Abstract
Background
Atrial fibrillation (AF) represents a major indication for oral anticoagulants (OAC) that contribute to spontaneous intracerebral hemorrhage (ICH). This study evaluated AF prevalence among patients with ICH, temporal trends, and early functional outcomes and death of patients.
Methods and Results
Patients with first‐ever ICH were prospectively recorded in the population‐based stroke registry of Dijon, France, (2006–2017). Association between AF and early outcome of patients with ICH (ordinal modified Rankin Scale score and death at discharge) were analyzed using ordinal and logistic regressions. Among 444 patients with ICH, 97 (21.9%) had AF, including 65 (14.6%) with previously known AF treated with OAC, and 13 (2.9%) with newly diagnosed AF. AF prevalence rose from 17.2% (2006–2011) to 25.8% (2012–2017) (P‐trend=0.05). An increase in the proportion of AF treated with OAC (11.3% to 17.5%, P‐trend=0.09) and newly diagnosed AF (1.5% to 4.2%, P‐trend=0.11) was observed. In multivariable analyses, after adjustment for premorbid OAC, AF was not significantly associated with ordinal modified Rankin Scale score (odds ratio [OR], 1.29; 95% CI, 0.69–2.42) or death (OR, 0.89; 95% CI, 0.40–1.96) in patients with ICH. Nevertheless, adjusted premorbid OAC use remained highly associated with a higher probability of death (OR, 2.53; 95% CI, 1.11–5.78).
Conclusions
AF prevalence and use of OAC among patients with ICH increased over time. Premorbid use of OAC was associated with poor outcome after ICH, thus suggesting a need to better identify ICH risk before initiating or pursuing OAC therapy in patients with AF, and to develop acute treatment and secondary prevention strategies after ICH in patients with AF.
Keywords: anticoagulants, atrial fibrillation, epidemiology, intracerebral hemorrhage, outcomes
Subject Categories: Atrial Fibrillation, Epidemiology, Intracranial Hemorrhage, Anticoagulants
Nonstandard Abbreviations and Acronyms
- ICH
intracerebral hemorrhage
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- OAC
oral anticoagulants
Clinical Perspective
What Is New?
The prevalence of atrial fibrillation (AF) reached 1 in 4 patients with intracerebral hemorrhage in the Dijon Stroke Registry for the 2012 to 2017 period.
The observed increase in AF prevalence among patients with intracerebral hemorrhage over time partly reflected a rise in the prevalence of previously anticoagulated patients with AF.
What Are the Clinical Implications?
With the ongoing aging population, and the expected increase in the burden of AF, our results highlight the urgent need for defining acute treatment and secondary prevention strategies after intracerebral hemorrhage in patients with AF.
Although spontaneous (non‐traumatic) intracerebral hemorrhage (ICH) accounts for only 15% of overall strokes, it is associated with half of stroke‐related deaths, and 42% of stroke related disability‐adjusted life‐years lost worldwide. 1 Because of the absence of major change in the acute treatment of ICH, early mortality of patients with ICH did not improve over the last 3 decades. 2 Oral anticoagulants (OAC) are important contributors of ICH, and since the main indication for their prescription is atrial fibrillation (AF), patients with ICH have frequently associated AF. Given the ongoing aging population, the burden of AF is increasing worldwide, 3 and this trend could lead to a rise in the incidence of OAC‐related ICH, as demonstrated in previous studies. 2 , 4
Therefore, using a stroke population‐based registry, this study aimed to assess the overall prevalence of AF among patients with ICH, time trends between 2006 and 2017, and to analyze associations between AF and early functional outcomes and death at discharge among patients with ICH, including the role of premorbid OAC use on these associations.
Methods
Data Source
Non‐traumatic non‐tumor‒related patients with ICH were retrieved from the Dijon Stroke Registry, a prospective population‐based study that complies with the defined criteria for conducting “ideal” incidence stroke studies, 5 and the guidelines for the reporting of incidence and prevalence studies in neuroepidemiology according to Standards of Reporting of Neurological Disorders. 6 The registry collects all cases of acute stroke and transient ischemic attack among residents of the city of Dijon (Burgundy, France, currently 156 000 inhabitants). The methodology of the Dijon Stroke Registry has been detailed in previous studies. 7 , 8 Briefly, case‐collection relies on multiple overlapping sources of information to identify hospitalized and not hospitalized cases of stroke. The final adjudication of cases is systematically made by senior neurologists trained in stroke ascertainment according to the World Health Organization diagnostic criteria 9 based on all information available. ICH location was determined through brain imaging as follows: lobar (frontal, temporal, parietal, and occipital); deep, when it originated from the lenticular or caudate nuclei, thalamus, or internal or external capsule; and infratentorial, when it originated from the brainstem or cerebellum. 2 , 10 ICH was classified as undetermined when the origin could not be reliably identified, as was the case in hemorrhages that overlapped 2 territories, or when data were missing.
Among the 2772 patients registered in the Dijon Stroke Registry between January 1, 2006 and December 31, 2017 (n=1373 for 2006–2011 and n=1399 for 2012–2017), only first‐ever ICH were included in the present study.
Data Collected
At registration, the following vascular risk factors and past medical history were collected 7 , 8 : hypertension (high blood pressure recorded in a patient’s medical history or patients under antihypertensive treatment), diabetes mellitus (glucose level ≥7.8 mmol/L reported in the medical record or patients taking insulin or oral hypoglycemic agents), hypercholesterolemia (total cholesterol level ≥5.7 mmol/L reported in the medical history or patients treated with lipid‐lowering therapy), smoking status (current smoker or past smoker), history of coronary heart disease, history of stroke or transient ischemic attack, chronic heart failure, and excessive alcohol consumption (defined as alcohol intake ≥3 units a day in men and ≥2 in women). AF (including atrial flutter) was defined as “known AF” if it was mentioned in the medical file of the patient before ICH, and “newly diagnosed AF” if it was diagnosed during the diagnostic work‐up of the ICH by ECG monitoring or further examinations. AF was registered whatever the ICH location. Prior‐to‐stroke treatment with oral anticoagulant treatments (vitamin K antagonists or direct oral anticoagulants) were recorded. Other premorbid therapies including antiplatelet agents and antihypertensive treatment were collected. The CHA2DS2VaSc score was calculated for each patient. 11
Severity at onset was quantified using the National Institutes of Health Stroke Scale (NIHSS) score either obtained at the first clinical examination, or retrospectively estimated on the basis of medical records and charts, as previously validated in the literature. 12 Post‐stroke outcome of patients was evaluated using the ordinal modified Rankin Scale (mRS) score at discharge from the acute care ward for hospitalized patients or at last clinical examination for non‐hospitalized patients.
