Abstract
Background
Hypertensive disorders of pregnancy (HDP) and pre‐pregnancy hypertension are associated with increased morbidity and mortality for the mother. Our aim was to investigate the relationships between HDP and pre‐pregnancy hypertension with maternal heart failure (HF) within 1 and 5 years of delivery and to examine racial/ethnic differences.
Methods and Results
We conducted a retrospective cohort study in South Carolina (2004–2016) involving 425 649 women aged 12 to 49 years (58.9% non‐Hispanic White [NHW], 31.5% non‐Hispanic Black [NHB], 9.6% Hispanic) with a live, singleton birth. Incident HF was defined by hospital/emergency department visit and death certificate data. Pre‐pregnancy hypertension and HDP (preeclampsia, eclampsia, or gestational hypertension) were based on hospitalization/emergency department visit and birth certificate data (i.e., gestational hypertension for HDP). The 425 649 women had pre‐pregnancy hypertension without superimposed HDP (pre‐pregnancy hypertension alone; 0.4%), HDP alone (15.7%), pre‐pregnancy hypertension with superimposed HDP (both conditions; 2.2%), or neither condition in any pregnancy (81.7%). Incident HF event rates per 1000 person‐years were higher in NHB than NHW women with HDP (HDP: 2.28 versus 0.96; both conditions: 4.30 versus 1.22, respectively). After adjustment, compared with women with neither condition, incident HF risk within 5 years of delivery was increased for women with pre‐pregnancy hypertension (HR,2.55, 95% CI: 1.31–4.95), HDP (HR,4.20, 95% CI: 3.66–4.81), and both conditions (HR,5.25, 95% CI: 4.24–6.50).
Conclusions
Women with HDP and pre‐pregnancy hypertension were at higher HF risk (highest for superimposed preeclampsia) within 5 years of delivery. NHB women with HDP had higher HF risk than NHW women, regardless of pre‐pregnancy hypertension.
Keywords: heart failure, hypertensive disorders of pregnancy, maternal outcomes, pre‐pregnancy hypertension, race/ethnicity
Subject Categories: Epidemiology, Pregnancy, Race and Ethnicity, Risk Factors, Women, Heart Failure, Preeclampsia, Hypertension
Nonstandard Abbreviations and Acronyms
- NHB
non‐Hispanic Black
- NHW
non‐Hispanic White
- R‐GINDEX
Revised‐Graduated Prenatal Care Utilization Index
- RUCA
Rural‐Urban Commuting Area
- SC DHEC
South Carolina Department of Health and Environmental Control
- SC RFA
South Carolina Revenue and Fiscal Affairs Office
Clinical Perspective
What is New?
Women with hypertensive disorders of pregnancy and pre‐pregnancy hypertension aged 12 to 49 years were at higher incident heart failure risk within 5 years of delivery in a large, retrospective cohort study conducted in South Carolina.
Racial/ethnic differences were observed with incident heart failure event rates highest among non‐Hispanic Black women who experienced hypertensive disorders of pregnancy as well as non‐Hispanic Black women who experienced both pre‐pregnancy hypertension and hypertensive disorders of pregnancy.
What Are the Clinical Implications?
In women identified to be at high‐risk of heart failure subsequent to hypertensive disorders of pregnancy and/or pre‐pregnancy hypertension exposure, clinical and public health prevention efforts are needed to reduce maternal morbidity and mortality.
Five to 10% of pregnancies are impacted by hypertensive disorders of pregnancy (HDP), which include gestational hypertension, preeclampsia, and eclampsia. 1 , 2 Pre‐pregnancy hypertension occurs in 1% to 4% of pregnancies and is a well‐known risk factor for preeclampsia. 3 , 4 , 5 HDP are of significant public health concern given their associations with increased maternal morbidity and mortality and potential long‐term risk for cardiovascular disease (CVD) and related events, 6 , 7 , 8 , 9 including development of CVD risk factors, 10 heart failure (HF), stroke, and myocardial infarction. 8 , 11 , 12 , 13 While racial disparities in the development of HDP exist, less is known about disparities in HDP‐related outcomes. 14 , 15 , 16 , 17 , 18
During pregnancy, pathological hemodynamic changes associated with pre‐pregnancy hypertension and HDP can lead to the development of acquired heart disease including HF, even when there is no history of CVD. In a recent meta‐analysis by Wu et al., an association between preeclampsia and subsequent HF risk was reported (risk ratio [RR],3.62, 95% CI: 2.25–5.85), with even higher risk when limited to studies controlling for potential confounders (RR,4.19, 95% CI: 2.09–8.38). 8 Additionally, in sensitivity analyses evaluating HF risk <1, 1 to 10, and >10 years after preeclampsia, the highest HF risk was observed 1 to 10 years after preeclampsia. 8
Racial disparities have been previously reported for the risk of HF. The cumulative incidence of HF prior to age 50 was higher among Black women (1.1%; 95% CI: 0.6–1.7) than White women (0.08%, 95% CI: 0.0–0.5) in the Coronary Artery Risk Development in Young Adults (CARDIA) study with a baseline age of 18 to 30 years and 20 years of follow‐up. 19 Several factors including hypertension are suspected to contribute to disparities in HF in late pregnancy or postpartum although more information is needed to better understand risk factors for HF in women of different racial/ethnic groups. 20
A limitation of past studies evaluating the association of preeclampsia and future HF is that many have not had the ability to adjust for potential confounders such as demographic factors (e.g., maternal age, ethnicity, education), socioeconomic status, smoking, and clinical or pregnancy characteristics (e.g., body mass index [BMI] or weight, parity, birth weight, gestational diabetes mellitus) including established cardiovascular risk factors such as diabetes mellitus and hypertension. 8 Further, 4 studies included in the meta‐analysis by Wu et al. that assessed HF risk controlled for three‐to‐four established cardiovascular risk factors. 8 As relationships exist for coronary artery disease and HF and for HDP and coronary heart disease, unmeasured confounders may be impacting HF risk subsequent to HDP. 21 Our study had the ability to adjust for four cardiovascular risk factors and a number of pregnancy‐related risk factors as well as to examine potential racial/ethnic differences. In this study, we investigated the relationships between HDP and pre‐pregnancy hypertension with incident maternal HF within 5 years after pregnancy, with a focus on racial/ethnic differences.
