Abstract
Background
Anxiety disorders are the most prevalent mental disorders and are an emerging risk factor for coronary artery disease and its complications. We determine the relationship between having a clinical diagnosis of an anxiety disorder and coronary endothelial dysfunction (CED) using invasive coronary reactivity testing across both sexes.
Methods and Results
Patients presenting with chest pain and nonobstructive coronary artery disease (stenosis <40%) at coronary angiography underwent an invasive assessment of CED. Patients were categorized as having a clinical diagnosis of an anxiety disorder at the time of coronary angiography by chart review. The frequency of CED was compared between patients with versus without an anxiety disorder and after stratifying patients by sex. Between 1992 and 2020, 1974 patients (mean age, 51.3 years; 66.2% women) underwent invasive coronary reactivity testing, of which 550 (27.9%) had a documented anxiety disorder at the time of angiography. There was a significantly higher proportion of patients with any type of CED in those with an anxiety disorder in all patients (343 [62.7%] versus 790 [56.4%]; P=0.011) that persisted in women but not in men. After adjusting for covariables, anxiety was significantly associated with any CED among all patients (odds ratio [95% CI], 1.36 [1.10–1.68]; P=0.004), and after stratifying by sex in women but not in men.
Conclusions
Anxiety disorders are significantly associated with CED in women presenting with chest pain and nonobstructive coronary artery disease. Thus, CED may represent a mechanism underpinning the association between anxiety disorders and coronary artery disease and its complications, highlighting the role of anxiety as a potential therapeutic target to prevent cardiovascular events.
Keywords: anxiety, chest pain, coronary endothelial dysfunction, ischemia
Subject Categories: Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- %ΔCADAch
percentage change in coronary artery diameter in response to acetylcholine
- %ΔCBFAch
percentage change in coronary blood flow in response to acetylcholine
- CBF
coronary blood flow
- CED
coronary endothelial dysfunction
Clinical Perspective
What Is New?
Anxiety disorders are significantly associated with coronary endothelial dysfunction in women presenting with chest pain and nonobstructive coronary artery disease.
What Are the Clinical Implications?
Coronary endothelial dysfunction may represent a mechanism underpinning the association between anxiety disorders and coronary artery disease and its complications, highlighting the role of anxiety as a potential therapeutic target to prevent cardiovascular events.
Impaired psychosocial health is an emerging risk factor for cardiovascular diseases (CVD), including coronary artery disease (CAD) and its complications. 1 Anxiety disorders are the most prevalent mental disorders, affecting nearly 1 in 5 adults in the United States, 2 with women being twice as likely to have an anxiety‐related disorder compared with men. 3 Anxiety has been associated with increased mortality in individuals with known CAD. 4 Furthermore, 2 recently published meta‐analyses confirmed that psychological factors, including anxiety, are associated with adverse CVD events in patients with 5 and without 6 ischemic heart disease at baseline. However, the role of sex on this association remains unclear.
There are several known differences in the manifestations of CAD between sexes. First, the prevalence of, and mortality from, CAD is higher in men, whereas women tend to develop CAD before menopause, on average 10 years after men. 9 Second, women have fewer traditional CVD risk factors than men. 10 Third, women present with signs and symptoms of ischemia, and tend to experience adverse CVD events, more frequently in the absence of obstructive CAD, which may be explained by a higher prevalence of functional vascular abnormalities, such coronary microvascular dysfunction and coronary endothelial dysfunction (CED). 11 , 12 , 13 CED characterizes early atherosclerosis, and is associated with CVD disease progression, as well as a several‐fold increased risk of ischemic cardiac events and stroke. 14 , 15 , 16 , 17 , 18 , 19
Although the principal target of the pathophysiologic effects of psychosocial risk factors on cardiovascular health appears to be the vasculature, and particularly the endothelium, 20 the precise mechanisms underpinning this relationship remain underrecognized, and challenging to address. Sex‐based differences in the prevalence and effects of anxiety could contribute to differences in vascular reactivity and endothelial function between men and women, which, in turn, may affect the differential presentation and progression of ischemic heart disease across sexes. Thus, endothelial dysfunction may explain, at least in part, some of the association between anxiety and adverse CVD events. The significance of the potential link between anxiety and CAD is underscored by the opportunity for early detection and intervention in early atherosclerotic disease. In the current study, we assess the relationship between having a clinical diagnosis of an anxiety disorder and CED using invasive coronary reactivity testing across both men and women, hypothesizing that anxiety is significantly associated with CED in women.
Methods
The authors of the current study are willing to make the data, methods used in the analysis, and materials used to conduct the research available to any researcher for purposes of reproducing the results or replicating the procedure.
Study Protocol
Patients were referred to our institution by their physician for clinically indicated coronary angiography for the assessment of chest pain or an abnormal stress test result. All patients were then evaluated by a cardiologist at our institution, and those with signs or symptoms of stable cardiac ischemic heart disease or an abnormal noninvasive stress test result were referred for a clinically indicated elective coronary angiogram. Patients with the following criteria were excluded: >40% diameter stenosis in major vessels; acute coronary syndrome; acute renal failure; uncontrolled hypertension; and left ventricular ejection fraction of ≤50% and left ventricular hypertrophy. 13 , 21 , 22 , 23 , 24 , 25 , 26 , 27 This retrospective cross‐sectional study was approved by the Mayo Clinic Institutional Review Board, and all study participants gave their informed consent.