Statistical Analysis
Characteristics were described according to the presence or absence of AF in participants with the first occurrence of ICH in 2006 to 2011, 2012 to 2017, and the overall 2006 to 2017 period. Prevalence of AF among patients with ICH was given through percentage for these periods. We assumed Poisson distribution for AF prevalence to calculate 95% CI. Time trends in AF prevalence was assessed using Poisson regression, treating time as a categorical variable (2012–2017 versus 2006–2011). Comparisons between categorical variables were performed using χ2 or Fisher tests and using Wilcoxon‐Mann‐Whitney test for quantitative variables. The association between AF and respectively mRS scores (using ordinal logistic regression, whom proportional odds assumptions were verified using score χ2 test) and death was studied using logistic regressions in a 3‐step modeling: a first model was adjusted for confounding factors described in the literature focusing on ICH outcomes 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 (age, sex, ICH location, time period of occurrence, vascular risk factors including hypertension, hypercholesterolemia, diabetes, smoking, excessive alcohol intake, and history of heart failure, stroke (other than ICH), transient ischemic attack, and coronary heart disease), and that were previously found to be associated with AF 23 , 24 or ICH outcomes but might not be along the causal pathway from AF to outcomes; 2 additional models, also adjusted for the above‐mentioned factors, were implemented to precise the role of severity, assessed by the NIHSS score (model 2), and premorbid use of OAC (model 3) on ICH outcomes as they both act as mediators, meaning these factors might be along the causal pathway from AF to ICH outcomes. ICH location was introduced in the models as it was found to be associated with ICH outcomes in several studies including among OAC‐related ICH. 25 , 26 , 27 Patients with missing data were excluded from logistic regressions. Statistical analyses were performed using SAS Guide version 9.4 software.
Ethics Approval
The Dijon Stroke Registry was approved by following national ethics boards: the Comité d’Evaluation des Registres (French National Committee of Registers), Santé Publique France (French Institute for Public Health Surveillance), and the Commission Nationale Informatique et Liberté (French data protection authority). In accordance with the French legislation, boards waived the need for written patient consent.
Data Availability Statement
Anonymized grouped data can be shared by request from a qualified investigator.
Results
Over the whole study period (2006–2017), 445 patients with a first‐ever acute ICH were recorded in the Dijon Stroke Registry. After excluding 1 patient in whom no information about AF was available, data of 444 patients with ICH were analyzed for this study, including 204 patients over period 2006 to 2011, and 240 patients over period 2012 to 2017.
Characteristics of patients are shown in Table 1. Over the whole study period (2006–2017), mean age at onset was 73.8±17 years, and women accounted for 52.2% of patients with ICH. A total of 97 (22%) patients with ICH had AF. AF was known before stroke in the majority of these cases (n=84, 87%). Among patients with previously known AF, 67% were receiving OAC. Compared with patients with ICH without AF, patients with AF were older (aged, 83.3 versus 71.8 years, P<0.0001), had higher prevalence of hypertension (78.3% versus 62.2%, P=0.003), hypercholesterolemia (37.1% versus 22.2%, P=0.003), chronic heart failure (17.5% versus 7.5%, P=0.003), and coronary heart disease (16.7% versus 6.3%, P=0.001) (Table 1). ICH location was similarly distributed among patients with ICH with or without AF (P=0.41). OAC were more frequently reported in patients with ICH with AF (72.2% versus 10.1%, P<0.0001). Patients with AF had a greater severity at ICH onset (median NIHSS 14 versus 8, P=0.03) as well as a poorer functional outcome: 81.5% of them had mRS ≥4 compared with 64.9% in patients without AF (Figure 1). In addition, mortality at discharge was higher in patients with ICH with AF (47.4% versus 30%, P<0.0001, Table 1).
Table 1.
Characteristics of Patients With ICH According to AF Prevalence and Prior AF, 2006 to 2017
| Time period | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006–2011 | 2012–2017 | Overall period (2006–2017) | ||||||||||
| All ICH | ICH with AF ‡ | ICH without AF | P value* | All ICH | ICH with AF ‡ | ICH without AF | P value* | All ICH | ICH with AF ‡ | ICH without AF | P value* | |
| n | 204 | 35 | 169 | 240 | 62 | 178 | 444 | 97 | 347 | |||
| Mean age, y (SD) | 75.0 (16) | 82.3 (9) | 73.8 (17) | 0.022 | 72.7 (18) | 83.6 (11) | 69.9 (20) | <0.0001 | 73.8 (17) | 83.3 (10) | 71.8 (18) | <0.0001 |
| Age <65 y, n (%) | 39 (19.1) | 2 (5.7) | 37 (21.9) | 0.027 | 67 (27.9) | 6 (9.7) | 61 (34.3) | 0.0002 | 106 (23.9) | 8 (8.2) | 98 (28.2) | <0.0001 |
| Women, n (%) | 113 (55.4) | 23 (65.7) | 90 (53.3) | 0.178 | 119 (49.6) | 33 (53.2) | 86 (48.3) | 0.506 | 232 (52.2) | 56 (57.7) | 176 (50.7) | 0.222 |
