Abstract
Background
There is limited research on hypertrophic cardiomyopathy (HCM), which is the most common inherited cardiac disorder, in diverse populations, including Black individuals. Current literature lacks comprehensive data on HCM disease expression, comorbidities, and outcomes in this historically disadvantaged group. The purpose of this study was to examine structural HCM characteristics, comorbidities, and outcomes in a Black and White cohort with HCM.
Methods and Results
The study was a subgroup analysis from a longitudinal, prospective study on HCM, with supplemental chart review. The sample included adults (≥18 years) with a clinical diagnosis of HCM, who self‐identified as Black/African American or White. The study sample comprised 434 individuals; 57 (13.1%) were Black, and 180 (41.5%) were women. Black patients were younger than White patients, 54.6 (13.4) versus 62.5 (14.8) years, P=0.001. Black patients were more likely to have sub‐basal and diffuse hypertrophy, 22 (38.6%) versus 56 (14.9%), P<0.001, 6 (10.5%) versus 15 (4%), P=0.017, mid‐LV obstruction, 7 (12.3%) versus 21 (5.5%), P=0.025, and cardiac fibrosis ≥15%, 10 (22.2%) versus 19 (8.8%), P=0.009, than White patients. Black patients were more likely to experience appropriate implantable cardioverter defibrillator interventions, 5 (38.5) versus 5 (6.8), P<0.001 and were more likely to have ≥2 sudden death risk factors. Comorbidities were largely similar between groups, though more Black participants had Class II obesity, 12 (21.8) versus 30 (8.1), P<0.001. Both groups had similar rates of genetic testing usage.
Conclusions
This study underscores the need for continued research of HCM in Black populations, including tailored approaches to diagnosis and precise evaluation of cardiac anatomy.
Keywords: health disparities, heart anatomy, hypertrophic cardiomyopathy, outcome, risk factor
Subject Categories: Cardiomyopathy, Hypertrophy
Nonstandard Abbreviations and Acronyms
- BB
beta blocker
- HCM
hypertrophic cardiomyopathy
- IRB
Institutional Review Board
- NYHA
New York Heart Association
Clinical Perspective
What Is New?
Black patients with hypertrophic cardiomyopathy (HCM) are underrepresented in HCM specialty care.
In this study, the Black cohort was more likely to have sub‐basal and diffuse hypertrophy, mid‐LV obstruction, and a high degree of cardiac fibrosis, all HCM characteristics associated with worse disease progression and prognosis.
Precise evaluation of cardiac anatomy is necessary in the diagnostic work‐up of Black patients with suspected HCM to tailor treatment approaches to optimize outcomes in this population; obesity was highly prevalent in both Black and White patients with HCM, and Black patients were more likely to have moderate obesity.
What Are the Clinical Implications?
Risk of preventable comorbidities, including obesity, should be routinely addressed with patients with HCM.
Genetic testing remains an integral component of HCM care and genetic testing use in Black populations should be further explored.
Hypertrophic cardiomyopathy (HCM) is the most common inherited cardiac disorder with a global prevalence of 1:200 to 1:500. 1 It is highly variable in disease expression, course and outcomes. 2 Despite an abundance of literature on disease expression, course, and outcomes there is a paucity of data on these variables in the context of different racial/ethnic groups. To date, the United States and European clinical HCM guidelines have not adequately addressed minority populations. 3 , 4 , 5 Research examining HCM expression, disease course, and outcomes across races and ethnic groups is sparse; especially in traditionally underserved populations, such as Hispanic/Latin and Black groups. The current literature exploring HCM differences between Black and White individuals is limited 6 , 7 , 8 , 9 , 10 ; a persistent challenge is the small overall numbers of Black participants with HCM in the cohorts studied. Further, the existing literature does not address rarer structural HCM characteristics in this population, characteristics that may have bearing on HCM disease course and outcomes. The purpose of this study was 2‐fold: to describe HCM disease expression in a Black cohort compared with a White cohort and to examine associations between structural HCM phenotype, comorbidities, and outcomes between groups.
Methods
This study was a subgroup analysis of an ongoing prospective longitudinal data set study from a specialized HCM center located in a large, urban, academic institution in the northeastern United States. The data that support the findings of this study are available from the corresponding author upon reasonable request. The primary study has institutional review board approval with informed consent given by all participants. The inclusion criteria for this analysis was adults (≥18 years), with a confirmed clinical diagnosis of HCM: unexplained left ventricular hypertrophy (LVH) of ≥15 mm, 5 that self‐identify as either Black/African American or White. 11 Individuals were excluded if they had had prior invasive interventions for HCM that would have altered their native cardiac structure, such as cardiac surgery, and alcohol septal ablation, and there was no imaging available before their intervention. The analysis also included medical records review to supplement missing data from the original data set.
Data Collection and Study Variables
We examined data for 502 consecutively enrolled subjects, who comprised referred patients to the specialty HCM center between July 2015 and November 2019. We excluded 68 individuals (67 White, 1 Black) with prior obstruction‐relieving interventions, such as myectomy and alcohol septal ablation, who had no available imaging of their native anatomy pre‐intervention for review. We analyzed data for 434 individuals, with 57 (13.1%) identifying as Black/American and 377 (86.9%) as White/Caucasian.
Demographic variables, including sex, age, and race/ethnicity and age at initial HCM center evaluation were examined. Age was used to identify trends in age at diagnosis between the population of interest and the comparison group. Data on New York Health Association (NYHA) class at the time of presentation for HCM care was also captured from the medical record. Descriptive data on cardiac structure were collected from imaging studies, including echocardiograms and cardiac magnetic resonance (CMR). Echocardiographic data were examined to describe LVH magnitude and distribution, ejection fraction, presence of obstruction and special anatomic features, such as apical aneurysms, anomalous papillary muscles, and complete systolic emptying. Individuals were considered obstructed if they had mid‐cavitary or left ventricular outflow tract gradients ≥30 mm Hg, whether at rest or with provocation (Valsalva, standing, or exercise). LVH distribution was categorized as sub‐basal, basal, and diffuse. Sub‐basal LVH included thickening in the apical, apical‐mid, and mid‐ventricular segments. Basal LVH constituted asymmetric septal hypertrophy of the basal septum segments which represent the most common, “typical” presentation of HCM. Diffuse LVH included thickening throughout all ventricular segments. CMR data were primarily examined for identifying the presence of fibrosis and quantifying fibrosis burden. Fibrosis burden was quantified using the Chan et al recommendations. 12 In addition, we also captured the ejection fraction and maximal LV wall thickness of those individuals who had undergone cardiac CMR testing. We also examined HCM related medications.