Consecutive patients presented to the cardiac catheterization laboratory in the fasting state, and all cardiovascular medications, including nitrates and calcium channel blockers, had been discontinued for at least 24 hours. Routine diagnostic coronary angiography was performed on all patients using standard clinical protocols. Angiograms were reviewed before the infusion of any pharmacological agents. In cases where the severity of stenosis was uncertain, online quantitative coronary angiography was used. All patients underwent evaluation of microvascular endothelial‐dependent and endothelial‐independent coronary flow reserve, as previously described. 28 , 29 Following intravenous administration of 5000 to 7000 U of heparin, a Doppler guidewire (Flowire; Volcano), 0.014 inches in diameter, within a 3F Slip‐Cath Infusion Catheter (Cook Medical) was positioned into the midportion of the left anterior descending coronary artery, 2 to 3 mm distal to the tip of the infusion catheter. This vessel was chosen for accessibility and because it supplies the largest territory of the myocardium. Heart rate and mean arterial blood pressure were continuously monitored throughout each procedure. 13 , 21 , 22 , 23 , 24 , 25 , 26 , 27
Baseline mean peak velocity was recorded using the intracoronary Doppler wire, after which acetylcholine was infused at concentrations of 10−6, 10−5, and 10−4 mol/L (to achieve estimated coronary bed concentrations of 10−8, 10−7, and 10−6 mol/L, respectively) for 3 minutes at each concentration to assess endothelial‐dependent function, as previously described. 13 , 21 , 22 , 23 , 24 , 25 , 26 , 27 Infusions were performed using a Harvard pump to maintain infusion rates of <1% of the estimated coronary blood flow (CBF). Doppler measurements of mean peak velocity were performed after each infusion, followed by repeated coronary angiography. Coronary artery diameter was measured at baseline and after the infusion with acetylcholine, by an independent investigator blinded to Doppler velocity data using a previously described computer‐based image analysis system. 30 , 31 Endothelial‐dependent CBF was then calculated using the following, as previously described 28 , 32 : CBF=(πmean peak velocity/2)(coronary artery diameter/2). 2 The maximal percentage increase in CBF in response to acetylcholine compared with the CBF at baseline was then calculated (percentage change in CBF in response to acetylcholine [%ΔCBFAch]). For quality control, all measurements were performed in the segment 5 mm distal to the tip of the Doppler wire; and following each infusion, the diameter was measured in the same segment of the vessel. 13 , 21 , 22 , 23 , 24 , 25 , 26 , 27
Definition of Terms
Impaired endothelial‐dependent macrovascular function was defined as a coronary artery diameter in response to acetylcholine (percentage change in coronary artery diameter in response to acetylcholine [%ΔCADAch]) of ≤−20%. Impaired endothelial‐dependent microvascular function was defined as a maximal percentage increase in CBF in response to any dose of acetylcholine compared with baseline CBF (%ΔCBFAch) of ≤50%. 13 , 21 , 22 , 23 , 24 CED was defined as the presence of impaired endothelial‐dependent macrovascular and/or microvascular dysfunction.
Patient Information
Data were collected on conventional cardiovascular risk factors, including hypertension, diabetes, hyperlipidemia, smoking status, and body mass index; biochemical parameters, including fasting blood glucose, hemoglobin A1c, serum total cholesterol, low‐density lipoprotein, high‐density lipoprotein, and triglycerides; and medication use, including antiplatelet and antihypertensive medication, statins, as well as psychotropic medication, including antidepressants and antipsychotics of any class. Smoking was categorized as a history of current smoking, former smoking, or never smoking; hyperlipidemia was defined by a documented history of hyperlipidemia, treatment with lipid‐lowering therapy, a low‐density lipoprotein cholesterol level above the target (<130 mg/dL for low‐risk patients, <100 mg/dL for moderate‐ to high‐risk patients, <70 mg/dL for very‐high‐risk patients, and <55 mg/dL for extreme high‐risk patients based on 10‐year atherosclerotic CVD risk), high‐density lipoprotein cholesterol <40 mg/dL in men or <50 mg/dL in women, or triglycerides >150 mg/dL. Type 2 diabetes was defined as a documented history of or treatment for type 2 diabetes, or a hemoglobin A1c of >6.5, if available. Hypertension was defined as a documented history of or treatment of the condition, a systolic blood pressure measurement of >130 mm Hg, or a diastolic blood pressure measurement of >90 mm Hg. All blood test results included in this study are based on blood samples obtained on the morning of the index procedure. A history of myocardial infarction was also documented and was diagnosed in the presence of at least 2 of the following: (1) typical chest pain for at least 20 minutes; (2) increased creatinine kinase (or the MB fraction) or troponin level; or (3) new ST‐segment elevation, Q waves, or left bundle‐branch block on ECG. Information was also collected on past medical history, including other vascular diseases (defined as a documented history of peripheral vascular disease, stroke, or transient ischemic attack). 13 , 21 , 22 , 23 , 24 Anxiety disorder was evaluated by patient chart review and was defined by a documented clinical diagnosis of any of the following disorders that were current at the time of coronary angiography: generalized anxiety disorder, panic disorder, phobic anxiety disorders, including social phobia, specific phobia, and agoraphobia, and posttraumatic stress disorder. All diagnoses were made in keeping with criteria established by the International Classification of Diseases, Tenth Revision (ICD‐10). 33
Statistical Analysis
Patients were retrospectively categorized on the basis of the presence or absence of a diagnosis of an anxiety disorder. The proportions of patients having abnormal endothelial‐dependent macrovascular function, measured using %ΔCADAch, abnormal endothelial‐dependent microvascular function, measured using %ΔCBFAch, and abnormal any CED were compared across groups in all patients and after stratifying by sex. Continuous variables are presented as a mean (SD), where data are normally distributed, and as a median (quartile 1–quartile 3) for skewed data. Categorical variables are presented as frequencies (percentages). Differences between groups were analyzed using Student t test and Wilcoxon rank‐sum test for continuous variables and Pearson χ 2 test for proportions. Univariable and multivariable logistic regression models were fitted to assess the association between having an anxiety disorder and endothelial‐dependent macrovascular or microvascular dysfunction, and any CED in all patients, and after stratifying by sex. Multivariable analyses were adjusted for conventional CVD risk factors, including age as a continuous variable, a history of hyperlipidemia, hypertension, diabetes, and smoking, and use of psychotropic medications as a categorical variable. A P<0.05 was considered significant, and all statistical analyses were performed using JMP 9 software (SAS Institute, Inc, Cary, NC).
Results
Sample Overview
Between 1992 and 2020, 1974 patients (mean age, 51.3 years; 66.2% women) underwent coronary angiography and invasive testing for CED. A total of 550 patients had a documented anxiety disorder at the time of angiography (27.9%). Table 1 summarizes the baseline characteristics of patients with and without an anxiety disorder. There was a higher proportion of White patients, individuals with diabetes and hyperlipidemia, and those taking psychotropic medications in those with an anxiety disorder versus those without. Study participants with an anxiety disorder also had, on average, lower values of total cholesterol, high‐density lipoprotein cholesterol, and low‐density lipoprotein cholesterol compared with participants without an anxiety disorder.
Table 1.