| AF, n (%) | 35 (17.2) | 35 (100) | 0 | 0 | 62 (25.8) | 62 (100) | 0 | 0 | 97 (21.9) | 97 (100) | 0 | 0 |
| Known AF, n (%) | 32 (15.7) | 32 (91.4) | 0 | 0 | 52 (21.7) | 52 (83.9) | 0 | 0 | 84 (18.9) | 84 (86.6) | 0 | 0 |
| Known AF treated with OAC, n(%) | 23 (11.3) | 23 (65.7) | 0 | 0 | 42 (17.5) | 42 (67.7) | 0 | 0 | 65 (14.6) | 65 (67.0) | 0 | 0 |
| Newly diagnosed AF, n (%) | 3 (1.5) | 3 (8.6) | 0 | 0 | 10 (4.2) | 10 (16.1) | 0 | 0 | 13 (2.9) | 13 (13.4) | 0 | 0 |
| ICH location, n (%) | 0.509 | 0.449 | 0.407 | |||||||||
| Lobar | 96 (48.7) | 15 (42.9) | 81 (50.0) | 121 (50.4) | 26 (41.9) | 95 (53.4) | 217 (49.7) | 41 (42.3) | 176 (51.8) | |||
| Deep | 77 (39.1) | 15 (42.9) | 62 (38.3) | 77 (32.1) | 23 (37.1) | 54 (30.3) | 154 (35.2) | 38 (39.2) | 116 (34.1) | |||
| Infratentorial | 18 (9.1) | 4 (11.4) | 14 (8.6) | 25 (10.4) | 7 (11.3) | 18 (10.1) | 43 (9.8) | 11 (11.3) | 32 (9.4) | |||
| Undetermined | 6 (3.1) | 1 (2.9) | 5 (3.1) | 17 (7.1) | 6 (9.7) | 11 (6.2) | 23 (5.3) | 7 (7.2) | 16 (4.7) | |||
| Missing | 7 (3.4) | 0 | 7 (4.1) | 0 | 0 | 0 | 7 (1.6) | 0 | 7 (2.0) | |||
| Premorbid treatment, n (%) | ||||||||||||
| Anticoagulants | 40 (19.6) | 24 (68.6) | 16 (9.5) | <0.0001 | 65 (27.1) | 46 (74.2) | 19 (10.7) | <0.0001 | 105 (23.6) | 70 (72.2) | 35 (10.1) | <0.0001 |
| Antiplatelet agents | 37 (18.1) | 4 (11.4) | 33 (19.5) | 0.259 | 44 (18.3) | 14 (22.6) | 30 (16.8) | 0.317 | 81 (18.2) | 18 (18.6) | 63 (18.2) | 0.928 |
| Antihypertensive treatments | 83 (40.7) | 15 (42.9) | 68 (40.2) | 0.774 | 115 (47.9) | 47 (75.8) | 68 (38.2) | <0.0001 | 198 (44.6) | 62 (63.9) | 136 (39.2) | <0.0001 |
| Medical history, n (%) | ||||||||||||
| Hypertension § | 146 (71.6) | 23 (65.7) | 123 (72.8) | 0.401 | 146 (60.8) | 53 (85.5) | 93 (52.2) | <0.0001 | 292 (65.8) | 76 (78.3) | 216 (62.2) | 0.003 |
| Hypercholesterolemia § | 49 (24.0) | 9 (25.7) | 40 (23..7) | 0.797 | 64 (26.7) | 27 (43.5) | 37 (20.8) | 0.0005 | 113 (25.4) | 36 (37.1) | 77 (22.2) | 0.003 |
| Diabetes § | 33 (16.3) | 5 (14.3) | 28 (16.7) | 0.729 | 26 (10.8) | 10 (16.1) | 16 (9.0) | 0.120 | 59 (13.3) | 15 (15.5) | 44 (12.7) | 0.482 |
| Missing | 1 (0.5) | 0 | 1 (0.6) | 0 | 0 | 0 | 1 (0.2) | 0 | 1 (0.3) | |||
| Smoking (current or former smoker) | 58 (29.3) | 7 (21.2) | 51 (30.9) | 0.265 | 66 (29.7) | 16 (28.6) | 50 (30.1) | 0.827 | 124 (29.5) | 23 (25.8) | 101 (30.5) | 0.392 |
| Missing | 6 (2.9) | 2 (5.7) | 4 (2.4) | 18 (7.5) | 6 (9.7) | 12 (6.7) | 24 (5.4) | 8 (8.2) | 16 (4.6) | |||
| Alcohol intake | 21 (10.4) | 2 (5.9) | 2 (11.4) | 0.341 | 28 (12.4) | 7 (12.5) | 21 (12.4) | 0.988 | 49 (11.5) | 9 (10.0) | 40 (11.9) | 0.615 |
| Missing | 3 (1.5) | 1 (2.9) | 2 (1.2) | 15 (6.3) | 6 (9.7) | 9 (5.1) | 18 (4.0) | 7 (7.2) | 11 (3.2) | |||
| Chronic heart failure | 25 (12.2) | 7 (20.0) | 18 (10.6) | 0.126 | 18 (7.5) | 10 (16.1) | 8 (4.5) | 0.003 | 43 (9.7) | 17 (17.5) | 26 (7.5) | 0.003 |
| Previous stroke | 43 (21.1) | 6 (17.1) | 37 (21.9) | 0.531 | 55 (22.9) | 17 (27.4) | 38 (21.4) | 0.328 | 98 (22.1) | 23 (23.7) | 75 (21.6) | 0.660 |
| Previous TIA | 7 (3.4) | 1 (2.9) | 6 (3.5) | 0.838 | 16 (6.7) | 7 (11.3) | 9 (5.1) | 0.134 | 23 (5.2) | 8 (8.2) | 15 (4.3) | 0.123 |
| Coronary heart diseases | 19 (9.3) | 6 (17.1) | 13 (7.7) | 0.080 | 19 (7.9) | 10 (16.4) | 9 (5.1) | 0.011 | 38 (8.6) | 16 (16.7) | 22 (6.3) | 0.001 |
| Missing | 0 | 0 | 0 | 1 (0.4) | 1 (1.6) | 0 | 1 (0.2) | 1 () | 0 | |||
| CHA2DS2VaSc score ≥2, % | 132 (84.2) | 24 (91.4) | 108 (82.3) | 0.199 | 139 (80.0) | 52 (96.8) | 87 (74.2) | <0.0001 | 271 (81.8) | 76 (94.9) | 195 (78.1) | <0.0001 |
| Missing | 1 (0.5) | 0 | 1 (0.6) | 0 | 0 | 0 | 1 (1.0) | 0 | 1 (0.3) | |||
| NIHSS score on admission, median (IQR) | 8.5 (4–21) | 11 (4–24) | 8 (4–19) | 0.243 | 11 (3–21) | 14 (5–23) | 8.5 (3.0–20) | 0.077 | 10 (4–21) | 14 (5–23) | 8 (4–20) | 0.031 |
| Modified Rankin Scale score at discharge, n (%) | 0.034 | 0.0003 | <0.0001 | |||||||||
| mRS 0 | 8 (3.9) | 0 | 8 (4.7) | 15 (6.2) | 0 | 15 (8.4) | 5.2 (23) | 0 | 23 (6.6) | |||
| mRS 1 | 19 (9.3) | 2 (5.7) | 17 (10.1) | 24 (10.0) | 2 (3.2) | 22 (12.4) | 43 (9.7) | 4 (4.1) | 39 (11.2) | |||
| mRS 2 | 26 (12.7) | 3 (8.6) | 23 (13.6) | 10 (4.2) | 3 (4.8) | 7 (3.9) | 36 (8.1) | 6 (6.2) | 30 (8.6) | |||
| mRS 3 | 14 (6.9) | 1 (2.9) | 13 (7.7) | 24 (10.0) | 7 (11.3) | 17 (9.6) | 38 (8.6) | 8 (8.2) | 30 (8.7) | |||
| mRS 4 | 58 (28.4) | 11 (31.4) | 47 (27.8) | 34 (14.2) | 4 (6.5) | 30 (16.9) | 92 (20.7) | 15 (15.5) | 77 (22.2) | |||
| mRS 5 | 15 (7.4) | 4 (11.4) | 11 (6.5) | 47 (19.6) | 14 (22.6) | 33 (18.5) | 62 (14.0) | 18 (18.6) | 44 (12.7) | |||
| mRS 6 | 64 (31.4) | 14 (40.0) | 50 (29.6) | 86 (35.8) | 32 (51.6) | 54 (30.3) | 150 (33.8) | 46 (47.4) | 104 (30.0) | |||
| Length of hospital stay ‖ , d (sd) | 19 (26) | 24 (37) | 19 (24) | 0.665 | 14 (15) | 16 (16) | 14 (15) | 0.268 | 17 (21) | 19 (25) | 16 (20) | |
Abbreviations: AF, atrial fibrillation; ICH, intracerebral hemorrhage; IQR, interquartile range; mRS, modified rankin score; TIA, transient ischemic attack; OAC, oral anticoagulants.