Genetic testing data were also captured, from data set entries and electronic chart review to identify patterns of genetic testing usage, as well as identify genetic testing yield; the distribution of pathogenic variants, likely‐pathogenic variants, variants of unknown significance, or negative results. This sample comprised of patients undergoing routine HCM clinical care; thus, genetic testing was performed via CLIA‐certified commercial laboratories using standard American College of Medical Genetics variant classification criteria. 13 Likely‐pathogenic variants and variants of unknown significance were further reviewed in ClinVar, 14 the National Institutes of Health‐supported, publicly accessible database of reported variants related to disease.
Outcome data included sudden HCM‐related death or appropriate defibrillator shock or anti‐tachycardia pacing, all‐cause mortality, and interventions. For this analysis, major HCM interventions included obstruction relieving interventions (cardiac surgery—myectomy±mitral valve repair/replacement, alcohol septal ablation, and mitral clip procedures), and implantable cardioverter defibrillator (ICD) placement for primary prevention. Traditional and enhanced sudden death risk factors 15 were described in those individuals with ICDs, including massive hypertrophy (LVH ≥30 mm), unexplained syncope, family history of sudden death in a first degree relative ≤50 years old, ventricular tachycardia on ambulatory monitoring, significant fibrosis ≥15 mm, LV ejection fraction less than 50% and apical aneurysm. 5
Comorbid conditions included hypertension, obesity class, and BMI, sleep apnea, atrial fibrillation, history of coronary artery disease, history of stroke/transient ischemic attack, and diabetes mellitus. These comorbid conditions were chosen as they are common within the general population and have implications for patients with HCM. 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Hypertension disproportionately and more severely affects Black individuals, 22 and contributes to the development of LVH. 16 , 22 Obesity is also more prevalent in the Black population overall 17 and can contribute independently to cardiac remodeling and development of LVH. 24
Statistical Analysis
Stata 16 software 25 was used for statistical analysis. Descriptive statistics were used to evaluate descriptive data. Means and SDs were calculated for normally distributed continuous variables, and medians and interquartile ranges were used for non‐normally distributed continuous variables. Frequency distributions were used for categorical variables. Associations were established with unpaired t‐tests for normally distributed continuous variables, Mann Whitney U tests for non‐normally distributed variables. Chi‐square tests were used for categorical variables, with Fisher exact tests used as needed for expected frequencies <5. We used ordinal and binomial logistic regression to examine relationships between race, HCM phenotype, and outcomes while controlling for comorbidities. For both aims significance was met if P value was ≤0.05 and CIs were 95% where appropriate.
Missing data points were excluded from analysis. Some parameters naturally had missing data as not every individual in the sample had undergone every testing modality. If the total number of analyzed individuals in certain parameters deviated from the original sample, this was noted.
Results
Demographics and Baseline Structural HCM Characteristics
A total of 434 individuals met the inclusion criteria with n (%); 57 (13.1%) were Black and 180 (41.5%) were women. Follow‐up 2.2 (1.2), mean (SD) years, Black participants were significantly younger than White participants, 54.6 (13.4) versus 62.5 (14.8) years, P=0.001. Men were overrepresented in both groups. Black participants were less likely to be categorized as functionally unlimited, or NYHA I, 13 (22.8%) versus 129 (35.5%), P=0.030. However, both groups had similar rates of advanced functional limitations, NYHA III–IV.
Echocardiography Data
Maximal LV wall thickness and ejection fraction were similar between the groups. However, there were significant differences in LVH distribution between the Black and White cohort. Sub‐basal and diffuse hypertrophy were significantly more prevalent in the Black cohort: sub‐basal—22 (38.6%) versus 56 (14.9%), P<0.001; diffuse—6 (10.5%) versus 15 (4%), P=0.017. Basal hypertrophy was significantly less prevalent in Black patients compared with White patients, 29 (50.9%) versus 306 (81.2), P<0.001. Both groups had similar proportions of non‐obstructed individuals and individuals with provokable obstruction (Valsalva, standing, or exercise). However, Black patients had significantly more mid‐ventricular obstruction, 7 (12.3%) versus 21 (5.5%), P=0.025 and less left ventricular outflow tract obstruction at rest, 17 (29.8%) versus 171 (45.1%), P=0.015. Black and White patients had similar prevalence of special anatomic features, such as anomalous papillary muscles and complete systolic emptying. There was a trend towards higher presence of apical aneurysms in Black patients, 17 (12.3%) versus 18 (4.8%), P=0.055, though this did not meet statistical significance (Figure; Table 1).
Figure 1. Apical akinetic aneurysm in a 65‐year‐old man with hypertrophic cardiomyopathy.