Methods
The data used for this study cannot be shared due to policies of the South Carolina (SC) Revenue and Fiscal Affairs (RFA) Office, Health and Demographics Section; and the SC Department of Health and Environmental Control (DHEC).
Study Design
We constructed a cohort from comprehensive statewide hospital discharge records and emergency department (ED) visit data available from the SC RFA Office, Health and Demographics Section. SC collects statewide administrative billing data on health information for individual encounters including inpatient discharges, ED visits, and other outpatient services requiring certificate of need. SC DHEC provided birth and death certificate data. Institutional review board approval was received and the requirement for consent was waived.
Study Population
Birth certificate data were used to identify live births that occurred in SC hospitals. Hospitalization and ED visit data were used to obtain maternal procedure and diagnosis codes, demographics, length of stay, and patient disposition. Death certificate data included the primary or underlying cause of death and comorbid causes of death for mothers, and demographic, payer, and clinical information related to current/previous pregnancies. SC RFA provided a unique identifier allowing for 97.5% linkage of birth certificate and maternal hospitalization/ED visit data.
Inclusion and Exclusion Criteria
We included single, liveborn infant, hospital deliveries in SC reported on birth certificates over a 13‐year period (2004–2016) with matched hospitalization/ED visit data. To have at least 1 year (maximum of 14 years) of follow‐up through 2017, we obtained an additional year of hospitalization/ED visit and mortality data. Figure 1 shows study inclusion and exclusion criteria. Because the study involved liveborn infants and a 1‐year minimum follow‐up, newborn deaths the day of delivery were excluded as were mothers residing out‐of‐state as they may not regularly seek care in SC. Women with comorbidities and risk factors were excluded (i.e., kidney transplant, prior diagnosis with HF, or congenital heart defect). In addition, women with a pre‐pregnancy weight of <92 or >320 lbs, pre‐pregnancy BMI of <16.0 or >52.8 kg/m2, or self‐reported “other race/ethnicity” were excluded from the study due to small numbers and concerns about data accuracy. We similarly excluded newborns with birth weights of <500 or >6000 g and deliveries with implausible size for gestational age. 21
Figure 1. Study inclusion and exclusion criteria flowchart.
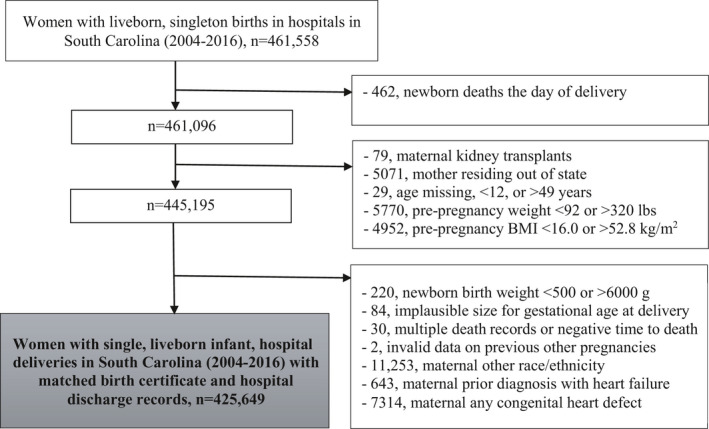
BMI indicates body mass index.
Definitions
Birth certificate data included information on maternal self‐reported race, ethnicity, education, primary payer, pre‐pregnancy smoking and BMI, smoking during pregnancy, BMI at delivery, pre‐pregnancy and gestational hypertension, and pre‐pregnancy and gestational diabetes mellitus. Women were categorized into non‐Hispanic White (NHW), non‐Hispanic Black (NHB), Hispanic, or other race, ethnicity based on self‐report. Prenatal care adequacy and utilization was measured by the Revised‐Graduated Prenatal Care Utilization Index (R‐GINDEX), which incorporates from the birth certificate the: (1) trimester and (2) gestational age prenatal care began, and (3) the total number of prenatal care visits. 22 , 23 The R‐GINDEX does not incorporate the quality of service received. Categories of the R‐GINDEX include: inadequate, intermediate, adequate, intensive, no care, or not able to calculate due to missing information.
International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD‐9‐CM/ICD‐10‐ CM) diagnosis and procedure codes identified the exposure, outcome, and covariates of interest (Table S1). Women from 4 exposure groups were included and referred to as: (1) superimposed preeclampsia termed pre‐pregnancy hypertension with superimposed HDP (“both conditions”), (2) HDP without pre‐pregnancy hypertension (“HDP alone”), (3) pre‐pregnancy hypertension without superimposed HDP (“pre‐pregnancy hypertension alone”), and (4) without pre‐pregnancy hypertension or HDP (“neither condition”). HDP was defined by diagnosis codes for gestational hypertension, preeclampsia, or eclampsia at delivery (ICD‐9‐CM/ICD‐10‐CM: 642.3–642.7; O11, O13–O15), or gestational hypertension as reported on birth certificates which was also selected for preeclampsia or eclampsia. Pre‐pregnancy hypertension was defined on birth certificates or by diagnosis codes at delivery (ICD‐9‐CM/ICD‐10‐CM: 642.0–642.2. 642.9; O10, O16). Birth certificates and/or delivery codes (ICD‐9‐CM/ICD‐10‐CM: 642.7; O11) defined pre‐pregnancy hypertension with superimposed HDP, with the understanding that this refers to preeclampsia. The index pregnancy was the first pregnancy in the data set for women with neither condition, and the first pregnancy with HDP and/or pre‐pregnancy hypertension for exposed women.