Between stroke with atrial fibrillation and stroke without atrial fibrillation.
Between stroke with known atrial fibrillation and stroke with newly diagnosed atrial fibrillation.
Refers to patients with both previously known atrial fibrillation and those with newly diagnosed atrial fibrillation.
Treated or not.
Among patients with intracerebral hemorrhage; hospitalized (436 of the 444 intracerebral hemorrhage).
Figure 1. Unadjusted distribution of modified Rankin Scores in intracerebral hemorrhage with and without atrial fibrillation for the period 2006 to 2017.
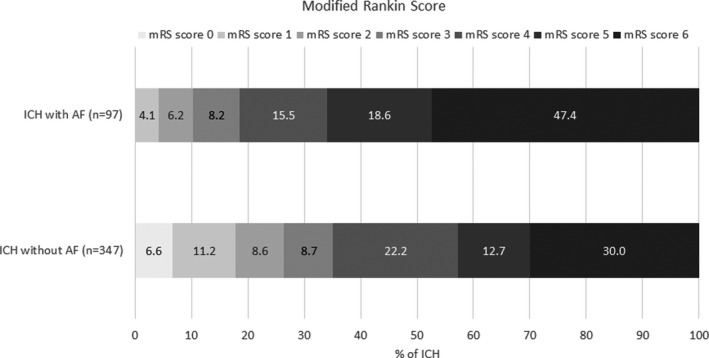
AF indicates atrial fibrillation; ICH, intracerebral hemorrhage; and mRS, modified Rankin Scale.
The prevalence of AF in patients with first‐ever ICH increased over time from 17.2% (95% CI, 12.3–23.9) for years 2006 to 2011 to 25.8% (95% CI, 20.1–33.1) in 2012 to 2017 (P‐trend=0.05). Concomitantly, the prevalence of patients with AF treated with OAC rose from 11.3% (95% CI, 7.5–17.0) to 17.5% (95% CI, 12.9–23.7) (P‐trend=0.09). Similarly, we observed a non‐significant increase in newly diagnosed AF in patients with ICH (1.5% [95% CI, 0.5–4.6] in 2006 to 2011 to 4.2 [95% CI, 2.2–7.7] in 2012 to 2017, P‐trend=0.11).
With regard to post‐stroke outcomes, AF was associated with higher mRS scores (univariate odds ratio [OR], 2.40; 95% CI, 1.58–3.64, P<0.0001), even after adjusting for confounding factors and NIHSS score (model 2: OR, 1.77; 95% CI, 1.06–2.96, P=0.03) (Table 2 and Figure 2). However, AF was no longer independently associated with mRS after adjusting for premorbid anticoagulants (model 3: OR, 1.29; 95% CI, 0.69–2.42, P=0.42). Finally, no significant association between AF and death were observed in multivariable analyses adjusted for NIHSS (model 2: OR, 1.41; 95% CI, 0.73–2.73, P=0.30; model 3: OR, 0.89; 95% CI, 0.40–1.96, P=0.76). According to interaction analyses, no significant interaction was found between AF and age for mRS, nor for death, and no interaction was found between AF and age according to OAC status for all outcomes.
Table 2.
Association Between AF and Respectively Higher Modified Rankin Scores and Death in Patients With ICH From the Dijon Stroke Registry, 2006 to 2017
| Effects | Modified Rankin scale scores (ordinal) | In‐hospital death | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |||
| AF (yes vs no) | 2.40 [1.58–3.64]* | 1.93 [1.23–3.05]* | 1.44 [1.0.6–2.96]* | 1.29 [0.69–2.42] | 2.11 [1.33–3.34]* | 1.69 [1.00–2.84]* | 1.41 [0.73–2.73] | 0.89 [0.40–1.96] |
| Severity | ||||||||
| NIHSS (for 1‐point increase) | 1.21 [1.17–1.24]* | … | 1.22 [1.19–1.26]* | 1.22 [1.19–1.26]* | 1.16 [1.13–1.20]* | … | 1.18 [1.14–1.22]* | 1.18 [1.14–1.22]* |
| Premorbid anticoagulant treatment | 2.35 [1.57–3.52]* | … | … | 1.74 [0.93–3.26] | 2.41 [1.54–3.78]* | … | … | 2.53 [1.11–5.78]* |
| Time period | ||||||||
| 2006–2011 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2012–2017 | 1.32 [0.94–1.84] | 1.31 [0.90–1.89] | 1.06 [0.71–1.57] | 1.03 [0.69–1.54] | 1.22 [0.82–1.82] | 1.20 [0.75–1.90] | 0.86 [0.48–1.54] | 0.85 [0.47–1.55] |
| Sex | ||||||||
| Men (reference) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Women | 1.10 [0.79–1.54] | 0.71 [0.48–1.05] | 0.73 [0.47–1.12] | 0.73 [0.47–1.12] | 1.26 [0.85–1.86] | 0.85 [0.51–1.40] | 0.84 [0.44–1.61] | 0.82 [0.42–1.58] |
| Age group, y | ||||||||
| <65 (reference) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 65–74 | 1.75 [1.03–2.99]* | 1.81 [1.02–3.22]* | 1.13 [0.61–2.11] | 1.12 [0.60–2.07] | 1.95 [0.93–4.09] | 2.04 [0.90–4.63] | 1.38 [0.50–3.83] | 1.33 [0.47–3.73] |
| 75–84 | 2.95 [1.88–4.64]* | 3.00 [1.78–5.06]* | 2.27 [1.28–4.01]* | 2.05 [1.16–3.65]* | 3.88 [2.10–7.16]* | 3.99 [1.94–8.19]* | 2.90 [1.16–7.26]* | 2.55 [1.00–6.46] |
| ≥85 | 3.70 [2.30–5.97]* | 3.88 [2.22–6.77]* | 4.45 [2.41–8.21]* | 4.23 [2.29–7.84]* | 3.87 [2.06–7.28]* | 4.00 [1.89–8.43]* | 3.42 [1.31–8.95]* | 3.18 [1.20–8.46]* |
| Vascular risk factors | ||||||||
| Hypertension (yes vs no) | 1.36 [0.96–1.92] | 0.94 [0.62–1.42] | 1.02 [0.65–1.60] | 1.01 [0.64–1.58] | 1.27 [0.84–1.94] | 0.96 [0.57–1.61] | 1.05 [0.54–2.03] | 1.00 [0.51–1.96] |
| Hypercholesterolemia (yes vs no) | 1.04 [0.71–1.53] | 0.92 [0.58–1.46] | 0.80 [0.48–1.33] | 0.82 [0.49–1.37] | 1.04 [0.67–1.64] | 1.05 [0.59–1.87] | 1.26 [0.58–2.73] | 1.37 [0.63–2.99] |
| Diabetes mellitus (yes vs no) | 0.84 [0.52–1.37] | 0.79 [0.45–1.38] | 0.53 [0.29–0.96]* | 0.52 [0.28–0.94]* | 0.77 [0.42–1.41] | 0.66 [0.32–1.39] | 0.50 [0.19–1.31] | 0.45 [0.17–1.19] |
| Smoking | 0.61 [0.42–0.88]* | 0.61 [0.39–0.95]* | 0.72 [0.44–1.17] | 0.70 [0.43–1.14] | 0.53 [0.33–0.86]* | 0.50 [0.27–0.95]* | 0.37 [0.16–0.86] | 0.36 [0.16–0.85] |
| Alcohol intake | 1.43 [0.84–2.45] | 2.09 [1.11–3.95]* | 2.00 [1.01–3.98]* | 2.13 [1.07–4.24]* | 1.46 [0.79–2.68] | 2.43 [1.09–5.41]* | 2.78 [0.99–7.77] | 3.20 [1.13–9.07]* |
| Heart failure (yes vs no) | 1.31 [0.74–2.31] | 1.33 [0.68–2.58] | 1.73 [0.84–3.56] | 1.64 [0.80–3.38] | 0.94 [0.48–1.84] | 0.93 [0.40–2.15] | 1.15 [0.41–3.24] | 1.11 [0.39–3.14] |
