Abstract
Background
Testosterone treatment is common in men, although risks for major cardiovascular events are unclear.
Methods and Results
A study was conducted in US male veterans, aged ≥40 years, with low serum testosterone and multiple medical comorbidities and without history of myocardial infarction, stroke, venous thromboembolism, prostate cancer, or testosterone treatment in the prior year. For the primary outcome, we examined if testosterone treatment was associated with a composite cardiovascular outcome (incident myocardial infarction, ischemic stroke, or venous thromboembolism). Testosterone use was modeled as intramuscular or transdermal and as current use, former use, and no use. Current testosterone users were compared with former users to reduce confounding by indication. The cohort consisted of 204 857 men with a mean (SD) age of 60.9 (9.9) years and 4.7 (3.5) chronic medical conditions. During follow‐up of 4.3 (2.8) years, 12 645 composite cardiovascular events occurred. In adjusted Cox regression analyses, current use of transdermal testosterone was not associated with risk for the composite cardiovascular outcome (hazard ratio [HR], 0.89; 95% CI, 0.76–1.05) in those without prevalent cardiovascular disease, and in those with prevalent cardiovascular disease was associated with lower risk (HR, 0.80; 95% CI, 0.70–0.91). In similar analyses, current use of intramuscular testosterone was not associated with risk for the composite cardiovascular outcome in men without or with prevalent cardiovascular disease (HR, 0.91; 95% CI, 0.80–1.04; HR, 0.98; 95% CI, 0.89–1.09, respectively).
Conclusions
In a large cohort of men without a history of myocardial infarction, stroke, or venous thromboembolism, testosterone treatment was not associated with increased risk for incident composite cardiovascular events.
Keywords: cohort study, myocardial infarction, stroke, testosterone, thrombosis
Subject Categories: Cardiovascular Disease, Myocardial Infarction, Ischemic Stroke, Thrombosis, Epidemiology
Nonstandard Abbreviations and Acronyms
- VA
Veterans Affairs
- VHA
Veterans Health Administration
Clinical Perspective
What Is New?
Using a database of a large, well‐characterized cohort, we examined the risk of both composite major cardiovascular outcomes (myocardial infarction, stroke, thrombosis) and respective components separately among men with low testosterone levels and without testosterone treatment at baseline, and examined risks associated with testosterone initiation.
Among men with low testosterone levels, the current use of intramuscular and transdermal testosterone (compared with previous use) was not associated with increased risk for composite or separate cardiovascular outcomes, regardless of whether they have cardiovascular disease or not.
What Are the Clinical Implications?
The results from this large cohort study provide some reassurance that testosterone treatment does not appear to be associated with significant risk for major cardiovascular events.
However, because of the observational study design and nonrandomization to treatment, residual confounding cannot be excluded, and a large, randomized, double‐blind, placebo‐controlled trial is needed to definitively assess the cardiovascular risks of testosterone.
Until a large, randomized trial has been completed, clinicians should follow testosterone treatment guidelines and carefully review specific risks and benefits of testosterone treatment with patients.
Testosterone prescriptions in the United States increased rapidly from 2000 to 2013 associated with direct‐to‐consumer advertising. 1 , 2 , 3 However, safety concerns developed after studies reported increased cardiovascular events, 4 , 5 , 6 increased cardiovascular events with intramuscular testosterone, 5 , 7 , 8 , 9 and that cardiovascular events occurred shortly after testosterone was initiated. 5 , 10 , 11 , 12 , 13 , 14 However, other studies failed to detect overall cardiovascular risks, 7 , 8 , 9 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 and regulatory agencies issued contradictory assessments of cardiovascular risks of testosterone treatment. 26 , 27 , 28 The conflicting results of prior studies may be attributable to cohorts with different cardiovascular morbidity and gonadal status (eugonadal, hypogonadal, or unknown). Another limitation has been the use of testosterone exposure models that characterized testosterone use as any use versus no use. 4 , 8 , 12 , 16 , 19 , 23 , 24 Although this model is often used in pharmaco‐epidemiologic studies, it is limited by an inability to account for intermittent or discontinued treatment or to ascertain whether cardiovascular events occurred during testosterone use. The objective of this study was to determine if testosterone treatment was associated with risk for major cardiovascular events in men with age‐related low testosterone. We examined the association of testosterone treatment with a composite cardiovascular end point composed of major, new‐onset cardiovascular events of myocardial infarction (MI), ischemic stroke, and venous thromboembolism (VTE). We addressed limitations of prior studies by modeling testosterone exposure as current use and former use to account for intermittent treatment and to ascertain if cardiovascular events occurred with current use of testosterone. We hypothesized that any potential risks associated with testosterone were attributable to a “drug‐in‐the‐blood” effect and that highest risk would occur during current use of testosterone.
Methods
Design and Data Source
We conducted a cohort study of men with low serum testosterone who were followed for testosterone initiation and incident cardiovascular events. We collected data from the Veterans Health Administration (VHA), the largest integrated healthcare system in the United States, and from the Centers for Medicare and Medicaid Services. The data from the VHA Corporate Data Warehouse included demographics, laboratory data, pharmacy data, diagnostic codes (International Classification of Diseases, Ninth Revision [ICD‐9]), and procedure codes (Current Procedural Terminology). 29 The Centers for Medicare and Medicaid Services data included diagnostic codes for MI, ischemic stroke, VTE, and prescription data (testosterone and anticoagulants). The Department of Veterans Affairs (VA) Institutional Review Board in Seattle, Washington, approved this study and waived the requirement for informed consent. Data sharing is not permitted by the VA. However, all source data for the study are available through the VA and can be accessed with proper VA Research and Development regulatory approvals.
Study Cohort
The cohort included male veterans aged 40 to 89 years with low serum testosterone measured between January 1, 2002, and December 31, 2011; at least 2 clinic visits in the prior year, no testosterone treatment or prostate‐specific antigen ≥4.0 ng/dL in the prior year; and no history of prostate or breast cancer as these are exclusions in testosterone treatment studies. Men were classified as having low serum testosterone if they had a testosterone measure flagged as low by the testing laboratory. Subjects with missing race, body mass index (BMI), or region were excluded from the analysis. Additional restrictions in the study cohort are specific to individual analyses; see Outcomes below.
Testosterone Exposure and Follow‐up Levels
Prescription data included testosterone formulation, fill date, and prescription duration. Testosterone formulations consisted of intramuscular and transdermal testosterone. Intramuscular formulations were primarily intermediate‐acting testosterone (enanthate or cypionate) and transdermal formulations were testosterone patch or gel. Intramuscular and transdermal testosterone formulations were tracked separately. For each formulation, we modeled time‐varying testosterone exposure as current use (current filled prescription), former use (prior prescription during the study, but no current filled prescription for that formulation), or no use (no testosterone prescription filled during the study). All subjects were nonusers of testosterone when they entered the cohort and became current users on the date a testosterone prescription was filled. Current use lasted for the prescription duration plus a 20% overrun of the prescription duration to account for refill time, noncompliance, or residual effects. Testosterone treatment status was continuously updated during the study, so that a subject could switch from current use to former use and then back to current use for each formulation. Follow‐up total testosterone levels were summarized for current testosterone users and nonusers who had testosterone levels assessed during the year after cohort entry.
