Abstract
Background
Redo mitral valve surgery is required in up to one‐third of patients and is associated with significant mortality and morbidity. Valve‐in‐valve transcatheter mitral valve replacement (ViV TMVR) is less invasive and could be considered in those at prohibitive surgical risk. Studies on comparative outcomes of ViV TMVR and redo surgical mitral valve replacement (SMVR) remain limited. Our study aimed to investigate the real‐world outcomes of the above procedures using the National Inpatient Sample database.
Methods and Results
We analyzed National Inpatient Sample data using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) from September 2015 to December 2018. A total of 495 and 2250 patients underwent redo ViV TMVR and SMVR, respectively. The patients who underwent ViV TMVR were older (77 versus 68 years, P<0.01). Adjusted mortality was higher in the redo SMVR group compared with the ViV TMVR group (7.6% versus <2.8%, P<0.01). Perioperative complications were higher among patients undergoing redo SMVR including blood transfusions (38% versus 7.6%, P<0.01) and acute kidney injury (36.7% versus 13.9%, P<0.01). Cost of care was higher (USD$57 172 versus USD$52 579, P<0.01), length of stay was longer (10 versus 3 days, P<0.01), and discharge to home was lower (20.3% versus 64.6%, P<0.01) in the SMVR group compared with the ViV TMVR group.
Conclusions
ViV TMVR is associated with lower mortality, periprocedural morbidity, and resource use compared with patients undergoing redo SMVR. ViV TMVR may be a viable option for some patients with mitral prosthesis dysfunction. Studies evaluating long‐term outcomes and durability of ViV TMVR are needed. A patient‐centered approach by the heart team, local institutional expertise, and careful preprocedure planning can help decision‐making about the choice of intervention for the individual patient.
Keywords: redo mitral valve surgery, redo valve replacement, transcatheter mitral valve in valve
Subject Categories: Catheter-Based Coronary and Valvular Interventions
Nonstandard Abbreviations and Acronyms
- NIS
National Inpatient Sample
- SMVR
surgical mitral valve replacement
- ViV TMVR
valve‐in‐valve transcatheter mitral valve replacement
Clinical Perspective
What Is New?
We report real‐world data on valve‐in‐valve transcatheter mitral valve replacement and redo surgical mitral valve replacement for degenerating bioprosthetic valves from a large national data set of 2745 patients.
In a matched cohort, valve‐in‐valve transcatheter mitral valve replacement is associated with lower mortality, morbidity, and resource use when compared with redo surgical mitral valve replacement.
What Are the Clinical Implications?
Valve‐in‐valve transcatheter mitral valve replacement may prove to be a viable treatment option for degenerating bioprosthetic valves, especially for prohibitive surgical risk patients.
There has been a steady rise in the volume of mitral valve surgeries in the United States, with a third of them being mitral valve replacement. 1 The use of biologic valve prostheses for mitral valve replacement has increased dramatically, from 16.8% in 1993 to 53.7% in 2013. 2 Bioprosthetic valves are preferred over mechanical valves because there are fewer thrombotic complications and avoidance of anticoagulation, but they degenerate over time needing reintervention. 3 Redo mitral valve surgery is required in up to one‐third of the patients and is associated with significant mortality and morbidity. 4 , 5 A dramatic improvement in reoperative mortality for mitral valve surgery has been observed in recent years. 6 Recent outcomes data on mitral valve reoperations from the Society of Thoracic Surgeons database showed operative mortality of 3.4% in patients with prior mitral valve surgery undergoing elective nonendocarditis operations. 7 Despite the overall improving trend in mortality, the risk of redo surgical mitral valve replacement (SMVR) can vary significantly from patient to patient, with some being at high risk or prohibitive risk. Learning from the experience with valve‐in‐valve transcatheter aortic valve replacement for patients with degenerative bioprosthetic valves who are at prohibitive surgical risk, redo valve‐in‐valve transcatheter mitral valve replacement (ViV TMVR) has emerged as a less invasive alternative and has been approved by the US Food and Drug Administration. 8 , 9 , 10
The outcomes data on the safety of ViV TMVR compared with redo mitral valve surgery remain limited to small retrospective studies and lack of randomized controlled trials. 11 , 12 Registry data from the STS/ACC TV Registry (Society of Thoracic Surgeons and American College of Cardiology Transcatheter Valve Therapy Registry) and the TMVR Registry (The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry Mitral Module) have shown feasibility, high rates of technical success, and excellent short‐term outcomes for ViV TMVR. 13 , 14 Therefore, our study aimed to investigate the trends of use, outcomes, and cost of care among patients requiring repeat mitral valve intervention in a real‐world population from the National Inpatient Sample (NIS) database.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Data
The NIS database from September 2015 to December 2018 was used. The NIS is developed through a Federal‐State‐Industry partnership through the Agency for Healthcare Research and Quality. The NIS has over 8 million admissions and represents a 20% sample of all participating hospital admissions. The NIS is compiled annually, which would allow the data to be used for analysis of disease trends over time. 15 Institutional review board approval and informed consent were not required for this study given the deidentified nature of the database and its public availability.
Study Design and Data Selection
We analyzed data using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes. ICD‐10‐CM was introduced in the fourth quarter of 2015. Therefore, NIS data from the fourth quarter of 2015 to 2018 were used (Data S1, Table S1). Patients undergoing redo mitral valve replacement were identified by using ICD‐10‐CM codes for prosthetic valve dysfunction (T82.01XA, T82.02XA, T82.03XA, T82.09XA, T82.221A, T82.222A, T82.223A, T82.228A, Z45.09, Z95.2 and T82.857). ViV TMVR (ICD‐10‐CM codes 02RG37H,02RG37Z, 02RG38H, 02RG38Z,02RG3JH, 02RG3JZ, 02RG3KH, and 02RG3KZ) or redo SMVR (ICD‐10‐CM codes 02RG07Z, 02RG08Z, 02RG0KZ, and 02RG0JZ) were selected. We excluded young patients (aged <50 years), those with infective endocarditis, and those undergoing concomitant coronary artery bypass graft surgery. Discharge weight provided was used for analysis. For cost of care, charge‐to‐cost ratio supplied by the Healthcare Cost and Utilization Project derived from the Centers for Medicare and Medicaid Services were applied to total hospital charges. Cost was also adjusted for inflation (January 2020). Charlson Comorbidity Index scores were calculated for each group to assess the comorbidity burden in the study groups. A flowchart of our selection of patients is shown in Figure 1.