Summary of Baseline Clinical Characteristics of Patients With and Without a Clinical Diagnosis of an Anxiety Disorder
| Characteristics |
Anxiety disorder (N=550; 27.9%) |
No anxiety disorder (N=1424; 72.1%) |
P Value |
|---|---|---|---|
| Age, mean (SD), y | 50.6 (12.0) | 51.5 (12.6) | 0.140 |
| Women, n (%) | 361 (65.6) | 946 (66.4) | 0.738 |
| White race, n (%) | 511 (92.9) | 1223 (85.9) | <0.001* |
| BMI, mean (SD), kg/m2 | 29.3 (6.5) | 29.0 (6.4) | 0.347 |
| Hypertension, n (%) | 238 (43.3) | 602 (42.3) | 0.688 |
| Diabetes, n (%) | 76 (13.8) | 139 (9.8) | 0.011* |
| Hyperlipidemia, n (%) | 331 (60.2) | 762 (53.5) | 0.007* |
| eGFR <60 mL/min per 1.73 m2, n (%) | 68 (12.4) | 203 (14.3) | 0.269 |
| History of MI, n (%) | 92 (17.2) | 208 (15.1) | 0.250 |
| History of vascular disease, n (%) | 61 (11.1) | 117 (8.2) | 0.050 |
| Family history of CAD, n (%) | 330 (60.0) | 785 (55.1) | 0.050 |
| Smoking status, n (%) | |||
| Never smoked | 285 (51.8) | 779 (54.7) | |
| Former smoker | 196 (35.6) | 476 (33.4) | |
| Current smoker | 68 (12.4) | 168 (11.8) | 0.632 |
| Total cholesterol, mean (SD), mg/dL | 181.4 (40.0) | 187.7 (44.4) | 0.003* |
| HDL‐C, mean (SD), mg/dL | 52.6 (17.0) | 54.7 (17.8) | 0.018* |
| LDL‐C, mean (SD), mg/dL | 102.5 (34.1) | 106.5 (38.6) | 0.030* |
| Triglycerides, mean (SD), mg/dL | 131.8 (86.3) | 133.9 (89.8) | 0.638 |
| Psychotropic medication, n (%) | 276 (50.2) | 457 (32.1) | <0.001* |
| Systolic blood pressure, mean (SD), mm Hg | 125.9 (17.1) | 125.8 (17.7) | 0.963 |
| Diastolic blood pressure, mean (SD), mm Hg | 75.7 (9.6) | 76.2 (10.1) | 0.291 |
BMI indicates body mass index; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and MI, myocardial infarction.
P value <0.05.
Proportion of CED in Patients With an Anxiety Disorder
Figure (A) through (C) demonstrate the proportion of patients with abnormal endothelial‐dependent macrovascular function, characterized as a %ΔCADAch <−20%, abnormal endothelial‐dependent microvascular function, characterized as a %ΔCBFAch <50%, and abnormal any CED in patients with versus those without an anxiety disorder across all patients, as well as separately in men and women. There was no significant difference in the proportion of patients with an abnormal %ΔCADAch (epicardial CED) between patients with anxiety disorders versus those without among all patients or in either men or women after stratifying by sex. There was a significantly higher proportion of patients with an abnormal %ΔCBFAch (microvascular CED) in the group of patients with an anxiety disorder in all patients (312 [57.1%] versus 712 [51.0%]; P=0.015), which remained after stratifying by sex in women but not men (206 [57.5%] versus 463 [49.8%]; P=0.013; and 106 [56.4%] versus 249 [53.3%]; P=0.476). Similarly, there was a significantly higher proportion of patients with any CED in those with versus without an anxiety disorder in all patients (343 [62.7%] versus 790 [56.4%]; P=0.011) that persisted in women but not in men (221 [61.6%] versus 504 [54.1%]; P=0.016; and 122 [64.9%] versus 286 [60.9%]; P=0.333).
Figure 1. Bar charts demonstrating the different proportions of patients with macrovascular, microvascular, and any coronary endothelial dysfunction in those with vs without an anxiety disorder.
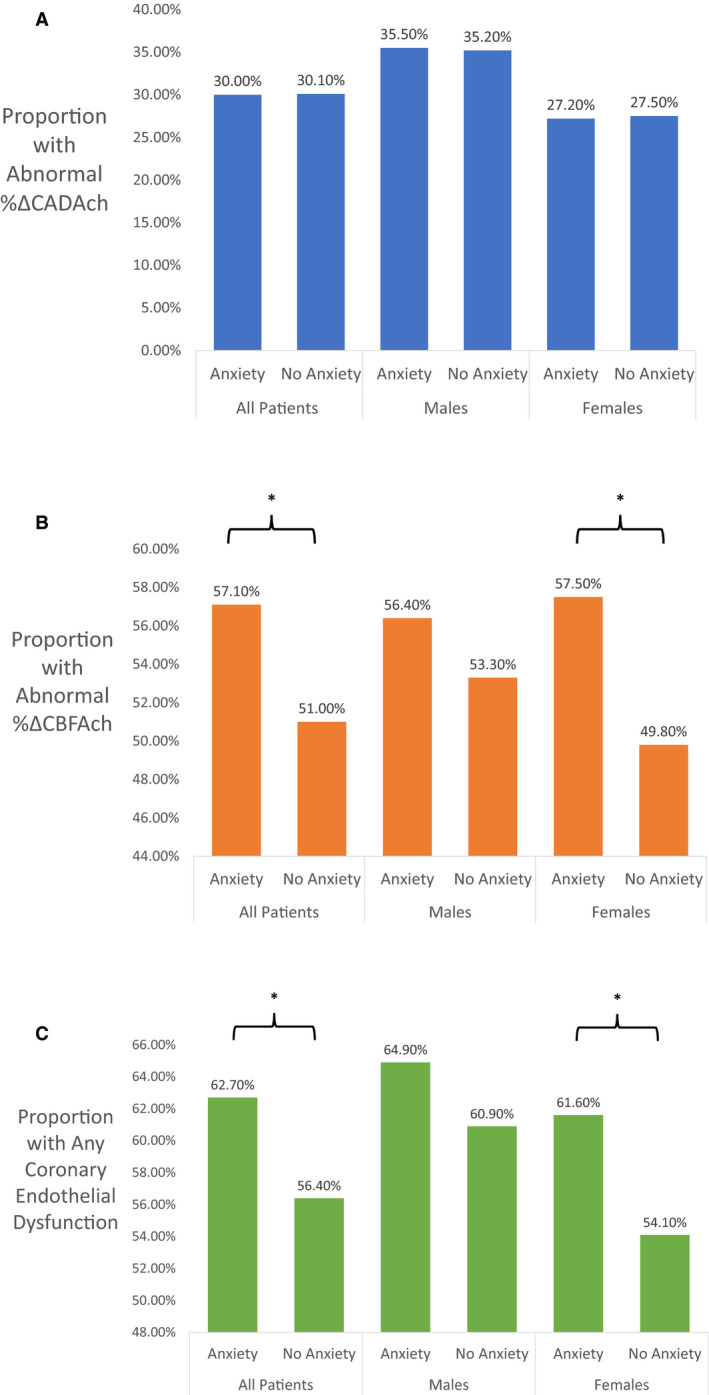
A, Proportion of patients with abnormal endothelial‐dependent coronary macrovascular function characterized as a percentage change in coronary artery diameter in response to acetylcholine (%ΔCADAch) of <−20% in patients with compared to without an anxiety disorder across all patients and after stratifying by sex. B, Proportion of patients with abnormal endothelial‐dependent coronary microvascular function characterized as a percentage change in coronary blood flow in response to acetylcholine (%ΔCBFAch) of <50% in patients with compared to without an anxiety disorder across all patients and after stratifying by sex. C, Proportion of patients with any coronary endothelial dysfunction in patients with compared to without an anxiety disorder across all patients and after stratifying by sex. *Signifies statistically significant difference.
