Abstract
Background
Takotsubo syndrome (TS) is a potentially life‐threatening acute cardiac syndrome with a clinical presentation similar to myocardial infarction and for which the natural history, management, and outcome remain incompletely understood. Our aim was to assess the relative short‐term mortality risk of TS, ST‐segment–elevation myocardial infarction (STEMI), and non‐STEMI (NSTEMI) and to identify predictors of in‐hospital complications and poor prognosis in patients with TS.
Methods and Results
This is an observational cohort study based on the data from the SCAAR (Swedish Coronary Angiography and Angioplasty Registry). We included all patients (n=117 720) who underwent coronary angiography in Sweden attributed to TS (N=2898 [2.5%]), STEMI (N=48 493 [41.2%]), or NSTEMI (N=66 329 [56.3%]) between January 2009 and February 2018. We compared patients with TS to those with NSTEMI or STEMI. The primary end point was all‐cause mortality at 30 days. Secondary outcomes were acute heart failure (Killip Class ≥2) and cardiogenic shock (Killip Class 4) at the time of angiography. Patients with TS were more often women compared with patients with STEMI or NSTEMI. TS was associated with unadjusted and adjusted 30‐day mortality risks lower than STEMI (adjusted hazard ratio [adjHR], 0.60; 95% CI, 0.48–0.76; P<0.001), but higher than NSTEMI (adjHR, 2.70; 95% CI, 2.14–3.41; P<0.001). Compared with STEMI, TS was associated with a similar risk of acute heart failure (adjHR, 1.26; 95% CI, 0.91–1.76; P=0.16) but a lower risk of cardiogenic shock (adjHR, 0.55; 95% CI, 0.34–0.89; P=0.02). The relative 30‐day mortality risk for TS versus STEMI and NSTEMI was higher for smokers than nonsmokers (adjusted P interaction STEMI=0.01 and P interaction NSTEMI=0.01).
Conclusions
The 30‐day mortality rate in TS was higher than in NSTEMI but lower than STEMI despite a similar risk of acute heart failure in TS and STEMI. Among patients with TS, smoking was an independent predictor of mortality.
Keywords: acute heart failure, cardiogenic shock, mortality rate, non–ST‐segment–elevation myocardial infarction, ST‐segment–elevation myocardial infarction, Swedish Coronary Angiography and Angioplasty Registry, Takotsubo syndrome
Subject Categories: Cardiomyopathy, Heart Failure, Myocardial Infarction
Nonstandard Abbreviations and Acronyms
- AHF
acute heart failure
- SCAAR
Swedish Coronary Angiography and Angioplasty Registry
- TS
Takotsubo syndrome
Clinical Perspective
What Is New?
In this nationwide study encompassing prospectively collected data from all patients who underwent coronary angiography in Sweden between January 2009 and February 2018, we show that the 30‐day mortality rate in Takotsubo syndrome was higher than in non–ST‐segment–elevation myocardial infarction but lower than in ST‐segment–elevation myocardial infarction despite a similar risk of acute heart failure in Takotsubo syndrome and ST‐segment–elevation myocardial infarction.
What Are the Clinical Implications?
Although patients with Takotsubo syndrome are at considerable risk of dying, Takotsubo syndrome appears to be a less malignant heart failure syndrome than ST‐segment–elevation myocardial infarction.
Takotsubo syndrome (TS) is an acute cardiac syndrome characterized by sudden onset of transient left ventricular dysfunction and wall‐motion abnormalities. 1 TS is often preceded by emotional or physical stress and predominantly affects older women. 2 TS is difficult to distinguish from acute myocardial infarction (MI) because of similar clinical, electrocardiographic, laboratory. and noninvasive imaging characteristics. 2 However, in contrast to MI, TS is not caused by coronary artery disease and does not cause irreparable myocardial damage. 3
Despite the transient nature of cardiac dysfunction in TS, death rates are reported to be similar in patients with TS as in patients with MI. 4 , 5 , 6 However, large studies that directly compare short‐term and long‐term prognoses for patients with TS versus MI are lacking. The aims of this study were to assess the relative short‐term and long‐term mortality risk of TS versus MI with or without ST‐elevation and to investigate clinical features and identify predictors of in‐hospital complications and poor prognosis in patients with TS.
METHODS
Databases and Patient Selection
We used data from the prospective SCAAR (Swedish Coronary Angiography and Angioplasty Registry) database, a national, comprehensive registry of all coronary angiographies performed in Sweden. Each catheterization procedure is described with ≈50 angiographic and up to 200 percutaneous coronary intervention variables, both demographic and procedure related. TS was included in the SCAAR in 2008. The SCAAR is sponsored by the Swedish state and does not have commercial interests. Detailed information about the SCAAR's organization and database have been reported previosuly. 7 , 8 The data will not be made available to other researchers for purposes of reproducing the results or replicating the procedure owing to restrictions in Swedish research ethics law. The study was approved by the ethics committee at the University of Gothenburg (Diarienumber [Dnr]. 759‐13, date of approval May 6, 2014).