Figure 1. Flowchart of derivation of the study cohort.
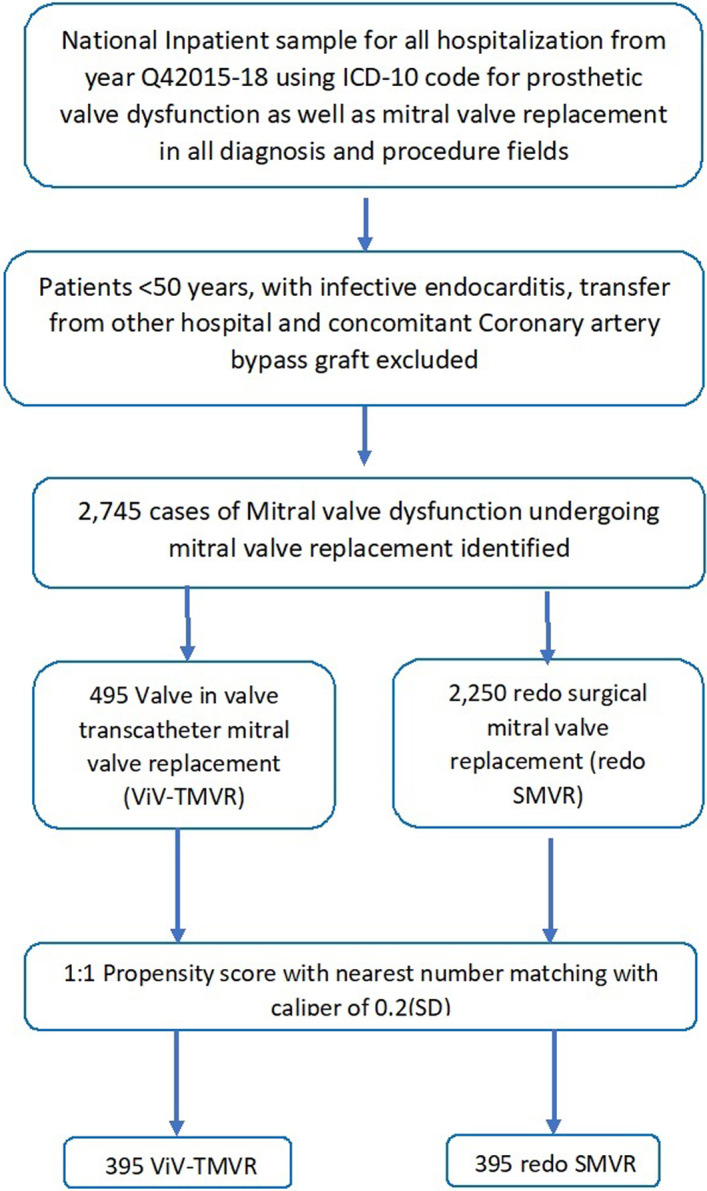
ICD‐10 indicates International Classification of Diseases, Tenth Revision; Q4, fourth quarter; SMVR, surgical mitral valve replacement; ViV TMVR, valve‐in‐valve transcatheter mitral valve replacement.
Study End Points
The primary outcomes of interest were in‐hospital mortality and periprocedural complications. Secondary end points were resource use and trends over time.
Statistical Analysis
Baseline characteristics were compared using a Pearsonχ2 exact test for categorical variables and independent‐samples t test for continuous variables. Categorical variables were presented as frequency and percentage, and continuous variables were reported as median with interquartile range (IQR).
Logistic regression analyses were performed for predictors of inpatient mortality using relevant demographic and clinical variables shown in Table 1. Cases with missing race or race other than White and Black, or Hispanic ethnicity, were categorized as other. Because of significant heterogeneity in the baseline characteristics of patients (Table 1), we developed a propensity score–matching model using logistic regression to derive 2 matched groups (redo SMVR versus ViV TMVR). A nearest neighbor 1:1 variable ratio, parallel, balanced propensity‐matching model was made using a caliper width of SD 0.2 (Figures S1–S4). Linear regression was used for trend analysis. For all analyses, a 2‐sided P value of 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 26 (IBM, Armonk, NY) and R version 3.5 (R Foundation for Statistical Computing, Vienna, Austria).
Table 1.
Basic Characteristics of the Patients Who Underwent Redo SMVR and ViV TMVR (2015–2018)
| Variable | Unmatched | Propensity matched | ||||
|---|---|---|---|---|---|---|
| Redo SMVR, 2250 | ViV TMVR, 495 | P value | Redo SMVR, 395 | ViV TMVR, 395 | SMD | |
| Age, y, median (IQR) | 68 (60–75) | 77 (70–83) | <0.01 | 75 (67–81) | 74 (68–80) | −0.075 |
| Women, n (%) | 1150 (51.1) | 260 (52.5) | 0.57 | 220 (55.7) | 240 (60.8) | 0.101 |
| Race/ethnicity, n (%) | ||||||
| White | 1585 (74.9) | 360 (80.9) | <0.01 | 280 (70.9) | 285 (72.2) | … |
| Black | 185 (8.7) | 50 (11.2) | 40 (10.1) | 35 (8.9) | 0.046 | |
| Hispanic | 140 (6.6) | 15 (3.4) | 10 (2.5) | 15 (3.8) | −0.052 | |
|
Other (Asians, native Americans or missing race information) |
205 (9.6) | 20 (4.5) | 65 (16.4) | 60 (15.2) | 0.090 | |
| Comorbidities and medical history | ||||||
| Deficiency anemia, n (%) | 100 (4.4) | <11 (<2.0) | 0.01 | <11 (<2.8) | 15 (3.8) | 0.061 |
| Atrial fibrillation, n (%) | 1565 (69.6) | 305 (61.6) | <0.01 | 255 (64.6) | 240 (60.8) | −0.082 |
| Congestive heart failure, n (%) | 1445 (64.2) | 415 (83.8) | <0.01 | 320 (81.0) | 305 (77.2) | −0.079 |
| Chronic obstructive pulmonary disease, n (%) | 575 (25.6) | 140 (28.3) | 0.21 | 110 (27.8) | 115 (29.1) | 0.029 |
| Coagulopathy, n (%) | 975 (43.3) | 80 (16.2) | <0.01 | 75 (19.0) | 110 (27.8) | 0.178 |
| Coronary artery disease, n (%) | 950 (42.2) | 345 (69.7) | <0.01 | 250 (63.3) | 225 (57.0) | −0.128 |
| Cerebrovascular disease, n (%) | 185 (8.2) | 30 (6.1) | 0.12 | 25 (6.3) | <11 (<2.8) | −0.139 |
| Diabetes mellitus, n (%) | 185 (8.2) | 40 (8.1) | 0.92 | 40 (10.1) | 50 (12.7) | 0.094 |
| Hypertension, n (%) | 1705 (75.8) | 405 (81.8) | 0.004 | 320 (81.0) | 320 (81.0) | 0 |
| Liver disease, n (%) | 140 (6.2) | 30 (6.1) | 0.89 | 25 (6.3) | 45 (11.4) | 0.209 |