Fatal and nonfatal incident HF was defined by ICD‐9‐CM/ICD‐10‐CM codes (402.01, 402.11, 402.91, 404.x1, 404.x3, 428; I11.0, 113.0, I13.2, I50.x) in the hospitalization/ED visit and death certificate data.
Statistical Analysis
Group differences for continuous variables were analyzed with analysis of variance (ANOVA). Categorical variables were analyzed with a Chi‐square test. Cox proportional hazards models provided hazard ratios (HRs) and corresponding 95% CIs to estimate incident HF events/deaths within the first year and up to 5 years after delivery. Models included time from delivery to the event/death. The proportional hazards assumption was met and checked with Schoenfeld residuals. Models controlled for sociodemographic (maternal age at delivery, race, ethnicity, education, urban/rural residence, median household income per year, payer during pregnancy, WIC [Women, Infants, and Children] eligibility during pregnancy), behavioral (smoking during pregnancy), and clinical characteristics (pre‐pregnancy BMI, change in BMI after delivery, pre‐pregnancy or gestational diabetes mellitus, gestational age at delivery, mode of delivery [Cesarean section, vaginal], induced labor, number of pregnancies prior to index pregnancy, previous Cesarean section, previous pre‐term delivery, R‐GINDEX). WIC is a U.S. federal grant program that provides nutrition education and counseling, supplemental nutritious foods, and health and social services screening and referrals. The models included a missing R‐GINDEX category and an unknown category for previous Cesarean section as this was a category on the birth certificate. Table S2 shows the proportion of complete data for variables included in the models overall and by race and ethnicity.
Because baseline rates of HF events differ across racial/ethnic groups, a joint analysis of race and ethnicity and exposure categories was performed with NHW women with neither condition serving as the reference group for comparison across groups. We also completed a secondary analysis which included interaction terms and provided results stratified by racial/ethnic group (see Table S3). While the overall models included Hispanic women, they were excluded from race‐ and ethnicity‐stratified models due to the small number of incident HF events (n=24). Analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC). 24 Graphs were created and the proportional hazards assumption checked with Stata Version 13. 25
Results
Our study included 425 649 eligible women (58.9% NHW, 31.5% NHB, 9.6% Hispanic) of the 461 558 women with births from 2004 to 2016 in SC. Of those, 414 000 (97.3%) complete cases were used in the Cox proportional hazards analysis. In at least 1 pregnancy, some women were diagnosed with pre‐pregnancy hypertension without superimposed HDP (pre‐pregnancy hypertension; 0.4%), no pre‐pregnancy hypertension with HDP (HDP; 15.7%), or pre‐pregnancy hypertension with superimposed HDP (both conditions; 2.2%). Women with neither condition throughout all pregnancies during the study period served as the control group (81.7%).
Table 1 displays characteristics overall and by exposure group. Compared with neither condition, women with pre‐pregnancy hypertension, HDP, or both conditions were more likely to be NHB, WIC eligible during pregnancy, diagnosed with pre‐pregnancy/gestational diabetes mellitus, and have a higher BMI pre‐pregnancy. Women with pre‐pregnancy hypertension, with and without HDP, were more likely to be older and have a lower income than women with neither condition. The R‐GINDEX varied by exposure group as women with pre‐pregnancy hypertension, HDP, and both conditions were less likely to receive inadequate prenatal care and more likely to receive intensive prenatal care than women with neither condition. Maternal characteristics are shown by race and ethnicity in Table S4.
Table 1.