| Stroke (yes vs no) | 1.16 [0.78–1.73] | 1.35 [0.87–2.11] | 1.43 [0.86–2.35] | 1.40 [0.84–2.31] | 0.83 [0.51–1.35] | 0.86 [0.49–1.50] | 0.54 [0.25–1.17] | 0.50 [0.23–1.10] |
| TIA (yes vs no) | 0.89 [0.42–1.87] | 0.67 [0.30–1.50] | 1.03 [0.41–2.54] | 0.96 [0.39–2.38] | 1.05 [0.43–2.53] | 1.17 [0.43–3.15] | 1.92 [0.45–8.15] | 1.84 [0.45–7.54] |
| Coronary heart disease (yes vs no) | 0.95 [0.53–1.71] | 0.63 [0.32–1.26] | 0.76 [0.36–1.61] | 0.76 [0.36–1.61] | 0.79 [0.38–1.64] | 0.54 [0.22–1.35] | 0.56 [0.16–1.96] | 0.61 [0.17–2.12] |
| ICH location | ||||||||
| Lobar (reference) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Deep | 1.56 [1.07–2.26]* | 1.58 [1.05–2.36]* | 1.04 [0.67–1.62] | 1.03 [0.66–1.61] | 1.66 [1.08–2.56]* | 1.89 [1.15–3.10]* | 1.83 [0.98–3.44] | 1.78 [0.94–3.36] |
| Infratentorial | 0.86 [0.48–1.53] | 0.85 [0.45–1.60] | 0.96 [0.49–1.87] | 0.96 [0.49–1.89] | 1.01 [0.50–2.07] | 1.09 [0.48–2.49] | 1.61 [0.55–4.78] | 1.58 [0.52–4.78] |
| Undertermined | 0.93 [0.44–1.99] | 0.89 [0.41–1.95] | 1.30 [0.53–3.22] | 1.30 [0.52–3.21] | 1.25 [0.50–3.09] | 1.45 [0.54–3.91] | 2.52 [0.60–10.5] | 2.34 [0.54–10.1] |
Model 1: adjusted for age, sex, intracerebral hemorrhage location, vascular risk factors, comorbidities; Model 2: model 1 adjusted for National Institutes of Health Stroke Scale; Model 3: model 2 adjusted for premorbid anticoagulants. Abbreviations: AF, atrial fibrillation; ICH, intracerebral hemorrhage; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and TIA, transient ischemic attack.
P value < 0.05.
Figure 2. Association between atrial fibrillation and respectively higher modified Rankin scores and death in patients with intracerebral hemorrhage from the Dijon Stroke Registry, 2006 to 2017.
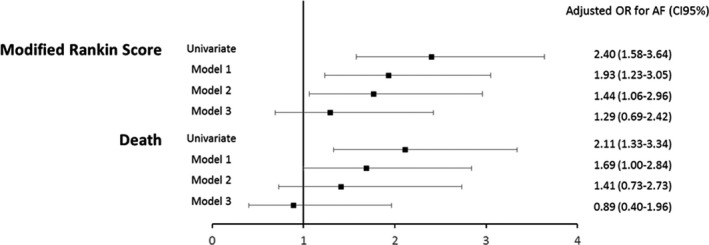
Model 1: adjusted for age, sex, intracerebral hemorrhage location, vascular risk factors, comorbidities. Model 2: Model 1 adjusted for National Institutes of Health Stroke Scale. Model 3: Model 2 adjusted for premorbid anticoagulants. AF indicates atrial fibrillation; and OR, odds ratio.
Discussion
This population‐based study demonstrated that AF is a frequent condition in patients with ICH and had been increasing over time. Hence, more than 1 in 6 patients with ICH during period 2006–2011, and 1 in 4 during period 2012–2017 had AF. Although AF was known before ICH in the large majority of patients with ICH with AF (86.6%), the proportion of newly diagnosed AF among patients with ICH with AF almost doubled between these 2 time periods (from 8.6% to 16.1%). There was a trend toward an increase in the proportion of patients with ICH with previously known AF treated with OAC, who accounted for two third of cases. Despite an apparent deleterious impact of AF on post‐ICH prognosis in univariate analyses, AF was not significantly associated with early poor functional outcome or death after considering confounding factors and the premorbid use of OAC.
In our study, the prevalence of AF in patients with ICH was consistent with that reported in population‐based and hospital‐based studies for the same time period, with numbers ranging from 16% to 31%. 28 , 29 , 30 , 31 Over the 2012 to 2017 period, 17.5% of ICH were previously anticoagulated for AF and 11% over the 2006 to 2011 period. A similar proportion was found in the PITCH (Prognosis of Intra‐Cerebral Hemorrhage) cohort study (10%) over the same time period (2004–2009). 32
The proportion of ICH with prior anticoagulation for AF rose between 2006 and 2017. Previous studies reported an increase in the incidence of overall ICH associated with antithrombotic use, irrespective of the indication for this therapy. 2 , 4 Of note, in a previous analysis of the Dijon Stroke Registry, we observed a 75% increase in the incidence of ICH in people aged >75 years between 1985 and 2008, mainly because of a rise in the incidence of lobar ICH. 2 Concomitantly, the prevalence of prior‐to‐ICH use of anticoagulants quadrupled among patients with ICH aged >75 years, thus indicating the continued importance of studying the optimal treatment strategies for this group of older individuals at risk for bleeding‐prone vasculopathies such as cerebral amyloid angiopathy. Our present findings highlight the fact that the increase in prior use of OAC in overall patients with ICH could be associated with the rise in the number of patients with AF receiving OAC. This result raises an important issue on a clinical point of view with regard to secondary prevention strategies. Indeed, a large majority of patients with ICH with AF were eligible to have an OAC prescription according to the CHA2DS2VaSc score that was ≥2 in 91.4% of them during period 2006 to 2011 and 96.8% during period 2012 to 2017. In the absence of current strong evidence‐based guidelines, there are therapeutic dilemmas around the net benefit of anticoagulation in this setting.