| Obesity, n (%) | 325 (14.4) | 55 (11.1) | 0.05 | 45 (11.4) | 60 (15.2) | 0.109 |
| Peripheral vascular disease, n (%) | 250 (11.1) | 90 (18.2) | <0.01 | 60 (15.2) | 70 (17.7) | 0.081 |
| Renal failure, n (%) | 670 (29.8) | 165 (33.3) | 0.12 | 125 (31.6) | 125 (31.6) | 0 |
| Smoking, n (%) | 165 (7.3) | 30 (6.1) | 0.32 | 30 (7.6) | 30 (7.6) | 0 |
| Charlson Comorbidity Index, median (IQR) | 5 (4–7) | 6 (5–8) | <0.01 | … | … | |
| Urban/rural, n (%) | ||||||
| Rural | <11 (<0.5) | <11 (<2.0) | 0.09 | … | <11 (<2.8) | … |
| Urban, nonteaching | 235 (10.4) | 40 (8.1) | 40 (10.1) | 20 (5.1) | −0.165 | |
| Urban, teaching | 2005 (89.1) | 455 (91.9) | 355 (89.9) | 365 (92.4) | 0.081 | |
| Hospital size, n (%) | ||||||
| Small | 195 (8.7) | 45 (9.1) | <0.01 | 45 (11.4) | 25 (6.3) | … |
| Medium | 455 (20.2) | 55 (11.1) | 50 (12.7) | 55 (13.9) | 0.032 | |
| Large | 1600 (71.1) | 395 (79.8) | 300 (75.9) | 315 (79.7) | 0.084 | |
| Primary payer, n (%) | ||||||
| Medicare | 1455 (64.7) | 395 (79.8) | <0.01 | 300 (75.9) | 320 (81.0) | … |
| Medicaid | 155 (6.9) | 20 (4.0) | 20 (5.1) | 20 (5.1) | 0 | |
| Private insurance | 575 (25.6) | 65 (13.1) | 65 (16.5) | 50 (12.7) | −0.087 | |
| Other | 65 (2.9) | 15 (3.0) | <11 (<2.8) | <11 (<2.8) | −0.078 | |
The <11 numbers are not reported per data‐provider recommendations.
IQR indicates interquartile range; SMD, standard mean difference; SMVR, surgical mitral valve replacement; and ViV TMVR, valve‐in‐valve transcatheter mitral valve replacement.
Results
Between September 2015 to December 2018, 2745 patients underwent redo mitral valve procedures. A total of 495 and 2250 patients underwent redo ViV TMVR and SMVR, respectively (Table 1). The patients undergoing ViV TMVR were older at 77 years (IQR, 70–83 years) versus 68 years (IQR, 60–75 years) and had higher White (80.9% versus 74.9%) and Black (11.2% versus 8.7%) representation (P<0.01 for all). Use of redo SMVR was higher among Hispanic patients (6.6% versus 3.4%) (P<0.01 for all). The median Charlson Comorbidity Index was higher in the ViV TMVR group compared with the redo SMVR group (6 versus 5, P<0.01). Over the study period, the proportion of redo SMVRs was reduced significantly from 87.8% to 77.6% (P<0.01) (Figure 2).
Figure 2. Trends in proportion of patients undergoing redo SMVR and ViV TMVR, fourth quarter (Q4) of 2015 to 2018.
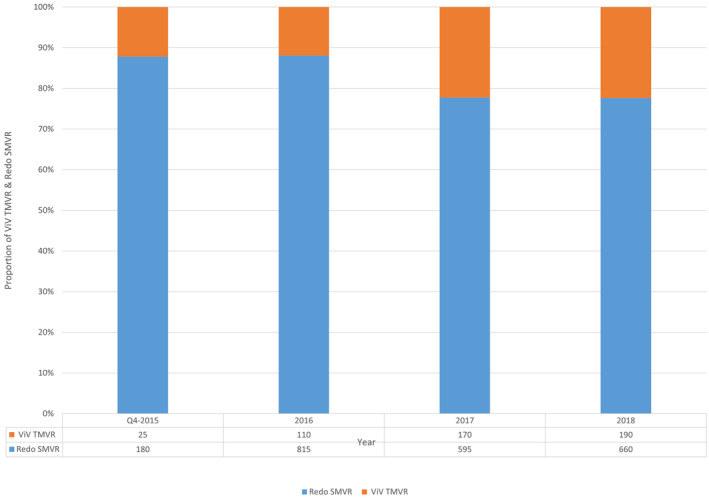
SMVR indicates surgical mitral valve replacement; and ViV TMVR, valve‐in‐valve transcatheter mitral valve replacement.
Clinical Outcomes in Matched Cohort
Adjusted mortality was much higher in the redo SMVR group (7.6%) as compared with the ViV TMVR group (<2.8%). A higher percentage of patients were discharged to home after ViV TMVR as compared with SMVR (64.6% versus 20.3%). Conversely, as compared with ViV TMVR, a higher number of patients undergoing redo SMVR were discharged to long‐term care facilities (44.3% versus 7.6%) (Table 2). Cost of care ($57 172 [IQR, $42 215–$86 803] versus $52 579 [IQR, $37 513–$69 408]) and length of stay (10 days [IQR, 7–16 days] versus 3 days [IQR, 1–8 days]) were considerably higher with SMVR. Rates of blood transfusion (38% versus 7.6%), acute kidney injury (36.7% versus 13.9%), and pneumonia (10.1% versus <2.8%) were higher in the SMVR group (Figure 3). Usage rate of extra corporeal membrane oxygenation was observed in the SMVR group (2.4% versus 0%, P<0.001). Residual atrial septal defect needing closure was needed in 8.9% of patients undergoing ViV TMVR.