We included all patients who underwent angiography attributed to ST‐segment–elevation myocardial infarction (STEMI), non‐STEMI (NSTEMI), or TS between January 2009 and February 2018. Patients were only included once (at the earlier time point) if they underwent coronary angiography attributed to STEMI, NSTEMI, or TS more than once during the course of the study period.
Definitions and Outcomes
The SCAAR uses standardized definitions across all hospitals, and all information is entered directly into the database by interventional cardiologists. Since 2009, the interventional cardiologist reports whether a patient has TS based on the criteria listed in Table S1, which are similar to the current European Society of Cardiology criteria 3 and other criteria used in previous studies related to TS. Patients without TS were classified as having either STEMI or NSTEMI based on the indication for coronary angiography as reported by the interventional cardiologist after percutaneous coronary intervention. Based on the reported Killip class at the time of angiography, we defined acute heart failure (AHF) as Killip Class ≥2 and cardiogenic shock as Killip Class 4. 8 The variables in the SCAAR are routinely validated against patient charts and are considered to have high accuracy, 9 and the TS variable has been specifically validated. 5 Coronary artery disease was defined as the presence of stenosis >50% in any coronary artery as reported in the SCAAR per visual assessment of the angiogram.
End Points
The primary end point was mortality at 30 days. Vital status and date of death were obtained from the Swedish National Population Registry until March 16, 2018. The SCAAR was merged with the Swedish National Population Registry according to Swedish personal identification numbers. Because use of personal identification numbers is mandatory, the population registry in Sweden has a high degree of completeness but is not reviewed or adjudicated to establish cardiac versus noncardiac causes of death. In‐hospital bleeding was defined as any of the following: puncture site hematoma or pseudo‐aneurysm, cardiac tamponade, drop in hemoglobin >20 g/L, or prolonged compression treatment, transfusion, or surgical intervention. 10
Statistical Analysis
Continuous variables are presented as median and interquartile range, and categorical variables as frequencies. Normal distribution of variables was assessed by inspecting the distribution of values on histograms and by the shapiro–wilk test. Intergroup differences in continuous variables were tested by linear regression. Differences in categorical variables were tested by logistic regression.
Imputation Protocol
Missing data were imputed with the multiple imputation chain–equation method 11 , 12 with 5 data sets. Calendar year, treating hospital, all variables in Table 1, and an event indicator were included in the imputation model. 13 Continuous variables were imputed by ordinary least squares multiple regression, binary variables by logistic regression, and categorical variables by multinomial logistic regression. The imputation procedure and subsequent analyses were done according to the protocol of Rubin 14 under the assumption that missing data are missing at random.
Table 1.
Patient Characteristics
| TS, N=2898 | STEMI, N=48 493 | NSTEMI, N=66 329 | P Value vs STEMI | P Value vs NSTEMI | |
|---|---|---|---|---|---|
| Age, y | 67.3±11.6 (2898) | 67.2±12.4 (48 493) | 68.7±11.2 (66 329) | 0.69 | <0.0001 |
| Age >75 y | 24.88 (721/2898) | 27.26 (13 221/48 493) | 29.87 (19 814/66 329) | 0.005 | <0.0001 |
| Female sex | 72.98 (2115/2898) | 29.80 (14 450/48 493) | 31.51 (20 901/66 329) | <0.0001 | <0.0001 |
| Diabetes mellitus | 13.30 (381/2865) | 15.38 (7349/47 779) | 22.62 (14 965/66 171) | 0.003 | <0.0001 |
| Insulin treatment | 5.31 (152/2862) | 6.55 (3120/47 650) | 11.15 (7371/66 081) | 0.009 | <0.0001 |
| Hypertension | 51.67 (1470/2845) | 46.29 (21 735/46 949) | 61.30 (40 399/65 903) | <0.0001 | <0.0001 |
| Smoking | |||||
| Previous smoker | 24.88 (721/2898) | 27.26 (13 221/48 493) | 29.87 (19 814/66 329) | 0.005 | <0.0001 |
| Current smoker | 16.13 (433/2684) | 28.79 (12 515/43 464) | 19.71 (12 594/63 903) | <0.0001 | <0.0001 |
| Hyperlipidemia | 33.46 (947/2830) | 22.95 (10 676/46 519) | 47.70 (31 381/65 783) | <0.0001 | <0.0001 |
| Previous MI | 12.62 (358/2837) | 12.18 (5731/47 034) | 22.61 (14 789/65 405) | 0.49 | <0.0001 |
| Previous PCI | 9.18 (266/2897) | 8.25 (3999/48 467) | 15.20 (10 080/66 314) | 0.08 | <0.0001 |
| Previous CABG | 3.35 (97/2898) | 3.23 (1564/48 468) | 8.88 (5887/66 322) | 0.72 | <0.0001 |
| Number of diseased vessels | |||||
| 1 | 13.59 (389/2863) | 47.33 (22 814/48 201) | 38.51 (25 490/66 186) | <0.0001 | <0.0001 |
| 2 | 7.23 (207/2863) | 28.54 (13 756/48 201) | 29.42 (19 469/66 186) | <0.0001 | <0.0001 |
| 3 | 5.48 (157/2863) | 16.33 (7869/48 201) | 19.65 (13 003/66 186) | <0.0001 | <0.0001 |
| Left main | 0.66 (19/2863) | 0.99 (479/48 201) | 1.35 (891/66 186) | 0.08 | 0.002 |
Data are provided as mean±SD (total number) or percentage (number/total number). CABG indicates coronary artery bypass grafting; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; and TS, Takotsubo syndrome.