Anxiety and CED: Univariable Analysis
Table 2 shows the associations between the presence of an anxiety disorder and CED in all patients and after stratifying by sex in univariable analyses. Anxiety was not associated with macrovascular CED (%ΔCADAch <−20%) among all patients or in either men or women after stratifying by sex. Anxiety was significantly associated with microvascular CED (%ΔCBFAch <50%) among all patients (odds ratio [OR] [95% CI], 1.28 [1.05–1.56]; P=0.015), and after stratifying by sex in women (OR [95% CI], 1.36 [1.07–1.74]; P=0.013) but not in men. Anxiety was significantly associated with any CED among all patients (OR [95% CI], 1.30 [1.06–1.59]; P=0.011), and after stratifying by sex in women (OR [95% CI], 1.36 [1.06–1.74]; P=0.016) but not in men.
Table 2.
Univariable Analyses of the Relationship Between the Presence of an Anxiety Disorder and Macrovascular, Microvascular, and Any CED Across All Patients and in Men and Women After Stratifying by Sex
| Odds ratio (95% CI) | Abnormal %ΔCADAch | P Value | Abnormal %ΔCBFAch | P Value | Any CED | P Value |
|---|---|---|---|---|---|---|
| All patients | ||||||
| No anxiety disorder (reference) |
N=427 1.00 |
N=712 1.00 |
N=790 1.00 |
|||
| Anxiety disorder |
N=165 0.99 (0.80–1.23) |
0.961 |
N=312 1.28 (1.05–1.56) |
0.015* |
N=343 1.30 (1.06–1.59) |
0.011* |
| Men | ||||||
| No anxiety disorder (reference) |
N=331 1.00 |
N=463 1.00 |
N=504 1.00 |
|||
| Anxiety disorder |
N=126 1.01 (0.71–1.44) |
0.958 |
N=206 1.13 (0.80–1.59) |
0.476 |
N=221 1.19 (0.84–1.69) |
0.333 |
| Women | ||||||
| No anxiety disorder (reference) |
N=129 1.00 |
N=249 1.00 |
N=286 1.00 |
|||
| Anxiety disorder |
N=51 0.98 (0.75–1.29) |
0.886 |
N=106 1.36 (1.07–1.74) |
0.013* |
N=122 1.36 (1.06–1.74) |
0.016* |
%ΔCADAch indicates percentage change in coronary artery diameter in response to acetylcholine; %ΔCBFAch, percentage change in coronary blood flow in response to acetylcholine; and CED, coronary endothelial dysfunction.
P value <0.05.
Anxiety and CED: Multivariable Analysis
Table 3 shows the associations between the presence of an anxiety disorder and CED in all patients and after stratifying by sex in multivariable analyses after adjusting for age and body mass index as continuous variables, and White race, hypertension, diabetes, hyperlipidemia, smoking, and use of psychotropic drugs as categorical variables. Anxiety was not associated with macrovascular CED among all patients or in either men or women after stratifying by sex. Anxiety was significantly associated with microvascular CED among all patients (OR [95% CI], 1.37 [1.11–1.68]; P=0.003), and after stratifying by sex in women (OR [95% CI], 1.44 [1.12–1.86]; P=0.005) but not in men. Anxiety was significantly associated with any CED among all patients (OR [95% CI], 1.36 [1.10–1.68]; P=0.004), and after stratifying by sex in women (OR [95% CI], 1.40 [1.08–1.81]; P=0.010) but not in men. The formal test for interaction with respect to sex, however, was not significant.
Table 3.
Multivariable Analyses of the Relationship Between the Presence of an Anxiety Disorder and Macrovascular, Microvascular, and Any CED Across All Patients and in Men and Women After Stratifying by Sex
| Odds ratio (95% CI) | Abnormal %ΔCADAch | P Value | Abnormal %ΔCBFAch | P Value | Any CED | P Value |
|---|---|---|---|---|---|---|
| All patients | ||||||
| No anxiety disorder (reference) |
N=427 1.00 |
N=712 1.00 |
N=790 1.00 |
|||
| Anxiety disorder |
N=165 1.01 (0.81–1.26) |
0.907 |
N=312 1.37 (1.11–1.68) |
0.003* |
N=343 1.36 (1.10–1.68) |
0.004* |
| Men | ||||||
| No anxiety disorder (reference) |
N=331 1.00 |
N=463 1.00 |
N=504 1.00 |
|||
| Anxiety disorder |
N=126 0.99 (0.69–1.43) |
0.966 |
N=206 1.28 (0.89–1.84) |
0.180 |
N=221 1.31 (0.90–1.89) |
0.155 |
| Women | ||||||
| No anxiety disorder (reference) |
N=129 1.00 |
N=249 1.00 |
N=286 1.00 |
|||
| Anxiety disorder |
N=51 1.01 (0.76–1.34) |
0.946 |
N=106 1.44 (1.12–1.86) |
0.005* |
N=122 1.40 (1.08–1.81) |
0.010* |
Multivariable analysis was adjusted for age and body mass index as continuous variables, and White race, hypertension, diabetes, hyperlipidemia, smoking, and use of psychotropic drugs as categorical variables. %ΔCADAch indicates percentage change in coronary artery diameter in response to acetylcholine; %ΔCBFAch, percentage change in coronary blood flow in response to acetylcholine; and CED, coronary endothelial dysfunction.
P value <0.05.
Discussion
In this large population of patients presenting with chest pain and nonobstructive CAD, we show for the first time that invasively determined CED, specifically microvascular CED, was more prevalent in individuals with a diagnosis of an anxiety disorder. This effect was driven by a significant association in women that was not present in men. Furthermore, we show that a current diagnosis of an anxiety disorder was significantly associated with microvascular CED and any CED in both univariable and multivariable analyses after adjusting for conventional cardiovascular risk factors and the use of psychotropic medications, a relationship that persisted in women but not men. Thus, the current study supports the concept that CED may play a mediating role in the presentation of ischemia in female patients with anxiety disorders, and highlights the need for early detection and potential role of anxiety as a therapeutic target to reduce the risk of CVD events.