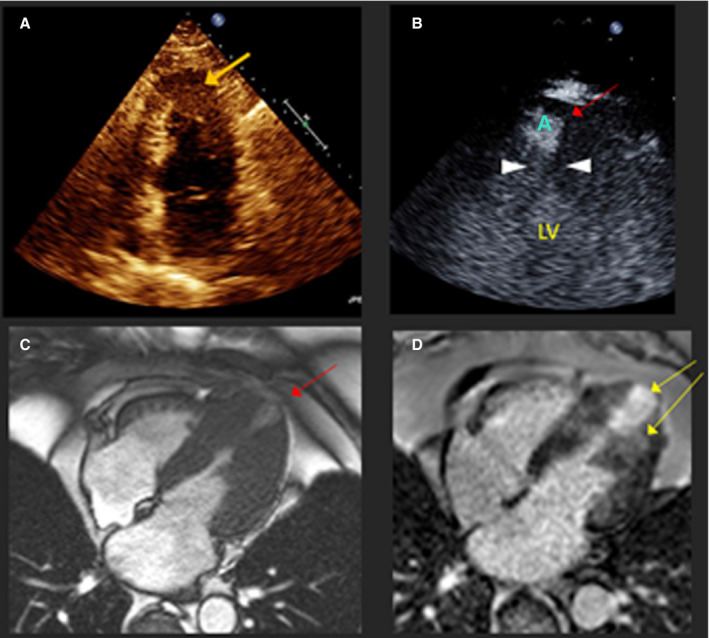
A, Apical 4‐chamber echocardiogram shows apical thickening (orange arrow) but aneurysm is not visualized due to foreshortening. Intravenous contrast is recommended for all patients with apical hypertrophic cardiomyopathy to detect aneurysm. B, Same patient after intravenous echo contrast. Red arrow, A=apical aneurysm; left ventricular cavity; white arrowheads point to mid‐left ventricular obstruction attributable to systolic complete emptying at that level. C, Same patient, 4‐chamber view cardiac magnetic resonance showing mid‐left ventricular hypertrophy, obstruction, and thin‐walled apical aneurysm. D, Delayed late gadolinium enhancement consistent with apical and mid‐left ventricular fibrosis, yellow arrows. CMR indicates cardiac magnetic resonance; and LV, left ventricular.
Table 1.
Demographics and Structural HCM Characteristics
| Sample n=434; Black, 57 [13.1], White, 377 [86.9] Patients | Black, n [%] | White, n [%] | P Value |
|---|---|---|---|
| Age (y), mean (SD) | 54.6 [13.4] | 62.5 [14.8] | 0.000 |
| Sex (men) | 30 [52.6] | 224 [59%] | 0.375 |
| NYHA Class I | 13 [28.8] | 129 [35.5] | 0.030 |
| NYHA Class II | 22 [38.6] | 124 [34.2] | 0.258 |
| NYHA Class III and IV | 22 [38.6] | 110 [30.3] | 0.105 |
| Max echocardiography LVH, mm, mean (SD) | 19.1 [5.9] | 18.4 [4.9] | 0.838 |
| Ejection fraction (echo), mean (SD) | 71.1 [6.1] | 69.6 [6.9] | 0.866 |
| LVH distribution (echo data) | |||
| Basal LVH | 29 [50.9] | 306 [81.2] | 0.000 |
| Sub‐basal LVH | 22 [38.6] | 56 [14.9] | 0.000 |
| Diffuse LVH | 6 [10.5] | 15 [4.0] | 0.017 |
| Special anatomic features [echo data] | |||
| Complete systolic emptying | 9 [15.8] | 30 [7.9] | 0.169 |
| Apical aneurysm | 7 [12.3] | 18 [4.75] | 0.055 |
| Anomalous papillary muscle | 10 [17.5] | 59 [15.6] | 0.916 |
| Obstruction (echo data) | |||
| No obstruction | 20 [35.1] | 116 [30.6] | 0.247 |
| LVOT obstruction at rest (≥30 mm Hg) | 17 [29.8] | 171 [45.1] | 0.015 |
| Provocable LVOT obstruction (Valsalva, standing, exercise) | 13 [22.8] | 71 [18.7] | 0.232 |
| Mid‐LV obstruction | 7 [12.3] | 21 [5.5] | 0.025 |
| Cardiac MRI data | |||
| CMR (had) | 45 [79.0] | 215 [57.6] | 0.009 |
| Max CMR LVH, mm, mean (SD) | 22.2 [6.4] | 18.9 [4.7] | 0.017 |
| Fibrosis (yes) | 36 [80.0] | 142 [67.3] | 0.121 |
| Fibrosis ≥15% (yes) | 10 [22.2] | 19 [8.8] | 0.009 |
CMR indicates cardiac magnetic resonance; HCM, hypertrophic cardiomyopathy; LVH, left ventricular hypertrophy; LVOT, left ventricular outflow tract; mid‐LV, mid‐ventricular; MRI, magnetic resonance imaging; and NYHA, New York Heart Association.
CMR Data
More Black than White patients had CMRs, 45 (79%) versus 215 (57.6%), P=0.009. In this subgroup, there were no differences in ejection fraction, though maximal LV wall thickness differed, with Black patients having a greater LV maximal thickness, mean (SD), 22.2 mm (6.4) versus 18.9 mm (4.7), P=0.017. The presence of cardiac fibrosis overall was noted in similar proportions between both groups. However, significantly more Black patients with HCM had fibrosis comprising ≥15% of the myocardium than White patients with HCM, 10 (22.2%) versus 19 (8.8%), P=0.009 (Table 1; Figure).
Medical Therapy
Beta blockers were the most common HCM‐related medications in both groups. However, the Black cohort was much more likely to be treated with non‐dihydropyridine calcium channel blockers alone, or in combination with beta blockers, 9 (15.8%) versus 17 (4.5%), P=0.003, and 15 (26.3%) versus 34 (9%), P<0.001, respectively. However, the Black HCM cohort was less likely to be treated with disopyramide±beta blockers±calcium channel blockers, than the White HCM cohort, 5 (8.8%) versus 77 (20.4%), P=0.036 (Table 2). This difference is consistent with the finding of lower prevalence of resting left ventricular outflow tract obstruction in the Black HCM cohort. The use of anticoagulants between groups was largely consistent with the prevalence of atrial fibrillation in the sample.
Table 2.
Distribution of Medical Therapy Between Groups
| Medical Therapy | Black, n (%) | White, n (%) | P Value |
|---|---|---|---|
| BB alone | 25 [43.6] | 215 [57.0] | 0.062 |
| CCB alone* | 9 [15.8] | 17 [4.5] | 0.003 |
| BB+CCB | 15 [26.3] | 34 [9.0] | <0.001 |
| Disopyramide±BB±CCB | 5 [8.8] | 77 [20.4] | 0.036 |
| None | 3 [5.3] | 34 [9.0] | 0.344 |
| Anticoagulants | 6 [10.5] | 92 [24.4] | 0.128 |
BB indicates beta‐blockers; and CCB, calcium‐channel blockers.
Fisher exact.
