Abstract
Background
Transesophageal echocardiogram is currently the standard preprocedural imaging for left atrial appendage occlusion. This study aimed to assess the additive value of preprocedural computed tomography (CT) planning versus stand‐alone transesophageal echocardiogram imaging guidance to left atrial appendage occlusion.
Methods and Results
We retrospectively reviewed 485 Watchman implantations at a single center to compare the outcomes of using additional CT preprocedural planning (n=328, 67.6%) versus stand‐alone transesophageal echocardiogram guidance (n=157, 32.4%) for left atrial appendage occlusion. The primary end point was the rate of successful device implantation without major peri‐device leak (>5 mm). Secondary end points included major adverse events, total procedural time, delivery sheath and devices used, risk of major peri‐device leak and device‐related thrombus at follow‐up imaging. A single/anterior‐curve delivery sheath was used more commonly in those who underwent CT imaging (35.9% versus 18.8%; P<0.001). Additional preprocedural CT planning was associated with a significantly higher successful device implantation rate (98.5% versus 94.9%; P=0.02), a shorter procedural time (median, 45.5 minutes versus 51.0 minutes; P=0.03) and a less frequent change of device size (5.6% versus 12.1%; P=0.01), particularly device upsize (4% versus 9.4%; P=0.02). However, there was no significant difference in the risk of major adverse events (2.1% versus 1.9%; P=0.87). Only 1 significant peri‐device leak (0.2%) and 5 device‐related thrombi were detected in follow‐up (1.2%) with no intergroup difference.
Conclusions
Additional preprocedural planning using CT in Watchman implantation was associated with a higher successful device implantation rate, a shorter total procedural time, and a less frequent change of device sizes.
Keywords: atrial fibrillation, computed tomography, left atrial appendage occlusion, three‐dimensional printing, transesophageal echocardiography
Subject Categories: Atrial Fibrillation, Echocardiography, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- LAA
left atrial appendage
- TEE
transesophageal echocardiogram
Clinical Perspective
What Is New?
Additional preprocedural planning in Watchman implantation using computed tomography was associated with a significantly higher rate of successful device implantation, a shorter total procedural time, and a less frequent need to change device sizes.
What Are the Clinical Implications?
A large‐scale randomized controlled trial comparing the 2 imaging modalities in different left atrial appendage occlusion devices is needed.
A streamlined computed tomography planning protocol without a 3‐dimensional–printed model should be developed to reduce cost and improve reproducibility.
Left atrial appendage (LAA) occlusion (LAAO) is a nonpharmacologic therapy for stroke prophylaxis in selected patients with nonvalvular atrial fibrillation (AF). 1 The anatomy of LAA is highly variable, and hence preprocedural imaging is important for procedural planning and device selection. 2 Two‐dimensional transesophageal echocardiogram (TEE) is currently the gold standard imaging modality for both pre‐ and intraprocedural guidance. 3 Previous studies found that 3‐dimensional cardiac computed tomography (CT) reported consistently larger LAA dimensions than TEE, 4 , 5 , 6 and a number of small comparative studies showed that preprocedural planning using 3‐dimensional CT was associated with more accurate device selection and improved procedural efficiency. 7 , 8 , 9 However, it was uncertain whether additional preprocedural planning using 3‐dimensional CT would impact on the LAAO procedural success, procedural safety, and subsequent occlusion outcomes. This study aimed to assess the additive value of preprocedural CT planning versus stand‐alone TEE imaging guidance to LAAO.
Methods
The authors declare that all supporting data are available within the article.
Study Design
We retrospectively reviewed all LAAO using the Watchman device (Boston Scientific, Marlborough, MA) performed at a single center from May 2015 to December 2019. All patients underwent preprocedural imaging before the actual procedure to assess the LAA anatomy. Patients with presence of a LAA thrombus or who were anatomically not suitable for the Watchman device according to the device instructions for use were excluded. 10 Preprocedural planning was performed using either CT or TEE according to the operator's preference. Preprocedural CT was obtained at least 72 hours before the actual procedure to reduce risk of contrast nephropathy, whereas preprocedural TEE was performed days/weeks before or on the same day as the actual procedure. Patients were then divided into 2 groups, using additional CT for preprocedural planning versus stand‐alone TEE‐guided LAAO, to compare the outcomes of the Watchman implantation. The study was approved by the Henry Ford Hospital institutional review board, and informed consent was waived for this retrospective analysis.