Psychosocial Risk Factors for CVD
Despite variations in study design, measures of exposure and outcomes, and patient characteristics, studies have shown significant associations between psychosocial risk factors and adverse clinical events. 34 , 35 Psychological distress, 36 vital exhaustion, 37 posttraumatic stress disorder, 38 depression, 39 and anxiety 4 have all been shown to be risk factors for CVD. Psychosocial stressors have in fact been shown to have an attributable CVD risk similar to that of diabetes, hyperlipidemia, hypertension, and cigarettes smoking. 40 , 41 Anxiety disorders affect nearly 1 in 5 adults in the United States, 2 and have been associated with increased mortality in individuals with known CAD, 4 and with adverse CVD events in patients with 5 and without 6 ischemic heart disease in both sexes. The principal site of transduction for the pathophysiologic effects of anxiety disorders on cardiovascular health is thought to be the vasculature, and particularly the endothelium, 20 although the precise mechanisms underpinning this relationship require further clarification.
The mechanism of the link between anxiety and CED may be multifactorial. Monkeys exposed to a new social group, and the inherent changes to social structure and environment, had increased endothelial cell damage and turnover in the thoracic aorta and coronary arteries, 42 and reduced NO availability in arteries with atherosclerosis. 43 Similarly, in humans, a public speaking task 44 and anger provocation 45 were shown to be associated with an increase in circulating endothelial cell‐derived microparticles, derived from the membranes of apoptotic endothelial cells. Mental stress more generally may also adversely influence endothelial cell function. In animal studies, acute and chronic stress was associated with lower levels of NO synthase mRNA expression, 46 , 47 leading to endothelial dysfunction. Mental stress is also associated with oxidative stress and the release of potent vasoconstrictors, such as endothelin 48 and angiotensin II, 49 as well as the upregulation of inflammatory cytokines, such as CRP (C‐reactive protein), interleukin‐1, interleukin‐6, and tumor necrosis factor‐α, 50 all of which contribute to endothelial dysfunction. Several studies have also shown a significant relationship between experimentally induced stress and increased peripheral vasoreactivity and microvascular endothelial dysfunction. 51 , 52 , 53 More important, in the current study, we show for the first time that this relationship extends to the coronary arteries of female patients, as anxiety disorders are significantly associated with invasively determined CED in women presenting with chest pain and nonobstructive CAD. CED is considered to be an early marker of atherosclerosis, and is associated with atherosclerotic disease progression, as well as a several‐fold increased risk of ischemic cardiac events and stroke. 14 , 15 , 16 , 17 , 18 , 19 Thus, the findings of the current study suggest that CED may be the underlying mechanism for the increased risk of cardiovascular events in individuals with a diagnosis of an anxiety disorder. Indeed, apical ballooning syndrome, a unique cardiomyopathy that occurs almost exclusively in women and that is typically preceded by strong emotional stress, has also been shown to be associated both CED 54 as well as peripheral endothelial dysfunction. 53 This further highlights the central role that endothelial dysfunction may play in the pathophysiology linking anxiety to CVD. Future prospective studies are required to determine the predictive ability of identifying patients presenting with chest pain with an anxiety disorder to help differentiate those who have CED. Given that the 1974 patients included in the current study sample were examined between 1992 and 2020, it will be possible to determine whether the women with anxiety disorder in this sample have experienced increased mortality and CVD events since study entry compared with those without a history of anxiety disorder. It would also be possible to determine whether those with increased microvascular CED are more likely to experience adverse CVD events, and if so, test whether that increased morbidity/mortality is mediating the adverse clinical outcomes in women with a history of anxiety disorder. We do not currently have data on incident CVD events during follow‐up in this study population, but it will be possible to collect such data and then determine whether the increased prevalence of CED in women with history of anxiety disorder found in the current study is one mediator of increased incident cardiovascular events in women with history of anxiety disorder who present with chest pain and nonobstructive CAD.
Sex‐Based Differences in the Relationship Between Anxiety and CED
Sex‐based differences in the manifestations of CAD are well known. The prevalence of, and mortality from, CAD is higher in men, 7 , 8 whereas women tend to develop CAD on average 10 years later than men before menopause. 9 Women also have fewer traditional CVD risk factors than men, 10 present with signs and symptoms of ischemia, and experience adverse CVD events more frequently in the absence of obstructive CAD, which may be explained by a higher prevalence of functional vascular abnormalities, such as CED. 11 , 12 , 13 We previously showed that hypothyroidism, 22 elevated uric acid levels, as an index of chronic inflammation, 55 and poor glycemic control in patients with diabetes 21 were more closely associated with microvascular CED in women than men. Sex‐based physiological differences may variably influence the impact of potentially injurious factors on vascular function and health, resulting in different clinical presentations and cardiovascular risk across sexes. The findings of the current study build further on this notion when we showed that the presence of an anxiety disorder is significantly associated with microvascular CED in women but not men.
Sex is an important determinant of a variety of psychosocial factors known to influence health. Sex is also known to influence lifestyle habits, such as eating patterns, smoking, alcohol use, and physical exercise. 56 Furthermore, feminine gender roles and personality traits, including anxiety, are associated with an increased risk of recurrent events after acute coronary syndrome. 57 A recent meta‐analysis showed that both men and women had a significant association between anxiety and incident ischemic heart disease, 6 with no differences across sexes. A further meta‐analysis showed that anxiety was associated with adverse events in men but not in women. 5 This discordance could in part be explained by including studies comprising younger populations, in whom CVD in general is less prevalent in women compared with men, and samples whose manifestation of ischemic heart disease was obstructive CAD, which is more common in men. These meta‐analyses also looked at a broad range of psychosocial risk factors, of which anxiety was only one, and for which the definitions and sample sizes varied significantly across studies. 5 , 6 Although anxiety disorders are typically more prevalent in women compared with men, 2 in the current study we found a similar frequency between sexes. Because of several psychosocial explanations, men typically seek treatment for emotional problems less often than women. 58 The men included in the current study would have had more frequent contact with healthcare professionals to evaluate their signs and symptoms of ischemia, and so may have been more likely to report and be identified with symptoms of anxiety disorders than may have occurred otherwise. The precise role that sex plays on the interplay between psychosocial factors, such as anxiety, and CVD requires further study.