Outcomes
The primary outcome was an incident composite cardiovascular end point, composed of incident MI, ischemic stroke, or VTE (pulmonary embolism or deep venous thrombosis). Men were excluded from the primary outcome if they had prior diagnoses of MI, ischemic stroke, or VTE. Secondary analyses considered separate outcomes of MI, ischemic stroke, and VTE. Men were excluded from the secondary analyses if they had a prior event of that type; for example, men with a history of MI were excluded from the MI analysis but were included in the VTE and stroke analyses. Cardiovascular outcomes were identified using inpatient diagnostic codes (from VHA and Centers for Medicare and Medicaid Services data), procedure codes, and medications (Table S1).
Medical Comorbidities
We characterized baseline and emerging medical conditions (diagnosed after cohort entry) using ICD‐9 diagnostic and procedure codes, Current Procedural Terminology procedure codes, medications, and laboratory results (Table S2). The medical comorbidities were time‐varying covariates that could change from absent to present over the course of the study but, once identified as present, were maintained as present. For example, once a patient was identified as having diabetes mellitus, they were classified as having diabetes mellitus for the remainder of the study. Medical comorbidities included chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, sexual dysfunction, hyperlipidemia, hypertension, major depression, morbid obesity, polycythemia, sleep apnea, smoking, and prevalent cardiovascular disease (CVD). Prevalent CVD included arrhythmia, angina, coronary artery disease, chronic heart failure, cardiomyopathy, peripheral vascular disease, cerebrovascular disease, transient ischemic attack, and cardiovascular procedures (angioplasty, bypass, stent placement). In secondary analyses for separate outcomes, prevalent CVD also included MI, ischemic stroke, or VTE except in analyses for that specific outcome. Finally, in analyses for VTE and the composite end point, we adjusted for malignancy (excluding basal cell skin cancer).
Statistical Analysis
We conducted Cox regression analyses to estimate the association between testosterone treatment and an incident composite cardiovascular end point and, in secondary analyses, the association of testosterone treatment with separate outcomes of incident MI, stroke, or VTE. The assumption of proportional hazards was confirmed via Schoenfeld residuals. Cohort entry was the date of the first low serum testosterone measure. Men were followed until a cardiovascular event, death, or end of follow‐up (September 30, 2012). Testosterone treatment was modelled separately for intramuscular and transdermal testosterone and updated continuously. Men who were current users of testosterone were compared with former users to reduce confounding by indication. In the regression analyses, we adjusted for baseline covariates and for emerging medical conditions that occurred following cohort entry. At baseline, we adjusted for age, race, region, year of cohort entry, BMI, hospitalization, medical comorbidities, and prevalent CVD. For conditions that occurred after baseline, we used time‐varying adjustments for hospitalization, medical comorbidities, and prevalent CVD. We also estimated how treatment effects varied by presence of CVD by including an interaction between time‐varying testosterone treatment status and CVD. In sensitivity analyses, we examined results using different definitions of current testosterone treatment that consisted of prescription duration plus an overrun ranging from 0% to 40% of the prescription duration. We also examined risk associated with testosterone initiation by comparing incidence rates for events in men with continuous treatment at 3, 6, and 12 months following testosterone initiation to the overall incidence rate associated with continuous testosterone treatment. Finally, we conducted an exploratory case‐crossover investigation for the composite cardiovascular outcome. This analysis used a subject as their own control and examined if current testosterone treatment is more likely at the time of the composite cardiovascular outcome than before the outcome. All analyses were conducted in R version 3.6.1. 30
Results
We identified 300 631 men, aged 40 to 89 years, with serum testosterone levels that were flagged as low per the testing laboratory. The mean threshold from the testing laboratories for low total testosterone was 220.85 ng/dL and for low free testosterone was 4.58 ng/dL. After a priori exclusions were applied, 204 857 (68%) men remained in the analytic cohort (Figure). Most testosterone measurements in the cohort were for total testosterone (78.0%) and free testosterone (20.8%), while 4.1% were for other testosterone measures. (Percentages add up to more than 100, as some men had multiple low testosterone measures.) Table 1 lists characteristics of testosterone nonusers and transdermal and intramuscular testosterone initiators in the analytic cohort. At baseline, the cohort had a mean (SD) age of 60.9 (9.9) years. The men in the cohort tended to be hypertensive (61.7%) and obese (mean BMI, 32.0 [6.7] kg/m2) and had chronic medical morbidity with a mean of 4.7 (3.5) chronic medical conditions, with 32.8% of men with diabetes mellitus and 39.6% with prevalent CVD. Over a mean follow‐up of 4.3 (2.8) years, 82 555 (40.3%) men filled a testosterone prescription. Of the testosterone‐treated men, 38% received only transdermal, 40% only intramuscular, and 22% received both intramuscular and transdermal testosterone at some point, but usually not concurrently. Testosterone treatment time was short, with a median cumulative treatment time of 4.8 months for transdermal and 11.1 months for intramuscular testosterone, with men spending more time as former users than current users of testosterone (Table 2). During follow‐up, serum total testosterone increased for nonusers, transdermal, and intramuscular users. Intramuscular testosterone users had a mean testosterone level within the physiologic range, while transdermal testosterone users and nonusers of testosterone had low or low‐normal mean testosterone levels (Table 3).
Figure 1. Study cohort inclusions and exclusions.
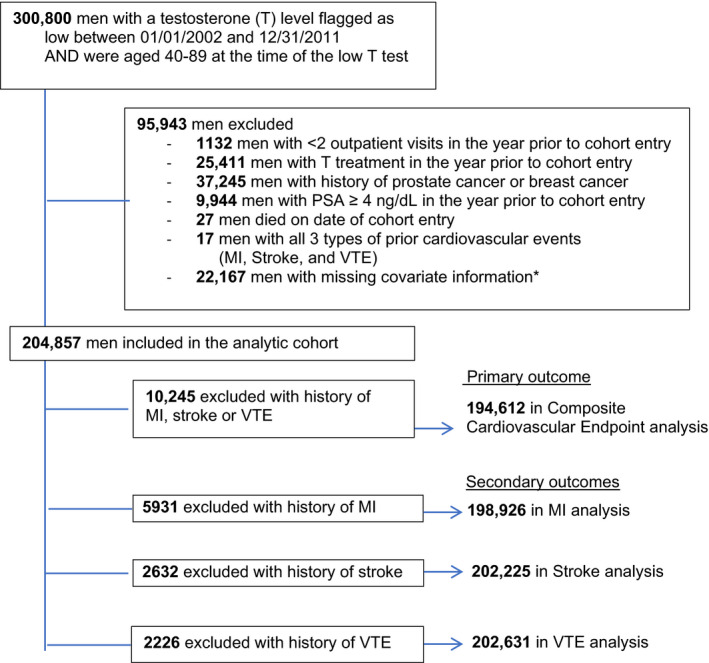
*Among 22 167 men with missing information: 20 957 were missing information on race, 1200 were missing information on BMI, 825 were missing information on region. These are overlapping groups, as some men were missing >1 covariate. BMI indicates body mass index; MI, myocardial infarction; PSA, prostate‐specific antigen; and VTE, venous thromboembolism.
Table 1.