Comorbidities
Both groups in this sample had a similar number of attendant comorbidities. There was a trend towards less atrial fibrillation and hyperlipidemia in Black patients but this did not reach statistical significance. Presence of a hypertension diagnosis was similar between groups. Body mass index (BMI) was available in 55 individuals of the Black HCM cohort and 372 of the White HCM cohort. Both groups had similar BMI, 30.4 (5.5) versus 28.8 (5.4), P=0.979. Overweight and obesity were highly prevalent in both groups, 47 (85.5%) of Black patients and 293 (78.8%) of White patients had a BMI of ≥25, P=0.125. However, Black patients had a significantly higher proportion of Class II obesity (BMI, 35.0–39.9) than White patients, 12 (21.8%) versus 30 (8.1%), P<0.001 (Table 3).
Table 3.
Comorbid Conditions
| Black, n [%] | White, n [%] | P Value | |
|---|---|---|---|
| No. of comorbidities, mean (SD) | 1.8 [1.4] | 1.9 [1.4] | 0.310 |
| Coronary artery disease | 4 [7.0] | 37 [9.9] | 0.501 |
| Stroke/transient ischemic attack | 2 [3.5] | 15 [4] | 0.865 |
| Sleep apnea | 4 [7.0] | 43 [11.5] | 0.312 |
| Hypertension | 32 [56.1] | 176 [47.6] | 0.201 |
| Atrial fibrillation | 6 [10.5] | 79 [21.1] | 0.061 |
| Hyperlipidemia | 22 [38.6] | 195 [52.1] | 0.057 |
| Diabetes mellitus | 7 [12.3] | 23 [6.2] | 0.090 |
| BMI, mean (SD) | 30.4 [5.5] | 28.8 [5.4] | 0.979 |
| Underweight (BMI, <19.9) | 1 [1.8] | 2 [0.5] | 0.148 |
| Normal weight (BMI, 20–24.9) | 7 [12.7] | 77 [20.7] | 0.082 |
| Overweight (BMI, 25–29.9) | 23 [41.82] | 150 [40.3] | 0.416 |
| Class I obesity (BMI, 30–34.9) | 10 [18.2] | 91 [24.5] | 0.149 |
| Class II obesity (BMI, 30–39.9) | 12 [21.8] | 30 [8.1] | 0.001 |
| Class III obesity (BMI, 40+) | 2 [3.64] | 22 [5.91] | 0.248 |
BMI indicates body mass index.
Outcomes and Interventions
In the follow‐up period (mean, 2.2 [1.2] years), there were no deaths among Black patients; 4 deaths (1 sudden death, 2 cardiac‐related but not sudden death, and 1 non‐cardiac) occurred in the White HCM cohort. These events occurred in older White patients (>age 70 years). Black and White patients had similar rates of ICD insertion for primary prevention, 13 (22.8%) versus 76 (20.2%), P=0.644. Of the individuals in both cohorts who had implanted ICDs, Black patients had a significantly higher rate of appropriate ICD interventions (anti‐tachycardia pacing or shock) than White patients, 5 (38.5%) versus 5 (6.6%), P<0.001 (Table 4). Of the 5 individuals in the Black HCM cohort who had appropriate ICD interventions, 3 individuals had ventricular tachycardia storm with multiple ICD shocks, with 1 individual requiring extra corporeal membrane oxygenation hemodynamic support and multiple antiarrhythmic agents before ventricular tachycardia was suppressed. More detailed HCM characteristics of individuals with appropriate ICD interventions are shown in Table 5.
Table 4.
Outcomes and Interventions
| Follow‐Up Period, 2.2 [1.2] y, Mean (SD) | Black, n [%] | White, n [%] | P Value |
|---|---|---|---|
| Outcomes | |||
| Appropriate ICD shocks/ATP | 5 [38.5] | 5 [6.8] | 0.001 |
| Sudden death from HCM | 0 [0] | 1 [0.3] | |
| Cardiac death | 0 [0] | 2 [0.5] | |
| Non‐cardiac death | 0 [0] | 1 [0.3] | |
| Interventions | |||
| ICD (primary prevention) | 13 [22.8] | 76 [20.2] | 0.644 |
| Obstruction relieving interventions | 15 [26.3] | 108 [28.7] | 0.716 |
| Type of obstruction‐relieving interventions | |||
| Myectomy | 15 [100] † | 101 [93.5] † | 0.940 |
| Alcohol septal ablation | 0 [0] | 4 [3.7] † | 0.568 |
| Mitral clip | 0 [0] | 3 [2.8] † | 0.655 |
ATP indicates anti‐tachycardia pacing; HCM, hypertrophic cardiomyopathy; and ICD, implantable cardioverter defibrillator.
Percentage calculations are based on n of individuals undergoing obstruction relieving interventions, n=15 Black individuals, n=108 White individuals.
Table 5.
Characteristics of Subgroup With Appropriate ICD Interventions
| Black, n=5 | White, n=5 | |
|---|---|---|
| Age, median [IQR] | 45 [44–64] | 48 [48–58] |
| Sex (men) | 2 [40.0] | 3 [60.0] |
| Massive LVH ≥30 mm | 2 [40.0] | 1 [20.0] |
| Syncope | 0 [0.0] | 1 [20.0] |
| SCD in family | 4 [80.0] | 0 [0.0] |
| VT on ambulatory monitoring | 2 [40.0] | 2 [40.0] |
| Apical aneurysm | 1 [20.0] | 1 [20.0] |
| HCM with LV systolic dysfunction EF<50% | 0 [0.0] | 0 [0.0] |
| Fibrosis ≥15% | 1 [20.0] | 0 [0.0] |
| Secondary prevention | 0 [0.0] | 1 [20.0] |
| Sudden death risk factors ≥2 | 2 [40.0] | 1 [20.0] |
| LVH distribution | ||
| Sub‐basal LVH | 0 [0.0] | 1 [20.0] |
| Basal LVH | 2 [40.0] | 3 [60.0] |
| Diffuse LVH | 3 [60.0] | 1 [20.0] |
| Obstruction | ||
| At rest (LVOT gradient ≥30 mm Hg) | 0 [0.0] | 3 [60.0] |
| With provocation | 1 [20.0] | 0 [0.0] |
| Mid‐LV obstruction | 1 [20.0] | 1 [20.0] |
| No obstruction | 3 [60.0] | 1 [20.0] |
| Genetic profile and corresponding genes | ||
| Pathogenic/likely‐pathogenic variant | 3 [60.0] [MYH7 and MYBPC3 x2]* | 1 [20.0] [MYH7] |
| Variant of unknown significance | 3 [60.0] [TNNI3, MYL3, AGL, SOS2]* | 1 [20.0] [MYBPC3] |
| No variant found | 0 [0.0] | 1 [20.0] |
| Did not have genetic testing | 1 [20.0] | 2 [40.0] |
EF indicates ejection fraction; AGL, amylo‐alpha‐1,6‐glucosidase, 4‐alpha‐glucanotransferase; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; IQR, interquartile ranges; LV, left ventricular; LVH, left ventricular hypertrophy; LVOT, left ventricular outflow tract; MYH7, myosin heavy chain 7; MYBPC3, myosin binding protein C3; MYL3, myosin light chain 3; SCD, sudden cardiac death; SOS2, SOS ras/rho guanine nucleotide exchange factor 2; TNNI3, troponin I3, cardiac type; and VT, ventricular tachycardia.