Microvascular Disease and Anxiety
In the current study, we show that anxiety disorders are associated with microvascular, but not macrovascular, CED. Previous studies have shown that environmental stress stimulates inflammatory responses, and the release of vasoactive substances leading to enhanced microvascular permeability in mesenteric vessels in an animal model. 59 Moreover, a recent meta‐analysis showed an association between indexes of microvascular dysfunction, including cerebral small‐vessel disease, retinal arteriolar diameter, albuminuria, and biomarkers of endothelial function, with the risk of developing late‐life depression. 60 Psychosocial risk factors, such as anxiety, may therefore preferentially affect the endothelium of the microcirculation. Indeed, in angiographically normal epicardial coronary arteries, mental stress is associated with vasodilatation and increased CBF in the setting of mental stress, 61 whereas local vasoconstriction is seen in segments with epicardial atherosclerotic disease. 62 The latter is associated with reductions in CBF above and beyond that explained by epicardial vasoconstriction alone, implicating the role of stress‐induced increases in the resistance of the coronary microcirculation. Thus, microvascular CED may form the underlying mechanism for ischemia in patients with anxiety disorders. In this way, anxiety could form a potential therapeutic target to reduce the risk of adverse CVD outcomes, particularly as studies of the clinical utility of psychological interventions, such as stress management 63 and meditation, 64 are emerging. In a randomized clinical trial, for example, group psychosocial stress management training in Swedish women with CAD was associated with a marked reduction in mortality. 65 Indeed, atypical chest pain mediated by CED could be the manifestation of anxiety in women, which, in turn, may lead to an impaired quality of life and recurrent presentations to hospital, 66 underscoring the need to address these symptoms early.
Study Limitations
This study has a few limitations. First, the current study is made up of patients presenting with chest pain who were referred for coronary angiography at a tertiary referral center and thus comprises a select population. Second, the cross‐sectional design of this study prohibits a causal association between anxiety and CED. Equally, we cannot establish CED as the underlying mechanism for the incidence of CVD events in patients with anxiety. Thus, the current study is hypothesis‐generating, and these additional questions would be better investigated in prospective clinical studies. Third, diagnosis of an anxiety disorder was made retrospectively through chart review and so we cannot exclude misclassification bias and heterogeneity in our sample. Although our multivariable analyses did adjust for the use of psychotropic medications, we did not evaluate the severity of anxiety at the time of coronary angiography, the presence of other potentially confounding psychosocial variables, the timing and duration of diagnosis, or the adequacy of control of symptoms at the time of angiography. However, all patients were evaluated as outpatients on an elective basis and so no patient had unstable symptoms or signs of anxiety that warranted acute hospitalization. We also did not have information on the use of benzodiazepines or cognitive‐behavioral therapy and other behavioral strategies to target anxiety.
Conclusions
Anxiety disorders are significantly associated with CED in women presenting with chest pain and nonobstructive CAD. CED may represent the underlying mechanism or potential therapeutic target for ischemia in patients with anxiety and could underpin the association between anxiety disorders and incident CVD events. Further prospective studies are required to evaluate the potential role of anxiety as a therapeutic target to reduce the risk of cardiovascular events.
Sources of Funding
None.
Disclosures
None.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M‐T, Corrà U, Cosyns B, Deaton C, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. DOI: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–1035. DOI: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015;17:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Celano CM, Millstein RA, Bedoya CA, Healy BC, Roest AM, Huffman JC. Association between anxiety and mortality in patients with coronary artery disease: a meta‐analysis. Am Heart J. 2015;170:1105–1115. DOI: 10.1016/j.ahj.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smaardijk VR, Maas A, Lodder P, Kop WJ, Mommersteeg PMC. Sex and gender‐stratified risks of psychological factors for adverse clinical outcomes in patients with ischemic heart disease: a systematic review and meta‐analysis. Int J Cardiol. 2020;302:21–29. DOI: 10.1016/j.ijcard.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 6. Smaardijk VR, Lodder P, Kop WJ, van Gennep B, Maas A, Mommersteeg PMC. Sex‐ and gender‐stratified risks of psychological factors for incident ischemic heart disease: systematic review and meta‐analysis. J Am Heart Assoc. 2019;8:e010859. DOI: 10.1161/JAHA.118.010859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart disease and stroke statistics‐2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. DOI: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 8. Bots SH, Peters SAE, Woodward M. Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ Glob Health. 2017;2:e000298. DOI: 10.1136/bmjgh-2017-000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Regitz‐Zagrosek V. Sex and gender differences in health: science & society series on sex and science. EMBO Rep. 2012;13:596–603. DOI: 10.1038/embor.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. AlBadri A, Wei J, Mehta PK, Shah R, Herscovici R, Gulati M, Shufelt C, Bairey MN. Sex differences in coronary heart disease risk factors: rename it ischaemic heart disease! Heart. 2017;103:1567–1568. DOI: 10.1136/heartjnl-2017-311921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Redberg RF, Cannon RO III, Bairey Merz N, Lerman A, Reis SE, Sheps DS; National Heart, Lung and Blood Institute, American College of Cardiology Foundation . Women's ischemic syndrome evaluation: current status and future research directions: report of the National Heart, Lung and Blood Institute workshop: October 2‐4, 2002: section 2: stable ischemia: pathophysiology and gender differences. Circulation. 2004;109:47–49. DOI: 10.1161/01.CIR.0000116207.38349.FF. [DOI] [PubMed] [Google Scholar]