Demographic, clinical characteristics of mothers at index pregnancy in South Carolina overall, by HDP and pre‐pregnancy hypertension status, 2004 to 2016* , † , ‡
| Characteristic n (%), mean±SD | Total | Neither pre‐preg hypertension nor HDP | Pre‐preg hypertension | HDP | Both pre‐preg hypertension and HDP | P‐value ‖ | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N=425 649 | N=347 930 (81.7) | N=1530 (0.4) | N=66 833 (15.7) | N=9356 (2.2) | |||||||
| Maternal age at delivery, y | 27.2 | ±6.0 | 27.1 | ±6.0 | 29.9 | ±6.4 | 27.2 | ±6.1 | 30.1 | ±6.2 | <0.0001 |
| <20 | 42 667 | (10.0) | 35 434 | (10.2) | 71 | (4.6) | 6727 | (10.1) | 435 | (4.6) | <0.0001 |
| 20 to 24 | 113 119 | (26.6) | 93 524 | (26.9) | 281 | (18.4) | 17 859 | (26.7) | 1455 | (15.6) | |
| 25 to 29 | 120 988 | (28.4) | 99 455 | (28.6) | 388 | (25.4) | 18 723 | (28.0) | 2422 | (25.9) | |
| 30 to 34 | 94 238 | (22.1) | 76 763 | (22.1) | 400 | (26.1) | 14 425 | (21.6) | 2650 | (28.3) | |
| 35 to 39 | 44 560 | (10.5) | 35 181 | (10.1) | 292 | (19.1) | 7279 | (10.9) | 1808 | (19.3) | |
| 40+ | 10 077 | (2.4) | 7573 | (2.2) | 98 | (6.4) | 1820 | (2.7) | 586 | (6.3) | |
| Race/ethnicity | |||||||||||
| Non‐Hispanic White | 250 506 | (58.9) | 208 794 | (60.0) | 708 | (46.3) | 36 873 | (55.2) | 4131 | (44.2) | <0.0001 |
| Non‐Hispanic Black | 134 220 | (31.5) | 102 676 | (29.5) | 715 | (46.7) | 25 924 | (38.8) | 4905 | (52.4) | |
| Hispanic | 40 923 | (9.6) | 36 460 | (10.5) | 107 | (7.0) | 4036 | (6.0) | 320 | (3.4) | |
| Education | |||||||||||
| Less than HS | 76 830 | (18.1) | 63 894 | (18.4) | 252 | (16.5) | 11 450 | (17.1) | 1234 | (13.2) | <0.0001 |
| HS graduate | 104 552 | (24.6) | 84 261 | (24.2) | 400 | (26.1) | 17 409 | (26.0) | 2482 | (26.5) | |
| Some college | 108 511 | (25.5) | 87 383 | (25.1) | 435 | (28.4) | 18 068 | (27.0) | 2625 | (28.1) | |
| ≥College graduate | 135 756 | (31.9) | 112 392 | (32.3) | 443 | (29.0) | 19 906 | (29.8) | 3015 | (32.2) | |
| Urban/rural (based on RUCA by zipcode of residence) | |||||||||||
| Urban | 313 755 | (73.7) | 256 703 | (73.7) | 1124 | (73.5) | 49 132 | (73.5) | 6796 | (72.6) | 0.05 |
| Rural | 111 894 | (26.3) | 91 227 | (26.2) | 406 | (26.5) | 17 701 | (26.5) | 2560 | (27.4) | |
| Annual household income | |||||||||||
| <$36 000 | 115 232 | (27.1) | 91 995 | (26.4) | 552 | (36.1) | 19 406 | (29.0) | 3279 | (35.0) | <0.0001 |
| $36 000 to <$54 000 | 200 773 | (47.2) | 163 364 | (47.0) | 565 | (36.9) | 32 607 | (48.8) | 4237 | (45.3) | |
| ≥$54 000 | 100 652 | (23.6) | 84 378 | (24.3) | 271 | (17.7) | 14 214 | (21.3) | 1789 | (19.1) | |
| Primary payer during pregnancy | |||||||||||
| Medicaid | 204 964 | (48.2) | 164 939 | (47.4) | 755 | (49.3) | 34 584 | (51.7) | 4686 | (50.1) | <0.0001 |
| Private | 169 942 | (39.9) | 138 778 | (39.9) | 643 | (42.0) | 26 566 | (39.7) | 3955 | (42.3) | |
| Self‐pay | 22 565 | (5.3) | 20 043 | (5.8) | 56 | (3.7) | 2223 | (3.3) | 243 | (2.6) | |
| Other | 24 996 | (5.9) | 21 489 | (6.2) | 55 | (3.6) | 3043 | (4.6) | 409 | (4.4) | |
| WIC eligibility during pregnancy | 218 525 | (51.3) | 179 592 | (50.6) | 842 | (55.0) | 36 506 | (54.6) | 5225 | (55.8) | <0.0001 |
| Pre‐pregnancy smoking | 64 555 | (15.2) | 53 004 | (15.2) | 232 | (15.2) | 9986 | (14.9) | 1333 | (14.2) | 0.02 |
| Smoking during pregnancy | 49 404 | (11.6) | 40 785 | (11.7) | 170 | (11.1) | 7444 | (11.1) | 1005 | (10.7) | <0.0001 |
| Pre‐pregnancy BMI (kg/m2) | 27.3 | ±6.8 | 26.5 | ±6.3 | 31.7 | ±8.0 | 30.1 | ±7.6 | 33.4 | ±7.9 | <0.0001 |
| Change in BMI at delivery | 4.9 | ±3.0 | 4.8 | ±2.9 | 4.4 | ±3.3 | 5.0 | ±3.3 | 4.5 | ±3.4 | <0.0001 |
| Gestational age at delivery | 38.4 | ±2.0 | 38.6 | ±1.8 | 37.8 | ±2.4 | 37.7 | ±2.4 | 37.0 | ±2.8 | <0.0001 |
| <28 wks | 2,123 | (0.5) | 1536 | (0.4) | 18 | (1.2) | 489 | (0.7) | 80 | (0.9) | <0.0001 |
| 28 to <34 wks | 7295 | (1.7) | 4374 | (1.3) | 65 | (4.2) | 2400 | (3.6) | 456 | (4.9) | |
| 34 to <37 wks | 27 359 | (6.4) | 19 375 | (5.6) | 157 | (10.3) | 6957 | (10.4) | 870 | (9.3) | |
| ≥37 wks | 377 974 | (88.8) | 321 400 | (92.4) | 1245 | (81.4) | 50 624 | (75.7) | 4705 | (50.3) | |