Two individuals had both pathogenic and variants of unknown significance.
All patients with ICDs had them implanted for primary prevention of sudden death and had at least 1 of the sudden death predictors as outlined by the most recent American Heart Association/American College of Cardiology HCM guidelines. 5 Specific sudden death risk factor data were available for 72 of the 76 White patients with ICDs and for all 13 Black individuals with ICDs. A higher proportion of the Black cohort had massive hypertrophy and ≥2 sudden death risk factors than the White cohort, 7(46.7%) versus 5(6.9%), P<0.001, and 10 (66.7%) versus 17 (23.6%), P=0.001. Other risk factors were not statistically significantly different in this subgroup analysis. Only 40 individuals of the White cohort had CMRs before ICD insertion, and 12 of 13 individuals of the Black ICD cohort. Sudden death risk factors for both groups are shown in Table 6. We evaluated additional clinical factors, beyond the guideline recognized factors, that may be associated with ICD implantation, such as LVH distribution and type of obstruction. However, none were significantly associated with ICD insertion. In Chi‐square analysis we did not find significant associations with phenotype, including LVH distribution, presence and type of obstruction, or fibrosis presence or burden, comorbidities, race, or sex.
Table 6.
Sudden Death Risk Factors in Those With ICDs
| ICD Insertion | Black, n [%] | White, n [%] | P Value |
|---|---|---|---|
| Individuals with ICD, n [%] of total cohort | 13 [22.8] | 76 [20.2] | |
| SCD factors documented, n [%] of cohort with ICD inserted | 13 [100] | 72 [94.7] | |
| Demographics and sudden death risk factors | |||
| Age (y), median [IQR]* | 57 [45–64] | 60 [47–65.5] | 0.685 |
| Sex (men) | 6 [46.2] | 45 [62.5] | 0.268 |
| Massive thickness, LVH ≥30 mm | 5 [38.5] | 5 [6.9] | 0.001 |
| Syncope ‡ | 1 [7.7] | 10 [13.9] | 0.468 |
| SCD in family ‡ | 5 [38.5] | 21 [29.2] | 0.357 |
| VT on ambulatory monitoring | 9 [69.2] | 35 [48.6] | 0.171 |
| Apical aneurysm ‡ | 3 [23.1] | 9 [12.5] | 0.267 |
| HCM with LV systolic dysfunction EF<50% ‡ | 0 [0] | 3 [4.2] | 0.604 |
| Individuals with CMR before ICD insertion | 12 [92.3] | 40 [55.6] | 0.012 |
| Fibrosis ≥15% ‡ | 4 [33.3] | 10 [25] | 0.409 |
| Secondary prevention | 0 [0] | 1 [1.4] | 0.847 |
| ≥2 sudden death risk factors ‡ | 8 [61.5] | 17 [23.6] | 0.010 |
CMR indicates cardiac magnetic resonance; EF, ejection fraction; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; IQR, interquartile ranges; LV, left ventricular; LVH, left ventricular hypertrophy; SCD, sudden cardiac death; and VT, ventricular tachycardia.
Mann‐Whitney U test.
Fisher exact test.
Black and White patients had similar proportions of obstruction‐relieving interventions. All Black patients in this study had myectomies as a method of obstruction‐relieving intervention and so did 93.5% of White patients. Five white patients underwent alcohol septal ablation or mitral clip procedure (Table 4).
Structural HCM Characteristics and NYHA Class
There was no statistically significant association between presence of fibrosis or significant fibrosis (≥15%) and LVH distribution in this sample. Further, there were no significant associations between fibrosis and type of obstruction. NYHA class was variable among those with significant fibrosis, without a clear pattern. Of the 29 individuals with fibrosis ≥15%, 11 (37.9%) were NYHA Class I, 8 (27.6%) were NYHA Class II, and 10 (34.5%) had NYHA Class III functional limitations. None had NYHA Class IV limitations. NYHA class was also variable among those with sub‐basal and diffuse hypertrophy. NYHA data were available in 74 of the 78 individuals with sub‐basal hypertrophy, 25 (33.8%) were NYHA class I, 22 (29.7%) NYHA Class II, 23 (31.1%) NYHA Class III, 4 (5.4%) NYHA Class IV. Among the 21 individuals with diffuse hypertrophy, 5 (23.8%) were NYHA Class I, 11 (52.4%) were NYHA Class II, and 5 (23.8%) were NYHA Class III. No individuals with diffuse hypertrophy had NYHA Class IV functional limitations. Neither fibrosis ≥15% nor LVH distribution had significant association with NYHA class.
Genetic Profile
Genetic testing data were available in 51 individuals of the Black HCM cohort and 376 individuals of the White HCM cohort. Less than half of each group had genetic testing, 23 (45.1%) Black patients versus 162 (43.1%) White patients, and there were no differences in genetic testing usage between groups. In absolute numbers, Black patients had more pathogenic, likely pathogenic, and variants of unknown significance than White patients, though this trend did not reach statistical significance (Table 7). In Black patients, pathogenic and likely pathogenic HCM variants were noted in the MYH7 and MBPC3 genes, whereas pathogenic and likely pathogenic HCM variants in White patients were noted in a wider variety of genes: ACTC1, GAA, MYH7, MBPC3, MYL3, TPM1,TNNI3, and TNNT2.
