Abstract
Background
In the United States, large disparities in cardiovascular health (CVH) exist in the general population, but little is known about the CVH status and its disparities among women of childbearing age (ie, 18–49 years).
Methods and Results
In this cross‐sectional study, we examined racial, ethnic, and geographic disparities in CVH among all women of childbearing age in the United States, using the 2011 to 2019 Behavioral Risk Factor Surveillance System. Life's Simple 7 (ie, blood pressure, glucose, total cholesterol, smoking, body mass index, physical activity, and diet) was used to examine CVH. Women with 7 ideal CVH metrics were determined to have ideal CVH. Among the 269 564 women of childbearing age, 13 800 (4.84%) had ideal CVH. After adjusting for potential confounders, non‐Hispanic Black women were less likely to have ideal CVH (odds ratio, 0.54; 95% CI, 0.46–0.63) compared with non‐Hispanic White women, and with significantly lower odds of having ideal metrics of blood pressure, blood glucose, body mass index, and physical activity. No significant difference in CVH was found between non‐Hispanic White and Hispanic women. Large geographic disparities with temporal variations were observed, with the age‐ and race‐adjusted ideal CVH prevalence ranging from 4.05% in the District of Columbia (2011) to 5.55% in Maine and Montana (2019). States with low ideal CVH prevalence and average CVH score were mostly clustered in the southern United States.
Conclusions
Large racial, ethnic, and geographic disparities in CVH exist among women of childbearing age. More efforts are warranted to understand and address these disparities.
Keywords: cardiovascular health, geographic disparities, racial disparities
Subject Categories: Women, Epidemiology, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- Add Health
the National Longitudinal Study of Adolescent to Adult Health
- BRFSS
the Behavioral Risk Factor Surveillance System
- CVH
cardiovascular health
- LS7
Life's Simple 7
Clinical Perspective
What Is New?
This is the first study examining racial, ethnic, and geographic disparities in cardiovascular health among women of childbearing age using a nationally representative sample.
Large geographic disparities with temporal variations in cardiovascular health exist among women of childbearing age.
Non‐Hispanic Black women have worse cardiovascular health compared with non‐Hispanic White women.
What Are the Clinical Implications?
More efforts are warranted to address these disparities to achieve and maintain ideal cardiovascular health among women of childbearing age.
Cardiovascular disease (CVD), the leading cause of death worldwide, contributed to ≈1 in every 5 deaths among women in the United States in 2017. 1 Conventional prevention strategies for CVD mainly focus on optimizing classical risk factors (eg, hypertension and diabetes mellitus). However, it is challenging to communicate a low absolute 10‐year CVD risk with young people efficiently. Life's Simple 7 (LS7) was introduced by the American Heart Association to assess cardiovascular health (CVH), 2 which reframed CVD prevention focus from disease to health. LS7 includes 7 metrics: 3 health factors (blood pressure, total cholesterol, and glucose) and 4 behavioral factors (body mass index [BMI], cigarette smoking, diet, and physical activity). Prior studies have shown that ideal CVH is not only associated with lower risks of subsequent CVD, 3 , 4 but also other health outcomes such as cancer, 5 cognitive impairment, 6 and depression. 7
In 2016, the American Heart Association initiated a new program, One Brave Idea, 8 with a goal to end coronary heart disease and its consequences. More recently, an interim target for the CVD endgame strategy named “50×50×50” was proposed, targeting on “≥50% segments of the population ≤50 years old by 2050 or sooner.” 9 At present, the prevalence of ideal CVH among the US population is estimated to be 50% at 10 years old and declines to <10% by 50 years old. 10 , 11 Thus, it is essential to decelerate loss of ideal CVH at earlier ages to achieve these goals. Compared with men, women of childbearing age experience events known to affect the risk of CVD, including pregnancy, 12 breastfeeding, 13 and menopause. 14 Furthermore, among women with children, a number of prepregnancy health conditions such as hypertension, diabetes mellitus, and obesity have been associated with not only women's future CVH, 15 , 16 but also the health of their children. 17 , 18 Therefore, achieving and maintaining ideal CVH among women of childbearing age is of critical value in achieving the American Heart Association's target of improving the population CVH.
Large racial, ethnic, and geographic disparities in CVH among the general population in the United States have been widely reported. 19 , 20 Previous studies observed larger racial and ethnic disparities in CVD‐related outcomes and important risk factors among women compared with men. 21 , 22 Meanwhile, emerging evidence suggested that efforts should be made throughout young adulthood to achieve better CVH. 23 , 24 Recently, Perak et al 25 found significant racial and ethnic disparities in CVH among pregnant women and several other studies reported disparities in hypertension and diabetes mellitus among women of childbearing age. 26 , 27 To our knowledge, disparities in CVH among women, particularly those of childbearing age, have not been systematically examined. Using data from the 2011 to 2019 Behavioral Risk Factor Surveillance System (BRFSS), we aim to examine both the racial, ethnic, and geographic disparities in CVH among women of childbearing age.