Although ICH has been longstanding regarded as a contraindication for OAC, a recent analysis from the PITCH cohort pointed out a high risk of long‐term occurrence of major ischemic events in ICH survivors. 33 At 5 years, the incidence of ischemic stroke was 9% whereas that of ICH recurrence was 4.9%. Interestingly, deep index ICH was associated with greater ischemic stroke events than ICH recurrences during follow‐up, whereas an inverse association was observed for lobar location. Anticoagulation during follow‐up was neither associated with hemorrhagic events nor with ischemic events. Patients with AF accounted for 11% of this cohort enrolled between 2004 and 2009 versus 26% in our study during 2012 to 2017 period, thus suggesting that this issue is getting greater in contemporary clinical practice. Although a recent meta‐analysis showed that OAC resumption after ICH was associated with decreased mortality, improved functional outcome, and decreased all‐cause stroke incidence in both lobar and deep ICH, 34 results of ongoing randomized clinical trials conducted on patients with ICH with AF are to be expected before drawing any definite conclusion (APACHE‐AF [Apixaban Versus Antiplatelet Drugs or No Antithrombotic Drugs after Anticoagulation‐Associated Intracerebral Haemorrhage in Patients With Atrial Fibrillation], 35 STATICH [Study of Antithrombotic Treatment after Intracerebral Hemorrhage], NASPAF‐ICH [Non‐VKA Anticoagulants for Stroke Prevention in Patients with AF and Previous Intracerebral Hemorrhage], SoSTART [Start or Stop Anticoagulants Randomised Trial] After Spontaneous Intracranial Haemorrhage, ASPIRE [Anticoagulation in ICH Survivors for Stroke Prevention and Recovery], ENRICH‐AF [Edoxaban for Intracranial Hemorrhage Survivors With Atrial Fibrillation], PRESTIGE‐AF [Prevention of Stroke in Intracerebral HaemorrhagE Survivors With Atrial Fibrillation]). 36 These results would probably also apply to patients with ICH with newly diagnosed AF who accounted for 4% of overall ICH cases in our study over the more recent time period.
Patients with ICH and AF had worse functional and vital outcomes compared with patients with ICH without AF. This observation remained significant for both outcomes after adjustments for confounding factors age, sex, ICH location, time period of occurrence, vascular risk factors, and comorbidities. The apparent increased probability of death and higher mRS score associated with AF was no longer observed after a further adjustment for severity through NIHSS score about death outcome but OR for AF remained high even non‐significant. It was only after adjustment for premorbid OAC that association between AF and mRS score was no more significant. A similar result was observed in a hospital‐based stroke registry, 37 which suggests a pivotal role of OAC on poor prognosis after ICH, as suspected in other works. 19 , 23 , 24 , 25 , 26 , 27 Several studies demonstrated larger ICH volumes among patients with premorbid anticoagulation compared with patients without 32 , 38 , 39 supporting the causal impact of premorbid OAC use on ICH outcomes among patients with AF but not AF itself. Although we did not evaluate ICH volume in our study, the observed greater severity at onset in patients with ICH and AF could reflect such an association. Nevertheless, the fact that the NIHSS score was added to our multivariable models indicated that the deleterious effect of OAC on post‐ICH prognosis could be mediated by other factors. Among these, OAC therapy has been shown to represent an important contributor of hematoma expansion in patients with ICH, 40 which is associated with a worse neurological outcome in turn. 41 Taken together, these results underline the increasing need for developing therapeutic strategies aiming at reducing such a complication in anticoagulated patients with AF and ICH so as to improve their prognosis.
Our study has several strengths. The Dijon Stroke Registry exhaustively recorded all stroke cases, including ICH, in a population‐based setting, thus ruling out the bias of hospital‐based collection of cases, and with few missing data. Although the epidemiology of ICH in Dijon could differ from that observed in other areas, our study came from real life experience and findings could be useful for stroke clinicians by underlying potential targets so as to improve ICH management. In addition, the population‐based methodology makes it possible for future comparisons with other similar studies. The long duration of the study with constant procedures for case‐collection allowed analyzing temporal trends. Several limitations should be acknowledged. Despite a large sample size given the population‐based methodology, study power was not sufficient to perform stratified analyses according to ICH locations or types of OAC. This could be of interest considering the debate about potential differential outcomes in patients with ICH according to the type of OAC therapy. Indeed, several studies compared characteristics of patients with ICH receiving vitamin K antagonists to those on direct oral anticoagulants with conflicting findings. 26 , 27 , 42 , 43 A recent collaborative multicenter pooled analysis did not conclude to differences between patients with ICH on vitamin K antagonists and patients with ICH on direct oral anticoagulants with regard to baseline ICH volume, hematoma expansion, 90‐day mortality, and functional outcome. 27 Finally, the cross‐sectional design did not allow us to investigate causality between AF and ICH outcomes.
To conclude, the prevalence of AF reached 1 in 4 patients with ICH in the Dijon Stroke Registry for the 2012 to 2017 period. The observed increase in AF prevalence among patients with ICH over time partly reflected a rise in the prevalence of previously anticoagulated patients with AF. Premorbid use of anticoagulants was a major contributor of the poor outcome observed in patients with ICH and AF. With the ongoing aging population, and the expected increase in the burden of AF, our results highlight the urgent need for defining acute treatment and secondary prevention strategies after ICH in patients with AF.
Sources of Funding
This work was supported by the Santé Publique France (French Institute for Public Health Surveillance), Institut National de la Santé et de la Recherche Médicale, and University Hospital of Dijon.