Figure 3. Procedural outcomes in redo SMVR and ViV TMVR in the adjusted cohort.
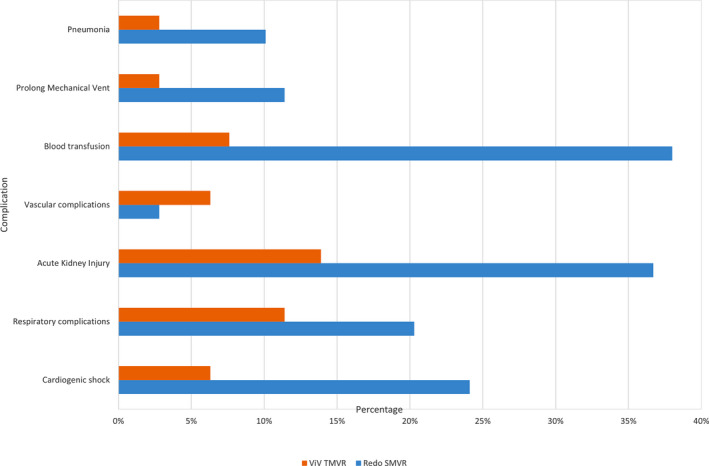
SMVR indicates surgical mitral valve replacement; and ViV TMVR, valve‐in‐valve transcatheter mitral valve replacement.
Temporal Trends
Over the study period, mortality in the redo SMVR group increased from 2.8% to 7.6% (P<0.01) and from 4.5% to 5.3% in the ViV TMVR group (P<0.01) (Figure 4). The median length of stay increased in the ViV TMVR group (1 day to 3 days, P<0.01) and marginally decreased for the redo SMVR group (8.5 days to 8 days, P<0.01) (Figure 5A). The median cost of stay remained similar during the study period in the ViV TMVR group ($49,279–$48.230, P<0.01) but increased for the redo SMVR group ($53,326–$57,338; P<0.01) (Figure 5B).
Figure 4. Trends in mortality in patients undergoing redo SMVR and ViV TMVR, fourth quarter 2015 to 2018.
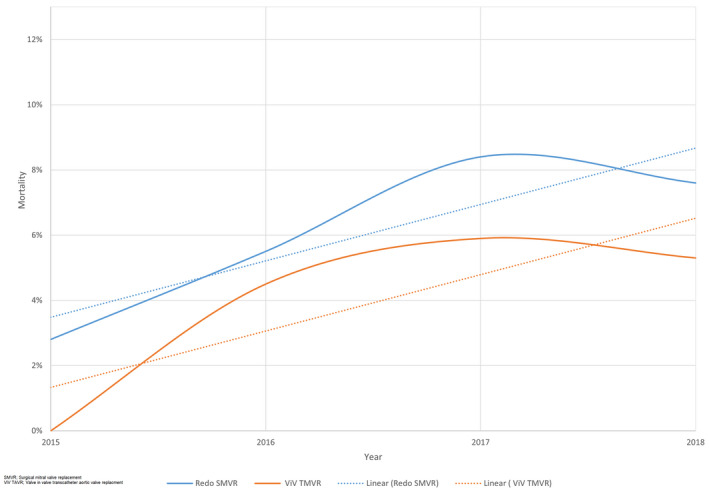
SMVR indicates surgical mitral valve replacement; and ViV TMVR, valve‐in‐valve transcatheter mitral valve replacement.
Figure 5. Trends in ViV TMVR and SMVR.
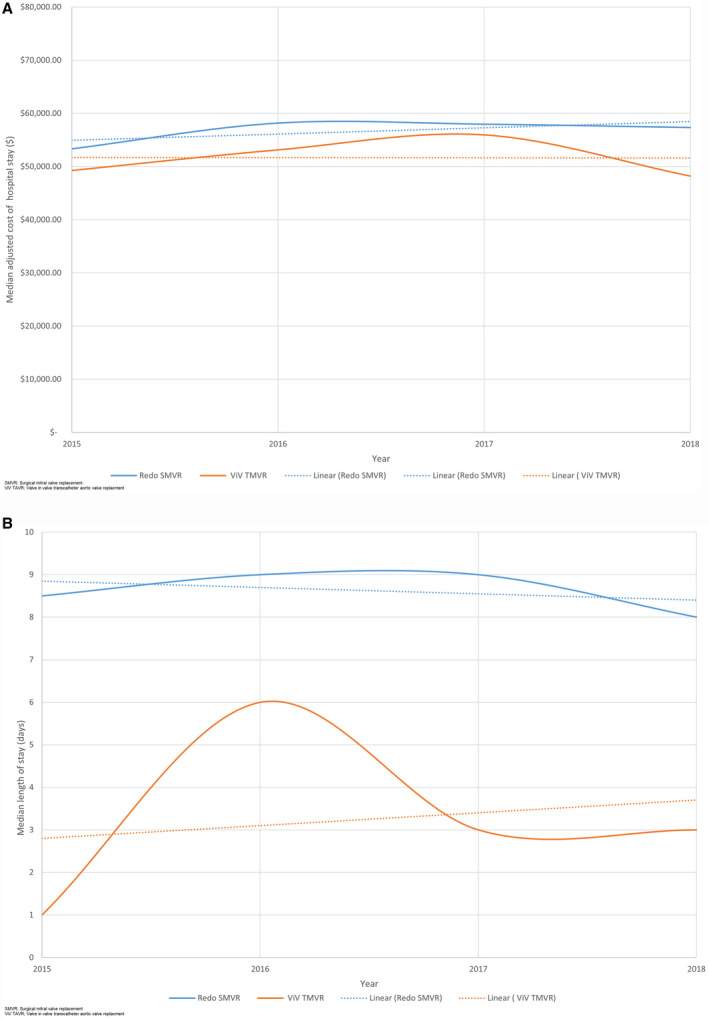
A, Trends in the length of stay in patients undergoing ViV TMVR and SMVR, fourth quarter (Q4) 2015 to 2018. B, Trends in cost of stay in patients undergoing ViV TMVR and SMVR, Q4 2015 to 2018. SMVR indicates surgical mitral valve replacement; and ViV TMVR, valve‐in‐valve transcatheter mitral valve replacement.