- 12. Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–1992. DOI: 10.1161/01.CIR.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 13. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8:1445–1453. DOI: 10.1016/j.jcin.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 14. Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. DOI: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 15. Reriani MK, Flammer AJ, Jama A, Lerman LO, Lerman A. Novel functional risk factors for the prediction of cardiovascular events in vulnerable patients following acute coronary syndrome. Circ J. 2012;76:778–783. DOI: 10.1253/circj.CJ-12-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr, Lerman A. Long‐term follow‐up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. DOI: 10.1161/01.CIR.101.9.948. [DOI] [PubMed] [Google Scholar]
- 17. Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–2809. DOI: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 18. Yoon MH, Reriani M, Mario G, Rihal C, Gulati R, Lennon R, Tilford JM, Lerman LO, Lerman A. Long‐term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. Int J Cardiol. 2013;168:1316–1321. DOI: 10.1016/j.ijcard.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lavi S, Bae JH, Rihal CS, Prasad A, Barsness GW, Lennon RJ, Holmes DR Jr, Lerman A. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525–1530. DOI: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah SM, Meadows JL, Burg MM, Pfau S, Soufer R. Effects of psychological stress on vascular physiology: beyond the current imaging signal. Curr Cardiol Rep. 2020;22:156. DOI: 10.1007/s11886-020-01406-x. [DOI] [PubMed] [Google Scholar]
- 21. Sara JD, Taher R, Kolluri N, Vella A, Lerman LO, Lerman A. Coronary microvascular dysfunction is associated with poor glycemic control amongst female diabetics with chest pain and non‐obstructive coronary artery disease. Cardiovasc Diabetol. 2019;18:22. DOI: 10.1186/s12933-019-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sara JD, Zhang M, Gharib H, Lerman LO, Lerman A. Hypothyroidism is associated with coronary endothelial dysfunction in women. J Am Heart Assoc. 2015;4:e002225. DOI: 10.1161/JAHA.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sara JDS, Corban MT, Prasad M, Prasad A, Gulati R, Lerman LO, Lerman A. Prevalence of myocardial bridging associated with coronary endothelial dysfunction in patients with chest pain and non‐obstructive coronary artery disease. EuroIntervention. 2020;15:1262–1268. DOI: 10.4244/EIJ-D-18-00920. [DOI] [PubMed] [Google Scholar]
- 24. Sara JDS, Prasad M, Zhang M, Lennon RJ, Herrmann J, Lerman LO, Lerman A. High‐sensitivity C‐reactive protein is an independent marker of abnormal coronary vasoreactivity in patients with non‐obstructive coronary artery disease. Am Heart J. 2017;190:1–11. DOI: 10.1016/j.ahj.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 25. Ahmad A, Corban MT, Toya T, Sara JD, Lerman B, Park JY, Lerman LO, Lerman A. Coronary microvascular endothelial dysfunction in patients with angina and nonobstructive coronary artery disease is associated with elevated serum homocysteine levels. J Am Heart Assoc. 2020;9:e017746. DOI: 10.1161/JAHA.120.017746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahmad A, Corban MT, Toya T, Verbrugge FH, Sara JD, Lerman LO, Borlaug BA, Lerman A. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;23:765–772. DOI: 10.1002/ejhf.2010. [DOI] [PubMed] [Google Scholar]
- 27. Reriani M, Sara JD, Flammer AJ, Gulati R, Li J, Rihal C, Lennon R, Lerman LO, Lerman A. Coronary endothelial function testing provides superior discrimination compared with standard clinical risk scoring in prediction of cardiovascular events. Coron Artery Dis. 2016;27:213–220. DOI: 10.1097/MCA.0000000000000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hasdai D, Cannan CR, Mathew V, Holmes DR Jr, Lerman A. Evaluation of patients with minimally obstructive coronary artery disease and angina. Int J Cardiol. 1996;53:203–208. DOI: 10.1016/0167-5273(95)02548-0. [DOI] [PubMed] [Google Scholar]
- 29. Lerman A, Holmes DR Jr, Bell MR, Garratt KN, Nishimura RA, Burnett JC Jr. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92:2426–2431. DOI: 10.1161/01.CIR.92.9.2426. [DOI] [PubMed] [Google Scholar]
- 30. Bell MR, Britson PJ, Chu A, Holmes DR Jr, Bresnahan JF, Schwartz RS. Validation of a new unix‐based quantitative coronary angiographic system for the measurement of coronary artery lesions. Cathet Cardiovasc Diagn. 1997;40:66–74. DOI: . [DOI] [PubMed] [Google Scholar]
- 31. Bove AA, Holmes DR Jr, Owen RM, Bresnahan JF, Reeder GS, Smith HC, Vlietstra RE. Estimation of the effects of angioplasty on coronary stenosis using quantitative video angiography. Cathet Cardiovasc Diagn. 1985;11:5–16. DOI: 10.1002/ccd.1810110103. [DOI] [PubMed] [Google Scholar]
- 32. Hasdai D, Gibbons RJ, Holmes DR Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. DOI: 10.1161/01.CIR.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 33. Bandelow B. Comparison of the DSM‐5 and ICD‐10: panic and other anxiety disorders. CNS Spectr. 2017;22:404–406. DOI: 10.1017/S1092852917000116. [DOI] [PubMed] [Google Scholar]
- 34. Dar T, Radfar A, Abohashem S, Pitman RK, Tawakol A, Osborne MT. Psychosocial stress and cardiovascular disease. Curr Treat Options Cardiovasc Med. 2019;21:23. DOI: 10.1007/s11936-019-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perk J, De Backer G, Gohlke H, Graham I, Reiner Ž, Verschuren WMM, Albus C, Benlian P, Boysen G, Cifkova R, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis. 2012;223:1–68. DOI: 10.1016/j.atherosclerosis.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 36. Russ TC, Stamatakis E, Hamer M, Starr JM, Kivimaki M, Batty GD. Association between psychological distress and mortality: individual participant pooled analysis of 10 prospective cohort studies. BMJ. 2012;345:e4933. DOI: 10.1136/bmj.e4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cohen R, Bavishi C, Haider S, Thankachen J, Rozanski A. Meta‐analysis of relation of vital exhaustion to cardiovascular disease events. Am J Cardiol. 2017;119:1211–1216. DOI: 10.1016/j.amjcard.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 38. Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: a meta‐analytic review. Am Heart J. 2013;166:806–814. DOI: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gan Y, Gong Y, Tong X, Sun H, Cong Y, Dong X, Wang Y, Xu X, Yin X, Deng J, et al. Depression and the risk of coronary heart disease: a meta‐analysis of prospective cohort studies. BMC Psychiatry. 2014;14:371. DOI: 10.1186/s12888-014-0371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi‐amorn C, Sato H, Yusuf S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:953–962. [DOI] [PubMed] [Google Scholar]
- 41. Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29:932–940. DOI: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 42. Strawn WB, Bondjers G, Kaplan JR, Manuck SB, Schwenke DC, Hansson GK, Shively CA, Clarkson TB. Endothelial dysfunction in response to psychosocial stress in monkeys. Circ Res. 1991;68:1270–1279. DOI: 10.1161/01.RES.68.5.1270. [DOI] [PubMed] [Google Scholar]