| Mode of delivery | |||||||||||
| Cesarean section | 144 850 | (34.0) | 108 988 | (31.3) | 698 | (45.6) | 30 096 | (45.0) | 5068 | (54.2) | <0.0001 |
| Vaginal | 280 787 | (66.0) | 238 932 | (68.7) | 832 | (54.4) | 36 735 | (55.0) | 4288 | (45.8) | |
| Induced labor | 62 259 | (14.6) | 46 373 | (13.3) | 240 | (15.7) | 13 812 | (20.7) | 1834 | (19.6) | <0.0001 |
| No. of pregnancies prior to index pregnancy | 0.8 | ±1.1 | 0.8 | ±1.1 | 1.2 | ±1.3 | 0.7 | ±1.0 | 0.9 | ±1.2 | <0.0001 |
| Previous Cesarean section | |||||||||||
| Yes | 23 562 | (5.5) | 17 817 | (5.1) | 133 | (8.7) | 4818 | (7.2) | 794 | (8.5) | <0.0001 |
| No | 174 670 | (41.0) | 141 813 | (40.8) | 568 | (37.1) | 28 632 | (42.8) | 3657 | (39.1) | |
| Unknown | 227 417 | (53.4) | 188 300 | (54.1) | 829 | (54.2) | 33 383 | (49.9) | 4905 | (52.4) | |
| Previous preterm birth | 11 116 | (2.6) | 8138 | (2.3) | 92 | (6.0) | 2350 | (3.5) | 536 | (5.7) | <0.0001 |
| Prenatal care, measured by R‐GINDEX | |||||||||||
| Inadequate | 74 911 | (17.6) | 63 713 | (18.3) | 212 | (13.9) | 9918 | (14.8) | 1068 | (11.4) | <0.0001 |
| Intermediate | 96 313 | (22.6) | 80 747 | (23.2) | 293 | (19.2) | 13 724 | (20.5) | 1549 | (16.6) | |
| Adequate | 6912 | (1.6) | 5487 | (1.6) | 28 | (1.8) | 1253 | (1.9) | 144 | (1.5) | |
| Intensive | 122 940 | (28.9) | 94 432 | (27.1) | 545 | (35.6) | 23 769 | (35.6) | 4194 | (44.8) | |
| No care | 3650 | (0.9) | 2973 | (0.9) | 12 | (0.8) | 591 | (0.9) | 74 | (0.8) | |
| Missing | 120 923 | (28.4) | 100 578 | (28.9) | 440 | (28.8) | 17 578 | (26.3) | 2327 | (24.9) | |
| Pre‐pregnancy or gestational diabetes mellitus | 27 999 | (6.6) | 18 270 | (5.3) | 315 | (20.6) | 7377 | (11.0) | 2037 | (21.8) | <0.0001 |
BMI indicates body mass index; HDP, Hypertensive disorders of pregnancy; HS, high school; no., number; pre‐preg, pre‐pregnancy; R‐GINDEX, Revised‐Graduated Prenatal Care Utilization Index; RUCA, Rural‐Urban Commuting Area; WIC, Women, Infants, and Children; and y, years.
Data include person‐level diagnostic codes at time of discharge or birth certificate. Hypertensive disorders of pregnancy (HDP) were defined as pre‐eclampsia, eclampsia, or gestational hypertension (ICD‐9/10‐CM: 642.3–642.7; O11, O13–O15) based on hospitalization/emergency department (ED) visit data, or gestational hypertension as reported on the birth certificate. Pre‐pregnancy hypertension was based on hospitalization/ED visit data (ICD‐9/10‐CM: 642.0–642.2, 642.9; O10, O16) or birth certificates. Pre‐pregnancy hypertension with superimposed HDP was based on hospitalization/ED visit data (ICD‐9/10‐CM: 642.7; O11) or a combination of the above diagnosis codes for HDP and pre‐pregnancy hypertension.
Pre‐pregnancy smoking, smoking during pregnancy, pre‐pregnancy BMI, BMI at delivery, gestational age at delivery, mode of delivery, induced labor, number of pregnancies prior to the index pregnancy, previous Cesarean section, pre‐pregnancy diabetes, and gestational diabetes mellitus were available from the birth certificate. Gestational diabetes mellitus was also defined using hospitalization/ED visit data based on ICD‐9‐CM and ICD‐10‐CM codes.
Variables with >0.1% missing data included: annual household income, n=8992; prenatal care as measured by R‐GINDEX, n=120 923; primary payer, n=3182; and WIC eligibility in pregnancy, n=6449.
Among women in the 4 exposure groups, characteristics are compared using P‐values calculated by chi‐square tests for categorical variables and analysis of variance (ANOVA) for continuous variables.
Figure 2 shows the cumulative hazard for incident HF following delivery by year, race and ethnicity, and exposure group. The hazard of incident HF was higher among NHB women than NHW women in each exposure group, with the highest hazards observed for NHB women with both conditions and NHB women with HDP.
Figure 2. Cumulative hazard of incident HF by years after delivery, race/ethnicity, and exposure group.
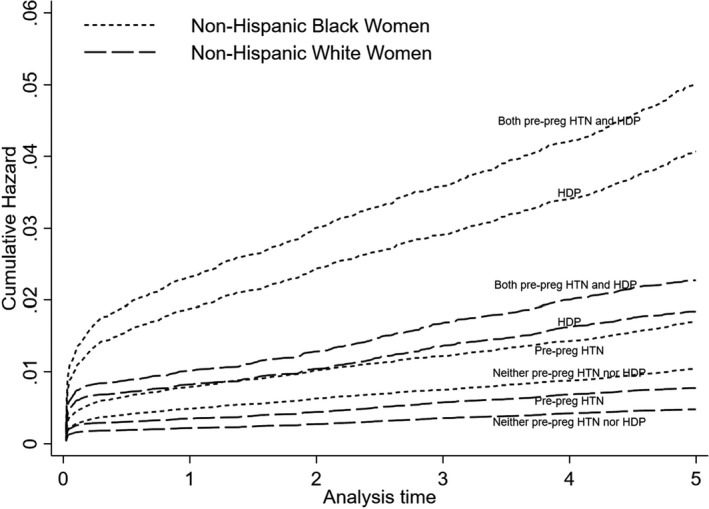
HDP indicates hypertensive disorders of pregnancy; pre‐preg HTN indicates pre‐pregnancy hypertension; and HF, heart failure.