Statistical Models
Cumulative mortality rates at 30 days and 5 years are presented for patients with TS, STEMI, and NSTEMI in the entire cohort as well as after matching each patient with TS to 5 patients with STEMI and 5 patients with NSTEMI based on sex (exact match) and age (±5 years) using a greedy matching algorithm. Landmark analysis was performed between 1 and 60 months. After multiple imputation of missing data, multivariable Cox proportional hazards and logistic regression models were used to adjust for differences in patient characteristics. All multivariable models were fit to the entire study cohort and were adjusted for variables in Table 1, calendar year, and treating hospital, with treating hospital included as a random effect variable to account for clustering of patients within hospitals. To incorporate the random effect for treating hospital, shared frailty Cox proportional hazards models were used to assess the adjusted risk of dying, and random intercept logistic regression was used to assess the risk of in‐hospital complications. Interaction terms were included to assess whether the association between TS (versus STEMI or NSTEMI) and outcome varied across subgroups (men versus women, smokers versus nonsmokers, and according to age). The assumption of proportional hazards was assessed by visual inspection of cumulative failure curves and Schoenfeld residuals and tested formally by an interaction term between TS (versus STEMI or NSTEMI) and time. Because of the nonproportional hazards between 0 and 5 years, separate Cox proportional hazards models were fit for the early (first month) and late (months 2–60) study period. all statistical analyses were performed using SAS (version 9.4). All tests were 2‐tailed, and P<0.05 was considered statistically significant.
Postestimation Diagnostics
Goodness‐of‐fit (calibration) for the models was assessed with the hosmer–lemeshow test. multicollinearity between the variables in the model was evaluated by calculating the variance inflation factor.
RESULTS
Patient Characteristics and Treatments
We identified 117 720 patients who underwent coronary angiography with TS on left ventricular angiography or echocardiography (N=2898 [2.5%]), STEMI (N=48 493 [41.2%]), or NSTEMI (N=66 329 [56.3%]) between January 2009 and February 2018. Vital status at 30 days was known for all patients. Patients with TS were more likely than patients with STEMI or NSTEMI to be women, and patients with TS and STEMI were on average younger and had a lower cardiovascular risk factor burden than patients with NSTEMI (Table 1).
Clinical Outcomes
30‐Day and 5‐Year Prognosis
Mortality at 30 days was highest among patients with STEMI followed by patients with TS and was lowest for patients with NSTEMI (Figure 1A). The 5‐year mortality risk was also highest in patients with STEMI but was similar for patients with NSTEMI and TS (Figure 1B). In a landmark analysis of patients who survived at least 30 days after the index procedure, patients with TS had lower mortality rates than patients with STEMI or NSTEMI (Figure 1C). When each patient with TS was age‐matched and sex‐matched to 5 patients with STEMI and 5 patients with NSTEMI, the relationship between TS, STEMI, and NSTEMI with regard to 30‐day and 5‐year mortality risks persisted (Figure S1A and S1B). However, in this age‐matched and sex‐matched cohort, patients with TS did not have significantly lower mortality rates than patients with STEMI or NSTEMI between 30 days and 5 years (Figure S1C).
Figure 1. Risk of dying for patients with Takotsubo syndrome compared with patients with STEMI and NSTEMI.