Preprocedural CT Protocol
The CT imaging acquisition and postprocessing protocol at our institution were previously described. 6 In short, contrast‐enhanced, cardiac CT angiographic acquisition was obtained using GE Discovery CT750 (GE Healthcare, Waukesha, WI) and image postprocessing was performed using Vitrea (Vital Images, Minnetonka, MN) and Mimics (Materialise, Leuven, Belgium). LAA dimensions were measured at the late ventricular systolic phase that corresponds with the maximal end‐diastolic filling for the LAA. Maximal and minimal diameters of the LAA landing zone, and the length/depth of the LAA from the landing zone to the distal LAA tip were measured (Figure). Device size was determined by the widest diameter of the landing zone measured by CT imaging and selection according to the Watchman instructions for use. Optimal C‐arm deployment angle, depth of deployment, and catheter tip positioning to optimize device implant coaxiality to the LAA were also determined by CT for procedural guidance. In addition, 3‐dimensional prints of patient's specific left atrial and LAA anatomy were generated to assist in bench‐test selection of catheter curvature for device implantation. However, device size testing or procedure simulation was not performed.
Figure . Case example of Watchman implantation using computed tomography (CT) for preprocedural planning.
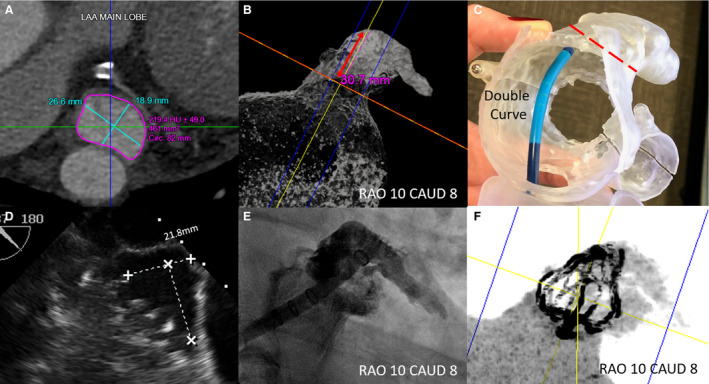
A, Left atrial appendage (LAA) landing zone dimensions measured by CT. B, The depth measured perpendicular to the LAA landing zone and the optimal fluoroscopic deployment projection determined by CT. C, In vitro testing to select the curvature of the delivery sheath, which achieved coaxiality to the LAA landing zone for optimal deployment. D, The landing zone size measured by intraprocedural TEE, which was consistently smaller than that by preprocedural CT. E, LAA angiogram at predetermined fluoroscopic projection. F, Postimplantation CT 3‐dimensional reconstruction.
Intraprocedural Imaging
All Watchman implantations were performed under real‐time TEE guidance. Two‐dimensional and 3‐dimensional LAA measurements were obtained after confirming mean LA pressure >10 mm Hg. For the additive CT group, the initial device size and choice of delivery sheath followed the preprocedural plan unless difficulty occurred intraprocedurally because of challenging transseptal access and ability to achieve device coaxiality. A CT‐derived LAA angiogram at the deployment projection was printed out as a road map. Intraprocedural LAA angiogram was obtained with reference to the road map before device implantation. On the other hand, in the stand‐alone TEE group, the choice of device size relied on the intraprocedural TEE measurements, and the choice of delivery sheath was decided by individual implanter after transseptal access and LAA angiogram.
End Points
The primary end point for the study was the rate of successful device implantation without major peri‐device leak (>5 mm). Secondary end points included major adverse events, total procedural time (defined by start of vascular access to vascular closure, ie, skin‐to‐skin time), radiation dose, total contrast used, number and types of delivery sheath used, number of devices used, number of partial recaptures, and risk of significant peri‐device leak (>5 mm) and device‐related thrombus at follow‐up imaging (45 days after implantation). Major adverse events included device embolization, procedural‐related myocardial infarction, procedural stroke, new pericardial effusion requiring intervention, surgical conversion, and procedural death.