Table 7.
Genetic Testing Usage and Results
| Black, n [%] | White, n [%] | P Value | |
|---|---|---|---|
| Genetic testing (yes) | 23 [45.1] | 162 [43.1] | 0.785 |
| Variant of unknown significance | 5 [21.7] | 26 [16.1] | 0.251 |
| Negative | 8 [34.8] | 87 [53.7] | 0.045 |
| Positive and likely pathogenic variants | 10 [43.5] | 49 [30.3] | 0.102 |
Discussion
Black patients represented 13.1% of the sample studied which was below the average population statistics of the study region. 26 However, this is consistent with the existing literature, where Black patients in the HCM study samples are also underrepresented. 6 , 8 , 9 , 10 Black participants are underrepresented in clinical research overall despite efforts to increase participation. 27 , 28 Women were also underrepresented in both study groups, which is also consistent with historical trends. 29 The reasons for this underrepresentation in the setting of HCM research have not been fully explored. However, most literature on HCM originates from specialty HCM centers and individuals must often navigate a complex and challenging healthcare system before they are seen in experienced HCM clinics. 5 , 30 Research exploring the social determinants of HCM health may provide insights into improving representation of Black individuals in the clinical and research setting.
Structural HCM Characteristics and Genetic Profiles
Black patients were more likely to have apical hypertrophy than their White counterparts, which is consistent with the existing literature. 6 , 8 However, Black patients were also more likely to have apical‐mid and mid‐ventricular LVH, as well as diffuse hypertrophy, which has previously not been reported in this population. As such, Black patients may be less likely to present with the “classic” HCM phenotype, which may contribute to delays in diagnosis and treatment. Further, while the existing literature shows that Black patients are less likely to be obstructed than White patients, we did not observe that in our sample. We found that Black individuals had significantly more mid‐ventricular obstruction, which is also a novel finding. The discrepancy between our findings and the existing literature may be because mid‐ventricular obstruction has been under‐evaluated and under‐recognized in existing research—echocardiograms often require the use of contrast which may not be a common practice in all echocardiography laboratories; thus, individuals with mid‐ventricular obstruction may be categorized as having no obstruction. 31 Our findings underscore the importance of differentiating and appropriately diagnosing mid‐ventricular obstruction given its association with poor outcomes. 32
More Black patients than White patients had CMR. Since baseline sudden death risk from HCM declines with age (especially after 60 years), 33 , 34 a possible explanation for this difference in usage may be related to the age difference between groups, as Black patients were significantly younger. Thus, it is possible that they were undergoing a more comprehensive sudden death risk evaluation. In this regard, Black patients were more likely to have significant fibrosis (≥15%), shown to be a risk for arrhythmia, and sudden death events. 12 , 34 , 35
Less than half of the study sample underwent genetic testing. This finding is consistent with the existing literature on HCM genetic testing usage in Black patients, which ranges from 38.7% to 50.4%, 6 , 8 though it is lower than expected for HCM genetic testing usage in White patients, which ranges between 50.4% to 62.1% in White patients. 8 , 10 Admittedly, the literature examining HCM genetic testing usage rates between Black and White HCM patients is itself significantly limited in number and may be specific to the study sites. It is possible that the more equitable use of genetic testing between groups in this sample may be because of the lower rate of HCM genetic testing usage in White patients rather than an increase in genetic testing usage in Black patients. The drivers in patient decision making for HCM genetic testing usage are not well understood and this lack of data may limit success in any future interventions aimed at improving the utilization of HCM genetic testing. Research examining the factors affecting decision making in genetic testing usage among both Black and White patients with HCM is warranted in general. However, this research is especially important for Black populations with HCM to reduce disparities beyond genetic testing usage alone.
Although Black patients had higher rates of pathogenic, likely pathogenic, and variants of unknown significance these did not meet statistical significance. It is possible that referral patterns may have affected the higher rates of these results among Black patients with HCM in this sample. It is important that genetic testing results for Black patients with HCM be closely examined. Genomic reference databases are not diverse, thus limiting precise variant interpretation in minority populations. 36 , 37 Further, there is precedent for reclassification of pathogenic HCM variants in a sample of Black patients with HCM, that were subsequently deemed benign. 7 In addition to the individuals with clinical HCM, genetic misclassification can affect family screening and impact the trajectory of family members that may be either cleared from screening or identified as having a high risk of developing HCM attributable to cascade screening based on inaccurate data. Improving genetic testing usage among Black patients with HCM will also benefit genomic reference databases by increasing representation of minority populations.
Outcomes and Sudden Death Risk Factors
Both groups in the study cohort had low mortality which is consistent with the existing literature. However, Black patients had a significantly higher rate of appropriate ICD ATP therapy or discharges. This is despite Black and White patients having similar rates of ICD insertion for primary prevention. We examined sudden risk factors for ICD insertion in both groups and Black patients were more likely to have massive LVH and have ≥2 sudden death risk factors than White patients. The proportion of Black and White individuals with ICDs who also had significant fibrosis did not differ, yet this may be because of the lower number of White patients who had undergone CMR before ICD insertion, limiting the statistical results. ICD interventions were not significantly associated with structural HCM characteristics, including fibrosis, or demographics. This may be because of the overall low number of events in the study groups. Racial disparities in the use of ICD implantation have narrowed overall 38 though they still exist. The reasons for this disparity are not well understood and require further evaluation, including decisional factors.
Efforts to refine models for sudden cardiac death risk from HCM and identify the most pertinent risk factors are ongoing. 15 , 39 One of the largest studies evaluating sudden death factors in HCM is the HCMR (Novel Markers of Prognosis in Hypertrophic Cardiomyopathy) registry (NCT01915615), a multi‐center longitudinal observational study with 2755 patients with HCM. 40 Registry participants comprised a low‐risk cohort. 41 Most participants did not have obstruction and few had either significant fibrosis (≥15%), 2%, apical aneurysms, 3%, or massive hypertrophy, 2.3%. 40 In contrast, both cohorts for the present study had a higher prevalence of these HCM characteristics. The sample in the HCMR registry was less diverse, with 7.5% Black patients that as we have shown have a higher incidence of ICD discharges. Further, clinical, echocardiography, and CMR data were only dichotomized between a White and minority group, with the minority group encompassing Black, Asian, Hispanic, and other individuals, in aggregate. Despite a much lower overall sample size, our study suggests there may be significant differences in sudden death risk factors between Black and White cohorts with HCM.