Subsequent Risk of Incident Heart Failure by HDP and Pre‐Pregnancy Hypertension Status
Compared with women with neither condition within the first year of delivery, the risk of incident HF was increased 4.39‐fold for pre‐pregnancy hypertension (95% CI: 1.93–9.97), 5.41‐fold for HDP (95% CI: 4.39–6.65), and 6.02‐fold for both conditions (95% CI: 4.38–8.27). Up to 5 years after delivery, incident HF risk was increased 2.55‐fold for pre‐pregnancy hypertension (95% CI: 1.31–4.95), 4.20‐fold for HDP (95% CI: 3.66–4.81), and 5.25‐fold for women with both conditions (95% CI: 4.24–6.50) compared with neither condition after covariate adjustment (Table 2).
Table 2.
Adjusted Hazard Ratios Comparing Women With and Without HDP by Pre‐Pregnancy Hypertension Status for Fatal and Nonfatal Incident Heart Failure Within the First Year of Delivery and Up to 5 Years Subsequent to Delivery
| Incident heart failure | |||
|---|---|---|---|
| Event | Event rate (95% CI)* | HR (95% CI) † | |
| The first year of delivery | |||
| Neither pre‐preg hypertension nor HDP | 172 | 0.49 (0.43–0.57) | Referent |
| Pre‐pregnancy hypertension | 6 | 3.94 (1.77–8.76) | 4.39 (1.93–9.97) |
| HDP | 247 | 3.71 (3.27–4.20) | 5.41 (4.39–6.65) |
| Both pre‐pregnancy hypertension and HDP | 62 | 6.66 (5.19–8.55) | 6.02 (4.38–8.27) |
| ≤5 y of delivery | |||
| Neither pre‐preg hypertension nor HDP | 442 | 0.25 (0.23–0.28) | Referent |
| Pre‐pregnancy hypertension | 9 | 1.18 (0.62–2.27) | 2.55 (1.31–4.95) |
| HDP | 480 | 1.44 (1.32–1.58) | 4.20 (3.66–4.81) |
| Both pre‐pregnancy hypertension and HDP | 130 | 2.81 (2.36–3.33) | 5.25 (4.24–6.50) |
HDP indicatesHypertensive disorders of pregnancy; HR, hazard ratio; and pre‐preg, pre‐pregnancy.
Per 1000 person‐years.
Adjusted for sociodemographic (maternal age, race/ethnicity, education, rural/urban residence, median income, payer, Women, Infants and Children [WIC]), behavioral (smoking during pregnancy), and clinical characteristics (pre‐pregnancy body mass index [BMI], change in BMI after delivery, pre‐pregnancy or gestational diabetes mellitus, gestational age at delivery, mode of delivery, induced labor, number of pregnancies prior to index pregnancy, previous Cesarean section, previous pre‐term delivery, Revised‐Graduated Prenatal Care Utilization Index [R‐GINDEX]).
Incident Heart Failure Among Women With HDP and/or Pre‐Pregnancy Hypertension With a Focus on Race and Ethnicity
Table 3 shows incident HF event rates and HF risk within 5 years of delivery by race and ethnic group. The event rates for incident HF differed by exposure status and race and ethnicity although the number of events was small for some groups; thus, race‐ and ethnicity‐specific findings are only presented for NHW and NHB women. Within 5 years of delivery, the incident HF event rate per 1000 person‐years for women with pre‐pregnancy hypertension was 0.85 (95% CI: 0.27–2.64) for NHW and 0.84 (95% CI: 0.27–2.61) for NHB women. The incident HF event rate for women with HDP was 0.96 (95% CI: 0.83–1.11) for NHW and 2.28 (95% CI: 2.03–2.56) for NHB women per 1000 person‐years. Among women with both conditions, the incident HF event rate was 1.22 (95% CI: 0.82–1.80) for NWH and 4.30 (95% CI: 3.55–5.22) for NHB women per 1000 person‐years. Figure 3 displays rates per 1000 person‐years for maternal incident HF events within 5 years of delivery among all women and by race and ethnic group for NHW and NHB women.
Table 3.
Adjusted Hazard Ratios Comparing Women With HDP With and Without and Pre‐Pregnancy Hypertension , and Those With Pre‐Pregnancy Hypertension Without HDP to Women With Neither HDP nor Pre‐Pregnancy Hypertension for Incident Heart Failure Stratified by Racial/Ethnic Group Within 5 Years of Delivery
| Incident heart failure | Non‐Hispanic White | Non‐Hispanic Black | ||||
|---|---|---|---|---|---|---|
| Event | Event rate (95% CI)* | HR (95% CI) † | Event | Event rate (95% CI)* | HR (95% CI) † | |
| ≤5 y of delivery | ||||||
| Neither pre‐preg hypertension nor HDP | 189 | 0.18 (0.16–0.21) | Referent | 244 | 0.48 (0.42–0.54) | 2.30 (1.88–2.82) |
| Pre‐preg hypertension | <5 | 0.85 (0.27–2.64) | 3.22 (1.03–10.1) | <5 | 0.84 (0.27–2.61) | 2.53 (0.82–8.11) |
| HDP | 176 | 0.96 (0.83–1.11) | 4.14 (3.35–5.13) | 293 | 2.28 (2.03–2.56) | 8.53 (6.96–10.5) |
| Both pre‐preg hypertension and HDP | 25 | 1.22 (0.82–1.80) | 4.07 (2.64–6.27) | 104 | 4.30 (3.55–5.22) | 11.3 (8.67–14.8) |
HDP indicates hypertensive disorders of pregnancy; HR, hazard ratio; and pre‐preg, pre‐pregnancy.