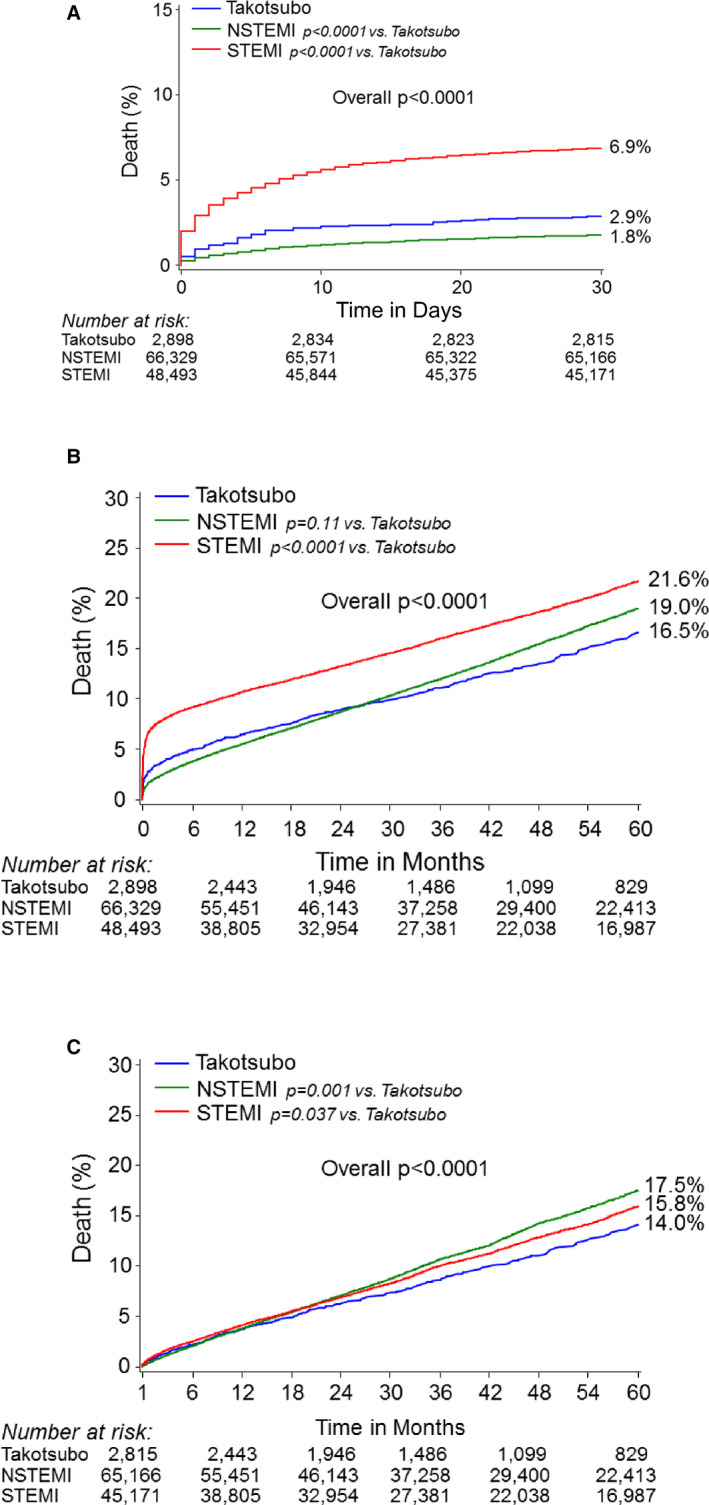
Kaplan‐Meier cumulative failure rates for patients with Takotsubo syndrome, NSTEMI, or STEMI: (A) risk of dying within 30 days, (B) risk of dying within 5 years, and (C) landmark analysis (risk of dying within 5 years for patients who were alive at 30 days). NSTEMI indicates non–ST‐segment–elevation myocardial infarction; and STEMI, ST‐segment–elevation myocardial infarction.
After multivariable adjustment, the adjusted short‐term (0–30 days) and longer term (>30 days–5 years) risks were lower for TS than STEMI, whereas the adjusted short‐term risk was higher in TS than NSTEMI (Figure 2).
Figure 2. Adjusted risk of dying for patients with TS compared with patients with STEMI and NSTEMI.
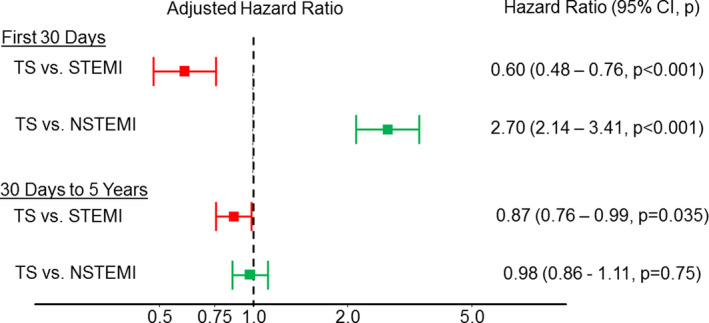
Adjusted hazard ratio for patients with TS, NSTEMI, or STEMI. Multivariable adjustment was done for the following covariate set: age, sex, diabetes mellitus, insulin‐treated diabetes mellitus, hypertension, hyperlipidemia, current smoker, previous smoker, prior myocardial infarction, prior percutaneous coronary intervention, and calendar year. Treating hospital was included in the model as a random effect. NSTEMI indicates non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction; and TS, Takotsubo syndrome.
A statistical interaction was present between TS and sex such that men with TS had an unadjusted risk of dying within 30 days that was similar to that of men with STEMI, whereas women with TS had a better unadjusted 30‐day prognosis than did women with STEMI (Table S2). In a landmark analysis of those who survived at least 30 days, TS was associated with a similar long‐term risk of dying as STEMI in men, whereas women with TS had a better prognosis than did women with STEMI (Table S2). Similarly, the short‐term and long‐term prognosis was relatively worse with TS versus STEMI or NSTEMI among smokers compared with nonsmokers (Table S3). There was also a relatively higher mortality risk associated with TS versus STEMI or NSTEMI among younger versus older patients (Table S4). After multivariable adjustment, the statistical interactions with regard to mortality risk between age and TS as well as between smoking status and TS persisted, whereas the interaction between TS and sex did not (Tables S2 through S4).