Medical Therapy and Interventions
The HCM‐specific medical therapy for both groups was consistent with current guideline recommendations. 5 Moreover, it was consistent with phenotypic presentation, specifically in the use of disopyramide, which was more prevalent in the White cohort, which was also more likely to have left ventricular outflow tract obstruction at rest. Calcium channel blocker therapy alone, or in combination with a beta blocker, was more common in the Black HCM cohort which was more likely to have diffuse and sub‐basal hypertrophy and mid‐LV obstruction. There is a need for contemporary examination of the use of verapamil in individuals with this less common HCM phenotype.
Comorbidities
Both groups had a similar number of comorbid conditions though Black patients were significantly younger. Further, there was no difference in prevalence of comorbidities between the 2 groups, except Class II obesity (BMI, 35–39.9) which was more prevalent in the Black cohort. Notably, despite the high prevalence of risk factors for coronary artery disease and stroke/transient ischemic attack, specifically obesity and hypertension in the general population, these conditions were relatively uncommon in both groups. Further, comorbidities were not associated with mortality outcomes, though it is likely that the low numbers of deaths and appropriate ICD interventions in the sample overall, limited statistical power for such analysis. More research is needed to explore comorbid conditions in Black patients with HCM, including the role of age and the degree of successful management of the attendant comorbidities.
Limitations
There were several limitations for this study. First, this was an analysis of an existing prospective database. Although there were missing datapoints in some of the variables of interest, this was minimized through chart reviews. The subjects enrolled in the study were individuals who were getting care at a specialty HCM center. The potential of referral bias limits the generalizability of the results to a wider HCM population who may not receive care at a specialty center. The subjects enrolled in the study had already navigated the healthcare system to obtain specialty care suggesting that patient characteristics may differ from a general patient population. Further, race was self‐identified in this sample. Thus, the inherent bias present in any self‐identification characterization was also present in our study. Further, we examined the use of HCM‐related medical therapy in the groups after they had established care in the HCM specialty center. It is possible that they had been on other therapy before presentation to specialty care. Additionally, we recognize that medical therapy is not static and may change depending on disease progression.
Conclusions
This study provides much needed data about the disease expression, course, and outcomes of Black patients with HCM, specifically addressing less common cardiac HCM characteristics that have not been reported in this population previously. The underrepresentation of Black participants in our study, which is consistent with the extant research, underscores the need for continued research of HCM in this population. Further, there is a need to explore HCM in Black patients in care settings beyond specialty HCM centers that can contribute to a wider scope of understanding of HCM course and outcomes in this population. To address health disparities experienced by Black patients with HCM our data suggests the need for tailored approaches for the diagnosis of this condition, including precise delineation of cardiac anatomy.
Sources of Funding
None.
Disclosures
Dr Arabadjian has received advisory panel fees from MyoKardia, Inc. Dr Sherrid has received consulting fees from Celltrion. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 10.
See Editorial by Lakdawala
References
- 1. Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65:1249–1254. DOI: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 2. Michels M, Olivotto I, Asselbergs FW, van der Velden J . Life‐long tailoring of management for patients with hypertrophic cardiomyopathy: awareness and decision‐making in changing scenarios. Neth Hearth J. 2017;25:186–199. DOI: 10.1007/s12471-016-0943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Authors/Task Force Members , Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. DOI: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 4. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2761–2796. DOI: 10.1161/CIR.0b013e318223e230. [DOI] [PubMed] [Google Scholar]
- 5. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2020;142:e533–e557. DOI: 10.1161/CIR.0000000000000938. [DOI] [PubMed] [Google Scholar]
- 6. Eberly LA, Day SM, Ashley EA, Jacoby DL, Jefferies JL, Colan SD, Rossano JW, Semsarian C, Pereira AC, Olivotto I, et al. Association of race with disease expression and clinical outcomes among patients with hypertrophic cardiomyopathy. JAMA Cardiol. 2020;5:83–91. DOI: 10.1001/jamacardio.2019.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manrai AK, Funke BH, Rehm HL, Olesen MS, Maron BA, Szolovits P, Margulies DM, Loscalzo J, Kohane IS. Genetic misdiagnoses and the potential for health disparities. N Engl J Med. 2016;375:655–665. DOI: 10.1056/NEJMsa1507092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sheikh N, Papadakis M, Panoulas VF, Prakash K, Millar L, Adami P, Zaidi A, Gati S, Wilson M, Carr‐White G, et al. Comparison of hypertrophic cardiomyopathy in Afro‐Caribbean versus white patients in the UK. Heart. 2016;102:1797–1804. DOI: 10.1136/heartjnl-2016-309843. [DOI] [PubMed] [Google Scholar]
- 9. Sorensen LL, Pinheiro A, Dimaano VL, Pozios I, Nowbar A, Liu H, Luo H‐C, Lin X, Olsen NT, Hansen TF, et al. Comparison of clinical features in blacks versus whites with hypertrophic cardiomyopathy. Am J Cardiol. 2016;117:1815–1820. DOI: 10.1016/j.amjcard.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 10. Wells S, Rowin EJ, Bhatt V, Maron MS, Maron BJ. Association between race and clinical profile of patients referred for hypertrophic cardiomyopathy. Circulation. 2018;137:1973–1975. DOI: 10.1161/CIRCULATIONAHA.117.032838. [DOI] [PubMed] [Google Scholar]
- 11. US Census Race and Ethnicity. Available at: https://www.census.gov/topics/population/race/about.html. Accessed April 30, 2021.
- 12. Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, et al. Prognostic value of quantitative contrast‐enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. DOI: 10.1161/CIRCULATIONAHA.113.007094. [DOI] [PubMed] [Google Scholar]
- 13. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier‐Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–423. DOI: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. ClinVar . 2020. Available at: https://www.ncbi.nlm.nih.gov/clinvar/. Accessed December 20, 2020.