Per 1000 person‐years.
Adjusted for sociodemographic (maternal age, education, rural/urban residence, median income, payer, Women, Infants and Children [WIC]), behavioral (smoking during pregnancy), and clinical characteristics (pre‐pregnancy body mass index [BMI], change in BMI after delivery, pre‐pregnancy or gestational diabetes mellitus, gestational age at delivery, mode of delivery, induced labor, number of pregnancies prior to index pregnancy, previous Cesarean section, previous pre‐term delivery, Revised‐Graduated Prenatal Care Utilization Index [R‐GINDEX].
Figure 3. Incident heart failure events (per 1000 person‐years) among women up to 5 years subsequent to delivery by race, ethnicity and exposure group.* .
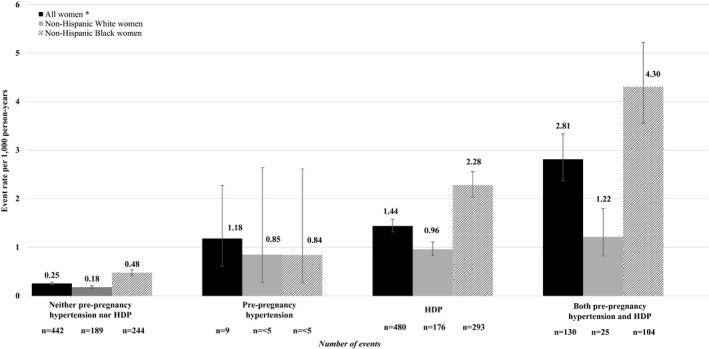
HDP indicates hypertensive disorders of pregnancy.
Peripartum cardiomyopathy, defined by diagnosis within 6 weeks of delivery, occurred among 14.4% (n=252; n=91 NHW, n=159 NHB, n=<5 Hispanic) of women with incident HF within 5 years of delivery versus 0.1% (n=105; n=41 NHW, n=61 NHB, n=<5 Hispanic) of women without. No women with incident HF within 5 years of delivery experienced peripartum death; however, peripartum death occurred among <5 women without the outcome of interest.
In a joint analysis of our 4 exposure categories and race and ethnic group, differences in incident HF risk were also observed (Table 3). Up to 5 years after delivery compared with NHW women with neither condition, the HR for incident HF risk among women with pre‐pregnancy hypertension was 2.53 for NHB (95% CI: 0.82–8.11) and 3.22 for NHW women (95% CI: 1.03–10.1). However, there were few events for each racial/ethnic group. For HDP, the HR for incident HF risk was 8.53 for NHB women (95% CI: 6.96–10.5) and 4.14 for NHW (95% CI: 3.35–5.13) women compared with NHW women with neither condition. For women with both conditions, the HR for incident HF risk was 11.3 for NHB (95% CI: 8.67–14.8) and 4.07 for NHW women (95% CI: 2.64–6.27) compared with NHW women with neither condition. The HR for incident HF risk for NHB women with neither condition was 2.30 (95% CI: 1.88–2.82) compared with NHW counterparts.
We did not find evidence of a significant interaction between the exposure and race and ethnic groups for incident HF (P=0.3452), but elected to provide race and ethnic‐stratified results regardless (Table S3). Within 5 years of delivery, incident HF risk was increased for women with HDP: NHW (HR,4.14; 95% CI: 3.31–5.17) and NHB women (HR,3.74; 95% CI: 3.12–4.49) compared with counterparts with neither condition. Similarly, among women with both conditions, incident HF risk was increased 4.11‐fold (95% CI: 2.64–6.40) for NHW and 4.88‐fold (95% CI: 3.78–6.29) for NHB women compared with those with neither condition, respectively. While race ethnicity‐ group specific HRs may be similar for women of different racial and ethnic groups, it is important to keep in mind that rates in the unexposed category are more than twice as high in NHB when compared with NHW women.
Discussion
In this diverse cohort with a large population of NHB women, we found significant associations between pre‐pregnancy hypertension and HDP with maternal incident HF (including both fatal and nonfatal) within 5 years of delivery. The highest risk was observed among women with both pre‐pregnancy hypertension and HDP (i.e., with superimposed preeclampsia), followed by women with HDP and women with pre‐pregnancy hypertension. While HRs for incident HF risk appeared higher during the first year after pregnancy, the increased relative risk remained higher through 5 years of follow‐up. In women with pre‐pregnancy hypertension as well as women with both pre‐pregnancy hypertension and HDP, there were a small number of events from which the increases were seen.
Racial/ethnic differences were evident for incident HF risk up to 5 years subsequent to delivery. In women with HDP, HF rates were substantially higher in NHB than NHW women (2.28 compared with 0.96 per 1000 person‐years). In women with both pre‐pregnancy hypertension and HDP, HF rates were also substantially higher in NHB than NHW women (4.30 compared with 1.22 per 1000 person‐years). We did not find evidence of a significant interaction between the exposure and racial and ethnic groups for incident HF (P=0.3452), but it is important to remember that incident HF rates in the unexposed category are more than twice as high in NHB than in NHW women. The absence of an interaction indicates that this difference holds across the 4 exposure categories. These racial and ethnic differences are important as NHB women experience more pregnancy‐related morbidity and mortality than NHW women. Clinical and public health prevention efforts are needed to reduce maternal morbidity and mortality in women at high risk of HF or HF death within 5 years of HDP and/or pre‐pregnancy hypertension.