AHF and Cardiogenic Shock
AHF at the time point of angiography occurred more often in TS or STEMI than NSTEMI, and TS carried a similar adjusted risk of AHF as STEMI (Table 2). In contrast, patients with TS had unadjusted and adjusted risks of cardiogenic shock that were higher than patients with NSTEMI and lower than patients with STEMI (Table 2). Among patients with AHF, 30‐day mortality was 12.4% for those with TS, 31.71% for those with STEMI (P<0.0001 versus TS), and 15.7% for those with NSTEMI (P=0.30 versus TS).
Table 2.
Unadjusted and Adjusted Risk of Acute Heart Failure and Cardiogenic Shock
| Incidence | P TS vs STEMI | P TS vs NSTENI | Adjusted* odds ratio (95% CI; P value) | ||||
|---|---|---|---|---|---|---|---|
| TS, % (n/N) | STEMI, % (n/N) | NSTEMI, % (n/N) | TS vs STEMI | TS vs NSTEMI | |||
| Acute heart failure | 9.8 (258/2631) | 9.8 (4466/45 736) | 3.1 (1410/45 736) | 0.94 | 0.0001 | 1.26 (0.91–1.76; P=0.16) | 4.06 (2.95–5.61; P<0.0001) |
| Cardiogenic shock | 1.1 (28/2603) | 3.1 (1410/45 736) | 0.4 (172/49 641) | 0.00001 | 0.0001 | 0.55 (0.34–0.89; P=0.02) | 6.92 (4.51–10.63; P<0.0001) |
NSTEMI indicates non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction; and TS, Takotsubo syndrome.
Random intercept multivariable logistic regression was adjusted for the following covariates: age, sex, diabetes mellitus, insulin‐treated diabetes mellitus, hypertension, hyperlipidemia, current smoker, previous smoker, prior myocardial infarction, prior percutaneous coronary intervention, prior coronary artery bypass grafting, and calendar year. Treating hospital was included the model as a random effect.
Independent Predictors of Mortality and In‐Hospital Complications Among Patients With TS
Among patients with TS, independent predictors of developing AHF or cardiogenic shock or dying within 30 days or 5 years are presented in Table 3. Current smoking was independently associated with worse short‐term and long‐term prognoses and was independently associated with increased risks of both AHF and cardiogenic shock. Male sex was also independently associated with worse short‐term and long‐term prognoses and an increased risk of cardiogenic shock. Concomitant coronary artery disease was associated with increased adjusted risks of short‐term mortality, AHF, and cardiogenic shock.
Table 3.
Independent Predictors of Adverse Clinical Outcomes Among Patients With Takotsubo Syndrome
| Outcome/predictor* | Adjusted hazard ratio † (95% CI; P Value) |
|---|---|
| 30‐d mortality | |
| Age, per y | 1.05 (1.02–1.07; P<0.001) |
| Current smoker | 2.29 (1.38–3.82; P=0.001) |
| Concomitant coronary artery disease | 2.04 (1.22–3.42; P=0.006) |
| 5‐y mortality | |
| Age, per y | 1.07 (1.05–1.08; P<0.001) |
| Current smoker | 2.21 (1.64–2.98; P<0.001) |
| Female sex | 0.68 (0.52–0.87; P=0.002) |
| Acute heart failure | |
| Current smoker | 2.11 (1.44–3.08; P=0.001) |
| Age, per y | 1.03 (1.02–1.04; P<0.001) |
| Hyperlipidemia | 0.63 (0.42–0.94; P=0.02) |
| Concomitant coronary artery disease | 1.82 (1.35–2.47; P=0.0001) |
| Cardiogenic shock | |
| Concomitant coronary artery disease | 2.40 (1.07–5.40; P=0.03) |
All models contained the following covariate set: age, sex, diabetes mellitus, insulin‐treated diabetes mellitus, hypertension, hyperlipidemia, current smoker, previous smoker, prior myocardial infarction, prior percutaneous coronary intervention, concomitant coronary artery disease, and calendar year. All models accounted for treating hospital by inclusion of hospital as a random effect in the model.
Refers to the hazard ratio (30‐day and 5‐year mortality) or odds ratio (acute heart failure and cardiogenic shock).
Regional Variations in TS Incidence and Prognosis
The reported incidence of TS was highest in the Western health care region whereas unadjusted and adjusted mortality for patients with TS was highest in the Southern health care region (Figure S2, Table S5). The adjusted 5‐year mortality risk increased, whereas the adjusted risk of AHF decreased, from North to South (Table S5). The incidence of TS varied slightly during the course of the study period (Figure S3).
Data Analysis and Postestimation Diagnostics
In the fully adjusted models, data were missing for 1 or several covariables in 11 241/117 720 (9.6%) of the patients (309/2898 [10.7%] of patients with TS). However, for only 1 variable—smoking status—was information missing for >5% patients (6.5%; Table 1). Postestimation analysis for the logistic regression models by the Hosmer–Lemeshow test showed adequate goodness of fit for the models (P>0.05). Squared covariate terms had no explanatory power in any of the models (link test; P>0.05). The average variance inflation factor was <5.0 for all models, indicating a lack of multicollinearity between the variables. Sex and age matching resulted in a cohort that was perfectly matched for sex, with a mean±SD age for patients with TS, STEMI, and NSTEMI of 67.7±11.2, 67.7±11.1, and 67.7±11.1 years, respectively.