Baseline Characteristics of Men in the Analytic Cohort
|
No use (N=122 302) |
Initiated with transdermal testosterone (N=43 502) |
Initiated with intramuscular testosterone (N=39 053) |
All (N=204 857) |
|
|---|---|---|---|---|
| Age, y, mean (SD) | 61.7 (10.2) | 59.8 (9.4) | 59.3 (9.2) | 60.9 (9.9) |
| Age, y, n (%) | ||||
| 40–49 | 14 228 (11.6) | 5969 (13.7) | 5684 (14.6) | 25 881 (12.6) |
| 50–59 | 37 748 (30.9) | 15 411 (35.4) | 14 433 (37.0) | 67 592 (33.0) |
| 60–69 | 44 315 (36.2) | 15 933 (36.6) | 13 924 (35.6) | 74 172 (36.2) |
| 70–79 | 18 470 (15.1) | 4775 (11.0) | 3985 (10.2) | 27 230 (13.3) |
| 80–89 | 7541 (6.2) | 1414 (3.3) | 1027 (2.6) | 9985 (4.9) |
| Cohort entry year, n (%) | ||||
| 2002–2003 | 18 539 (15.2) | 6412 (14.7) | 7111 (18.2) | 32 062 (15.7) |
| 2004–2005 | 15 903 (13.0) |
6246 (14.3) |
5525 (14.2) | 27 674 (13.5) |
| 2006–2007 | 18 147 (14.8) | 6733 (15.5) | 6332 (16.2) | 31 212 (15.2) |
| 2007–2009 | 27 229 (22.3) | 10 252 (23.6) | 8575 (22.0) | 46 056 (22.5) |
| 2010–2011 | 42 484 (34.7) | 13 859 (31.9) | 11 510 (29.5) | 67 853 (33.1) |
| Region of United States, n (%) | ||||
| Western | 27 686 (22.6) | 7068 (16.2) | 12 830 (32.9) | 47 584 (23.3) |
| Upper Midwest | 12 923 (10.6) | 5297 (12.2) | 2490 (6.4) | 20 710 (10.1) |
| Upper middle and eastern | 22 321 (18.3) | 5746 (13.2) | 8090 (20.7) | 36 157 (17.6) |
| Northeastern | 16 349 (13.4) | 7352 (16.9) | 2036 (5.2) | 25 737 (12.6) |
| Southern | 43 023 (35.2) | 18 039 (41.5) | 13 607 (34.8) | 74 669 (36.4) |
| BMI, mean (SD) | 31.5 (6.7) | 32.6 (6.7) | 32.8 (6.7) | 32.0 (6.7) |
| BMI, n (%) | ||||
| <18.5 | 1405 (1.1) | 343 (0.8) | 223 (0.6) | 1971 (1.0) |
| 18.5–24.9 | 15 844 (13.0) | 4059 (9.3) | 3263 (8.4) | 23 166 (11.4) |
| 25–29.9 | 37 139 (30.4) | 11 895 (27.2) | 10 623 (27.2) | 59 657 (29.1) |
| 30–34.9 | 36 079 (29.5) | 13 558 (31.8) | 12 425 (31.8) | 62 062 (30.3) |
| >35 | 31 835 (26.0) | 13 647 (31.4) | 12 519 (32.1) | 58 001 (28.3) |
| Race, n (%) | ||||
| White | 97 589 (79.8) | 35 548 (81.7) | 33 158 (84.9) | 166 295 (81.2) |
| Black | 21 102 (17.3) | 6722 (15.5) | 4609 (11.8) | 32 433 (15.8) |
| Other * | 3611 (3.0) | 6722 (2.8) | 1286 (3.3) | 6129 (3.0) |
| Prevalent cardiovascular disease, n (%) | 50 292 (41.1) | 16 481 (37.9) | 14 441 (37.0) | 81 214 (39.6) |
| Arrhythmia | 20 813 (17.0) | 6309 (14.5) | 5869 (15.0) | 32 991 (16.1) |
| Cardiomyopathy | 3681 (3.0) | 1146 (2.6) | 814 (2.1) | 5641 (2.8) |
| Coronary artery disease or angina | 32 055 (26.2) | 10 390 (23.9) | 8914 (22.8) | 51 359 (25.1) |
| Ischemic cerebrovascular disease or TIA | 4690 (3.8) | 1494 (3.4) | 1137 (2.9) | 7321 (3.6) |
| Chronic heart failure | 9949 (8.1) | 2978 (6.8) | 2177 (5.6) | 15 104 (7.4) |
| Peripheral vascular disease | 11 626 (9.5) | 3539 (8.1) | 2885 (7.4) | 18 050 (8.8) |
| Myocardial infarction | 3904 (3.2) | 1127 (2.6) | 900 (2.3) | 5931 (2.9) |
| Stroke, ischemic | 1800 (1.5) | 490 (1.1) | 342 (0.9) | 2632 (1.3) |
| VTE (DVT and PE) | 1175 (1.0) | 388 (0.9) | 248 (0.6) | 1811 (0.9) |
| Medical comorbidities, n (%) | ||||
| Chronic kidney disease | 6837 (5.6) | 1790 (4.1) | 1376 (3.5) | 10 003 (4.9) |
| Chronic lung disease | 24 643 (20.2) | 8239 (18.9) | 7166 (18.4) | 40 048 (19.5) |
| Diabetes mellitus | 40 983 (33.5) | 14 058 (32.3) | 12 063 (30.9) | 67 104 (32.8) |
| Erectile dysfunction | 46 158 (37.7) | 18 585 (42.7) | 16 790 (43.0) | 81 533 (39.8) |
| Hospitalization in prior year | 20 421 (16.7) | 6240 (14.3) | 4952 (12.7) | 31 613 (15.4) |
| Hyperlipidemia | 36 008 (29.4) | 13 558 (31.2) | 11 531 (29.5) | 61 097 (29.8) |
| Hypertension | 76 665 (62.7) | 26 661 (61.3) | 23 118 (59.2) | 126 444 (61.7) |
| Major depression | 19 284 (15.8) | 7923 (18.2) | 7388 (18.9) | 34 595 (16.9) |
| Malignancy | 17 667 (14.5) | 5813 (13.4) | 4750 (12.2) | 28 230 (13.8) |
| Morbid obesity | 8497 (7.0) | 3467 (8.0) | 3095 (7.9) | 15 059 (7.3) |
| Polycythemia | 447 (0.4) | 130 (0.3) | 146 (0.4) | 723 (0.4) |
| Sleep apnea | 18 627 (15.2) | 7806 (17.9) | 7145 (18.3) | 33 578 (16.4) |
| Smoking | 35 963 (29.4) | 12 424 (28.6) | 10 957 (28.1) | 59 344 (29.0) |
| No. of comorbidities, mean (SD) | 4.8 (3.6) | 4.7 (3.5) | 4.5 (3.4) | 4.7 (3.5) |
| Serum total testosterone, ng/dL, mean (SD) | 210 (126) | 174 (83) | 173 (76) | 195 (111) |
Table 2.
Duration of Cumulative Testosterone Treatment Times
| Cumulative testosterone treatment time | Transdermal | Intramuscular |
|---|---|---|
| Current use, mo | n=49 834 | n=50 845 |
| Median (IQR) | 4.8 (2.4–10.9) | 11.1 (4.8–22.9) |
| Mean (SD) | 9.5 (12.7) | 17.4 (18.4) |
| Former use, mo | n=49 104 | n=49 515 |
| Median (IQR) | 27.8 (12.4–55.9) | 20.3 (7.0–47.7) |
| Mean (SD) | 37.4 (31.1) | 31.3 (30.7) |
Transdermal‐treated men had a shorter duration of testosterone treatment than men who were treated with intramuscular testosterone.