Methods
Study Population
We used data from a nationally representative survey, the BRFSS, which are publicly available through the Centers for Disease Control and Prevention at https://www.cdc.gov/brfss/index.html. Informed consent was obtained from the participants by the BRFSS. Codes will be made freely available to those who request it. BRFSS is conducted annually using a multistage sampling design with random digit dialing, collecting data on risk health behaviors, chronic health status, and healthcare services use from US residents at the state and local levels. We used data from 2011 to 2019 (ie, 2011, 2013, 2015, 2017, and 2019) since data before 2011 used a different weighting method and information on all 7 CVH metrics were available only in odd years. Among 1 332 097 women in the BRFSS 2011 to 2019, a total of 269 564 women of childbearing age (ie, 18–49 years) 28 were identified after excluding those who were enrolled in Guam, Puerto Rico, and Virgin Islands (n=23 116), women over 49 years old (n=883 089), or those with missing information on race and ethnicity (n=5227) or any of the 7 CVH metrics (n=151 101). A flowchart of the inclusion and exclusion criteria is provided in Figure S1.
Assessment of CVH
We categorized each CVH metric as ideal (1 point) or nonideal (0 point) on the basis of the American Heart Association criteria of the LS7, 2 and an overall CVH score ranging from 0 to 7 was calculated, with a higher CVH score indicating better CVH. 20 , 29 Women with a CVH score of 7 were determined to have ideal CVH. 29 Table S1 shows the detailed criteria. Specifically, participants were asked separately whether they have ever been told by a doctor or other health professional that they have high blood pressure, diabetes mellitus, or high cholesterol, and those who answered “No” to each question were defined as having ideal condition in the corresponding metric. BMI was calculated using self‐reported height (meter) and weight (kilogram), and a value between 18.5 and 25 was considered as ideal. Ideal smoking status was defined as having smoked a lifetime total of <100 cigarettes or smoked at least 100 cigarettes but currently not smoking at all. According to self‐reported frequency and duration of moderate and vigorous activities, total minutes of physical activity per week were calculated. Participants were considered to be physically active if they met the recommendations (≥150 minutes of moderate activity, ≥75 minutes of vigorous activity, or ≥150 minutes of the combination of both activities per week). Participants were asked several separate questions on how often (ie, times per day/week/month) they drink or eat different types of food (ie, fruit juices, fruits, dark green vegetables, potatoes/beans, orange‐colored vegetables, and other vegetables in the 2011 to 2015 surveys, and fruits, juices, dark green vegetables, French fries, potatoes, and other vegetables in the 2017 and 2019 surveys), and average daily consumption of fruits and vegetables was calculated on the basis of their answers to these questions. Ideal diet was assigned if a participant reported ≥5 servings of daily fruit and vegetable consumption.
Assessment of Racial and Ethnicity
Race and ethnicity were obtained on the basis of self‐reports and categorized into non‐Hispanic White, non‐Hispanic Black, Hispanic, and others (including multiracial individuals).
Covariates
Participants' sociodemographic status were obtained based on self‐reported questionnaires, including age (18–24, 25–34, 35–44, and 45–49 years old), education (less than high school, high school or equivalent, some college, and college/graduate or above), marital status (never married, married or living with partner, and previously married), annual household income (<25 000, 25 000–50 000, and ≥50 000), pregnancy status (current, never, and ever) and history of CVD (a dichotomous variable indicating previous diagnosis of heart attack, stroke, angina, or coronary heart disease). Missing values were coded as an additional category for each covariate.
Statistical Analysis
Descriptive analyses were performed to examine the distributions of ideal CVH and CVH score by covariates. Weighted logistic and linear regression models were fitted to examine racial and ethnic disparities in ideal CVH, individual CVH metrics, and CVH score. Three sets of models were used, including a set of unadjusted models; a set of models crudely adjusted for age; and a set of models additionally adjusted for education, annual household income, marital status, history of CVD, pregnancy status, and survey year. Odds ratios or beta coefficients with 95% CIs were calculated. To examine geographic disparities in ideal CVH, we calculated age‐ and race‐adjusted prevalence of ideal CVH and average CVH score at the state level. Furthermore, the SaTScan software was employed to identify statistically significant spatial clusters using the rsatscan package. 30 Both ideal CVH prevalence and CVH score were categorized into quintiles in the maps. In addition, we assessed the potential temporal variations in geographic disparities. All analyses were conducted using the “survey” package in R 3.5.1 after accounting for the complex survey design.
This study was approved by the Institutional Review Board at the University of Florida (IRB201903278).
Results
Table 1 shows the sociodemographic characteristics of the study sample by CVH status and the corresponding CVH scores. Among the 269 564 women of childbearing age, a total of 13 800 (4.84%) women had ideal CVH. The mean CVH score was 4.53 (95% CI, 4.52–5.54). Compared with women with ideal CVH status, those who had nonideal CVH were more likely to be 45 to 49 years old, non‐Hispanic Black or Hispanic, previously married or never married, of lower education levels, of lower income, or have a history of CVD, confirming known risk factors. Consistently, lowest CVH scores were also seen among similar subgroups of women.
Table 1.