DISCUSSION
In this study, we assessed the relative short‐term and long‐term mortality risk of TS versus MI with or without ST‐elevation and investigated clinical features and predictors of in‐hospital complications and worse prognosis among patients with TS. We analyzed data from the SCAAR on all patients with TS, STEMI, or NSTEMI who underwent coronary angiography in Sweden between January 2009 and February 2018, thus including 1 of the largest TS cohorts in the literature consisting of 2898 patients. We showed that (1) the 30‐day mortality risk for TS was higher than NSTEMI and lower than STEMI; (2) the risk of AHF was higher for TS than for NSTEMI or NSTEMI, whereas the risk of cardiogenic shock was between what we found for STEMI and NSTEMI; (3) the relative risk of dying associated with TS versus STEMI or NSTEMI may be particularly high for men, younger patients, and smokers; and (4) smoking was the strongest independent risk factor of adverse outcomes among patients with TS.
Our study shows that patients with TS have a short‐term risk of dying that exceeds that of patients with NSTEMI. A substantial short‐term risk of death in TS was also reported in a recent study of 1750 patients with TS from the International Takotsubo Registry, although in that study no direct comparison was made to patients with MI. 4 A relatively high short‐term risk of dying can be expected in TS attributed to the extensive degree of transient left ventricular hypokinesia and akinesia that is often observed. 15 However, in our study, even if patients with TS had a considerable short‐term risk of dying, their risk was lower than for patients with STEMI. The mortality rate was lower for TS than for STEMI even though the incidence of AHF, the most common cause of in‐hospital death among patients with MI, 16 was similar for both groups. Because TS was associated with at least as high a risk of AHF but a lower risk of dying it is possible that AHF that occurs in the setting of TS is a less severe AHF syndrome than AHF in the setting of STEMI. Consistent with this notion is our observation that despite having a similar risk of AHF than patients with STEMI, patients with TS had a considerably lower risk of cardiogenic shock, the most severe and life‐threatening form of decompensated AHF. 17 Also consistent with the notion that TS is a less severe form of AHF than found with STEMI are previous reports that TS is associated with relatively preserved cardiac output and end‐organ perfusion compared with STEMI despite more widespread cardiac dysfunction and low blood pressure. 18 , 19 Patients with TS often survive when more than half of the left ventricle is akinetic, 15 a degree of akinesia that would invariably lead to progressive cardiogenic shock and death if it had occurred in the setting of STEMI. 20 Future studies are necessary to determine the extent to which the differences in the clinical AHF phenotype observed in TS versus STEMI are related to intracardiac factors such as regional systolic and diastolic function versus extracardiac factors such as endothelial function and ventriculoarterial coupling. 21
In addition to a substantial short‐term mortality rate, the long‐term mortality rate for patients with TS was almost 3% annually. Although among the patients who were alive at 30 days, the long‐term mortality rate for TS was significantly lower than for STEMI or NSTEMI, the adjusted risk was similar for TS and NSTEMI. The long‐term mortality rate for patients with TS in our study is also consistent with previously reported rates. 4 , 5 Hence, TS appears to be associated with long‐term mortality despite the fact that cardiac function recovers completely in most of these patients. 3 The extent to which this long‐term risk relates to cardiac or extracardiac factors or both remains to be established.
TS is known to be less common in men than women, but among patients with TS the death rates have been reported to be higher for men. 4 , 22 Male sex was an independent risk factor for death among patients with TS also in our study, and the relative mortality risk in TS versus STEMI was higher for men than women. The excess mortality among men in TS has been suggested to be related in part to a higher proportion of physical versus emotional stressors among men than women. 4 Our data did not allow us to assess the relative contribution of sex‐related differences in the type of TS stressor to the excess mortality observed in men. However, we could show that after adjustment for other cardiovascular risk factors the mortality risks in TS versus STEMI for men and women were no longer significantly different. Some of the excess risk for men versus women with TS may therefore be mediated by sex‐related differences in other risk factors. One important risk factor that has been suggested to mediate part of the difference in outcome between male and female patients with TS is concomitant coronary artery disease. 23 Concomitant coronary artery disease was relatively prevalent among patients with TS in our study and appeared to be an important risk factor as it was associated with an increased risk of acute mortality, AHF, and cardiogenic shock.
Younger patients are less likely than older patients to develop TS, and older age was an independent risk factor for dying among patients with TS in our study and in previous studies. 24 However, in our study the association between age and mortality risk was weaker in TS than STEMI and NSTEMI. Why the relative risk of TS versus STEMI or NSTEMI decreased with increasing age is not apparent from our results or from any previous work. One could speculate that a relatively high risk among the few younger patients who were hospitalized with TS was observed because younger patients with milder forms of TS did not seek medical care. It has been hypothesized that the TS cases observed in clinic represent the most severe cases and that many cases are asymptomatic or do not result in sufficiently pronounced symptoms to prompt the patient to seek medical care. 25 Younger patients may better tolerate milder forms of TS.