Statistical Analysis
SPSS version 24 (IBM, Armonk, NY) and Microsoft Excel 365 (Microsoft Corp., Redmond, WA) were used for all statistical analyses. Categorical variables were summarized as percentages. Normally distributed continuous data were expressed as mean±SD and skewed data as median (interquartile range). Unpaired Student t test was used to compare means of 2 independent samples and independent samples median test was used to compare medians. Differences between groups were assessed using the chi‐square test for independence. In case the frequency of an observation was <5 in the contingency table, Fisher's exact test was used instead. A multiple logistic regression test was used to identify independent predictors of successful device implantation, change of device size, and device upsize. Statistical significance was defined as P<0.05.
Results
From May 2015 to December 2019, a total of 485 LAAOs were performed using the Watchman device, including 328 (67.6%) cases who underwent additional CT for preprocedural planning and 157 (32.4%) cases using stand‐alone TEE for guidance. The baseline patient characteristics were shown in Table 1. Patients in the additive CT group had a significantly lower body mass index (28.7±6.2 versus 30.9±6.8; P=0.001) and higher HAS‐BLED score (3.2±1.0 versus 2.9±1.0; P=0.006); otherwise, there were no significant difference in their baseline clinical profiles, including the baseline creatinine level. The maximum landing zone diameters measured by intraprocedural TEE between the 2 groups were similar (22.4±3.7 mm versus 22.4±3.6 mm; P=0.96). In the additive CT group, the maximal landing zone diameter measured by CT was 2.8±2.4 mm larger than that measured by intraprocedural 2‐dimensional TEE, which was consistent with previously published data 4 , 5 , 6 (Table 2).
Table 1.
Baseline Patient Characteristics
| Overall (n=485) | Additive CT (n=328) | Stand‐Alone TEE (n=157) | P Value | |
|---|---|---|---|---|
| Age | 77.5±8.5 | 77.5±8.7 | 77.4±8.2 | 0.93 |
| Sex, male | 265 (54.6) | 180 (54.9) | 85 (54.1) | 0.88 |
| BMI, kg/m2 | 29.4±6.4 | 28.7±6.2 | 30.9±6.8 | 0.001 |
| CHADS2‐VASc Score | 4.5±1.5 | 4.5±1.5 | 4.5±1.3 | 0.85 |
| HAS‐BLED score | 3.1±1.0 | 3.2±1.0 | 2.9±1.0 | 0.006 |
| History of CHF | 232 (47.8) | 165 (50.3) | 67 (42.7) | 0.12 |
| History of hypertension | 438 (90.3) | 296 (90.2) | 142 (90.4) | 0.94 |
| History of DM | 198 (40.8) | 125 (38.1) | 73 (46.5) | 0.08 |
| History of Stroke/TIA | 179 (36.9) | 130 (39.6) | 49 (31.2) | 0.07 |
| History of prior bleeding | 404 (83.3) | 272 (82.9) | 132 (84.1) | 0.75 |
| History of ICH | 68 (14) | 45 (13.7) | 23 (14.6) | 0.78 |
| History of gastrointestinal bleed | 231 (45.9) | 150 (45.7) | 81 (51.6) | 0.23 |
| History of renal impairment* | 56 (11.5) | 40 (12.2) | 16 (10.2) | 0.52 |
| LVEF, % | 55.9±10.7 | 55.6±11.2 | 56.4±9.5 | 0.42 |
| Concomitant procedures | 20 (4.1) | 13 (4.0) | 7 (4.5) | 0.80 |
| Baseline creatinine, mg/dL | 1.1 (0.5) | 1.085 (0.50) | 1.1 (0.58) | 0.70 |
| Maximum ostium diameter (intraprocedural TEE), mm † | 22.4±3.9 | 22.4±3.7 | 22.4±3.6 | 0.96 |
Data were presented as mean ±SD or median (interquartile range) or n (%). BMI indicates body mass index; CHF, congestive heart failure; CT, computed tomography; DM, diabetes mellitus; ICH, intracranial hemorrhage; LVEF, left ventricular ejection fraction; TEE, transesophageal echocardiogram; and TIA, transient ischemic attack.
Renal dialysis, renal transplant, creatinine >2.26 mg/dL or >200 µmol/L.
For those with successful Watchman implantation.
Table 2.
