncertain. Additionally, 2 of these trials studied sotagliflozin, which is unique, being a dual SGLT1/2 inhibitor. The RCTs that studied sotagliflozin were stopped early because of a loss of sponsor funding; this agent may never become available for patient use. 55 , 56 Future RCTs should assess the impact of SGLT inhibitors on HF hospitalization and cardiovascular death in patients with AF, particularly in those who were excluded from previous trials.
Limitations
The main limitation of this systematic review and meta‐analysis relates to the ascertainment of both prevalent and incident AF in the included studies. We urge caution when interpreting these findings because the studies captured in this review were not specifically designed to assess AF and therefore did not provide the level of details that are available in most contemporary HF and AF studies including burden and symptoms of AF. Compared with contemporary population‐wide analyses of the incidence and recurrence of AF in patients with and those without diabetes mellitus, the relatively low rate of AF events suggests that AF was likely underestimated. 67 Most trials only reported AF events that met the criteria for serious adverse event as described above. Although our pooled analysis of total AF events also showed a consistent reduction in AF with SGLT inhibitors, further research prospectively assessing for total AF incidence, prevalence, arrhythmia burden, and symptomatology is needed to definitively characterize the role and impact of SGLT inhibitors on AF. Similar to the previously published report from the DECLARE‐TIMI 58 trial, AF events in our systematic review and meta‐analysis came from adverse event reporting and MedDRA classification in all studies. 24 Higher rates of AF events may have been driven in part by arrhythmias detected during HF hospitalizations, which were more common in patients allocated to placebo. For studies of patients with a past history of AF, the proportion of patients with different patterns of AF (paroxysmal, persistent, and permanent) is not known. AF pattern impacts baseline risk of HF events, and treatment effect may vary for different AF patterns. 68
CONCLUSIONS
SGLT inhibitors may reduce the incidence or recurrence of AF. In patients with type 2 diabetes mellitus, SGLT inhibitors may reduce a composite of HF hospitalization or cardiovascular death, both in patients with and without AF. Appropriately designed RCTs are needed to clarify their role in preventing AF and reducing HF hospitalization and cardiovascular death in patients with AF.
Sources of Funding
Pandey is supported by the McMaster Medical Student Research Excellence Award (MAC RES) from the Michael G. DeGroote School of Medicine, McMaster University.
Disclosures
Dr Belley‐Cote reports grants from Bayer and grants from Roche, outside the submitted work. Dr Conen reports consulting fees from Roche Diagnostics outside of the current work. Dr Verma reports grants from Amarin, grants and personal fees from Amgen, grants and personal fees from AstraZeneca, grants and personal fees from Bayer, grants and personal fees from Boehringer‐Ingelheim, personal fees from Bristol‐Myers Squibb, grants and personal fees from Eli Lilly, personal fees from EOCI Pharmacomm Ltd, grants and personal fees from HLS Therapeutics, grants and personal fees from Janssen, grants and personal fees from Merck, personal fees from Novartis, grants and personal fees from Novo Nordisk, grants from Pfizer, grants from PhaseBio, grants and personal fees from Sanofi, personal fees from Sun Pharmaceuticals, personal fees from Toronto Knowledge Translation Working Group, outside the submitted work. Dr Gerstein reports grants and personal fees from Sanofi, grants and personal fees from Eli Lilly, grants and personal fees from Novo Nordisk, grants from AstraZeneca, grants from Merck, personal fees from Abbott, personal fees from Pfizer, personal fees from Boehringer Ingelheim, personal fees from DKSH, personal fees from Zuellig, sitting on a Data Safety Monitoring Board for Covance and sitting on a Data Safety Monitoring Board for Kowa, outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Figures S1–S3
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.022222
For Sources of Funding and Disclosures, see page 9.