Sociodemographic Characteristics by CVH Status and Distribution of CVH Score Among Women of Childbearing Age, BRFSS 2011 to 2019 (n=269 564)
| Characteristics | Ideal CVH | Nonideal CVH | Total | CVH Score |
|---|---|---|---|---|
|
No. % (95% CI)* |
No. % (95% CI)* |
No. % (95% CI)* |
Mean (95% CI) † |
|
| Overall | 13 800 | 255 764 | 269 564 | 4.53 |
| 4.8 (4.7–5.0) | 95.2 (95.0–95.3) | 100 | (4.52–4.54) | |
| Age, y | ||||
| 18–24 | 1345 | 23 784 | 25 129 | 4.94 |
| 18.7 (17.2–20.2) | 16.8 (16.5–17.1) | 16.9 (16.6–17.2) | (4.92–4.96) | |
| 25–34 | 3687 | 67 097 | 70 784 | 4.65 |
| 30.9 (29.3–32.5) | 30.7 (30.4–31.1) | 30.7 (30.4–31.1) | (4.63–4.66) | |
| 35–44 | 5606 | 100 295 | 105 901 | 4.42 |
| 35.2 (33.6–36.7) | 35.0 (34.6–35.3) | 35.0 (34.6–35.3) | (4.40–4.44) | |
| 45–49 | 3162 | 64 588 | 67 750 | 4.16 |
| 15.2 (14.2–16.2) | 17.5 (17.3–17.8) | 17.4 (17.2–17.7) | (4.14–4.19) | |
| Race/Ethnicity | ||||
| Non‐Hispanic White | 11 123 | 180 466 | 191 589 | 4.59 |
| 67.5 (65.8–69.3) | 58.7 (58.3–59.1) | 59.1 (58.8–59.5) | (4.58–4.60) | |
| Non‐Hispanic Black | 552 | 27 186 | 27 738 | 4.19 |
| 6.6 (5.7–7.5) | 13.7 (13.4–13.9) | 13.3 (13.1–13.6) | (4.16–4.21) | |
| Hispanic | 1092 | 27 787 | 28 879 | 4.48 |
| 14.6 (13.2–16.1) | 18.3 (18.0–18.6) | 18.1 (17.8–18.5) | (4.45–4.50) | |
| Other ‡ race/ethnicity | 1033 | 20 325 | 21 358 | 4.78 |
| 11.2 (9.8–12.6) | 9.3 (9.0–9.5) | 9.4 (9.1–9.6) | (4.74–4.81) | |
| Education | ||||
| Less than high school | 185 | 13 153 | 13 338 | 3.94 |
| 3.2 (2.5–3.9) | 10.0 (9.7–10.3) | 9.7 (9.4–9.9) | (3.90–3.98) | |
| High school | 1260 | 51 526 | 52 786 | 4.27 |
| 12.5 (11.2–13.7) | 22.4 (22.1–22.7) | 21.9 (21.6–22.2) | (4.25–4.29) | |
| Some college or associate of arts degree | 2888 | 75 687 | 78 575 | 4.49 |
| 30.7 (29.0–32.5) | 33.5 (33.2–33.9) | 33.4 (33.0–33.7) | (4.47–4.51) | |
| College graduate or above | 9452 | 115 213 | 124 665 | 4.9 |
| 53.5 (51.8–55.2) | 33.9 (33.6–34.3) | 34.9 (34.6–35.2) | (4.89–4.92) | |
| Missing | 15 | 185 | 200 | 4.33 |
| 0.1 (0.0–0.2) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | (3.92–4.75) | |
| Annual household income | ||||
| <$25 000 | 1291 | 55 478 | 56 769 | 4.1 |
| 12.5 (11.3–13.7) | 24.5 (24.2–24.8) | 23.9 (23.6–24.3) | (4.08–4.12) | |
| $25 000–$50 000 | 1905 | 51 823 | 53 728 | 4.42 |
| 15.0 (13.8–16.3) | 19.9 (19.6–20.2) | 19.7 (19.4–20.0) | (4.40–4.45) | |
| ≥%50 000 | 9468 | 126 492 | 135 960 | 4.79 |
| 62.1 (60.4–63.7) | 45.2 (44.9–45.6) | 46.1 (45.7–46.4) | (4.78–4.80) | |
| Missing | 1136 | 21 971 | 23 107 | 4.6 |
| 10.4 (9.3–11.5) | 10.3 (10.1–10.5) | 10.3 (10.1–10.6) | (4.57–4.63) | |
| Marital status | ||||
| Married/living with partner | 10 007 | 155 933 | 165 940 | 4.59 |
| 64.8 (63.1–66.5) | 56.7 (56.3–57.1) | 57.1 (56.7–57.5) | (4.58–4.60) | |
| Previously married | 1308 | 40 690 | 41 998 | 4.07 |
| 8.0 (7.1–8.9) | 12.8 (12.5–13.0) | 12.5 (12.3–12.7) | (4.04–4.10) | |
| Never married | 2456 | 58 594 | 61 050 | 4.61 |
| 27.0 (25.3–28.6) | 30.3 (29.9–30.7) | 30.1 (29.8–30.5) | (4.60–4.63) | |
| Missing | 29 | 547 | 576 | 4.55 |
| 0.2 (0.1–0.3) | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | (4.30–4.81) | |
| History of CVD | ||||
| No. | 13 669 | 247 411 | 261 080 | 4.57 |
| 99.3 (99.1–99.5) | 97.0 (96.9–97.1) | 97.1 (97.0–97.2) | (4.56–4.58) | |
| Yes | 115 | 7434 | 7549 | 3.32 |
| 0.6 (0.4–0.8) | 2.6 (2.5–2.7) | 2.5 (2.4–2.6) | (3.26–3.38) | |
| Missing | 16 | 919 | 935 | 3.44 |
| 0.1 (0.0–0.2) | 0.4 (0.3–0.4) | 0.3 (0.3–0.4) | (3.16–3.72) | |
| Pregnancy status | ||||
| Currently pregnant | 447 | 7121 | 7568 | 4.72 |