Predictors of Mortality
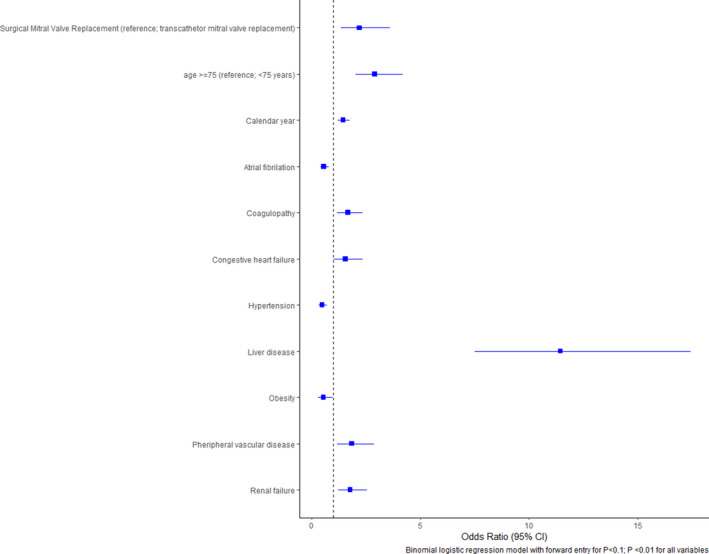
Logistic regression showed redo SMVR was associated with higher mortality (odds ratio [OR], 2.2 [95% CI, 1.3–3.6]; P<0.01). Factors associated with higher mortality included liver disease (OR, 11.4 [95% CI, 7.5–17.4]; P<0.01), age >75 years (OR, 2.9 [95% CI, 2–4.2]; P<0.01), peripheral vascular disease (OR, 1.8 [95% CI, 1.2–2.9]; P<0.01), and renal failure (OR, 1.8 [95% CI, 1.2–2.5]; P<0.01) (Figure 6).
Figure 6. Predictors of mortality in redo mitral valve replacement.
Discussion
We report the following main findings in our contemporary real‐world study of outcomes with redo SMVR compared with ViV TMVR. (1) The odds of in‐hospital mortality were significantly higher for patients undergoing redo SMVR. (2) Redo SMVR was associated with increased periprocedural complications. (3) The length of stay and cost of stay were significantly higher for patients with redo SMVR when compared with ViV TMVR. (4) A minority of patients underwent additional tricuspid surgery in the SMVR group and iatrogenic atrial septal defect closure in the ViV TMVR group.
Our study provides data on the outcomes and use of resources of ViV TMVR compared with surgical SMVR from a real‐world sample using the NIS database. The first ViV TMVR for failed bioprosthetic mitral valve was reported in 2010, which was performed by a transseptal approach. 16 With advancements in imaging technology with regard to preprocedural planning using computed tomography, intraprocedural echocardiography guidance, and the adaptation of the transcatheter aortic valves to mitral location, TMVR has emerged as an alternative to conventional redo mitral valve surgery in patients at high or prohibitive surgical risk. 11 , 17 , 18 In our nationally representative sample of hospitalizations in the United States, the use of ViV TMVR for the treatment of degenerated bioprosthetic valves has increased steadily over the years from 2015 to 2018, which represents adoption of this technique at a national level in the United States. Our findings are similar to the registry data that showed ViV TMVR use has increased over the years in patients with high or prohibitive surgical risk with demonstrated safety and feasibility of the procedure. 13 , 14 , 19 , 20
Similar to other studies, the phenotype of patients undergoing ViV TMVR group was older, with higher burden of comorbidities, indicating that a greater proportion of patients may be at high surgical risk. Despite the high comorbidity burden in the ViV TMVR group, in‐hospital mortality was significantly higher in the redo SMVR group. Multiple previous studies have reported that redo SMVR is associated with higher periprocedural mortality. 21 , 22 Despite the higher periprocedural mortality of redo SMVR, it has been reported that 30‐day and 1‐year mortality is comparable between the 2 groups. 11 , 23 However, the findings of these studies should be interpreted with caution, because they were small retrospective studies with limited sample size (Simonetto et al, n=78; Kamioka et al, n=121). 11 , 23 The mortality rate of 5.3% in our study in the TMVR group was similar to the in‐hospital mortality of 6.3% reported between 2013 and 2017 from the STS/ACC TV Registry and all‐cause mortality of 6.2% in the ViV TMVR group from the multicenter TMVR Registry. 14 ViV TMVR is predominantly performed via a transapical approach because of ease of technique. However, there has been an increase in the use of transseptal access over time. 19 , 24 , 25 Although not evaluated in our study, recent data show transseptal access to be associated with lower mortality compared with transapical access and needs confirmation in larger studies. 24
Complications like acute kidney injury contribute to morbidity and have been studied extensively in patients undergoing mitral valve surgery. 12 , 26 Risk factors for acute kidney injury include the presence of multiple comorbidities, low left ventricular systolic function and cardiorenal syndrome, and postoperative low cardiac output state. Registry data suggest that acute kidney injury also occurs in approximately 5% of patients undergoing ViV TMVR. 13 Redo mitral valve surgery poses a nontrivial risk of complete heart block requiring permanent pacemaker placement. 26 , 27 On the contrary, the risk of complete heart block with ViV TMVR appears to be trivial to nonexistent. 13 Our study complements the findings of previous studies that reported higher use of resources in terms of length and cost of stay along with greater proportion of nonhome discharges in patients undergoing redo SMVR. Despite the lower mean age and the Society of Thoracic Surgeons Predicted Risk Mortality score, total procedure time, intensive care unit stay, and total hospital stay were significantly higher in the surgical cohort compared with ViV TMVR in the study reported by Kamioka et al. 11
We report the rate of concomitant tricuspid valve surgery at 2.7% in the redo SMVR group. Right‐sided valve involvement and right ventricular dysfunction are seen in patients with mitral valve disease. The prognostic impact of significant tricuspid regurgitation in those with left‐sided valve disease and after transcatheter interventions of mitral and aortic valves is well established. 28 , 29 , 30 Presence of moderate tricuspid regurgitation is associated with worse prognosis in patients after redo valve surgery. 29 Societal guidelines recommend correction of tricuspid pathology at the time of left‐sided valve surgery, and hence, a proportion of redo SMVR patients undergo tricuspid valve surgery. 31 Transcatheter management of tricuspid regurgitation is evolving and may become an option for those undergoing ViV TMVR with significant tricuspid regurgitation. The use of transseptal access for ViV TMVR can result in iatrogenic atrial septal defect, and a minority need closure. The rates of atrial septal defect closure were 7% in the multicenter transcatheter valve therapy registry and are similar to the rates of atrial septal defect closure of 8% in our study. 13
Patient selection for either ViV TMVR or redo SMVR should be individualized to optimize outcomes. Heart teams should also acknowledge the lack of data on long‐term outcomes and durability of ViV TMVR. Limited data show gradients at 1 year, which averaged around 7 mm Hg after the ViV TMVR. Mean gradients are lower with the larger‐size Sapien valve compared with smaller‐size Sapien valve. Furthermore, the 1‐year mortality was higher with use of a smaller‐size (20 and 23 mm) Sapien 3 valve compared with the larger‐size valves (26 or 29 mm) for those undergoing ViV TMVR. 24 Nonetheless, in the absence of advanced heart failure and futility, institutional expertise, anatomic factors such as size of the prosthesis, and risk of left ventricular outflow tract obstruction, the need for revascularization and presence of significant tricuspid regurgitation should guide patient selection.