Disclosures
Yannick Béjot reports personal fees from BMS, Pfizer, Medtronic, Amgen, Servier, and Boehringer‐Ingelheim, outside the submitted work. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Feigin VL, Krishnamurthi RV, Parmar P, Norrving BO, Mensah GA, Bennett DA, Barker‐Collo S, Moran AE, Sacco RL, Truelsen T, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. 2015;45:161–176. DOI: 10.1159/000441085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Béjot Y, Cordonnier C, Durier J, Aboa‐Eboulé C, Rouaud O, Giroud M. Intracerebral haemorrhage profiles are changing: results from the Dijon population‐based study. Brain. 2013;136:658–664. DOI: 10.1093/brain/aws349. [DOI] [PubMed] [Google Scholar]
- 3. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y‐H, McAnulty JH, Zheng Z‐J, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. DOI: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lovelock CE, Molyneux AJ, Rothwell PM. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population‐based study. Lancet Neurol. 2007;6:487–493. DOI: 10.1016/S1474-4422(07)70107-2. [DOI] [PubMed] [Google Scholar]
- 5. Feigin V, Norrving B, Sudlow CLM, Sacco RL. Updated criteria for population‐based stroke and transient ischemic attack incidence studies for the 21st century. Stroke. 2018;49:2248–2255. DOI: 10.1161/STROKEAHA.118.022161. [DOI] [PubMed] [Google Scholar]
- 6. Bennett DA, Brayne C, Feigin VL, Barker‐Collo S, Brainin M, Davis D, Gallo V, Jetté N, Karch A, Kurtzke JF, et al. Development of the standards of reporting of neurological disorders (STROND) checklist: a guideline for the reporting of incidence and prevalence studies in neuroepidemiology. Neurology. 2015;85:821–828. DOI: 10.1212/WNL.0000000000001866. [DOI] [PubMed] [Google Scholar]
- 7. Graber M, Garnier L, Mohr S, Delpont B, Blanc‐Labarre C, Vergely C, Giroud M, Bejot Y. Influence of pre‐existing mild cognitive impairment and dementia on post‐stroke mortality. The Dijon stroke registry. Neuroepidemiology. 2019;1–8. DOI: 10.1159/000497614. [DOI] [PubMed] [Google Scholar]
- 8. Giroud M, Delpont B, Daubail B, Blanc C, Durier J, Giroud M, Bejot Y. Temporal trends in sex differences with regard to stroke incidence: the Dijon stroke registry (1987–2012). Stroke. 2017;48:846–849. DOI: 10.1161/STROKEAHA.116.015913. [DOI] [PubMed] [Google Scholar]
- 9. The world health organization monica project (monitoring trends and determinants in cardiovascular disease): A major international collaboration . Who monica project principal investigators. J Clin Epidemiol. 1988;41:105–114. DOI: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 10. Bejot Y, Grelat M, Delpont B, Durier J, Rouaud O, Osseby GV, Hervieu‐Begue M, Giroud M, Cordonnier C. Temporal trends in early case‐fatality rates in patients with intracerebral hemorrhage. Neurology. 2017;88:985–990. DOI: 10.1212/WNL.0000000000003681. [DOI] [PubMed] [Google Scholar]
- 11. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. DOI: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 12. Williams LS, Yilmaz EY, Lopez‐Yunez AM. Retrospective assessment of initial stroke severity with the NIH stroke scale. Stroke. 2000;31:858–862. DOI: 10.1161/01.STR.31.4.858. [DOI] [PubMed] [Google Scholar]
- 13. Bhalla A, Wang Y, Rudd A, Wolfe CD. Differences in outcome and predictors between ischemic and intracerebral hemorrhage: the south London stroke register. Stroke. 2013;44:2174–2181. DOI: 10.1161/STROKEAHA.113.001263. [DOI] [PubMed] [Google Scholar]
- 14. James ML, Grau‐Sepulveda MV, Olson DM, Smith EE, Hernandez AF, Peterson ED, Schwamm LH, Bhatt DL, Fonarow GC. Insurance status and outcome after intracerebral hemorrhage: findings from get with the guidelines‐stroke. J Stroke Cerebrovasc Dis. 2014;23:283–292. DOI: 10.1016/j.jstrokecerebrovasdis.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 15. Palm F, Henschke N, Wolf J, Zimmer K, Safer A, Schröder RJ, Inselmann G, Brenke C, Becher H, Grau AJ. Intracerebral haemorrhage in a population‐based stroke registry (LuSSt): incidence, aetiology, functional outcome and mortality. J Neurol. 2013;260:2541–2550. DOI: 10.1007/s00415-013-7013-0. [DOI] [PubMed] [Google Scholar]
- 16. Zaganas I, Halpin AP, Oleinik A, Alegakis A, Kotzamani D, Zafiris S, Chlapoutaki C, Tsimoulis D, Giannakoudakis E, Chochlidakis N, et al. A comparison of acute hemorrhagic stroke outcomes in 2 populations: the Crete‐Boston study. Stroke. 2011;42:3640–3642. DOI: 10.1161/STROKEAHA.111.632174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiu D, Peterson L, Elkind MSV, Rosand J, Gerber LM, Silverstein MD. Comparison of outcomes after intracerebral hemorrhage and ischemic stroke. J Stroke Cerebrovasc Dis. 2010;19:225–229. DOI: 10.1016/j.jstrokecerebrovasdis.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 18. Leker RR, Khoury ST, Rafaeli G, Shwartz R, Eichel R, Tanne D. Prior use of statins improves outcome in patients with intracerebral hemorrhage: prospective data from the National Acute Stroke Israeli Surveys (NASIS). Stroke. 2009;40:2581–2584. DOI: 10.1161/STROKEAHA.108.546259. [DOI] [PubMed] [Google Scholar]
- 19. Mustanoja S, Strbian D, Putaala J, Meretoja A, Curtze S, Haapaniemi E, Sairanen T, Hietikko R, Siren J, Kaste M, et al. Association of prestroke statin use and lipid levels with outcome of intracerebral hemorrhage. Stroke. 2013;44:2330–2332. DOI: 10.1161/STROKEAHA.113.001829. [DOI] [PubMed] [Google Scholar]
- 20. Zhou J, Zhang Y, Arima H, Zhao Y, Zhao H, Zheng D, Tian Y, Liu Y, Huang Q, Yang J. Sex differences in clinical characteristics and outcomes after intracerebral haemorrhage: results from a 12‐month prospective stroke registry in Nanjing, china. BMC neurology. 2014;14:172. DOI: 10.1186/s12883-014-0172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen YW, Li CH, Yang CD, Liu CH, Chen CH, Sheu JJ, Lin SK, Chen AC, Chen PK, Chen PL, et al. Low cholesterol level associated with severity and outcome of spontaneous intracerebral hemorrhage: results from Taiwan stroke registry. PLoS One. 2017;12:e0171379. DOI: 10.1371/journal.pone.0171379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christensen MC, Broderick J, Vincent C, Morris S, Steiner T. Global differences in patient characteristics, case management and outcomes in intracerebral hemorrhage: the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Cerebrovasc Dis (Basel, Switzerland). 2009;28:55–64. DOI: 10.1159/000219298. [DOI] [PubMed] [Google Scholar]