References
- 1. Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, Connolly SJ. Prevention of atrial fibrillation with angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: a meta‐analysis. J Am Coll Cardiol. 2005;45:1832–1839. DOI: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 3. Celik T. Angiotensin receptor blockers for the prevention of atrial fibrillation recurrences: unending hot debate. J Atr Fibrillation. 2010;3:306–306. DOI: 10.4022/jafib.v2i2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dorian P, Angaran P. Beta‐blockers and atrial fibrillation: hypertension and other medical conditions influencing their use. Can J Cardiol. 2014;30:S38–S41. [DOI] [PubMed] [Google Scholar]
- 5. Gorenek B, Pelliccia A, Benjamin EJ, Boriani G, Crijns HJ, Fogel RI, Van Gelder IC, Halle M, Kudaiberdieva G, Lane DA, et al. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Eur J Prev Cardiol. 2017;24:4–40. DOI: 10.1177/2047487316676037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fauchier L, Villejoubert O, Clementy N, Bernard A, Pierre B, Angoulvant D, Ivanes F, Babuty D, Lip GY. Causes of death and influencing factors in patients with atrial fibrillation. Am J Med. 2016;129:1278–1287. DOI: 10.1016/j.amjmed.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 7. Marijon E, Le Heuzey J‐Y, Connolly S, Yang S, Pogue J, Brueckmann M, Eikelboom J, Themeles E, Ezekowitz M, Wallentin L, et al. Causes of death and influencing factors in patients with atrial fibrillation: clinical perspective. Circulation. 2013;128:2192–2201. DOI: 10.1161/CIRCULATIONAHA.112.000491. [DOI] [PubMed] [Google Scholar]
- 8. Fauchier L, Samson A, Chaize G, Gaudin AF, Vainchtock A, Bailly C, Cotte FE. Cause of death in patients with atrial fibrillation admitted to French hospitals in 2012: a nationwide database study. Open Heart. 2015;2:e000290. DOI: 10.1136/openhrt-2015-000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan G‐A, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS) the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. DOI: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 10. Rienstra M, Damman K, Mulder BA, Van Gelder IC, McMurray JJ, Van Veldhuisen DJ. Beta‐blockers and outcome in heart failure and atrial fibrillation: a meta‐analysis. JACC Heart Fail. 2013;1:21–28. DOI: 10.1016/j.jchf.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 11. Chaugai S, Sherpa LY, Sepehry AA, Arima H, Wang DW. Effect of RAAS blockers on adverse clinical outcomes in high CVD risk subjects with atrial fibrillation: a meta‐analysis and systematic review of randomized controlled trials. Medicine (Baltimore). 2016;95:e4059. DOI: 10.1097/MD.0000000000004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JGF, Lip GYH, Coats AJS, Andersson B, Kirchhof P, et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual‐patient data meta‐analysis. Lancet. 2014;384:2235–2243. DOI: 10.1016/S0140-6736(14)61373-8. [DOI] [PubMed] [Google Scholar]
- 13. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo‐Jack S, Pratley R, Greenberg M, Wang S, Huyck S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta‐analysis. JAMA Cardiol. 2021;6:148–158. DOI: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh AK, Singh R. Heart failure hospitalization with SGLT‐2 inhibitors: a systematic review and meta‐analysis of randomized controlled and observational studies. Expert Rev Clin Pharmacol. 2019;12:299–308. DOI: 10.1080/17512433.2019.1588110. [DOI] [PubMed] [Google Scholar]
- 15. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;39:31–39. DOI: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 16. Butler J, Zannad F, Filippatos G, Anker SD, Packer M. Totality of evidence in trials of sodium‐glucose co‐transporter‐2 inhibitors in the patients with heart failure with reduced ejection fraction: implications for clinical practice. Eur Heart J. 2020;41:3398–3401. DOI: 10.1093/eurheartj/ehaa731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pozzoli M, Cioffi G, Traversi E, Pinna GD, Cobelli F, Tavazzi L. Predictors of primary atrial fibrillation and concomitant clinical and hemodynamic changes in patients with chronic heart failure: a prospective study in 344 patients with baseline sinus rhythm. J Am Coll Cardiol. 1998;32:197–204. DOI: 10.1016/S0735-1097(98)00221-6. [DOI] [PubMed] [Google Scholar]
- 18. McKibbon KA, Wilczynski NL, Haynes RB, Hedges T. Retrieving randomized controlled trials from medline: a comparison of 38 published search filters. Health Info Libr J. 2009;26:187–202. DOI: 10.1111/j.1471-1842.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 19. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 20. Li KQ, Rice K. Improved inference for fixed‐effects meta‐analysis of 2 × 2 tables. Res Synth Methods. 2020;11(3):387–396. [DOI] [PubMed] [Google Scholar]
- 21. Greenland S, Thomas DC. On the need for the rare disease assumption in case‐control studies. Am J Epidemiol. 1982;116:547–553. [DOI] [PubMed] [Google Scholar]
- 22. Borenstein M, Higgins JP. Meta‐analysis and subgroups. Prev Sci. 2013;14:134–143. DOI: 10.1007/s11121-013-0377-7. [DOI] [PubMed] [Google Scholar]
- 23. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–998. DOI: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE‐TIMI 58 Trial. Circulation. 2020;141:1227–1234. DOI: 10.1161/CIRCULATIONAHA.119.044183. [DOI] [PubMed] [Google Scholar]