Our study is constrained by the inherent limitations of the NIS database. The NIS is an administrative claim‐based database that uses ICD‐10‐CM codes for diagnosis, which may be subject to error. NIS collects data on inpatient discharges, and each admission is registered as an independent event. NIS samples are not designed to follow patients longitudinally, so long‐term outcomes could not be assessed from the present data set. Like any retrospective database study, association does not mean causation, and a conclusion should be drawn cautiously. Because of the inherent shortcomings of the NIS database, we are unable to assess variables such as type of valve, valve area, and echocardiographic data. The proportion of patients with prior mitral valve repair is not known because of the lack of a distinct ICD code for the entity. Data on the rates of residual mitral regurgitation, left ventricular outflow tract obstruction, and route of access (transapical versus transseptal) were not available. Similarly, pathology of tricuspid valve and right‐heart function could not be assessed. We could also not assess short‐term outcomes such as valve thrombosis and postprocedure gradients, and mid‐ and long‐term outcomes, because of the limitations of the database. The long‐term durability of ViV TMVR also needs to be demonstrated in future studies. The NIS provides insights into the national experience with redo SMVR and ViV TMVR. However, it is important to mention that outcomes vary depending on the center where the procedure is performed, with some centers reporting an operative mortality as low as 0.75% after isolated redo mitral valve replacement surgery. 32 Moreover, a more contemporary Society of Thoracic Surgeons database analysis of mitral valve reoperations reported an operative mortality of 3.4% for elective nonendocarditis‐related mitral valve surgery. 7
Conclusions
We report real‐world data on in‐hospital outcomes of ViV TMVR and redo SMVR for degenerated bioprosthetic valves in the mitral location. ViV TMVR is associated with lower mortality, periprocedural morbidity, and resource use compared with patients with redo SMVR. Early data point toward elevated mean gradients, especially with use of smaller‐size valves for ViV TMVR. A patient‐centered approach by the heart team, local institutional expertise, and careful preprocedure planning can guide decision‐making about the choice of intervention for the individual patient. More data on the durability and long‐term outcomes of ViV TMVR are needed to extend its candidacy beyond inoperable or prohibitive surgical risk.
Sources of Funding
None.
Disclosures
None.
Table 2.
Clinical Outcomes and Hospital Use in Patients Who Underwent Redo SMVR and ViV TMVR (2015–2018)
| Variable | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Redo SMVR, 2250 | ViV TMVR, 495 | P value | Redo SMVR, 395 | ViV TMVR, 395 | P value | |
| In‐hospital mortality, n (%) | 150 (6.7) | 25 (5.1) | 0.18 | 30 (7.6) | <11 (<2.8) | 0.001 |
| Home discharge, n (%) | 655 (29.1) | 295 (59.6) | <0.01 | 80 (20.3) | 255 (64.6) | <0.01 |
| Skilled nursing care, n (%) | 640 (28.4) | 45 (9.1) | 175 (44.3) | 30 (7.6) | ||
| Home with home health, n (%) | 800 (35.6) | 125 (25.3) | 110 (27.8) | 95 (24.1) | ||
| Cardiogenic shock, n (%) | 460 (20.4) | 45 (9.1) | <0.01 | 95 (24.1) | 25 (6.3) | <0.01 |
| Respiratory complications, n (%) | 305 (13.6) | 60 (12.1) | 0.4 | 80 (20.3) | 45 (11.4) | <0.01 |
| Acute kidney injury, n (%) | 695 (30.9) | 85 (17.2) | <0.01 | 145 (36.7) | 55 (13.9) | <0.01 |
| Stroke, n (%) | 80 (3.6) | <11 (<2.2) | 0.12 | … | <11 (<2.8) | … |
| Vascular complications, n (%) | 40 (1.8) | 30 (6.1) | <0.01 | <11 (<2.8) | 25 (6.3) | 0.01 |
| Blood transfusion, n (%) | 795 (35.3) | 35 (7.1) | <0.01 | 150 (38.0) | 30 (7.6) | <0.01 |
| Cardiac arrest with CPR, n (%) | 15 (0.7) | <11 (<2.2) | 0.004 | … | … | … |
| Prolong mechanical ventilation, n (%) | 165 (7.3) | <11 (<2.2) | <0.01 | 45 (11.4) | <11 (<2.8) | <0.01 |
| Pneumonia, n (%) | 145 (6.4) | <11 (<2.2) | <0.01 | 40 (10.1) | <11 (<2.8) | <0.01 |
| Urinary tract infection, n (%) | 160 (7.1) | 25 (5.1) | 0.1 | 40 (10.1) | 25 (6.3) | 0.05 |
| Pericardial effusion, n (%) | 30 (1.3) | 15 (3.0) | 0.01 | <11 (<2.8) | <11 (<2.8) | 1 |
| PPM, n (%) | 200 (8.9) | <11 (<2.2) | <0.01 | 15 (3.8) | <11 (<2.8) | 0.31 |
| ECMO, n (%) | 70 (3.1) | … | … | <11 (<2.8) | … | … |
| LVAD, n (%) | 20 (0.9) | … | 0.04 | … | … | … |
| ASD transcatheter repair, n (%) | … | 40 (8.1) | <0.01 | … | 35 (8.9) | <0.01 |
| Tricuspid valve replacement, n (%) | 65 (2.9) | … | <0.01 | … | 15 (3.8) | <0.01 |
| Length of stay, d, median (IQR) | 9 (7–15) | 3 (1–8) | <0.01 | 10 (7–16) | 3 (1–8) | <0.01 |
| Cost of hospitalization, $, median (IQR) | 57 800 (42 925–89 776) | 51 675 (3869–67 626) | <0.01 | 57 172 (42 215–86 803) | 52 579 (37 513–69 408) | <0.01 |
The <11 numbers are not reported per data‐provider recommendations.