| 3.2 (2.7–3.6) | 3.3 (3.1–3.4) | 3.2 (3.1–3.4) | (4.67–4.76) | |
| Ever pregnant | 4208 | 73 995 | 78 203 | 4.49 |
| 25.2 (23.8–26.5) | 26.5 (26.2–26.8) | 26.4 (26.1–26.7) | (4.47–4.51) | |
| Never pregnant | 7492 | 140 548 | 148 040 | 4.6 |
| 64.1 (62.6–65.6) | 61.8 (61.5–62.2) | 61.9 (61.6–62.3) | (4.59–4.61) | |
| Missing | 1653 | 34 100 | 35 753 | 4.11 |
| 7.6 (6.9–8.3) | 8.5 (8.3–8.6) | 8.4 (8.2–8.6) | (4.08–4.14) | |
| Year | ||||
| 2011 | 3684 | 60 342 | 64 026 | 4.51 |
| 20.7 (19.5–21.9) | 18.8 (18.5–19.0) | 18.9 (18.6–19.1) | (4.49–4.53) | |
| 2013 | 3038 | 53 993 | 57 031 | 4.49 |
| 19.0 (17.7–20.2) | 18.4 (18.1–18.6) | 18.4 (18.1–18.7) | (4.47–4.51) | |
| 2015 | 2543 | 42 898 | 45 441 | 4.55 |
| 19.9 (18.6–21.2) | 17.7 (17.5–18.0) | 17.8 (17.6–18.1) | (4.53–4.57) | |
| 2017 | 2621 | 52 455 | 55 076 | 4.57 |
| 22.1 (20.6–23.6) | 23.1 (22.8–23.4) | 23.0 (22.7–23.4) | (4.55–4.59) | |
| 2019 | 1914 | 46 076 | 47 990 | 4.52 |
| 18.3 (16.8–19.8) | 22.0 (21.7–22.4) | 21.9 (21.5–22.2) | (4.50–4.54) | |
BRFSS indicates the Behavioral Risk Factor Surveillance System; CVD, cardiovascular diseases; CVH, cardiovascular health.
Number of participants and weighted percentage with 95% CI.
Weighted average CVH score with 95% CI.
Other includes American Indian or Alaska Native, Asian, and Pacific Islander.
Table 2 shows the racial and ethnic disparities in CVH among women of childbearing age. Racial and ethnic disparities were observed for both the presence of ideal CVH and CVH scores. In the crude and age‐adjusted models, non‐Hispanic Black and Hispanic women demonstrated significantly lower odds of ideal CVH and lower CVH scores compared with non‐Hispanic White women. After further controlling for education, income, marital status, history of CVD, pregnancy status, and survey year, significantly lower odds of ideal CVH were still observed in non‐Hispanic Black women (odds ratio, 0.54; 95% CI, 0.46–0.63) but not in Hispanic women. Similarly in the fully adjusted model, non‐Hispanic Black race was associated with a 0.22‐point lower CVH score (95% CI, −0.25 to −0.20), while Hispanic race (β coefficient, 0.13; 95% CI, 0.11–0.16) and other races/ethnicities (β coefficient, 0.12; 95% CI, 0.08–0.16) were associated a higher CVH score, as compared with those who are non‐Hispanic White. Table S2 shows the racial and ethnic disparities in each of the individual CVH metrics.
Table 2.
Racial Disparities in CVH Among Women of Childbearing Age, BRFSS 2011 to 2019 (n=269 564)
| CVH status | Unadjusted | Age‐Adjusted | Fully Adjusted* |
|---|---|---|---|
| Odds Ratio (95% CI) † | |||
| Ideal CVH | |||
| Non‐Hispanic White | Reference | Reference | Reference |
| Non‐Hispanic Black | 0.42 (0.36 to 0.49) | 0.42 (0.36 to 0.48) | 0.54 (0.46 to 0.63) |
| Hispanic | 0.70 (0.62 to 0.78) | 0.68 (0.61 to 0.77) | 1.03 (0.91 to 1.16) |
| Other race/ethnicity | 1.05 (0.91 to 1.22) | 1.04 (0.90 to 1.20) | 1.00 (0.86 to 1.16) |
| β Coefficient (95% CI) † | |||
|---|---|---|---|
| CVH score | |||
| Non‐Hispanic White | Reference | Reference | Reference |
| Non‐Hispanic Black | −0.40 (−0.43 to −0.37) | −0.43 (−0.45 to −0.40) | −0.22 (−0.25 to −0.20) |
| Hispanic | −0.11 (−0.14 to −0.08) | −0.16 (−0.19 to −0.13) | 0.13 (0.11 to 0.16) |
| Other ‡ race/ethnicity | 0.19 (0.15 to 0.23) | 0.14 (0.11 to 0.18) | 0.12 (0.08 to 0.16) |
BRFSS indicates the Behavioral Risk Factor Surveillance System; CVD, cardiovascular disease; and CVH, cardiovascular health.
Models adjusted for age, education, income, marital status, history of CVD, pregnancy status, and year.