Table 3.
Serum Total Testosterone Levels
| Total testosterone, ng/dL | Testosterone treatment status | ||
|---|---|---|---|
|
No use N=29 954 |
Transdermal N=5953 |
Intramuscular N=7337 |
|
| Baseline, mean (SD) | 195 (105) | 165 (81) | 166 (71) |
| Baseline, median (IQR) | 190 (145–230) | 165 (119–210) | 170 (125–210) |
| Follow‐up, mean (SD) | 276 (167) | 312 (212) | 455 (321) |
| Follow‐up, median (IQR) | 249 (182–330) | 260 (173–399) | 372 (215–620) |
Among treated men, testosterone measures were those obtained during the first year of current treatment. Among untreated men, testosterone levels were those obtained during the first year following cohort entry. If there were multiple levels obtained during the time period, levels were averaged. IQR indicates interquartile range.
Primary and Secondary Outcomes
During the study, 12 645 composite cardiovascular events occurred. In current and former transdermal users with (without) prevalent CVD, incidence rates were 15.74 and 20.73 (8.68 and 9.62) per 1000 person‐years, respectively. In current and former intramuscular users with (without) prevalent CVD, incidence rates were 19.16 and 21.29 (10.03 and 11.03) per 1000 person‐years, respectively (Table 4). In fully adjusted Cox regression analyses, current compared with former transdermal use was associated with lower risk of composite cardiovascular events in those with prevalent CVD (hazard ratio [HR], 0.80; 95% CI, 0.70–0.91), and no increased risk was detected in those without prevalent CVD (HR, 0.89; 95% CI, 0.76–1.05), nor in the comparison of current to former intramuscular users with or without prevalent CVD (HR, 0.98; 95% CI, 0.89–1.09; HR, 0.91; 95% CI, 0.80–1.04, respectively). In secondary analyses of separate outcomes of MI, ischemic stroke, and VTE, current transdermal users with prevalent CVD had a lower risk for MI and VTE compared with former users (HR, 0.80; 95% CI, 0.67–0.91; HR, 0.72; 95% CI, 0.55–0.94, respectively). No increased risk of MI, ischemic stroke, or VTE were detected in transdermal users without prevalent CVD, nor in current versus former intramuscular users with or without prevalent CVD (Table 5). In a sensitivity analysis that varied the exposure window for current testosterone (Table S3), our results were largely unchanged, and we found no increased risks associated with testosterone initiation (Table S4). The results of an exploratory case‐crossover investigation for the composite cardiovascular outcome also supported our conclusions and detected no increased risk associated with current testosterone treatment (Data S1).
Table 4.
Cardiovascular Event IRs per 1000 person‐years
| Transdermal treatment | PY/1000 | Events | IR | Unadjusted HR | |
|---|---|---|---|---|---|
| Composite cardiovascular end point* | Former | 139.8 | 2103 | 15.04 | 1.00 (ref) |
| Current | 36.9 | 434 | 11.77 | 0.78 (0.7–0.87) | |
| No use | 631.2 | 10108 | 16.01 | 1.07 (1.02–1.12) | |
| MI | Former | 144.6 | 1210 | 8.37 | 1.00 (ref) |
| Current | 37.9 | 246 | 6.49 | 0.78 (0.68–0.90) | |
| No use | 651.9 | 6030 | 9.25 | 1.12 (1.05–1.19) | |
| Stroke | Former | 148.1 | 618 | 4.17 | 1.00 (ref) |
| Current | 38.5 | 131 | 3.41 | 0.84 (0.69–1.01) | |
| No use | 666.7 | 3047 | 4.57 | 1.11 (1.02–1.22) | |
| VTE † | Former | 148.3 | 558 | 3.76 | 1.00 (ref) |
| Current | 38.4 | 106 | 2.76 | 0.69 (0.56–0.85) | |
| No use | 670.1 | 2361 | 3.52 | 0.91 (0.83–1.00) |
| Intramuscular Treatment | |||||
|---|---|---|---|---|---|
| Composite cardiovascular endpoint* | Former | 117.8 | 1902 | 16.14 | 1.00 (ref) |
| Current | 68.9 | 975 | 14.15 | 0.88 (0.82–0.95) | |
| No use | 621.2 | 9768 | 15.72 | 0.98 (0.93–1.03) | |
| MI | Former | 121.8 | 1123 | 9.22 | 1.00 (ref) |
| Current | 70.5 | 575 | 8.16 | 0.90 (0.81–0.99) | |
| No use | 642.2 | 5788 | 9.01 | 0.99 (0.93–1.05) | |
| Stroke | Former | 124.9 | 569 | 4.56 | 1.00 (ref) |
| Current | 72.0 | 252 | 3.50 | 0.80 (0.69–0.93) | |
| No use | 656.4 | 2975 | 4.53 | 1.03 (0.94–1.13) | |
| VTE † | Former | 125.4 | 461 | 3.68 | 1.00 (ref) |
| Current | 71.9 | 228 | 3.17 | 0.83 (0.71–0.97) | |
| No use | 659.4 | 2336 | 3.54 | 0.94 (0.85–1.04) | |
IR indicates incidence rate; and MI, myocardial infarction.
Composite cardiovascular end point was composed of MI, ischemic stroke, and VTE.
VTE: venous thromboembolism, composed of pulmonary embolism and deep vein thrombosis.
Table 5.