Left Atrial Appendage Sizing Analysis by CT Versus TEE for the Same Patient
| CT | TEE | Mean Difference | P Value | |
|---|---|---|---|---|
| Maximum ostium diameter, mm | 25.2 ± 3.7 | 22.4 ± 3.8 | +2.8 (+2.2 to +3.4) | <0.001 |
| Minimum ostium diameter, mm | 20.3 ± 4.0 | 17.0 ± 3.3 | +3.4 (+2.8 to +4.0) | <0.001 |
| Maximum depth, mm | 29.7 ± 6.8 | 29.2 ± 5.4 | + 0.5 (−0.4 to +1.5) | 0.28 |
Data are presented as mean±SD or mean difference (95% CI). CT indicates computed tomography; and TEE, transesophageal echocardiogram.
Procedural Features and Outcomes
Patients' who had additive CT for preprocedural planning had a significantly higher rate of successful device implantation with <5 mm peri‐device leak than using stand‐alone TEE (98.5% versus 94.9%, P=0.02; Table 3). There was no significant difference in the risk of major adverse events between the 2 groups (2.1% versus 1.9%; P=0.87). Total procedural time was shorter in the additive CT group than the stand‐alone TEE group (median, 45.5 minutes [36.75–59.00] versus 51.0 minutes [39.00–66.50]; P=0.03; Table 4). The total contrast used was significantly more in the additive CT group (59.2±34.5 mL versus 51.5±35.9 mL; P=0.05). The additive CT group used more anterior or single curve delivery sheath (35.9% versus 18.8%; P<0.001) than the stand‐alone TEE group (single curve, 11.1% versus 7.7%; double curve, 64.1% versus 81.2%; anterior curve, 24.8% versus 16.8%; P<0.001). Moreover, the devices used between the 2 groups were significantly different (Table 4), with fewer 21‐mm devices (7.4% versus 12.8%) and more 33 mm devices (18.6% versus 10.1%) used in the additive CT group. It was also less common to change device size after initial deployment in the additive CT group than the stand‐alone TEE group (5.6% versus 12.1%; P=0.01), with significantly more device upsizing in the stand‐alone TEE group (4% versus 9.4%; P=0.02). With multiple logistic regression analysis, it was found that additional CT had a significant association with successful device implantation (odds ratio [OR], 3.63; 95% CI, 1.06–12.5; P=0.041), stand‐alone TEE had a significant association with change of device size (OR, 2.34; 95% CI, 1.17–4.62; P=0.016), stand‐alone TEE (OR, 2.51; 95% CI, 1.14–5.52; P=0.022), and history of hypertension (OR, 0.33; 95% CI, 0.12–0.87; P=0.025) had significant associations with device upsize.
Table 3.
Procedural Outcomes and Complications
| Overall (n=485) | Additive CT (n=328) | Stand‐Alone TEE (n=157) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|---|
| Successful device implantation | 472 (97.3) | 323 (98.5) | 149 (94.9) | 3.45 (1.11–11.1) | 0.02 |
| Major adverse events | 10 (2.1) | 7 (2.1) | 3 (1.9) | 0.87 | |
| Device embolization | 0 | 0 | 0 | 0.99 | |
| Procedural related MI | 0 | 0 | 0 | 0.99 | |
| Procedural stroke | 1 (0.2) | 1 (0.3) | 0 | 0.99 | |
| New pericardial effusion | 7 (1.4) | 5 (1.5) | 2 (1.3) | 0.99 | |
| Pericardial effusion requiring intervention | 4 (0.8) | 3 (0.9) | 1 (0.6) | 0.99 | |
| Surgical conversion | 0 | 0 | 0 | 0.99 | |
| Peri‐procedural death | 2 (0.4) | 1 (0.3) | 1 (0.6) | 0.54 |
Data are presented as n (%). CT indicates computed tomography; MI, myocardial infarction; and TEE, transesophageal echocardiogram.
Table 4.