Weighted odds ratio and β coefficient with 95% CI. ‡ Other includes American Indian or Alaska Native, Asian, and Pacific Islander.
Other includes American Indian or Alaska Native, Asian, and Pacific Islander.
Figure shows the overall age‐ and race‐adjusted prevalence of ideal CVH and CVH scores by state. Geographic disparities in ideal CVH were observed among women of childbearing age, with the prevalence of ideal CVH ranging from 4.16% to 5.50%. Overall, the lowest ideal CVH prevalence was observed in Mississippi (4.16%), followed by the District of Columbia (4.27%) and Georgia (4.34%), while the highest ideal CVH prevalence was found in Montana (5.50%), followed by a prevalence of 5.49% in Vermont, South Dakota, and Maine. A total of 19 states had an ideal CVH prevalence below the average prevalence of 4.84%. Five statistically significant spatial clusters of low ideal CVH prevalence were observed, locating in the Southwest, South, Midwest, and Mid‐Atlantic, with the largest cluster located in the southern United States. The average CVH scores ranged from 4.43 (Mississippi) to 4.66 (Hawaii), and 14 states showed a CVH score below the average value of 4.53. Consistently, 3 significant spatial clusters of low CVH scores were identified in the same regions, with the largest cluster located in the South, Midwest, and Mid‐Atlantic.
Figure 1. Age‐ and race‐adjusted and weighted prevalence of ideal cardiovascular health (CVH) and CVH score among women of childbearing age, BRFSS 2011 to 2019 (n=269 564).
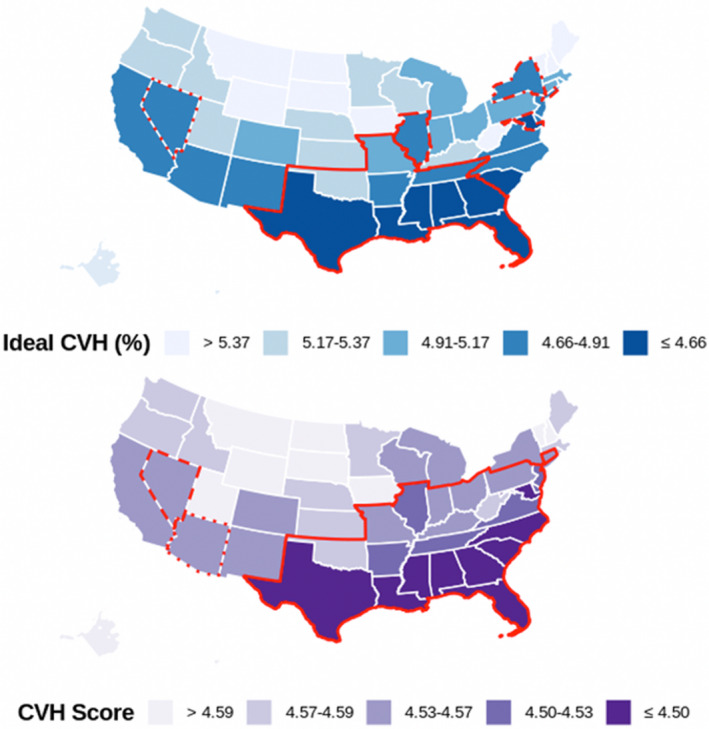
Large geographic disparities with significant temporal variations in CVH among women of childbearing age were observed (Tables S3 and S4), with the age‐ and race‐adjusted ideal CVH prevalence ranging from 4.05% in the District of Columbia (2011) to 5.55% in Maine and Montana (2019). Consistent with the overall patterns, the largest spatial clusters of both low ideal CVH prevalence and low average CVH scores were identified in the South, Midwest, and Mid‐Atlantic across the years (Figures S2 and S3). Table S5 shows the age‐ and race‐adjusted prevalence with 95% CI of ideal status on individual CVH metrics by state, and Figure S4 shows the spatial clusters of each individual CVH metric.
Discussion
To our knowledge, this is the first study examining both racial, ethnic, and geographic disparities in CVH among women of childbearing age using a nationally representative sample. Using the BRFSS 2011 to 2019, we observed large disparities in CVH among women of childbearing age. Non‐Hispanic Black women have worse CVH compared with non‐Hispanic White women, and are especially disadvantaged in maintaining ideal status on blood pressure, glucose, BMI, and physical activity. No significant difference in overall CVH were found between non‐Hispanic White and Hispanic women, despite significant differences in individual CVH metrics such as glucose, BMI, and physical activity. In addition, we also observed large geographic disparities with temporal variations. To note, states with worse CVH were mainly clustered in the South, especially in the historical “Stroke Belt.” 31 Compared with findings in the general population, 29 women of childbearing age had a slightly higher overall CVH score.