Cardiovascular Outcomes by Testosterone Treatment Status and Prevalent Cardiovascular Disease
| No prevalent cardiovascular disease* | Prevalent cardiovascular disease* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Transdermal testosterone | PY/1000 | Events | IR | Unadjusted HR | Adjusted HR † | PY/1000 | Events | IR | Unadjusted HR | Adjusted HR † | |
| Composite cardiovascular end point ‡ | Former | 71.5 | 688 | 9.62 | 1.00 (ref) | 1.00 (ref) | 68.3 | 1415 | 20.73 | 1.00 (ref) | 1.00 (ref) |
| Current | 20.7 | 180 | 8.68 | 0.89 (0.75–1.05) | 0.89 (0.76–1.05) | 16.1 | 254 | 15.74 | 0.74 (0.64–0.84) | 0.80 (0.70–0.91) § | |
| No use | 332.2 | 3424 | 10.31 | 1.07 (0.98–1.16) | 1.02 (0.94–1.11) | 299.0 | 6684 | 22.35 | 1.06 (1–1.12) | 1.03 (0.97–1.09) | |
| MI | Former | 71.5 | 359 | 5.02 | 1.00 (ref) | 1.00 (ref) | 73.1 | 851 | 11.64 | 1.00 (ref) | 1.00 (ref) |
| Current | 20.7 | 95 | 4.58 | 0.91 (0.73–1.15) | 0.92 (0.73–1.15) | 17.2 | 151 | 8.78 | 0.74 (0.62–0.88) | 0.80 (0.67–0.95) § | |
| No use | 332.2 | 1860 | 5.60 | 1.12 (1–1.25) | 1.06 (0.95–1.19) | 319.8 | 4170 | 13.04 | 1.11 (1.03–1.19) | 1.07 (0.99–1.15) | |
| Stroke | Former | 71.5 | 168 | 2.35 | 1.00 (ref) | 1.00 (ref) | 76.6 | 450 | 5.88 | 1.00 (ref) | 1.00 (ref) |
| Current | 20.7 | 45 | 2.17 | 0.93 (0.67–1.3) | 0.96 (0.69–1.34) | 17.7 | 86 | 4.85 | 0.82 (0.65–1.03) | 0.90 (0.72–1.14) | |
| No use | 332.2 | 865 | 2.60 | 1.12 (0.95–1.32) | 1.06 (0.90–1.25) | 334.5 | 2182 | 6.52 | 1.10 (1–1.22) | 1.05 (0.94–1.16) | |
| VTE | Former | 71.5 | 172 | 2.40 | 1.00 (ref) | 1.00 (ref) | 76.8 | 386 | 5.03 | 1.00 (ref) | 1.00 (ref) |
| Current | 20.7 | 42 | 2.03 | 0.78 (0.56–1.1) | 0.75 (0.53–1.05) | 17.6 | 64 | 3.63 | 0.66 (0.5–0.86) | 0.72 (0.55–0.94) § | |
| No use | 332.2 | 739 | 2.22 | 0.89 (0.75–1.05) | 0.86 (0.73–1.02) | 337.9 | 1622 | 4.80 | 0.91 (0.81–1.02) | 0.93 (0.83–1.04) | |
| Intramuscular testosterone | |||||||||||
| Composite cardiovascular end point ‡ | Former | 59.1 | 652 | 11.03 | 1.00 (ref) | 1.00 (ref) | 58.7 | 1250 | 21.29 | 1.00 (ref) | 1.00 (ref) |
| Current | 37.8 | 379 | 10.03 | 0.89 (0.79–1.01) | 0.91 (0.80–1.04) | 31.1 | 596 | 19.16 | 0.88 (0.8–0.98) | 0.98 (0.89–1.09) | |
| No use | 327.6 | 3261 | 9.96 | 0.89 (0.81–0.96) | 0.82 (0.75–0.89) | 293.6 | 6507 | 22.16 | 1.03 (0.97–1.09) | 0.96 (0.90–1.02) | |
| MI | Former | 59.1 | 349 | 5.90 | 1.00 (ref) | 1.00 (ref) | 62.7 | 774 | 12.34 | 1.00 (ref) | 1.00 (ref) |
| Current | 37.8 | 211 | 5.58 | 0.93 (0.79–1.11) | 0.95 (0.80–1.13) | 32.7 | 364 | 11.14 | 0.89 (0.79–1.01) | 0.99 (0.87–1.12) | |
| No use | 327.6 | 1754 | 5.35 | 0.89 (0.79–1) | 0.84 (0.75–0.94) | 314.6 | 4034 | 12.82 | 1.03 (0.95–1.11) | 0.97 (0.89–1.04) | |
| Stroke | Former | 59.1 | 158 | 2.67 | 1.00 (ref) | 1.00 (ref) | 65.8 | 411 | 6.25 | 1.00 (ref) | 1.00 (ref) |
| Current | 37.8 | 90 | 2.38 | 0.90 (0.69–1.17) | 0.95 (0.73–1.23) | 34.2 | 162 | 4.73 | 0.77 (0.64–0.92) | 0.87 (0.73–1.05) | |
| No use | 327.6 | 830 | 2.53 | 0.95 (0.8–1.13) | 0.86 (0.72–1.02) | 328.8 | 2145 | 6.52 | 1.05 (0.95–1.17) | 0.94 (0.84–1.04) | |
| VTE | Former | 59.1 | 155 | 2.62 | 1.00 (ref) | 1.00 (ref) | 66.3 | 306 | 4.62 | 1.00 (ref) | 1.00 (ref) |
| Current | 37.8 | 82 | 2.17 | 0.77 (0.59–1.01) | 0.77 (0.59–1.00) | 34.2 | 146 | 4.27 | 0.87 (0.71–1.06) | 0.97 (0.79–1.18) | |
| No use | 327.6 | 716 | 2.19 | 0.79 (0.66–0.94) | 0.71 (0.60–0.85) | 331.9 | 1620 | 4.88 | 1.02 (0.9–1.15) | 0.94 (0.83–1.07) | |
HR indicates hazard ratio; MI, myocardial infarction; PY, person‐years; VTE, venous thromboembolism.
Cardiovascular disease includes arrhythmia, angina, coronary artery disease, chronic heart failure, cardiomyopathy, peripheral vascular disease, cerebrovascular disease, transient ischemic attack, and cardiovascular procedures. In secondary analyses for separate cardiovascular outcomes, it also included MI, stroke, or VTE except in analyses for that specific outcome.
Fully adjusted for age, race, geographic region, year of cohort entry, body mass index, hospitalization, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, sexual dysfunction, hyperlipidemia, hypertension, major depression, morbid obesity, polycythemia, sleep apnea, smoking, and cardiovascular disease. Adjustment for the composite end point and VTE also included malignancy (excluding basal cell skin cancer).
The composite cardiovascular endpoint was comprised of MI, ischemic stroke, and VTE.
p<0.05.
Discussion
In a large cohort of US veterans, aged ≥40 years, with low testosterone and multiple medical comorbidities, we examined cardiovascular risks of current versus former use of transdermal and intramuscular testosterone in multivariable adjusted Cox regression analyses. Over a mean follow‐up of 4.3 years, we detected 12 645 incident composite cardiovascular events and found no consistent association of transdermal or intramuscular testosterone with increased risk for an incident composite cardiovascular end point or separate events of incident MI, ischemic stroke, or VTE. We had hypothesized that risk would be greatest during current testosterone treatment, but we did not detect this. In our analyses, we observed that in men with prevalent CVD, current transdermal testosterone was associated with a lower risk of composite cardiovascular events, MI, and VTE compared with former use of transdermal testosterone. We did not hypothesize that testosterone treatment would protect against the risk of cardiovascular events and do not believe the observed association is causal, but rather the result of residual confounding or multiple comparisons. Furthermore, this association was not seen in transdermal‐treated men without prevalent CVD nor in men treated with intramuscular testosterone.