Procedural Characteristics of Successful Watchman Implantations
| Overall (n=472) | Additive CT (n=323) | Stand‐Alone TEE (n=149) | Difference | P Value | |
|---|---|---|---|---|---|
| Total procedural time (skin to skin), min | 48 (37, 61) | 45.5 (37, 59) | 52.5 (39, 66) | –7.0 | 0.03 |
| Total radiation dose, mGy | 219 (126, 418) | 239.0 (139, 427) | 176 (90, 373) | +63 | 0.03 |
| Total contrast used, mL | 50.0 (30, 75) | 50.0 (35, 76) | 40.0 (20, 75) | +10 | 0.30 |
| Odds radio (95% CI) | |||||
| Delivery sheath used | <0.001 | ||||
| Single curve | 39 (8.3) | 36 (11.1) | 3 (2.0) | ||
| Double curve | 328 (69.5) | 207 (64.1) | 121 (81.2) | ||
| Anterior curve | 105 (22.2) | 80 (24.8) | 25 (16.8) | ||
| Anterior or single curve | 144 (30.5) | 116 (35.9) | 28 (18.8) | ||
| Number of delivery sheath used | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.91 | |
| Change of delivery Sheath | 23 (4.9) | 16 (5.0) | 7 (4.7) | 0.99 | |
| Device implanted | 0.02 | ||||
| 21 mm | 43 (9.1) | 24 (7.4) | 19 (12.8) | ||
| 24 mm | 112 (23.7) | 84 (26.0) | 28 (18.8) | ||
| 27 mm | 142 (30.1) | 91 (28.2) | 51 (34.2) | ||
| 30 mm | 100 (21.2) | 64 (19.8) | 36 (24.2) | ||
| 33 mm | 75 (15.9) | 60 (18.6) | 15 (10.1) | ||
| Number of device used | 1 (1, 1) | 1 (1, 1) | 1 (1, 1) | 0.84 | |
| Change of device size | 36 (7.6) | 18 (5.6) | 18 (12.1) | 2.3 (1.1–4.6) | 0.02 |
| Upsize | 27 (5.7) | 13 (4) | 14 (9.4) | 2.4 (1.1–5.4) | 0.031 |
| Downsize | 9 (1.9) | 5 (1.5) | 4 (2.7) | 0.47 | |
| Number of partial recapture | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0.99 | |
| Need of partial recapture | 112 (23.7) | 77 (23.8) | 35 (23.5) | 0.79 |
Data are presented as median (25th percentile, 75th percentile) or n (%). CT indicates computed tomography; mGy, milligray; and TEE, transesophageal echocardiogram.
Follow‐Up Clinical and Imaging Outcomes
The overall follow‐up imaging rate was high, with no significant difference between the 2 groups (overall, 93.2%; additive CT group 93.8% versus stand‐alone TEE group, 91.9%; P=0.46; Table 5). A majority of patients in the additive CT group (68.4%) received CT as follow‐up imaging, whereas the stand‐alone TEE group received TEE (81.9%) instead. There was only 1 significant peri‐device leak (>5 mm; 0.2%) and 5 device‐related thrombi detected (1.2%), with no difference between the 2 groups.
Table 5.
Clinical and Imaging Follow‐Up Outcomes of Successful Watchman Implantations at 45 Days
| Overall (n=472) | Additive CT (n=323) | Stand‐Alone TEE (n=149) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|---|
| 45‐d clinical follow‐up | 440 (93.2) | 303 (93.8) | 137 (91.9) | 0.44 | |
| 45‐d imaging follow‐up | <0.001 | ||||
| No follow‐up imaging | 51 (10.8) | 35 (10.8) | 16 (10.7) | ||
| CT | 232 (49.2) | 221 (68.4) | 11 (7.4) | ||
| TEE | 189 (40.0) | 67 (20.7) | 122 (81.9) | ||
| Significant PDL (>5 mm) | 1 (0.2) | 1 (0.3) | 0 | 0.99 | |
| Any PDL | 79 (18.8) | 37 (12.9) | 42 (31.3) | 0.32 (0.2–0.54) | <0.001 |
| DRT | 5 (1.2%) | 4 (1.4%) | 1 (0.7%) | 0.99 |
Data are presented as n (%). CT indicates computed tomography; DRT, device‐related thrombus; PDL, peri‐device leak; and TEE, transesophageal echocardiogram.
Discussion
The main finding of our study was that additional preprocedural planning using CT when compared with stand‐alone TEE in LAAO using the Watchman device was associated with a higher rate of successful device implantation, a shorter total procedural time, and a less frequent need to change device sizes. Besides, the additive CT group also had significant differences in the choice of delivery sheath used and the size of device implanted compared with the stand‐alone TEE group.