- 15. Maron MS, Rowin EJ, Wessler BS, Mooney PJ, Fatima A, Patel P, Koethe BC, Romashko M, Link MS, Maron BJ. Enhanced American College of Cardiology/American Heart Association strategy for prevention of sudden cardiac death in high‐risk patients with hypertrophic cardiomyopathy. JAMA Cardiol. 2019;4:644–657. DOI: 10.1001/jamacardio.2019.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoon SS, Fryar CD, Carroll MD. Hypertension Prevalence and Control Among Adults: United States, 2011–2014. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2015. [Google Scholar]
- 17. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 18. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. DOI: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 19. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S , Després J‐P, Fullerton HJ, Howard VJ, et al. Executive summary: heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:434–441. DOI: 10.1161/CIR.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 20. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. 2017;70:2022–2035. DOI: 10.1016/j.jacc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 21. Bibbins‐Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FAR, Herzstein J, Kemper AR, Krist AH, Kurth AE, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:407–414. DOI: 10.1001/jama.2016.20325. [DOI] [PubMed] [Google Scholar]
- 22. Williams SK, Ravenell J, Seyedali S, Nayef S, Ogedegbe G. Hypertension treatment in blacks: discussion of the U.S. clinical practice guidelines. Prog Cardiovasc Dis. 2016;59:282–288. DOI: 10.1016/j.pcad.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woodiwiss AJ, Norton GR. Obesity and left ventricular hypertrophy: the hypertension connection. Curr Hypertens Rep. 2015;17:28. DOI: 10.1007/s11906-015-0539-z. [DOI] [PubMed] [Google Scholar]
- 24. Fumagalli C, Maurizi N, Day SM, Ashley EA, Michels M, Colan SD, Jacoby D, Marchionni N, Vincent‐Tompkins J, Ho CY, et al. Association of obesity with adverse long‐term outcomes in hypertrophic cardiomyopathy. JAMA Cardiol. 2020;5:65–72. DOI: 10.1001/jamacardio.2019.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corp S . Stata Statistical Software: Release 16. College Station, TX: StataCorp, LLC; 2019. [Google Scholar]
- 26. US Census . Quick facts. Available at: https://www.census.gov/quickfacts. Accessed April 30, 2021.
- 27. George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104:e16–e31. DOI: 10.2105/AJPH.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21:879–897. DOI: 10.1353/hpu.0.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pilote L, Raparelli V. Participation of women in clinical trials: not yet time to rest on our laurels. J Am Coll Cardiol. 2018;71:1970–1972. DOI: 10.1016/j.jacc.2018.02.069. [DOI] [PubMed] [Google Scholar]
- 30. Cook NL, Ayanian JZ, Orav EJ, Hicks LS. Differences in specialist consultations for cardiovascular disease by race, ethnicity, gender, insurance status, and site of primary care. Circulation. 2009;119:2463–2470. DOI: 10.1161/CIRCULATIONAHA.108.825133. [DOI] [PubMed] [Google Scholar]
- 31. Malcolmson JW, Hamshere SM, Joshi A, O'Mahony C, Dhinoja M, Petersen SE, Sekhri N, Mohiddin SA. Doppler echocardiography underestimates the prevalence and magnitude of mid‐cavity obstruction in patients with symptomatic hypertrophic cardiomyopathy. Catheter Cardiovasc Interv. 2018;91:783–789. DOI: 10.1002/ccd.27143. [DOI] [PubMed] [Google Scholar]
- 32. Elsheshtawy MO, Mahmoud AN, Abdelghany M, Suen IH, Sadiq A, Shani J. Left ventricular aneurysms in hypertrophic cardiomyopathy with midventricular obstruction: a systematic review of literature. Pacing Clin Electrophysiol. 2018;41:854–865. DOI: 10.1111/pace.13380. [DOI] [PubMed] [Google Scholar]
- 33. Lorenzini M, Anastasiou Z, O’Mahony C, Guttman OP, Gimeno JR, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Garcia‐Pavia P, et al. Mortality among referral patients with hypertrophic cardiomyopathy vs the general European population. JAMA Cardiol. 2020;5:73–80. DOI: 10.1001/jamacardio.2019.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maron BJ, Rowin EJ, Casey SA, Garberich RF, Maron MS. What do patients with hypertrophic cardiomyopathy die from? Am J Cardiol. 2016;117:434–435. DOI: 10.1016/j.amjcard.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 35. Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert E‐M, Nassenstein K, Schlosser T, Sabin GV, Sechtem U, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–887. DOI: 10.1016/j.jacc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 36. Landry LG, Ali N, Williams DR, Rehm HL, Bonham VL. Lack of diversity in genomic databases is a barrier to translating precision medicine research into practice. Health Aff. 2018;37:780–785. DOI: 10.1377/hlthaff.2017.1595. [DOI] [PubMed] [Google Scholar]
- 37. Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016;538:161. DOI: 10.1038/538161a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel NJ, Edla S, Deshmukh A, Nalluri N, Patel N, Agnihotri K, Patel A, Savani C, Patel N, Bhimani R, et al. Gender, racial, and health insurance differences in the trend of implantable cardioverter‐defibrillator (ICD) utilization: a United States experience over the last decade. Clin Cardiol. 2016;39:63–71. DOI: 10.1002/clc.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rowin EJ, Maron BJ, Haas TS, Garberich RF, Wang W, Link MS, Maron MS. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol. 2017;69:761–773. DOI: 10.1016/j.jacc.2016.11.063. [DOI] [PubMed] [Google Scholar]
- 40. Neubauer S, Kolm P, Ho CY, Kwong RY, Desai MY, Dolman SF, Appelbaum E, Desvigne‐Nickens P, DiMarco JP, Friedrich MG, et al. Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM registry. J Am Coll Cardiol. 2019;74:2333–2345. DOI: 10.1016/j.jacc.2019.08.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sherrid MV, Massera D. Risk stratification and hypertrophic cardiomyopathy subtypes. J Am Coll Cardiol. 2019;74:2346–2349. DOI: 10.1016/j.jacc.2019.09.020. [DOI] [PubMed] [Google Scholar]