Our study provides evidence for an important association between smoking and prognosis in TS that has not been previously reported. Smoking was an independent predictor of 30‐day and 5‐year mortality as well as in‐hospital AHF among patients with TS. Furthermore, the relative mortality risk of TS versus STEMI or NSTEMI was disproportionally higher among smokers than nonsmokers. This is consistent with the notion of TS as an AHF syndrome in which alterations at the level of the peripheral vasculature is as important as alterations at the level of the heart 18 , 19 , 26 because the peripheral vasculature is affected by several components of cigarette smoke. 27 , 28 For example, smoking accelerates arterial stiffening 28 and worsens endothelial function, 27 which may interfere with ventriculo‐arterial coupling and worsen cardiac output in AHF. 21 Irrespective of the underlying mechanisms, the observed association between smoking and prognosis in TS merits further investigation.
Lastly, the reported prevalence of TS in the catheterization laboratory among patients with suspected ACS differed across the different health care regions in Sweden, and the regional variation in prognosis was more pronounced for patients with TS than for patients with NSTEMI or STEMI. To our knowledge, this study is the first to reliably assess regional differences in the incidence and prognosis of TS within a country, which was made possible by the nationwide and complete coverage of the SCAAR. The extent to which these observed differences relate to reporting differences, socioeconomic factors, rural–urban differences, climate and other geographical factors, and regional health care policies remains to be established.
Strengths and Limitations
The SCAAR is considered unique among large‐scale registries because of its nationwide coverage and high‐quality data. Another strength of SCAAR compared with other nationwide registries such as the US nationwide in‐patient sample SCAAR, is the routine and systemic validation of SCAAR data. 9 Therefore, our study provides unique real‐world data from a large detailed cohort of patients. Irrespective of the nationwide coverage and high‐quality data of the SCAAR, this is an observational study and provides only evidence of association, not cause, as we cannot exclude selection bias and residual confounding; the SCAAR does not collect data on the potential precipitating stressors for patients with TS, and we did not have data on cause‐specific mortality. Lastly, we lack detailed data on in‐hospital management and medications at discharge and during the following 5 years.
CONCLUSIONS
The 30‐day mortality rate in TS was higher than in NSTEMI but lower than in STEMI despite a similar risk of AHF in TS and STEMI. Among 2898 patients with TS, smoking was an independent predictor of short‐term mortality and in‐hospital complications.
Sources of Funding
This work was supported by the Swedish Scientific Council and the Swedish Heart and Lung Foundation.
Disclosures
None.
Supporting information
Tables S1–S5
Figures S1–S3
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.017290.
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Hurst RT, Prasad A, Askew JW III, Sengupta PP, Tajik AJ. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging. 2010;3:641–649. doi: 10.1016/j.jcmg.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 2. Tsuchihashi K, Ueshima K, Uchida T, Oh‐mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris‐Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/S0735-1097(01)01316-X [DOI] [PubMed] [Google Scholar]
- 3. Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, et al. Current state of knowledge on Takotsubo syndrome: a position statement from the Taskforce on Takotsubo syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424 [DOI] [PubMed] [Google Scholar]
- 4. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761 [DOI] [PubMed] [Google Scholar]
- 5. Redfors B, Vedad R, Angeras O, Ramunddal T, Petursson P, Haraldsson I, Ali A, Dworeck C, Odenstedt J, Ioaness D, et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction—a report from the SWEDEHEART registry. Int J Cardiol. 2015;185:282–289. doi: 10.1016/j.ijcard.2015.03.162 [DOI] [PubMed] [Google Scholar]
- 6. Tornvall P, Collste O, Ehrenborg E, Jarnbert‐Petterson H. A case‐control study of risk markers and mortality in takotsubo stress cardiomyopathy. J Am Coll Cardiol. 2016;67:1931–1936. doi: 10.1016/j.jacc.2016.02.029 [DOI] [PubMed] [Google Scholar]
- 7. Ramunddal T, Hoebers LP, Henriques JP, Dworeck C, Angeras O, Odenstedt J, Ioanes D, Olivecrona G, Harnek J, Jensen U, et al. Prognostic impact of chronic total occlusions: a report from SCAAR (Swedish Coronary Angiography and Angioplasty Registry). JACC Cardiovasc Interv. 2016;9:1535–1544. doi: 10.1016/j.jcin.2016.04.031 [DOI] [PubMed] [Google Scholar]