New Gold Standard
CT has become the gold standard for device sizing in transcatheter aortic valve replacement. The LAA has a more complex anatomy and existing data consistently showed that the maximal LAA landing zones measured by CT were significantly bigger than that measured by TEE, 4 , 5 , 6 and device sizing according to CT measurements was more accurate. 7 , 8 , 9 Our study supported these findings and was the first to show that additional CT preprocedural planning was associated with a higher rate of successful device implantation. In fact, the rate of successful device implantation in our CT cohort was significantly higher than that reported in published US studies, with a numerically lower median procedural time (Table 6). In line with previous studies, CT measurements yield larger LAA dimensions and more accurate device sizing than TEE, which resulted in less frequent need to upsize the device, as illustrated by our study. In addition, we believed that a more tailored choice of delivery sheath and fluoroscopic coaxial deployment projection obtained by preprocedural CT contributed to the improved rate of successful device implantation and procedural efficiency. Most operators preferentially used a double‐curve delivery sheath and right anterior oblique 20 to 30 caudal 20 to 30 projection for device deployment. From our analysis by in vitro testing in a patient‐specific 3‐dimensional–printed model, coaxial alignment with the LAA ostium was better achieved by a single‐curve or anterior‐curve delivery sheath in a substantial proportion of patients. As a result, >30% of the cases in the additive CT group used a single/anterior‐curve delivery sheath for device deployment with an overall 5% delivery sheath change only. We also found that, as in coplanar projection during valve deployment in transcatheter aortic valve replacement, a CT‐derived individualized fluoroscopy projection angle that aligns the LAA ostium further improved deployment accuracy and occlusion result. Paradoxically, more contrast was used in the patients with additional CT preprocedural planning. We speculated that more contrast was used to obtain a LAA angiogram similar to the CT‐derived road map, that is, more forceful or repeated injections to fill the whole LAA, before device implantation. However, there was no significant difference in the risk of major adverse events, as the overall risk reported in our study was low (Table 6). 11 , 12 , 13 , 14 , 15 Whether additive CT would improve the safety of Watchman implantation in lower volume or new implant sites remains to be proven. In addition, the overall risk of device‐related thrombus was significantly lower than that reported in recent series, 16 whether this was related to additive CT planning, implantation technique, or postimplantation antithrombotic remained unclear. Finally, the adoption of additional CT preprocedural planning±follow‐up imaging modality could reduce the need to subject patients to multiple TEE, and thus improve patient comfort, reduce the risk of esophageal injury, aspiration, and sedation. On the other hand, contrast CT carries the risk of contrast nephropathy. However, by excluding patients with severe chronic kidney disease, spacing out contrast exposure and adequate prehydration, which is the standard for any kind of LAA imaging, the risk of contrast nephropathy could be minimized. Further analysis to compare the cost‐effectiveness of additional preprocedural CT planning versus stand‐alone TEE for LAAO, taking into account the reduced lab time. In the era of COVID, TEE—being an aerosol‐generating procedure—can spread the virus and pose a risk for echocardiographers, personnel, and patients. Avoiding multiple TEEs during the pandemic could potentially reduce the risk of spreading infection and reduce the use of personal protective equipment and resources.
Table 6.
Comparison of Procedural Outcomes With Other Major Clinical Studies or Registry
| Henry Ford Additive CT Cohort | Henry Ford Stand‐Alone TEE Cohort | PROTECT‐AF | PREVAIL | CAP | CAP‐2 | Post‐FDA Approval Registry | NCDR Registry | |
|---|---|---|---|---|---|---|---|---|
| Number of procedures | 328 | 157 | 463 | 269 | 566 | 579 | 3822 | 38 158 |
| Implantation success, % | 98.5 |
94.9 P=0.02* |
90.9 P<0.001* |
95.1 P=0.0187* |
94.4 P=0.0028* |
94.8 P=0.0060* |
95.6 P=0.0125* |
98.3 † |
| Procedure time, min, median (1st–3rd quartile) | 46 (37–59) | 51 (39–67) | 51 (37–71) | 52 (40–73) | 46 (34–62) | 55 (39–80) | 50 (36–66) | N/A |
| Number of device used, mean | 1.3 | 1.3 | 1.6 | 1.5 | 1.4 | 1.4 | 1.38 | N/A |
| Pericardial tamponade, % | 0.9 | 0.6 | 4.3 | 1.9 | 1.4 | 1.9 | 1.02 | 1.39 |
| Device embolization, % | 0 | 0 | 0.6 | 0.7 | 0.2 | 0 | 0.24 | 0.07 |
Aggregate data combined from PROTECT‐AF, PREVAIL, CAP, CAP2, and the Post‐FDA Approval Experience. CAP indicates continued access to PROTECT‐AF; CAP2, continued access to PREVAIL; CT, computed tomography; FDA, Food and Drug Administration; LAA, left atrial appendage; N/A, not available; NCDR, National Cardiovascular Data Registry; PREVAIL, Prospective Randomized Evaluation of the Watchman LAA Closure Device in Patients With Atrial Fibrillation Versus Long Term Warfarin Therapy; PROTECT‐AF, Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation; and TEE, transesophageal echocardiogram.