Prior Studies
Results of prior cohort studies examining the association of testosterone treatment with cardiovascular events have been conflicting. In studies that examined the risks of testosterone treatment for a composite cardiovascular end point, several studies reported that testosterone treatment was associated with increased risk, 4 , 12 , 14 while others reported either no overall risk 9 , 25 or a decreased risk 18 , 19 for a composite outcome. However, these studies are difficult to compare as none of them defined the composite cardiovascular end point in the same way (Table S5). All the studies included MI in the composite end point, but they differed on inclusion of stroke, unstable angina, transient ischemic attack, coronary revascularization, sudden cardiac death, postoperative mortality, and total mortality. We defined the composite end point as MI, VTE, and ischemic stroke, as these are major adverse cardiovascular events that have a clinically significant impact. We did not include revascularization procedures, as these may be influenced by local practice patterns; or total mortality and postoperative mortality, as they include noncardiovascular mortality; or angina and transient ischemic attack, as these may be more prone to ascertainment bias than major cardiovascular events such as MI and stroke. In studies of testosterone treatment and risk for separate cardiovascular events, most studies found that testosterone treatment had no overall association with MI 8 , 13 , 15 , 18 , 24 or was associated with lower MI risk. 17 , 19 One study reported higher MI risk, but that study may have been biased, as it assumed that clinicians prescribing testosterone would not be influenced if a patient had a recent MI. 5 In studies of testosterone treatment and risk for stroke, testosterone treatment either had no association with stroke 18 or was associated with lower stroke risk. 16 , 17 , 19 Finally, in studies of testosterone treatment and VTE risk, most found no overall association with VTE, 7 , 11 , 22 , 23 while one reported higher VTE risk. 31
The pathophysiologic basis for an association of testosterone treatment with cardiovascular risk is unclear, as testosterone and its metabolites have complex effects on the cardiovascular system. 32 , 33 , 34 , 35 Testosterone treatment could increase cardiovascular risk by increased platelet aggregation and hematocrit, which increase viscosity and vascular shear force; increased coronary artery noncalcified plaque volume; exacerbation of severe untreated sleep apnea; or increased levels of estradiol, a metabolite of testosterone, which may induce thrombosis. 32 , 36 , 37 , 38 , 39 In contrast, testosterone treatment could have potential protective effects by a decrease in cardiovascular risk factors such as metabolic syndrome and central obesity; decreased exercise‐induced cardiac ischemia; or increased release of endothelial nitric oxide, which causes vasodilation. 33 , 35 , 40
The conflicting results from different studies about the association of testosterone with cardiovascular events may be related to heterogeneity in databases 41 that were used, with some having limited information on medical comorbidities 5 , 7 , 8 , 13 , 22 and gonadal status. 8 , 12 , 13 , 15 , 22 Other factors include methodologic issues such as studies 16 , 23 with immortal time bias 42 , 43 and other studies with time‐dependent confounding attributable to failure to adjust for covariate changes over time. 8 , 15 , 16 , 23 This study avoided these methodologic limitations and differed from others in 2 aspects of the analytic approach:
Testosterone exposure. Several cohort studies modeled testosterone exposure as any use versus no use. 4 , 8 , 16 , 19 , 23 However, such a model cannot ascertain whether cardiovascular events occurred when subjects were currently treated with testosterone. Furthermore, the any‐use /no‐use model may lead to an overestimation of testosterone exposure, which could bias results. We modeled testosterone exposure as current use, former use, and no use to more accurately assess the association between current testosterone exposure and cardiovascular events and to account for intermittent treatment. We also modelled testosterone exposure separately by transdermal and intramuscular formulations because of significant differences in pharmacokinetics and serum levels between transdermal and intramuscular formulations. 44 , 45
Reference group. Many cohort studies compared testosterone‐treated to ‐untreated men. However, in cohort studies, untreated subjects are not an ideal reference group, as the treated and untreated groups are likely to differ because of the nonrandomization to treatment. 46 Statistical methods attempt to adjust for this, but there may be residual confounding caused by differences in confounders that are not captured in the data set, such as family history or health habits. For example, studies of hormone replacement therapy in women found that beneficial effects associated with hormone replacement therapy were later found to be attributable to a healthy‐user effect in which women on hormone replacement therapy had better health habits, compliance, and medical follow‐up than untreated women. 47 , 48 , 49 Thus, to decrease the risk for confounding, we compared current users of testosterone to former users as both had been selected to initiate testosterone treatment at some point during the study. Another approach is to compare treated men with men treated with a different but comparable medication. However, this approach was not possible, as there is no comparable alternative medication for men with low testosterone.
Limitations
There are several limitations to our study. The study population consisted of US male veterans with multiple medical comorbidities, including a high prevalence of obesity, hypertension, and diabetes mellitus, and these results may not be applicable to healthier men with fewer comorbidities and lower cardiovascular risk. Subjects had 1 low serum testosterone measure, but 2 levels and a clinical assessment are recommended before testosterone treatment. 37 , 50 We did not have information on manifestations of testosterone deficiency or indications for testosterone treatment and assumed that men started testosterone on the fill date and were compliant with treatment but could not ensure this. Cardiovascular outcomes and medical comorbidities were ascertained with ICD‐9 codes, procedure codes, and laboratory and pharmacy data and not via chart review. We excluded outpatient deep venous thrombosis diagnoses since the accuracy for outpatient deep venous thrombosis diagnoses is low. We did not examine testosterone dosage regimens in our analyses. There were a small number of follow‐up serum testosterone levels, as we limited these to current users and nonusers in the first year following cohort entry. The mean follow‐up time was short (4.3 years). However, testosterone treatment time itself was short (median cumulative treatment time of 4.8 months for transdermal and 11.1 months for intramuscular testosterone) and in several studies, adverse cardiovascular events occurred within a relatively short time following testosterone treatment. 5 , 7 , 8 , 9 , 10 , 11 Our findings should also be interpreted in the context of multiple comparisons undertaken to examine composite (primary analysis) and separate cardiovascular outcomes (secondary analyses), each by testosterone formulation and prevalent cardiovascular disease status. Finally, because of the observational study design, these results do not imply causality, and residual confounding cannot be excluded.
Strengths
Despite these limitations, our study has several strengths. First, we had a large cohort with a high number of testosterone‐treated men (82 555) and cardiovascular events, which enhanced our ability to detect a safety signal. In contrast, other studies had a relatively low number of testosterone‐treated men and cardiovascular events. 4 , 11 , 18 , 24 We restricted our analyses to men who had no history of the specific cardiovascular outcome to decrease heterogeneity among subjects and reduce the possibility that cardiovascular events were related to recurrent disease, independent of testosterone treatment. Another strength was that VHA diagnoses were supplemented with Centers for Medicare and Medicaid Services diagnoses to capture acute cardiovascular events that were treated in non‐VHA settings. Other strengths include a new‐user design 51 and detailed models of testosterone exposure that allowed us to characterize treatment status by formulation and distinguish current treatment from former treatment. In contrast, many cohort studies used an any‐use/no‐use model of testosterone treatment and were unable to ascertain if cardiovascular events occurred when subjects were currently treated with testosterone. 4 , 8 , 12 , 16 , 19 , 23 , 24 Finally, we attempted to minimize the risk for confounding by using former users (rather than nonusers) as the reference group and continuously adjusted for changes in medical covariates to adjust for differences between groups. Another approach to adjust for differences between groups is propensity score analysis, but this approach does not offer advantages in studies that have a large number of subjects and events. 52
Conclusions
In a large cohort of US male veterans, aged ≥40 years, with low testosterone and multiple medical comorbidities, we did not detect increased risk for composite or separate cardiovascular events of MI, stroke, or VTE with current use compared with former use of transdermal or intramuscular testosterone. Given the large study size and detailed characterization of testosterone exposure, this study provides some reassurance that testosterone treatment does not appear to pose a significant risk for major cardiovascular events of MI, stroke, or thrombosis. However, a large, carefully designed prospective, randomized, double‐blind, placebo‐controlled trial is needed to definitively assess the cardiovascular risks associated with testosterone treatment. Until such a trial is completed, clinicians should follow recommended guidelines regarding testosterone treatment and carefully review potential risks and benefits of treatment in men who have unequivocal hypogonadism. 37 , 50
Sources of Funding
This work was supported by National Institutes of Health grant R01 AG042934‐01.