ASD indicates atrial septal defect; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; LVAD, left ventricular assist device; PPM, permanent pacemaker; SMVR, surgical mitral valve replacement; and ViV TMVR, valve‐in‐valve transcatheter mitral valve replacement.
Supporting information
Data S1
Table S1
Figures S1–S4
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.020948
For Sources of Funding and Disclosures, see page 10.
References
- 1. Gammie JS, Chikwe J, Badhwar V, Thibault DP, Vemulapalli S, Thourani VH, Gillinov M, Adams DH, Rankin JS, Ghoreishi M, et al. Isolated mitral valve surgery: the society of thoracic surgeons adult cardiac surgery database analysis. Ann Thorac Surg. 2018;106:716–727. DOI: 10.1016/j.athoracsur.2018.03.086. [DOI] [PubMed] [Google Scholar]
- 2. Goldstone AB, Chiu P, Baiocchi M, Lingala B, Patrick WL, Fischbein MP, Woo YJ. Mechanical or biologic prostheses for aortic‐valve and mitral‐valve replacement. N Engl J Med. 2017;377:1847–1857. DOI: 10.1056/NEJMoa1613792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones JM, O’Kane H, Gladstone DJ, Sarsam MAI, Campalani G, MacGowan SW, Cleland J, Cran GW. Repeat heart valve surgery: Risk factors for operative mortality. J Thorac Cardiovasc Surg. 2001;122:913–918. DOI: 10.1067/mtc.2001.116470. [DOI] [PubMed] [Google Scholar]
- 4. Thourani VH, Weintraub WS, Guyton RA, Jones EL, Williams WH, Elkabbani S, Craver JM. Outcomes and long‐term survival for patients undergoing mitral valve repair versus replacement: Effect of age and concomitant coronary artery bypass grafting. Circulation. 2003;108:298–304. DOI: 10.1161/01.CIR.0000079169.15862.13. [DOI] [PubMed] [Google Scholar]
- 5. Potter DD, Sundt TM, Zehr KJ, Dearani JA, Daly RC, Mullany CJ, McGregor CGA, Puga FJ, Schaff HV, Orszulak TA. Risk of repeat mitral valve replacement for failed mitral valve prostheses. Ann Thorac Surg. 2004;78:67–72. DOI: 10.1016/j.athoracsur.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 6. Mehaffey HJ, Hawkins RB, Schubert S, Fonner C, Yarboro LT, Quader M, Speir A, Rich J, Kron IL, Ailawadi G. Contemporary outcomes in reoperative mitral valve surgery. Heart. 2018;104:652–656. DOI: 10.1136/heartjnl-2017-312047. [DOI] [PubMed] [Google Scholar]
- 7. Kilic A, Acker MA, Gleason TG, Sultan I, Vemulapalli S, Thibault D, Ailawadi G, Badhwar V, Thourani V, Kilic A. Clinical outcomes of mitral valve reoperations in the United States: an analysis of the society of thoracic surgeons national database. Ann Thorac Surg. 2019;107:754–759. DOI: 10.1016/j.athoracsur.2018.08.083. [DOI] [PubMed] [Google Scholar]
- 8. Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S, Barbanti M, Latib A, Schaefer U, Rodés‐Cabau J, et al. Investigators for the V‐VIDR. transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162–170. DOI: 10.1001/jama.2014.7246. [DOI] [PubMed] [Google Scholar]
- 9. Schäfer U, Bader R, Frerker C, Schewel D, Thielsen T, Schmoeckel M, Kreidel F, Kuck KH. Balloon‐expandable valves for degenerated mitral xenografts or failing surgical rings. EuroIntervention. 2014;10:260–268. DOI: 10.4244/EIJV10I2A42. [DOI] [PubMed] [Google Scholar]
- 10. Wilbring M, Alexiou K, Tugtekin SM, Sill B, Hammer P, Schmidt T, Simonis G, Matschke K, Kappert U. Transapical transcatheter valve‐in‐valve implantation for deteriorated mitral valve bioprostheses. Ann Thorac Surg. 2013;95:111–117. DOI: 10.1016/j.athoracsur.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 11. Kamioka N, Babaliaros V, Morse MA, Frisoli T, Lerakis S, Iturbe JM, Binongo J, Corrigan F, Yousef A, Gleason P, et al. Comparison of clinical and echocardiographic outcomes after surgical redo mitral valve replacement and transcatheter mitral valve‐in‐valve therapy. JACC Cardiovasc Interv. 2018;11:1131–1138. DOI: 10.1016/j.jcin.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 12. Murzi M, Berti S, Gasbarri T, Trianni G, Maffei S, Solinas M, Dvir D, Cerillo AG. Transapical transcatheter mitral valve‐in‐valve implantation versus minimally invasive surgery for failed mitral bioprostheses. Interact Cardiovasc Thorac Surg. 2017;25(1):57–61. DOI: 10.1093/icvts/ivx067. [DOI] [PubMed] [Google Scholar]
- 13. Yoon S‐H, Whisenant BK, Bleiziffer S, Delgado V, Dhoble A, Schofer N, Eschenbach L, Bansal E, Murdoch DJ, Ancona M, et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J. 2019;40:441–451. DOI: 10.1093/eurheartj/ehy590. [DOI] [PubMed] [Google Scholar]
- 14. Guerrero M, Vemulapalli S, Xiang Q, Wang DD, Eleid M, Cabalka AK, Sandhu G, Salinger M, Russell H, Greenbaum A, et al. Thirty‐Day outcomes of transcatheter mitral valve replacement for degenerated mitral bioprostheses (Valve‐in‐Valve), failed surgical rings (Valve‐in‐Ring), and native valve with severe mitral annular calcification (valve‐in‐mitral annular calcification) in the United States: Data from the society of thoracic surgeons/American College of Cardiology/Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2020;13:e008425. DOI: 10.1161/CIRCINTERVENTIONS.119.008425. [DOI] [PubMed] [Google Scholar]