P value when compared with Henry Ford Additive CT cohort.
NCDR reported success rate among those with device deployed, device not attempted were excluded (7% of procedures were cancelled/aborted for multiple reasons).
Future Directions
Although device size prediction accuracy by CT was proven to be high, it was uncertain which parameter (maximal dimension, perimeter‐derived diameter, or area‐derived diameter) performs the best. In fact, the best parameter could be device specific, 7 as in the case of transcatheter valves, and should be determined next with increasing CT experience in LAAO. Additionally, the combination of preprocedural CT and intraprocedural intracardiac echocardiography might further reduce the need of general anesthesia, intubation, and multiple TEEs; improve patient comfort; and make LAAO more minimalistic. Moreover, with an expected increase in the array of LAAO devices available in the United States, the value of CT in preprocedural planning for optimal device size and choice could not be overlooked. With CT‐based preprocedural planning, digital simulation, and in vitro simulation in 3‐dimensional–printed models, LAAO could be more personalized and become safer and more efficient.
Study Limitations
First, this was a single‐center retrospective study without randomization. Choice of additive CT versus stand‐alone TEE for preprocedural planning could be biased by the individual operator's preference. LAAO outcome could be affected by the individual operator's experience, which was not adjusted for in our study. Second, patients screened as not eligible for the Watchman device were not available for analysis, and hence whether additional preprocedural CT screened out more unfavorable LAA anatomy for the Watchman device than stand‐alone TEE remained uncertain. Third, in our additive CT cohort, a 3‐dimensional–printed left atrium and LAA model was generated for a majority of patients for bench testing of delivery sheath alignment. This approach involved extra resources and expertise and might not be feasible in other sites. A completely CT‐based approach to determine the optimal choice of delivery sheath is under validation, and hopefully to simplify our approach and increase the reproducibility. Finally, only the Watchman device was used in our study, and it was uncertain whether the result could be generalized to other LAAO devices.
Conclusions
Additional preprocedural planning using CT when compared with stand‐alone TEE in LAAO using the Watchman device was associated with a higher rate of successful device implantation, a shorter total procedural time, and a less frequent need to change device sizes.
Sources of Funding
None.
Disclosures
Dr Wang has served as a consultant to Edwards Lifesciences and Boston Scientific, and received research grant support from Boston Scientific assigned to employer Henry Ford Health System. Dr W. O'Neill has served as a consultant for Abiomed, Edwards Lifesciences, Medtronic, Boston Scientific, Abbott Vascular, and St. Jude Medical; and serves on the Board of Directors of Neovasc Inc. Dr Eng is a clinical proctor for Edwards Lifesciences, Medtronic, and Boston Scientific. Dr B. O'Neill has served as a consultant and received research support from Edwards Lifesciences. Dr Frisoli is a clinical proctor for Edwards Lifesciences, Abbott, Boston Scientific, and Medtronic. The remaining authors have no disclosures to report.
Acknowledgments
Dr So received the Sir David Todd Memorial Scholarship from the Hong Kong College of Physicians for his overseas fellowship in structural heart disease. We thank Angle Lai (Chinese University of Hong Kong) for data analysis.
(J Am Heart Assoc. 2021;10:e020615. DOI: 10.1161/JAHA.120.020615.)
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Chak‐yu So, Email: kentso987@gmail.com.
Dee Dee Wang, Email: dwang2@hfhs.org.