The majorities of studies examining racial and ethnic disparities in CVH among childbearing‐aged women focused only on 1 or 2 CVH metrics. Britton et al 27 found large racial and ethnic disparities in dysglycemia among women of reproductive age using data from the Add Health (National Longitudinal Study of Adolescent to Adult Health) study, where non‐Hispanic Black women had the highest prevalence of both diabetes mellitus and prediabetes (15.0% and 38.5%, respectively) compared with other races/ethnicities (4.5%–7.5% and 16.6%–27.8%). Another study using the 1999 to 2008 National Health and Nutrition Examination Survey also suggested that the dysglycemia prevalence was roughly twice as high in both non‐Hispanic Black and Mexican American women of childbearing age with BMI <25 kg/m2 compared with non‐Hispanic White women. 32 Similar results were also observed for comorbid hypertension and diabetes mellitus, 26 overweight, and obesity, 33 where non‐Hispanic Black women of childbearing age were more likely to have unfavorable status compared with women of other races/ethnicities. Consistent with previous findings, our study also found that non‐Hispanic Black women of childbearing age are less likely to have ideal status on blood pressure, blood glucose, and BMI. These 3 factors along with physical activity are likely to be the main contributors to the racial and ethnic disparities among non‐Hispanic Black women in overall CVH. Although no significant racial and ethnic disparities in overall CVH were found for Hispanic women, our study showed that Hispanic women of childbearing age were less likely to have ideal status on glucose, BMI, and physical activity. Disparities in these individual CVH metrics were also found in the general population. For example, using the 1999 to 2012 National Health and Nutrition Examination Survey, Pool et al 19 found that non‐Hispanic Black women are less likely to have ideal status on blood pressure, fasting glucose, BMI, and physical activity than non‐Hispanic White women, while Mexican American women had worse status on glucose, BMI, and physical activity.
Notably, compared with non‐Hispanic White women, non‐Hispanic Black and Hispanic women were found to have higher odds of ideal smoking status and ideal diet. Similar results on smoking status were also observed in previous studies. 19 , 34 Non‐Hispanic Black and Mexican American women generally had higher scores for smoking status than non‐Hispanic White women, but there was no significant difference in diet across the 3 racial and ethnic groups. One possible explanation for the inconsistent results on diet could be the different measurement methods.
We observed large racial and ethnic disparities in overall CVH among women of childbearing age, which are consistent with previous studies focusing on the general population. 3 , 10 , 19 , 35 For instance, Shay et al 10 found that in the general population, non‐Hispanic Black and Hispanic women are less likely to have ideal CVH compared with non‐Hispanic White women. Similarly, Pool et al 19 found significantly lower CVH scores among non‐Hispanic Black (difference=0.93) and Hispanic women (difference=0.71) compared with non‐Hispanic White women in the 2011 to 2012 National Health and Nutrition Examination Survey. In the present study, lower odds of ideal CVH and significantly lower CVH scores were also observed for non‐Hispanic Black women in comparison with non‐Hispanic White women. However, we did not find significant disparities in overall CVH among Hispanic women of childbearing age. Furthermore, Hispanic race was found to be associated with a slightly increased CVH score. This indicates that the disparities among Hispanic women may be largely explained by socioeconomic factors, including household income and education.
Similar to the large geographic disparities in CVH observed in the general population, 29 , 36 we also found geographic disparities among women of childbearing age, with worse CVH observed generally in the southern United States. Fang et al 29 found large geographic variations in the prevalence of ideal CVH in the general population, ranging from 1.2% in Oklahoma to 6.9% in the District of Columbia in 2009. Substantial differences in CVH status by state were also reported by Gebreab et al, 36 who found that the prevalence of poor CVH was the highest in Louisiana (17.2%) and lowest in Colorado (6.0%) in 2011. However, these findings are inconsistent with our results since previous studies used 1‐year data from the BRFSS, and the prevalence they reported was adjusted by age only. In this study, we leveraged data during 2011 to 2019 and found that the overall age‐ and race‐adjusted ideal CVH prevalence ranges from 4.16% in Mississippi to 5.50% in Maine among women of childbearing age, with majority of the states showing significant temporal variations in average CVH score over the study period.
This study has several strengths. First, we used multiyear data from a nationally representative survey to ensure the generalizability of the findings. Second, both racial, ethnic, and geographic disparities in CVH among women of childbearing age were examined, with additional analyses conducted on individual CVH metrics. Nevertheless, several limitations also need to be noted. First, our analyses controlled only for a limited number of socioeconomic factors such as education and household income. It is possible that racial and ethnic disparities in CVH may be affected by other factors that were not included in the analysis. Second, the BRFSS assessed CVH metrics based on self‐reports, which may lead to measurement bias and potential misclassifications. Specifically, the 3 health factors (ie, blood pressure, total cholesterol, and glucose) were derived from a binary response from the participants, while ideally they should be measured as continuous variables. In addition, BMI was found to be underreported in the BRFSS, particularly among women 20 to 39 years old. On the other hand, self‐reported diagnoses of chronic conditions, physical activity measures, and tobacco smoking measures from the BRFSS data were supported by previous validity assessment, 37 which are comparable to those from other national surveys such as the National Health Interview Survey and National Health and Nutrition Examination Survey. 38 , 39 Third, the overall CVH score was calculated by summing all 7 metrics as defined by the LS7, assuming each metric contributed equally. In this case, any given score may represent different combinations of presence or absence of CVH metrics. While more efforts are warranted to specify the contribution of each metric, such as assigning weights to individual metrics according to their health effects, previous studies showed that CVH measured by LS7 is a stable and strong predictor of CVD outcomes. 3 , 24 , 40 Finally, although women with missing data on race and ethnicity or any CVH metric were excluded from this study, this may lead to minimal selection bias attributable to the incorporation of sampling weights in the analyses. 41
Conclusions
Large racial, ethnic, and geographic disparities in CVH exist among women of childbearing age. Non‐Hispanic Black women are less likely to have ideal CVH, which may be largely driven by their poor status on blood glucose, BMI, and physical activity. Women residing in the southern United States also have worse CVH in general. The results showed the urgent need to better understand the underlying factors contributing to the CVH disparities among women of childbearing age with longitudinal data and to develop targeted interventions to improve CVH among women.