- 8. Fröbert O, Lagerqvist BO, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angerås O, Calais F, Danielewicz M, et al. Thrombus aspiration during ST‐segment elevation myocardial infarction. N Engl J Med. 2013;369:1587–1597. doi: 10.1056/NEJMoa1308789 [DOI] [PubMed] [Google Scholar]
- 9. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish Web‐system for enhancement and development of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617–1621. doi: 10.1136/hrt.2010.198804 [DOI] [PubMed] [Google Scholar]
- 10. Sahlen A, Varenhorst C, Lagerqvist B, Renlund H, Omerovic E, Erlinge D, Wallentin L, James SK, Jernberg T. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J. 2016;37:3335–3342. doi: 10.1093/eurheartj/ehw284 [DOI] [PubMed] [Google Scholar]
- 11. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 12. Angeras O, Albertsson P, Karason K, Ramunddal T, Matejka G, James S, Lagerqvist B, Rosengren A, Omerovic E. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J. 2013;34:345–353. doi: 10.1093/eurheartj/ehs217 [DOI] [PubMed] [Google Scholar]
- 13. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998. doi: 10.1002/sim.3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubin DB. Inference and missing data. Biometrika. 1976;63:581–590. doi: 10.1093/biomet/63.3.581 [DOI] [Google Scholar]
- 15. Eitel I, von Knobelsdorff‐Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992 [DOI] [PubMed] [Google Scholar]
- 16. Steg PG, Dabbous OH, Feldman LJ, Cohen‐Solal A, Aumont MC, López‐Sendón J, Budaj A, Goldberg RJ, Klein W, Anderson FA Jr. Determinants and prognostic impact of heart failure complicating acute coronary syndromes: observations from the Global Registry of Acute Coronary Events (GRACE). Circulation. 2004;109:494–499. doi: 10.1161/01.CIR.0000109691.16944.DA [DOI] [PubMed] [Google Scholar]
- 17. Redfors B, Angerås O, Råmunddal T, Dworeck C, Haraldsson I, Ioanes D, Petursson P, Libungan B, Odenstedt J, Stewart J, et al. 17‐year trends in incidence and prognosis of cardiogenic shock in patients with acute myocardial infarction in western Sweden. Int J Cardiol. 2015;185:256–262. doi: 10.1016/j.ijcard.2015.03.106 [DOI] [PubMed] [Google Scholar]
- 18. Redfors B, Shao Y, Ali A, Sun B, Omerovic E. Rat models reveal differences in cardiocirculatory profile between Takotsubo syndrome and acute myocardial infarction. J Cardiovasc Med (Hagerstown). 2015;16:632–638. doi: 10.2459/JCM.0000000000000088 [DOI] [PubMed] [Google Scholar]
- 19. Singh K, Neil CJ, Nguyen TH, Stansborough J, Chong CR, Dawson D, Frenneaux MP, Horowitz JD. Dissociation of early shock in takotsubo cardiomyopathy from either right or left ventricular systolic dysfunction. Heart Lung Circ. 2014;23:1141–1148. doi: 10.1016/j.hlc.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 20. Page DL, Caulfield JB, Kastor JA, DeSanctis RW, Sanders CA. Myocardial changes associated with cardiogenic shock. N Engl J Med. 1971;285:133–137. doi: 10.1056/NEJM197107152850301 [DOI] [PubMed] [Google Scholar]
- 21. Mentz RJ, O'Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol. 2016;13:28–35. doi: 10.1038/nrcardio.2015.134 [DOI] [PubMed] [Google Scholar]
- 22. Krishnamoorthy P, Garg J, Sharma A, Palaniswamy C, Shah N, Lanier G, Patel NC, Lavie CJ, Ahmad H. Gender differences and predictors of mortality in Takotsubo cardiomyopathy: analysis from the National Inpatient Sample 2009–2010 database. Cardiology. 2015;132:131–136. doi: 10.1159/000430782 [DOI] [PubMed] [Google Scholar]
- 23. Napp LC, Cammann VL, Jaguszewski M, Szawan KA, Wischnewsky M, Gili S, Knorr M, Heiner S, Citro R, Bossone E, et al. Coexistence and outcome of coronary artery disease in Takotsubo syndrome. Eur Heart J. 2020;41:3255–3268. doi: 10.1093/eurheartj/ehaa210 [DOI] [PubMed] [Google Scholar]
- 24. Schneider B, Sechtem U. Influence of age and gender in Takotsubo syndrome. Heart Fail Clin. 2016;12:521–530. doi: 10.1016/j.hfc.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 25. Madias JE. Are there mild forms of Takotsubo syndrome? Int J Cardiol. 2016;211:25–26. doi: 10.1016/j.ijcard.2016.02.152 [DOI] [PubMed] [Google Scholar]
- 26. Redfors B, Ali A, Shao Y, Lundgren J, Gan LM, Omerovic E. Different catecholamines induce different patterns of takotsubo‐like cardiac dysfunction in an apparently afterload dependent manner. Int J Cardiol. 2014;174:330–336. doi: 10.1016/j.ijcard.2014.04.103 [DOI] [PubMed] [Google Scholar]
- 27. Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, Fiore MC, Stein JH. Effects of smoking and smoking cessation on endothelial function: 1‐year outcomes from a randomized clinical trial. J Am Coll Cardiol. 2010;55:1988–1995. doi: 10.1016/j.jacc.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tomiyama H, Hashimoto H, Tanaka H, Matsumoto C, Odaira M, Yamada J, Yoshida M, Shiina K, Nagata M, Yamashina A. Continuous smoking and progression of arterial stiffening: a prospective study. J Am Coll Cardiol. 2010;55:1979–1987. doi: 10.1016/j.jacc.2009.12.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S3