References
- 1. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. DOI: 10.1161/CIR.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7:1251–1265. DOI: 10.1016/j.jcmg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 3. Mobius‐Winkler S, Majunke N, Sandri M, Mangner N, Linke A, Stone GW, Dahnert I, Schuler G, Sick PB. Percutaneous left atrial appendage closure: technical aspects and prevention of periprocedural complications with the watchman device. World J Cardiol. 2015;7:65–75. DOI: 10.4330/wjc.v7.i2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nucifora G, Faletra FF, Regoli F, Pasotti E, Pedrazzini G, Moccetti T, Auricchio A. Evaluation of the left atrial appendage with real‐time 3‐dimensional transesophageal echocardiography: implications for catheter‐based left atrial appendage closure. Circ Cardiovasc Imaging. 2011;4:514–523. DOI: 10.1161/CIRCIMAGING.111.963892. [DOI] [PubMed] [Google Scholar]
- 5. Saw J, Fahmy P, Spencer R, Prakash R, McLaughlin P, Nicolaou S, Tsang M. Comparing measurements of CT angiography, TEE, and fluoroscopy of the left atrial appendage for percutaneous closure. J Cardiovasc Electrophysiol. 2016;27:414–422. DOI: 10.1111/jce.12909. [DOI] [PubMed] [Google Scholar]
- 6. Wang DD, Eng M, Kupsky D, Myers E, Forbes M, Rahman M, Zaidan M, Parikh S, Wyman J, Pantelic M, et al. Application of 3‐dimensional computed tomographic image guidance to WATCHMAN implantation and impact on early operator learning curve: single‐center experience. JACC Cardiovasc Interv. 2016;9:2329–2340. DOI: 10.1016/j.jcin.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 7. Chow DH, Bieliauskas G, Sawaya FJ, Millan‐Iturbe O, Kofoed KF, Sondergaard L, De Backer O. A comparative study of different imaging modalities for successful percutaneous left atrial appendage closure. Open Heart. 2017;4:e000627. DOI: 10.1136/openhrt-2017-000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eng MH, Wang DD, Greenbaum AB, Gheewala N, Kupsky D, Aka T, Song T, Kendall BJ, Wyman J, Myers E, et al. Prospective, randomized comparison of 3‐dimensional computed tomography guidance versus TEE data for left atrial appendage occlusion (PRO3DLAAO). Catheter Cardiovasc Interv. 2018;92:401–407. DOI: 10.1002/ccd.27514. [DOI] [PubMed] [Google Scholar]
- 9. Rajwani A, Nelson AJ, Shirazi MG, Disney PJS, Teo KSL, Wong DTL, Young GD, Worthley SG. CT sizing for left atrial appendage closure is associated with favourable outcomes for procedural safety. Eur Heart J Cardiovasc Imaging. 2017;18:1361–1368. DOI: 10.1093/ehjci/jew212. [DOI] [PubMed] [Google Scholar]
- 10. Saw J, Lempereur M. Percutaneous left atrial appendage closure: procedural techniques and outcomes. JACC Cardiovasc Interv. 2014;7:1205–1220. DOI: 10.1016/j.jcin.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 11. Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation. 2011;123:417–424. DOI: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 12. Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, Halperin JL, Holmes D; Investigators PA . Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3‐year follow‐up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) Trial. Circulation. 2013;127:720–729. DOI: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 13. Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long‐term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. DOI: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 14. Reddy VY, Gibson DN, Kar S, O'Neill W, Doshi SK, Horton RP, Buchbinder M, Gordon NT, Holmes DR. Post‐approval U.S. experience with left atrial appendage closure for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2017;69:253–261. DOI: 10.1016/j.jacc.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 15. Freeman JV, Varosy P, Price MJ, Slotwiner D, Kusumoto FM, Rammohan C, Kavinsky CJ, Turi ZG, Akar J, Koutras C, et al. The NCDR left atrial appendage occlusion registry. J Am Coll Cardiol. 2020;75:1503–1518. DOI: 10.1016/j.jacc.2019.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fauchier L, Cinaud A, Brigadeau F, Lepillier A, Pierre B, Abbey S, Fatemi M, Franceschi F, Guedeney P, Jacon P, et al. Device‐related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. 2018;71:1528–1536. DOI: 10.1016/j.jacc.2018.01.076. [DOI] [PubMed] [Google Scholar]