Sources of Funding
This work was supported by the Scientist Development Grant (17SDG33630165) from the American Heart Association. All conclusions are the authors' own and do not necessarily reflect the opinion of the American Heart Association.
Disclosures
None.
Supporting information
Tables S1–S5
Figures S1–S4
(J Am Heart Assoc. 2021;10:e020138. DOI: 10.1161/JAHA.120.020138.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020138
For Sources of Funding and Disclosures, see page 8.
References
- 1. Centers for Disease Control and Prevention . Underlying cause of death. 1999‐2017 on CDC wonder online database, released December, 2018. Data are from the multiple cause of death files, 1999‐2017, as compiled from data provided by the 57 vital statistics jurisdictions through the vital statistics cooperative program. 2019. Available at: http://wonder.cdc.gov/ucd‐icd10.html. View in Article. Accessed February 15, 2019.
- 2. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. DOI: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3. Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. DOI: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44:1909–1914. DOI: 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ogunmoroti O, Allen NB, Cushman M, Michos ED, Rundek T, Rana JS, Blankstein R, Blumenthal RS, Blaha MJ, Veledar E, et al. Association between Life's Simple 7 and noncardiovascular disease: the Multi‐Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2016;5:e003954. DOI: 10.1161/JAHA.116.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thacker EL, Gillett SR, Wadley VG, Unverzagt FW, Judd SE, McClure LA, Howard VJ, Cushman M. The American Heart Association Life's Simple 7 and incident cognitive impairment: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2014;3:e000635. DOI: 10.1161/JAHA.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kronish IM, Carson AP, Davidson KW, Muntner P, Safford MM. Depressive symptoms and cardiovascular health by the American Heart Association’s definition in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. PLoS One. 2012;7:e52771. DOI: 10.1371/journal.pone.0052771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cranley J, MacRae CA. A new approach to an old problem: one brave idea. Circ Res. 2018;122:1172–1175. DOI: 10.1161/CIRCRESAHA.118.310941. [DOI] [PubMed] [Google Scholar]
- 9. Labarthe D, Lloyd‐Jones DM. 50× 50× 50: cardiovascular health and the cardiovascular disease endgame. Circulation. 2018;138:968–970. DOI: 10.1161/CIRCULATIONAHA.118.035985. [DOI] [PubMed] [Google Scholar]
- 10. Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd‐Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125:45–56. DOI: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ning H, Labarthe DR, Shay CM, Daniels SR, Hou L, Van Horn L, Lloyd‐Jones DM. Status of cardiovascular health in US children up to 11 years of age: the National Health and Nutrition Examination Surveys 2003–2010. Circ Cardiovasc Qual Outcomes. 2015;8:164–171. DOI: 10.1161/CIRCOUTCOMES.114.001274. [DOI] [PubMed] [Google Scholar]
- 12. Ramlakhan KP, Johnson MR, Roos‐Hesselink JW. Pregnancy and cardiovascular disease. Nat Rev Cardiol. 2020;17:718–731. DOI: 10.1038/s41569-020-0390-z [DOI] [PubMed] [Google Scholar]
- 13. Martin RM, Ben‐Shlomo Y, Gunnell D, Elwood P, Yarnell JW, Smith GD. Breast feeding and cardiovascular disease risk factors, incidence, and mortality: the Caerphilly study. J Epidemiol Community Health. 2005;59:121–129. DOI: 10.1136/jech.2003.018952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosano G, Vitale C, Marazzi G, Volterrani M. Menopause and cardiovascular disease: the evidence. Climacteric. 2007;10:19–24. DOI: 10.1080/13697130601114917. [DOI] [PubMed] [Google Scholar]
- 15. Samuels‐Kalow ME, Funai EF, Buhimschi C, Norwitz E, Perrin M, Calderon‐Margalit R, Deutsch L, Paltiel O, Friedlander Y, Manor O, et al. Prepregnancy body mass index, hypertensive disorders of pregnancy, and long‐term maternal mortality. Am J Obstet Gynecol. 2007;197:490.e491–490.e496. DOI: 10.1016/j.ajog.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, Gillman MW. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016;4:1025–1036. DOI: 10.1016/S2213-8587(16)30217-0. [DOI] [PubMed] [Google Scholar]
- 17. Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, Broekman BF. Influence of maternal obesity on the long‐term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53–64. DOI: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chou H‐H, Chiou M‐J, Liang F‐W, Chen L‐H, Lu T‐H, Li C‐Y. Association of maternal chronic disease with risk of congenital heart disease in offspring. CMAJ. 2016;188:E438–E446. DOI: 10.1503/cmaj.160061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pool LR, Ning H, Lloyd‐Jones DM, Allen NB. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999–2012. J Am Heart Assoc. 2017;6:e006027. DOI: 10.1161/JAHA.117.006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gebreab SY, Davis SK, Symanzik J, Mensah GA, Gibbons GH, Diez‐Roux AV. Geographic variations in cardiovascular health in the United States: contributions of state‐and individual‐level factors. J Am Heart Assoc. 