- 43. Williams JK, Kaplan JR, Manuck SB. Effects of psychosocial stress on endothelium‐mediated dilation of atherosclerotic arteries in cynomolgus monkeys. J Clin Invest. 1993;92:1819–1823. DOI: 10.1172/JCI116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spicer J, Shimbo D, Johnston N, Harlapur M, Purdie‐Vaughns V, Cook J, Fu J, Burg MM, Wager TD. Prevention of stress‐provoked endothelial injury by values affirmation: a proof of principle study. Ann Behav Med. 2016;50:471–479. DOI: 10.1007/s12160-015-9756-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shimbo D, Rosenberg LB, Chaplin W, Zhao S, Goldensohn ER, Cholankeril M, Fu J, Hong SB, Jelic S, Burg MM. Endothelial cell activation, reduced endothelial cell reparative capacity, and impaired endothelial‐dependent vasodilation after anger provocation. Int J Cardiol. 2013;167:1064–1065. DOI: 10.1016/j.ijcard.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Custodis F, Gertz K, Balkaya M, Prinz V, Mathar I, Stamm C, Kronenberg G, Kazakov A, Freichel M, Böhm M, et al. Heart rate contributes to the vascular effects of chronic mental stress: effects on endothelial function and ischemic brain injury in mice. Stroke. 2011;42:1742–1749. DOI: 10.1161/STROKEAHA.110.598607. [DOI] [PubMed] [Google Scholar]
- 47. Chung IM, Kim YM, Yoo MH, Shin MK, Kim CK, Suh SH. Immobilization stress induces endothelial dysfunction by oxidative stress via the activation of the angiotensin II/its type I receptor pathway. Atherosclerosis. 2010;213:109–114. DOI: 10.1016/j.atherosclerosis.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 48. Friedman EH. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive patients. Circulation. 1997;95:1667–1668. [PubMed] [Google Scholar]
- 49. Kosunen KJ. Plasma renin activity, angiotensin II, and aldosterone after mental arithmetic. Scand J Clin Lab Invest. 1977;37:425–429. DOI: 10.3109/00365517709091502. [DOI] [PubMed] [Google Scholar]
- 50. Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, Wing A, Goldberg EL, Youm Y‐H, Brown CW, Elsworth J, et al. Inflammasome‐driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature. 2017;550:119–123. DOI: 10.1038/nature24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mehta PK, Hermel M, Nelson MD, Cook‐Wiens G, Martin EA, Alkhoder AA, Wei J, Minissian M, Shufelt CL, Marpuri S, et al. Mental stress peripheral vascular reactivity is elevated in women with coronary vascular dysfunction: results from the NHLBI‐sponsored cardiac autonomic nervous system (CANS) study. Int J Cardiol. 2018;251:8–13. DOI: 10.1016/j.ijcard.2017.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O'Neal WT, Sullivan S, et al. Hemodynamic, catecholamine, vasomotor and vascular responses: determinants of myocardial ischemia during mental stress. Int J Cardiol. 2017;243:47–53. DOI: 10.1016/j.ijcard.2017.05.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martin EA, Prasad A, Rihal CS, Lerman LO, Lerman A. Endothelial function and vascular response to mental stress are impaired in patients with apical ballooning syndrome. J Am Coll Cardiol. 2010;56:1840–1846. DOI: 10.1016/j.jacc.2010.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patel SM, Lerman A, Lennon RJ, Prasad A. Impaired coronary microvascular reactivity in women with apical ballooning syndrome (Takotsubo/stress cardiomyopathy). Eur Heart J Acute Cardiovasc Care. 2013;2:147–152. DOI: 10.1177/2048872613475891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prasad M, Matteson EL, Herrmann J, Gulati R, Rihal CS, Lerman LO, Lerman A. Uric acid is associated with inflammation, coronary microvascular dysfunction, and adverse outcomes in postmenopausal women. Hypertension. 2017;69:236–242. DOI: 10.1161/HYPERTENSIONAHA.116.08436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mommersteeg PM, Arts L, Zijlstra W, Widdershoven JW, Aarnoudse W, Denollet J. Impaired health status, psychological distress, and personality in women and men with nonobstructive coronary artery disease: sex and gender differences: the twist (TWEESTEDEN Mild Stenosis) study. Circ Cardiovasc Qual Outcomes. 2017;10:e003387. DOI: 10.1161/CIRCOUTCOMES.116.003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pelletier R, Khan NA, Cox J, Daskalopoulou SS, Eisenberg MJ, Bacon SL, Lavoie KL, Daskupta K, Rabi D, Humphries KH, et al. Sex versus gender‐related characteristics: which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol. 2016;67:127–135. DOI: 10.1016/j.jacc.2015.10.067. [DOI] [PubMed] [Google Scholar]
- 58. Moller‐Leimkuhler AM. Barriers to help‐seeking by men: a review of sociocultural and clinical literature with particular reference to depression. J Affect Disord. 2002;71:1–9. DOI: 10.1016/S0165-0327(01)00379-2. [DOI] [PubMed] [Google Scholar]
- 59. Wilson LM, Baldwin AL. Effects of environmental stress on the architecture and permeability of the rat mesenteric microvasculature. Microcirculation. 1998;5:299–308. DOI: 10.1111/j.1549-8719.1998.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 60. van Agtmaal MJM, Houben A, Pouwer F, Stehouwer CDA, Schram MT. Association of microvascular dysfunction with late‐life depression: a systematic review and meta‐analysis. JAMA Psychiatry. 2017;74:729–739. DOI: 10.1001/jamapsychiatry.2017.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ Jr, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. N Engl J Med. 1991;325:1551–1556. DOI: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 62. Arrighi JA, Burg M, Cohen IS, Kao AH, Pfau S, Caulin‐Glaser T, Zaret BL, Soufer R. Myocardial blood‐flow response during mental stress in patients with coronary artery disease. Lancet. 2000;356:310–311. DOI: 10.1016/S0140-6736(00)02510-1. [DOI] [PubMed] [Google Scholar]
- 63. Blumenthal JA, Sherwood A, Smith PJ, Watkins L, Mabe S, Kraus WE, Ingle K, Miller P, Hinderliter A. Enhancing cardiac rehabilitation with stress management training: a randomized, clinical efficacy trial. Circulation. 2016;133:1341–1350. DOI: 10.1161/CIRCULATIONAHA.115.018926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Levine GN, Lange RA, Bairey‐Merz CN, Davidson RJ, Jamerson K, Mehta PK, Michos ED, Norris K, Ray IB, Saban KL, et al. Meditation and cardiovascular risk reduction: a scientific statement from the American Heart Association. J Am Heart Assoc. 2017;6:e002218. DOI: 10.1161/JAHA.117.002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Orth‐Gomer K, Schneiderman N, Wang HX, Walldin C, Blom M, Jernberg T. Stress reduction prolongs life in women with coronary disease: the Stockholm women's intervention trial for coronary heart disease (SWITCHD). Circ Cardiovasc Qual Outcomes. 2009;2:25–32. DOI: 10.1161/CIRCOUTCOMES.108.812859. [DOI] [PubMed] [Google Scholar]
- 66. Olson MB, Kelsey SF, Matthews K, Shaw LJ, Sharaf BL, Pohost GM, Cornell CE, McGorray SP, Vido D, Bairey Merz CN. Symptoms, myocardial ischaemia and quality of life in women: results from the NHLBI‐sponsored WISE study. Eur Heart J. 2003;24:1506–1514. DOI: 10.1016/S0195-668X(03)00279-3. [DOI] [PubMed] [Google Scholar]