- 25. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. DOI: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 26. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. DOI: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 27. Böhm M, Slawik J, Brueckmann M, Mattheus M, George JT, Ofstad AP, Inzucchi SE, Fitchett D, Anker SD, Marx N, et al. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA‐REG OUTCOME trial. Eur J Heart Fail. 2020;22:126–135. DOI: 10.1002/ejhf.1663. [DOI] [PubMed] [Google Scholar]
- 28. Bailey CJ, Gross JL, Hennicken D, Iqbal N, Mansfield TA, List JF. Dapagliflozin add‐on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double‐blind, placebo‐controlled 102‐week trial. BMC Med. 2013;11:1. DOI: 10.1186/1741-7015-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC; EMPA‐REG RENAL Trial Investigators . Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–384. DOI: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 30. Bode B, Stenlöf K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract. 2013;41:72–84. DOI: 10.3810/hp.2013.04.1020. [DOI] [PubMed] [Google Scholar]
- 31. Cannon CP, Pratley R, Dagogo‐Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. DOI: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 32. Cefalu WT, Leiter LA, de Bruin TW, Gause‐Nilsson I, Sugg J, Parikh SJ. Dapagliflozin’s effects on glycemia and cardiovascular risk factors in high‐risk patients with type 2 diabetes: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study with a 28‐week extension. Diabetes Care. 2015;38:1218–1227. DOI: 10.2337/dc14-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Danne T, Cariou B, Buse JB, Garg SK, Rosenstock J, Banks P, Kushner JA, McGuire DK, Peters AL, Sawhney S, et al. Improved time in range and glycemic variability with sotagliflozin in combination with insulin in adults with type 1 diabetes: a pooled analysis of 24‐week continuous glucose monitoring data from the inTandem program. Diabetes Care. 2019;42:919–930. DOI: 10.2337/dc18-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double‐blind, placebo‐controlled, phase 3 trial. Diabetes Care. 2010;33:2217–2224. DOI: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frías JP, Guja C, Hardy E, Ahmed A, Dong F, Öhman P, Jabbour SA. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION‐8): a 28 week, multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004–1016. DOI: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
- 36. Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, Fulcher GR, Pozzilli P, Gesty‐Palmer D, Lapuerta P, Simó R, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 2017;377:2337–2348. DOI: 10.1056/NEJMoa1708337. [DOI] [PubMed] [Google Scholar]
- 37. Grunberger G, Camp S, Johnson J, Huyck S, Terra SG, Mancuso JP, Jiang ZW, Golm G, Engel SS, Lauring B. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther. 2018;9:49–66. DOI: 10.1007/s13300-017-0337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haering HU, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, Meinicke T, Woerle HJ, Broedl UC. Empagliflozin as add‐on to metformin plus sulphonylurea in patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;110:82–90. DOI: 10.1016/j.diabres.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 39. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou F‐F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. DOI: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 40. Kovacs CS, Seshiah V, Merker L, Christiansen AV, Roux F, Salsali A, Kim G, Stella P, Woerle HJ, Broedl UC. Empagliflozin as add‐on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther. 2015;37:1773–1788. DOI: 10.1016/j.clinthera.2015.05.511. [DOI] [PubMed] [Google Scholar]
- 41. Leiter LA, Cefalu WT, de Bruin TW, Gause‐Nilsson I, Sugg J, Parikh SJ. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study with a 28‐week extension. J Am Geriatr Soc. 2014;62:1252–1262. DOI: 10.1111/jgs.12881. [DOI] [PubMed] [Google Scholar]
- 42. Mathieu C, Ranetti AE, Li D, Ekholm E, Cook W, Hirshberg B, Chen H, Hansen L, Iqbal N. Randomized, double‐blind, phase 3 trial of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009–2017. DOI: 10.2337/dc15-0779. [DOI] [PubMed] [Google Scholar]
- 43. Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Ways K, Desai M, Shaw W, Capuano G, Alba M, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium–glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–411. DOI: 10.2337/dc14-1237. [DOI] [PubMed] [Google Scholar]
- 44. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. DOI: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 45. Wilding JPH, Charpentier G, Hollander P, González‐Gálvez G, Mathieu C, Vercruysse F, Usiskin K, Law G, Black S, Canovatchel W, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267–1282. DOI: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pollock C, Stefánsson B, Reyner D, Rossing P, Sjöström CD, Wheeler DC, Langkilde AM, Heerspink HJ. Albuminuria‐lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2019;7:429–441. DOI: 10.1016/S2213-8587(19)30086-5. [DOI] [PubMed] [Google Scholar]