- 23. Allan V, Honarbakhsh S, Casas JP, Wallace J, Hunter R, Schilling R, Perel P, Morley K, Banerjee A, Hemingway H. Are cardiovascular risk factors also associated with the incidence of atrial fibrillation? A systematic review and field synopsis of 23 factors in 32 population‐based cohorts of 20 million participants. Thromb Haemost. 2017;117:837–850. DOI: 10.1160/TH16-11-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens ACJW, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e000102. DOI: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roh D, Boehme A, Young C, Roth W, Gutierrez J, Flaherty M, Rosand J, Testai F, Woo D, Elkind MSV. Hematoma expansion is more frequent in deep than lobar intracerebral hemorrhage. Neurology. 2020;95:e3386–e3393. DOI: 10.1212/WNL.0000000000010990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilson D, Charidimou A, Shakeshaft C, Ambler G, White M, Cohen H, Yousry T, Al‐Shahi Salman R, Lip GYH, Brown MM, et al. Volume and functional outcome of intracerebral hemorrhage according to oral anticoagulant type. Neurology. 2016;86:360–366. DOI: 10.1212/WNL.0000000000002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson D, Seiffge DJ, Traenka C, Basir G, Purrucker JC, Rizos T, Sobowale OA, Sallinen H, Yeh S‐J, Wu TY, et al. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology. 2017;88:1693–1700. DOI: 10.1212/WNL.0000000000003886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Overvad TF, Andersen SD, Larsen TB, Lip GYH, Søgaard M, Skjøth F, Nielsen PB. Incidence and prognostic factors for recurrence of intracerebral hemorrhage in patients with and without atrial fibrillation: a cohort study. Thromb Res. 2020;191:1–8. DOI: 10.1016/j.thromres.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 29. Horstmann S, Rizos T, Jenetzky E, Gumbinger C, Hacke W, Veltkamp R. Prevalence of atrial fibrillation in intracerebral hemorrhage. Eur J Neurol. 2014;21:570–576. DOI: 10.1111/ene.12215. [DOI] [PubMed] [Google Scholar]
- 30. Roquer J, Rodríguez‐Campello A, Jiménez‐Conde J, Cuadrado‐Godia E, Giralt‐Steinhauer E, Vivanco Hidalgo RM, Soriano C, Ois A. Sex‐related differences in primary intracerebral hemorrhage. Neurology. 2016;87:257–262. DOI: 10.1212/WNL.0000000000002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prats‐Sánchez L, Guisado‐Alonso D, Painous C, Fayos F, Pascual‐Goñi E, Delgado‐Mederos R, Martínez‐Domeño A, Camps‐Renom P, Martí‐Fàbregas J. Insular damage, new‐onset atrial fibrillation and outcome after acute intracerebral hemorrhage. Eur J Neurol. 2018;25:491–496. DOI: 10.1111/ene.13522. [DOI] [PubMed] [Google Scholar]
- 32. Dequatre‐Ponchelle N, Hénon H, Pasquini M, Rutgers MP, Bordet R, Leys D, Cordonnier C. Vitamin K antagonists–associated cerebral hemorrhages. Stroke. 2013;44:350–355. DOI: 10.1161/STROKEAHA.112.672303. [DOI] [PubMed] [Google Scholar]
- 33. Casolla B, Moulin S, Kyheng M, Hénon H, Labreuche J, Leys D, Bauters C, Cordonnier C. Five‐year risk of major ischemic and hemorrhagic events after intracerebral hemorrhage. Stroke. 2019;50:1100–1107. DOI: 10.1161/STROKEAHA.118.024449. [DOI] [PubMed] [Google Scholar]
- 34. Biffi A, Kuramatsu JB, Leasure A, Kamel H, Kourkoulis C, Schwab K, Ayres AM, Elm J, Gurol ME, Greenberg SM, et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017;82:755–765. DOI: 10.1002/ana.25079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Nieuwenhuizen KM, van der Worp HB, Algra A, Kappelle LJ, Rinkel GJ, van Gelder IC, Schutgens RE, Klijn CJ. Apixaban versus antiplatelet drugs or no antithrombotic drugs after anticoagulation‐associated intracerebral haemorrhage in patients with atrial fibrillation (APACHE‐AF): study protocol for a randomised controlled trial. Trials. 2015;16:393. DOI: 10.1186/s13063-015-0898-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. COCROACH . The University of Edinburgh. https://www.ed.ac.uk/clinicalbrain‐sciences/research/so‐start/for‐collaborators. Accessed August 15.
- 37. Roquer J, Vivanco‐Hidalgo RM, Prats‐Sánchez LL, Martínez‐Domeño A, Guisado‐Alonso D, Cuadrado‐Godia E, Giralt Steinhauer E, Jiménez‐Conde J, Rodríguez‐Campello A, Martí‐Fàbregas J, et al. Interaction of atrial fibrillation and antithrombotics on outcome in intracerebral hemorrhage. Neurology. 2019;93:e1820–e1829. DOI: 10.1212/WNL.0000000000008462. [DOI] [PubMed] [Google Scholar]
- 38. Flaherty ML, Tao H, Haverbusch M, Sekar P, Kleindorfer D, Kissela B, Khatri P, Stettler B, Adeoye O, Moomaw CJ, et al. Warfarin use leads to larger intracerebral hematomas. Neurology. 2008;71:1084–1089. DOI: 10.1212/01.wnl.0000326895.58992.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cucchiara B, Messe S, Sansing L, Kasner S, Lyden P. Hematoma growth in oral anticoagulant related intracerebral hemorrhage. Stroke. 2008;39:2993–2996. DOI: 10.1161/STROKEAHA.108.520668. [DOI] [PubMed] [Google Scholar]
- 40. Al‐Shahi Salman R, Frantzias J, Lee RJ, Lyden PD, Battey TWK, Ayres AM, Goldstein JN, Mayer SA, Steiner T, Wang X, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta‐analysis of individual patient data. Lancet Neurol. 2018;17:885–894. DOI: 10.1016/S1474-4422(18)30253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leira R, Dávalos A, Silva Y, Gil‐Peralta A, Tejada J, Garcia M, Castillo J. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–467. DOI: 10.1212/01.WNL.0000133204.81153.AC. [DOI] [PubMed] [Google Scholar]
- 42. Inohara T, Xian Y, Liang LI, Matsouaka RA, Saver JL, Smith EE, Schwamm LH, Reeves MJ, Hernandez AF, Bhatt DL, et al. Association of intracerebral hemorrhage among patients taking non‐vitamin K antagonist vs vitamin K antagonist oral anticoagulants with in‐hospital mortality. JAMA. 2018;319:463–473. DOI: 10.1001/jama.2017.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Purrucker JC, Haas K, Rizos T, Khan S, Wolf M, Hennerici MG, Poli S, Kleinschnitz C, Steiner T, Heuschmann PU, et al. Early clinical and radiological course, management, and outcome of intracerebral hemorrhage related to new oral anticoagulants. JAMA Neurology. 2016;73:169–177. DOI: 10.1001/jamaneurol.2015.3682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized grouped data can be shared by request from a qualified investigator.