Disclosures
Dr Walsh is a consultant for Boston Scientific. Dr Matsumoto has obtained research support from AbbVie; is on advisory boards for AbbVie; and is an editor for UpToDate. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S5
References 4, 9, 12, 14, 18,19, 25
Acknowledgments
We acknowledge the support of the Department of Veterans Affairs, with use of resources and facilities at the VA Puget Sound Health Care System, the VA Informatics and Computing Infrastructure (VINCI), VA Health Services Research and Development Service (VA HSR RES 13‐457), and the Seattle Epidemiologic Research and Information Center of the Cooperative Studies Program within the VA Office of Research and Development, Seattle, Washington (Project Numbers SDR 02‐237 and 98‐004). We also acknowledge support for VA/ Centers for Medicare and Medicaid Services data provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, and VA Information Resource Center (VIReC) (Project Numbers SDR 02‐237 and 98‐004). The contents of this article do not represent the views of the US Department of Veterans Affairs or the US government.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020562
For Sources of Funding and Disclosures, see page 11.
Footnotes
Other includes Alaskan / American Indian, Asian, Hawaiian/Pacific Islander.
References
- 1. Layton JB, Li D, Meier CR, Sharpless JL, Sturmer T, Jick SS, Brookhart MA. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835–842. DOI: 10.1210/jc.2013-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Layton JB, Kim Y, Alexander GC, Emery SL. Association between direct‐to‐consumer advertising and testosterone testing and initiation in the United States, 2009–2013. JAMA. 2017;317:1159–1166. DOI: 10.1001/jama.2016.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baillargeon J, Kuo YF, Westra JR, Urban RJ, Goodwin JS. Testosterone prescribing in the United States, 2002–2016. JAMA. 2018;320:200–202. DOI: 10.1001/jama.2018.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vigen R, O'Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310:1829–1836. DOI: 10.1001/jama.2013.280386. [DOI] [PubMed] [Google Scholar]
- 5. Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, Fraumeni JF Jr, Hoover RN. Increased risk of non‐fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014;9:e85805. DOI: 10.1371/journal.pone.0085805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta‐analysis of placebo‐controlled randomized trials. BMC Med. 2013;11:108. DOI: 10.1186/1741-7015-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H, Benoit K, Wang W, Motsko S. Association between use of exogenous testosterone therapy and risk of venous thrombotic events among exogenous testosterone treated and untreated men with hypogonadism. J Urol. 2016;195:1065–1072. DOI: 10.1016/j.juro.2015.10.134. [DOI] [PubMed] [Google Scholar]
- 8. Li H, Mitchell L, Zhang X, Heiselman D, Motsko S. Testosterone therapy and risk of acute myocardial infarction in hypogonadal men: an administrative health care claims study. J Sex Med. 2017;14:1307–1317. DOI: 10.1016/j.jsxm.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 9. Layton JB, Li D, Meier CR, Sharpless JL, Sturmer T, Brookhart MA. Injection testosterone and adverse cardiovascular events: a case‐crossover analysis. Clin Endocrinol. 2018;88:719–727. DOI: 10.1111/cen.13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, et al. Adverse events associated with testosterone administration. N Eng J Med. 2010;363:109–122. DOI: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martinez C, Suissa S, Rietbrock S, Katholing A, Freedman B, Cohen AT, Handelsman DJ. Testosterone treatment and risk of venous thromboembolism: population based case‐control study. BMJ. 2016;355:i5968. DOI: 10.1136/bmj.i5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wallis CJ, Lo K, Lee Y, Krakowsky Y, Garbens A, Satkunasivam R, Herschorn S, Kodama RT, Cheung P, Narod SA, et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: an intention‐to‐treat observational cohort study. Lancet Diabetes Endocrinol. 2016;4:498–506. DOI: 10.1016/S2213-8587(16)00112-1. [DOI] [PubMed] [Google Scholar]
- 13. Etminan M, Skeldon SC, Goldenberg SL, Carleton B, Brophy JM. Testosterone therapy and risk of myocardial infarction: a pharmacoepidemiologic study. Pharmacotherapy. 2015;35:72–78. DOI: 10.1002/phar.1534. [DOI] [PubMed] [Google Scholar]
- 14. Loo SY, Azoulay L, Nie R, Dell'Aniello S, Yu OHY, Renoux C. Cardiovascular and cerebrovascular safety of testosterone replacement therapy among aging men with low testosterone levels: a cohort study. Am J Med. 2019;132:1069–1077. DOI: 10.1016/j.amjmed.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 15. Baillargeon J, Urban RJ, Kuo YF, Ottenbacher KJ, Raji MA, Du F, Lin YL, Goodwin JS. Risk of myocardial infarction in older men receiving testosterone therapy. Ann Pharmacother. 2014;48:1138–1144. DOI: 10.1177/1060028014539918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma R, Oni OA, Gupta K, Chen G, Sharma M, Dawn B, Sharma R, Parashara D, Savin VJ, Ambrose JA, et al. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36:2706–2715. DOI: 10.1093/eurheartj/ehv346. [DOI] [PubMed] [Google Scholar]
- 17. Tan RS, Cook KR, Reilly WG. Myocardial infarction and stroke risk in young healthy men treated with injectable testosterone. Int J Endocrinol. 2015;2015:970750. DOI: 10.1155/2015/970750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson JL, May HT, Lappe DL, Bair T, Le V, Carlquist JF, Muhlestein JB. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated health care system. Am J Cardiol. 2016;117:794–799. DOI: 10.1016/j.amjcard.2015.11.063. [DOI] [PubMed] [Google Scholar]
- 19. Cheetham TC, An J, Jacobsen SJ, Niu F, Sidney S, Quesenberry CP, VanDenEeden SK. Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern Med. 2017;177:491–499. DOI: 10.1001/jamainternmed.2016.9546. [DOI] [PubMed] [Google Scholar]
- 20. Sharma R, Oni OA, Gupta K, Sharma M, Sharma R, Singh V, Parashara D, Kamalakar S, Dawn B, Chen G, et al. Normalization of testosterone levels after testosterone replacement therapy is associated with decreased incidence of atrial fibrillation. J Am Heart Assoc. 2017;6:e004880. DOI: 10.1161/JAHA.116.004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maggi M, Wu FCW, Jones TH, Jackson G, Behre HM, Hackett G, Martin‐Morales A, Balercia G, Dobs AS, Arver STE, et al. Testosterone treatment is not associated with increased risk of adverse cardiovascular events: results from the Registry of Hypogonadism in Men (RHYME). Int J Clin Pract. 2016;70:843–852. DOI: 10.1111/ijcp.12876. [DOI] [PubMed] [Google Scholar]
- 22. Baillargeon J, Urban RJ, Morgentaler A, Glueck CJ, Baillargeon G, Sharma G, Kuo YF. Risk of venous thromboembolism in men receiving testosterone therapy. Mayo Clin Proc. 2015;90:1038–1045. DOI: 10.1016/j.mayocp.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 23. Sharma R, Oni OA, Chen G, Sharma M, Dawn B, Sharma R, Parashara D, Savin VJ, Barua RS, Gupta K. Association between testosterone replacement therapy and the incidence of DVT and pulmonary embolism: a retrospective cohort study of the veterans administration database. Chest. 2016;150:563–571. DOI: 10.1016/j.chest.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 24. Oni OA, Dehkordi SHH, Jazayeri MA, Sharma R, Sharma M, Masoomi R, Sharma R, Gupta K, Barua RS. Relation of testosterone normalization to mortality and myocardial infarction in men with previous myocardial infarction. Am J Cardiol. 2019;124:1171–1178. DOI: 10.1016/j.amjcard.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 25. Argalious MY, Steib J, Daskalakis N, Mao G, Li M, Armanyous S, Roselli E. Association of testosterone replacement therapy and the incidence of a composite of postoperative in‐hospital mortality and cardiovascular events in men undergoing cardiac surgery. Anesth Analg. 2020;130:890–898. DOI: 10.1213/ANE.0000000000004115. [DOI] [PubMed] [Google Scholar]
- 26. European Medicines Agency (EMA) . No consistent evidence of an increased risk of heart problems with testosterone medicines. 2014. https://www.ema.europa.eu/en/documents/referral/testosterone‐article‐31‐referral‐no‐consistent‐evidence‐increased‐risk‐heart‐problems‐testosterone_en.pdf. Last accessed Jan 31, 2020.