2015;4:e001673. DOI: 10.1161/JAHA.114.001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. DOI: 10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanchi R, Perlman SE, Chernov C, Wu W, Tabaei BP, Trinh‐Shevrin C, Islam N, Seixas A, Rodriguez‐Lopez J, Thorpe LE. Gender and race disparities in cardiovascular disease risk factors among New York City adults: New York City Health and Nutrition Examination Survey (NYC Hanes) 2013–2014. J Urban Health. 2018;95:801–812. DOI: 10.1007/s11524-018-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu K, Daviglus ML, Loria CM, Colangelo LA, Spring B, Moller AC, Lloyd‐Jones DM. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (young) Adults (CARDIA) study. Circulation. 2012;125:996–1004. DOI: 10.1161/CIRCULATIONAHA.111.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the aha definition, and relation to cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. DOI: 10.1016/j.jacc.2010.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perak AM, Ning H, Khan SS, Van Horn LV, Grobman WA, Lloyd‐Jones DM. Cardiovascular health among pregnant women, aged 20 to 44 years, in the United States. J Am Heart Assoc. 2020;9:e015123. DOI: 10.1161/JAHA.119.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Britton LE, Berry DC, Hussey JM. Comorbid hypertension and diabetes among US women of reproductive age: prevalence and disparities. J Diabetes Complications. 2018;32:1148–1152. DOI: 10.1016/j.jdiacomp.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Britton LE, Hussey JM, Crandell JL, Berry DC, Brooks JL, Bryant AG. Racial/ethnic disparities in diabetes diagnosis and glycemic control among women of reproductive age. J Womens Health (Larchmt). 2018;27:1271–1277. DOI: 10.1089/jwh.2017.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anyalechi GE, Hong J, Kreisel K, Torrone E, Boulet S, Gorwitz R, Kirkcaldy RD, Bernstein K. Self‐reported infertility and associated pelvic inflammatory disease among women of reproductive age—National Health and Nutrition Examination Survey, United States, 2013–2016. Sex Transm Dis. 2019;46:446–451. DOI: 10.1097/OLQ.0000000000000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fang J, Yang Q, Hong Y, Loustalot F. Status of cardiovascular health among adult Americans in the 50 states and the District of Columbia, 2009. J Am Heart Assoc. 2012;1:e005371. DOI: 10.1161/JAHA.112.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rsatscan KK. Tools, classes, and methods for interfacing with satscan stand‐alone software. 2015. Available at: https://CRAN.R‐project.org/package=rsatscan. Accessed February 1, 2021
- 31. Howard G, Howard VJ. Twenty years of progress toward understanding the stroke belt. Stroke. 2020;51:742–750. DOI: 10.1161/STROKEAHA.119.024155. [DOI] [PubMed] [Google Scholar]
- 32. Marcinkevage JA, Alverson C, Narayan KV, Kahn HS, Ruben J, Correa A. Race/ethnicity disparities in dysglycemia among us women of childbearing age found mainly in the nonoverweight/nonobese. Diabetes Care. 2013;36:3033–3039. DOI: 10.2337/dc12-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J. 2009;13:268. DOI: 10.1007/s10995-008-0340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Caleyachetty R, Echouffo‐Tcheugui JB, Muennig P, Zhu W, Muntner P, Shimbo D. Association between cumulative social risk and ideal cardiovascular health in US adults: NHANES 1999–2006. Int J Cardiol. 2015;191:296–300. DOI: 10.1016/j.ijcard.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 35. Brown AF, Liang L‐J, Vassar SD, Escarce JJ, Merkin SS, Cheng E, Richards A, Seeman T, Longstreth W Jr. Trends in racial/ethnic and nativity disparities in cardiovascular health among adults without prevalent cardiovascular disease in the United States, 1988 to 2014. Ann Intern Med. 2018;168:541. DOI: 10.7326/M17-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gebreab SY, Davis SK, Symanzik J, Mensah GA, Gibbons GH, Diez‐Roux AV. Geographic variations in cardiovascular health in the United States: contributions of state‐ and individual‐level factors. J Am Heart Assoc. 2015;4:e001673. DOI: 10.1161/JAHA.114.001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pierannunzi C, Hu SS, Balluz L. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004–2011. BMC Med Res Methodol. 2013;13:1–14. DOI: 10.1186/1471-2288-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nelson DE, Powell‐Griner E, Town M, Kovar MG. A comparison of national estimates from the National Health Interview Survey and the Behavioral Risk Factor Surveillance System. Am J Public Health. 2003;93:1335–1341. DOI: 10.2105/AJPH.93.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fahimi M, Link M, Mokdad A, Schwartz DA, Levy P. Peer reviewed: tracking chronic disease and risk behavior prevalence as survey participation declines: statistics from the Behavioral Risk Factor Surveillance System and other national surveys. Prev Chronic Dis. 2008;5:A80. [PMC free article] [PubMed] [Google Scholar]
- 40. Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. DOI: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mirel LB, Burt V, Curtin LR, Zhang C. Different approaches for non‐response adjustments to statistical weights in the continuous NHANES (2003–04). Federal Committee on Statistical Methodology Research Conference. 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S4