- 47. Pratley RE, Eldor R, Raji A, Golm G, Huyck SB, Qiu Y, Sunga S, Johnson J, Terra SG, Mancuso JP, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: the VERTIS FACTORIAL randomized trial. Diabetes Obes Metab. 2018;20:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC; EMPA‐REG Mono Trial Investigators . Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–219. DOI: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 49. Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ; EMPA‐REG BASALTM Trial Investigators . Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78‐week randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2015;17:936–948. DOI: 10.1111/dom.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosenstock J, Frias J, Páll D, Charbonnel B, Pascu R, Saur D, Darekar A, Huyck S, Shi H, Lauring B, et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab. 2018;20:520–529. DOI: 10.1111/dom.13103. [DOI] [PubMed] [Google Scholar]
- 51. Søfteland E, Meier JJ, Vangen B, Toorawa R, Maldonado‐Lutomirsky M, Broedl UC. Empagliflozin as add‐on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24‐week randomized, double‐blind, parallel‐group trial. Diabetes Care. 2017;40:201–209. DOI: 10.2337/dc16-1347. [DOI] [PubMed] [Google Scholar]
- 52. Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S. Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405–415. DOI: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 53. Yale J‐F, Bakris G, Cariou B, Nieto J, David‐Neto E, Yue D, Wajs E, Figueroa K, Jiang J, Law G, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–1027. DOI: 10.1111/dom.12348. [DOI] [PubMed] [Google Scholar]
- 54. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. DOI: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 55. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384:117–128. DOI: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 56. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. 2021;384:129–139. DOI: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 57. Monaco A. Empagliflozin and Atrial Fibrillation Treatment (EMPA‐AF). October 12, 2020. In: ClinicalTrials.gov [Internet]. Bethesda, MD: U.S. National Library of Medicine; 2000. Available at: https://clinicaltrials.gov/ct2/show/NCT04583813; ClinicalTrials.gov Identifier: NCT04583813. [last updated October 14, 2020; Accessed March 21, 2021]. [Google Scholar]
- 58. Li WJ, Chen XQ, Xu LL, Li YQ, Luo BH. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta‐analysis of 16 randomized controlled trials. Cardiovasc Diabetol. 2020;19:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Okunrintemi V, Mishriky BM, Powell JR, Cummings DM. Sodium‐glucose co‐transporter‐2 inhibitors and atrial fibrillation in the cardiovascular and renal outcome trials. Diabetes Obes Metab. 2021;23:276–280. DOI: 10.1111/dom.14211. [DOI] [PubMed] [Google Scholar]
- 60. Lee S, Zhou J, Chang C, Liu T, Chang D, Wong WT, Leung KS, Wai AK, Cheung BM, Tse G, et al. Comparative effects of sodium glucose cotransporter 2 (SGLT2) inhibitors and dipeptidyl peptidase‐4 (DPP4) inhibitors on new‐onset atrial fibrillation and stroke outcomes. medRxiv. January 1, 2021.
- 61. Bonora BM, Raschi E, Avogaro A, Fadini GP. SGLT‐2 inhibitors and atrial fibrillation in the Food and Drug Administration adverse event reporting system. Cardiovasc Diabetol. 2021;20:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia. 2018;61:2108–2117. DOI: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 63. Tuttle KR, Brosius FC III, Cavender MA, Fioretto P, Fowler KJ, Heerspink HJL, Manley T, McGuire DK, Molitch ME, Mottl AK, et al. SGLT2 inhibition for CKD and cardiovascular disease in type 2 diabetes: report of a scientific workshop sponsored by the National Kidney Foundation. Am J Kidney Dis. 2021;77:94–109. DOI: 10.1053/j.ajkd.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 64. Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co‐transporter‐2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high‐fat diet/streptozotocin‐induced diabetic rats. Cardiovasc Diabetol. 2019;18:1–4. DOI: 10.1186/s12933-019-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, Barron B, Mayoux E, Rector RS, Whaley‐Connell A, et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol. 2017;16:1–5. DOI: 10.1186/s12933-016-0489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Peng X, Li L, Zhang M, Zhao Q, Wu K, Bai R, Ruan Y, Liu N. Sodium‐glucose cotransporter 2 inhibitors potentially prevent atrial fibrillation by ameliorating ion handling and mitochondrial dysfunction. Front Physiol. 2020;11:912. DOI: 10.3389/fphys.2020.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bisson A, Bodin A, Fauchier G, Herbert J, Angoulvant D, Ducluzeau PH, Lip GY, Fauchier L. Sex, age, type of diabetes and incidence of atrial fibrillation in patients with diabetes mellitus: a nationwide analysis. Cardiovasc Diabetol. 2021;20:1–1. DOI: 10.1186/s12933-021-01216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Steinberg BA, Li Z, O’Brien EC, Pritchard J, Chew DS, Bunch TJ, Mark DB, Nabutovsky Y, Greiner MA, Piccini JP. Atrial fibrillation burden and heart failure: data from 39,710 individuals with cardiac implanted electronic devices. Heart Rhythm. 2021;18:709–716. DOI: 10.1016/j.hrthm.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Figures S1–S3