- 27. US Federal Drug Administration Drug safety communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use . 2015. https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐drug‐safety‐communication‐fda‐cautions‐about‐using‐testosterone‐products‐low‐testosterone‐due. Last accessed Aug 8, 2019. [DOI] [PubMed]
- 28. Health Canada. Summary safety review‐testosterone replacement products‐eHalth Canada . 2014. https://hpr‐rps.hres.ca/reg‐content/summary‐safety‐review‐detail.php?linkID=SSR000582020. Last accessed Jan 31, 2020.
- 29. US Department of Veterans Affairs . 172VA10P2: VHA Corporate Data Warehouse – VA. 79 FR 4377. Last updated December 15, 2017.
- 30. R Core Team . R: a language and environment for statistical computing. 2018. https://www.R‐project.org/. Last accessed Jan 19, 2019.
- 31. Walker RF, Zakai NA, MacLehose RF, Cowan LT, Adam TJ, Alonso A, Lutsey PL. Association of testosterone therapy with risk of venous thromboembolism among men with and without hypogonadism. JAMA Intern Med. 2020;180:190–197. DOI: 10.1001/jamainternmed.2019.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gagliano‐Juca T, Basaria S. Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol. 2019;16:555–574. DOI: 10.1038/s41569-019-0211-4. [DOI] [PubMed] [Google Scholar]
- 33. Perusquia M, Greenway CD, Perkins LM, Stallone JN. Systemic hypotensive effects of testosterone are androgen structure‐specific and neuronal nitric oxide synthase‐dependent. Am J Physiol Regul Integr Comp Physiol. 2015;309:R189–R195. DOI: 10.1152/ajpregu.00110.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chistiakov DA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Role of androgens in cardiovascular pathology. Vasc Health Risk Manag. 2018;14:283–290. DOI: 10.2147/VHRM.S173259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohler ER, Ellenberg SS, Lewis CE, Wenger NK, Budoff MJ, Lewis MR, Barrett‐Connor E, Swerdloff RS, Stephens‐Shields A, Bhasin S, et al. The effect of testosterone on cardiovascular biomarkers in the testosterone trials. J Clin Endocrinol Metab. 2018;103:681–688. DOI: 10.1210/jc.2017-02243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER, Wenger NK, Bhasin S, Barrett‐Connor E, Swerdloff RS, Stephens‐Shields A, Cauley JA, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317:708–716. DOI: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone therapy in men with hypogonadism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2018;103:1715–1744. DOI: 10.1210/jc.2018-00229. [DOI] [PubMed] [Google Scholar]
- 38. Simon T, Yon B, de Jonage‐Canonico M, Oger E, Wahl D, Conard J, Meyer G, Emmerich J, Barrellier MT, Guiraud A, et al. Indicators of lifetime endogenous estrogen exposure and risk of venous thromboembolism. J Thromb Haemost. 2006;4:71–76. DOI: 10.1111/j.1538-7836.2005.01693.x. [DOI] [PubMed] [Google Scholar]
- 39. Sandset PM. Mechanisms of hormonal therapy related thrombosis. Thromb Res. 2013;131:S4–S7. DOI: 10.1016/S0049-3848(13)70009-4. [DOI] [PubMed] [Google Scholar]
- 40. Webb CM, McNeill JG, Hayward CS, de Zeigler D, Collins P. Effects of testosterone on coronary vasomotor regulation in men with coronary heart disease. Circulation. 1999;100:1690–1696. DOI: 10.1161/01.CIR.100.16.1690. [DOI] [PubMed] [Google Scholar]
- 41. Madigan D, Ryan PB, Schuemie M, Stang PE, Overhage JM, Hartzema AG, Suchard MA, DuMouchel W, Berlin JA. Evaluating the impact of database heterogeneity on observational study results. Am J Epidemiol. 2013;178:645–651. DOI: 10.1093/aje/kwt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prada‐Ramallal G, Takkouche B, Figueiras A. Bias in pharmacoepidemiologic studies using secondary health care databases: a scoping review. BMC Med Res Methodol. 2019;19:53. DOI: 10.1186/s12874-019-0695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dekkers OM, Groenwold RHH. When observational studies can give wrong answers: the potential of immortal time bias. Eur J Endocrinol. 2020;184:E1–E4. DOI: 10.1530/EJE-20-1124. [DOI] [PubMed] [Google Scholar]
- 44. Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51:1335–1339. DOI: 10.1210/jcem-51-6-1335. [DOI] [PubMed] [Google Scholar]
- 45. Swerdloff RS, Wang C, Cunningham G, Dobs A, Iranmanesh A, Matsumoto AM, Snyder PJ, Weber T, Longstreth J, Berman N. Long‐term pharmacokinetics of transdermal testosterone gel in hypogonadal men. J Clin Endocrinol Metab. 2000;85:4500–4510. DOI: 10.1210/jc.85.12.4500. [DOI] [PubMed] [Google Scholar]
- 46. Patorno E, Patrick AR, Garry EM, Schneeweiss S, Gillet VG, Bartels DB, Masso‐Gonzalez E, Seeger JD. Observational studies of the association between glucose‐lowering medications and cardiovascular outcomes: addressing methodological limitations. Diabetologia. 2014;57:2237–2250. DOI: 10.1007/s00125-014-3364-z. [DOI] [PubMed] [Google Scholar]
- 47. Gleason CE, Dowling NM, Friedman E, Wharton W, Asthana S. Using predictors of hormone therapy use to model the healthy user bias: how does healthy user status influence cognitive effects of hormone therapy? Menopause. 2012;19:524–533. DOI: 10.1097/gme.0b013e318238ff2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shrank WH, Patrick AR, Brookhart MA. Healthy user and related biases in observational studies of preventive interventions: a primer for physicians. J Gen Intern Med. 2011;26:546–550. DOI: 10.1007/s11606-010-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol. 1996;143:971–978. DOI: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- 50. Diem SJ, Greer NL, MacDonald R, McKenzie LG, Dahm P, Ercan‐Fang N, Estrada A, Hemmy LS, Rosebush CE, Fink HA, et al. Efficacy and safety of testosterone treatment in men: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2020;172:105–118. DOI: 10.7326/M19-0830. [DOI] [PubMed] [Google Scholar]
- 51. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. DOI: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 52. Shah BR, Laupacis A, Hux JE, Austin PC. Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol. 2005;58:550–559. DOI: 10.1016/j.jclinepi.2004.10.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5
References 4, 9, 12, 14, 18,19, 25