- 15. Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the National Inpatient Sample. JAMA ‐ J Am Med Assoc. 2017;318:2011–2018. DOI: 10.1001/jama.2017.17653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Webb JG, Wood DA, Ye J, Gurvitch R, Masson J‐B, Rodés‐Cabau J, Osten M, Horlick E, Wendler O, Dumont E, et al. Transcatheter valve‐in‐valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121:1848–1857. DOI: 10.1161/CIRCULATIONAHA.109.924613. [DOI] [PubMed] [Google Scholar]
- 17. Guerrero M, Salinger M, Pursnani A, Pearson P, Lampert M, Levisay J, Russell H, Feldman T. Transseptal transcatheter mitral valve‐in‐valve: A step by step guide from preprocedural planning to postprocedural care. Catheter Cardiovasc Interv. 2018;92:E185–E196. DOI: 10.1002/ccd.27128. [DOI] [PubMed] [Google Scholar]
- 18. Hamid NB, Khalique OK, Monaghan MJ, Kodali SK, Dvir D, Bapat VN, Nazif TM, Vahl T, George I, Leon MB, et al. Transcatheter valve implantation in failed surgically inserted bioprosthesis review and practical guide to echocardiographic imaging in valve‐in‐valve procedures. JACC Cardiovasc Imaging. 2015;8:960–979. DOI: 10.1016/j.jcmg.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 19. Urena M, Himbert D, Brochet E, Carrasco JL, Iung B, Nataf P, Vahanian A. Transseptal transcatheter mitral valve replacement using balloon‐expandable transcatheter heart valves: a step‐by‐step approach. JACC Cardiovasc Interv. 2017;10:1905–1919. DOI: 10.1016/j.jcin.2017.06.069. [DOI] [PubMed] [Google Scholar]
- 20. Werner N, Kilkowski C, Sutor D, Weisse U, Schneider S, Zahn R. Transcatheter mitral valve implantation (TMVI) Using Edwards SAPIEN 3 prostheses in patients at very high or prohibitive surgical risk: a single‐center experience. J Interv Cardiol 2020;2020:9485247. DOI: 10.1155/2020/9485247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ejiofor JI, Hirji SA, Ramirez‐Del Val F, Norman AV, McGurk S, Aranki SF, Shekar PS, Kaneko T. Outcomes of repeat mitral valve replacement in patients with prior mitral surgery: A benchmark for transcatheter approaches. J Thorac Cardiovasc Surg. 2018;156:619–627.e1. DOI: 10.1016/j.jtcvs.2018.03.126. [DOI] [PubMed] [Google Scholar]
- 22. Akins CW, Buckley MJ, Daggett WM, Hilgenberg AD, Vlahakes GJ, Torchiana DF, Madsen JC. Risk of reoperative valve replacement for failed mitral and aortic bioprostheses. Ann Thorac Surg. 1998;65:1545–1552. DOI: 10.1016/S0003-4975(98)00301-4. [DOI] [PubMed] [Google Scholar]
- 23. Simonetto F, Purita PAM, Malerba M, Barbierato M, Pascotto A, Mangino D, Zanchettin C, Tarantini G, Gerosa G, D'Onofrio A, et al. Surgical redo versus transseptal or transapical transcatheter mitral valve‐in‐valve implantation for failed mitral valve bioprosthesis. Catheter Cardiovasc Interv. 2021;97:714–722. DOI: 10.1002/ccd.29324. [DOI] [PubMed] [Google Scholar]
- 24. Whisenant B, Kapadia SR, Eleid MF, Kodali SK, McCabe JM, Krishnaswamy A, Morse M, Smalling RW, Reisman M, Mack M, et al. One‐year outcomes of mitral valve‐in‐valve using the SAPIEN 3 transcatheter heart valve. JAMA Cardiol. 2020;5:1245–1252. DOI: 10.1001/jamacardio.2020.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paradis JM, Del TM, Puri R, Rodés‐Cabau J. Transcatheter valve‐in‐valve and valve‐in‐ring for treating aortic and mitral surgical prosthetic dysfunction. J Am Coll Cardiol. 2015;66:2019–2037. DOI: 10.1016/j.jacc.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 26. Vohra HA, Whistance RN, Roubelakis A, Burton A, Barlow CW, Tsang GMK, Livesey SA, Ohri SK. Outcome after redo‐mitral valve replacement in adult patients: A 10‐year single‐centre experience. Interact Cardiovasc Thorac Surg. 2012;14:575–579. DOI: 10.1093/icvts/ivs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kho J, Ioannou A, O’Sullivan KE, Jones M. Permanent pacemaker implantation rates following cardiac surgery in the modern era. Ir J Med Sci. 2020;189:1289–1294. DOI: 10.1007/s11845-020-02254-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hahn RT, Asch F, Weissman NJ, Grayburn P, Kar S, Lim S, Ben‐Yehuda O, Shahim B, Chen S, Liu M, et al. Impact of tricuspid regurgitation on clinical outcomes: The COAPT trial. J Am Coll Cardiol. 2020;76:1305–1314. DOI: 10.1016/j.jacc.2020.07.035. [DOI] [PubMed] [Google Scholar]
- 29. Fukunaga N, Okada Y, Konishi Y, Murashita T, Kanemitsu H, Koyama T. Impact of tricuspid regurgitation after redo valvular surgery on survival in patients with previous mitral valve replacement. J Thorac Cardiovasc Surg. 2014;148:1983–1988. DOI: 10.1016/j.jtcvs.2013.08.089. [DOI] [PubMed] [Google Scholar]
- 30. Mangieri A, Montalto C, Pagnesi M, Jabbour RJ, Rodés‐Cabau J, Moat N, Colombo A, Latib A. Mechanism and implications of the tricuspid regurgitation: from the pathophysiology to the current and future therapeutic options. Circ Cardiovasc Interv. 2017;10. DOI: 10.1161/CIRCINTERVENTIONS.117.005043. [DOI] [PubMed] [Google Scholar]
- 31. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Muñoz D, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2786. DOI: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 32. Javadikasgari H, Chemtob RA, Gillinov AM, Pettersson GB, Lowry AM, Desai MY, Svensson LG, Blackstone EH, Wierup P. Outcomes of mitral valve re‐replacement for bioprosthetic structural valve deterioration. J Thorac Cardiovasc Surg. 2020;S0022–5223(20):32455–32457. DOI: 10.1016/j.jtcvs.2020.08.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1
Figures S1–S4