While previous studies have investigated the relationship between HDP and cardiovascular outcomes including HF, definition of the exposure and/or outcome, adjustment for covariates, and length of follow‐up has varied. 8 , 12 , 13 , 14 , 15 , 26 , 27 , 28 , 29 , 30 , 31 Further, evidence is lacking on potential racial/ethnic disparities in HF and other CVD outcomes among women with HDP. Many studies examining the risk of CVD subsequent to preeclampsia have not been able to adjust for multiple, established cardiovascular risk factors and other confounders. 8 Four cardiovascular risk factors (i.e., maternal age, pre‐pregnancy BMI, smoking during pregnancy, pre‐pregnancy or gestational diabetes mellitus) were adjusted for in our study in addition to several pregnancy‐related risk factors (e.g., number of pregnancies prior to index pregnancy, previous pre‐term delivery, induced labor). Pre‐pregnancy hypertension was also considered by including 4 exposure groups of women who experienced HDP and/or pre‐pregnancy hypertension as well as women who did not experience either condition.
With regard to the duration between delivery and maternal incident HF outcomes, studies that have examined HF events up to 5 years after delivery indicate elevated risk persists 5 years after pregnancy; however, few studies have examined HF events more than 5 years after delivery. A recent study by Garovic et al. in Minnesota investigated the association of HDP and several conditions including congestive HF with 36.2 (interquartile range [IQR]: 23.5 to 38.2 years) and 35.8 years median follow‐up (IQR: 13.7 to 37.9 years) for women with a history of HDP and referent women, respectively. 32 The risk of congestive HF was increased among women with HDP compared with referent women matched by age and parity (HR,2.11, 95% CI: 1.19–3.76). 32 Similar cumulative hazards of congestive HF were found for women with HDP and referent women until around 13 years, after which the hazard of congestive HF became higher among women with HDP than referent women. 32 In addition, Chen et al. (2018) assessed the relationship between HDP and HF within 5, 6 to 10, and more than 10 years after delivery in Taiwan (with a mean follow‐up of 5.72 years). 33 A higher incidence rate ratio (IRR) for HF was observed for women with HDP compared with the control cohort that varied slightly by follow‐up period with an IRR of 4.14 (95% CI: 4.07–4.27) for 6 to 10 years and an IRR of 5.32 (95% CI: 5.21–5.43) for more than 10 years following delivery. 33
Our study provides more information on HF among women with history of HDP or pre‐pregnancy hypertension and racial/ethnic differences. Women exposed to HDP may have underlying risk factors such as hypertension or overweight/obese BMI that could later manifest as CVD. However, as preeclampsia and CVD have common risk factors, more research is needed to determine their temporality. Pathophysiologic changes seen in preeclampsia include placental malperfusion, hypoxia, and oxidative stress, which for the early‐onset type is associated with incomplete spiral artery remodeling, although the heterogeneous pathogenesis is not completely understood. 34 , 35 , 36 Endothelial dysfunction and vasoconstriction can lead to hypertension and end‐organ hypoperfusion, with the resulting cardiovascular risk factors raising the risk of CVD later in life. 37
Even in the absence of hypertensive disease, NHB women may not tolerate well the physiological cardiovascular changes of pregnancy predisposing them to HF. Physiological effects may be present in NHB women with racism exposure throughout their life, such as dysfunctions in stress‐response that can lead to vascular changes during pregnancy. 38 , 39 The cardiovascular system is strained by an increased BMI, and in pregnancy, obesity has been linked to elevated blood pressure, dyslipidemia, hyperinsulinemia, and endothelial impairment. 40 , 41 Among non‐pregnant women in the U.S., NHB women have higher rates of obesity than those of other racial/ethnic groups including NHW women. 42 The highest prevalence of pre‐pregnancy obesity has been observed among NHB women and American Indian/Alaskan Native women. 43 , 44
Previous studies have described the association of preeclampsia with CVD and specifically HF. Associations were observed between preeclampsia (OR=4.1, 95% CI: 2.9–5.8) and gestational hypertension (OR=2.6, 95% CI: 1.5–4.5) with HF in the year following delivery by a NYC study. 45 A UK prospective study with a median of 7 years follow‐up reported an association between long‐term HF risk and history of HDP (HR,1.7, 95% CI: 1.04–2.60) following covariate adjustment. 11 The disparities identified in risk factors for HDP and vascular disease among racial/ethnic groups may also be evident for maternal outcomes. This study provides evidence for such differences in maternal pregnancy‐related CVD outcomes with the inclusion of 4 exposure groups focusing on HDP and pre‐pregnancy hypertension.
Strengths and Limitations
Limitations of our study include possible underestimation of HF incidence as HF was captured by hospital/ED visit and death certificate data given that many diagnoses are made as outpatients. As data were not available on type of HDP (e.g., gestational hypertension, preeclampsia), severity, early onset, or duration, we were unable to investigate relationships with subsequent HF risk. However, we adjusted for continuous gestational age at delivery, which potentially may be correlated with severity of HDP. We also adjusted for previous pre‐term delivery. Due to the data sources, we could not differentiate between HF with preserved versus reduced ejection fraction, assess the potential effect of residual hypertension after delivery, or discriminate gestational hypertension from preeclampsia. It was therefore not possible to individually examine the effect of each disorder on maternal incident HF subsequent to delivery. The use of ICD‐9/10‐CM diagnosis codes for preeclampsia and pre‐pregnancy hypertension has previously been reported to vary in accuracy, 46 and the number of women with pre‐pregnancy hypertension and/or preeclampsia may be underestimated. The inability to identify women who may have moved out of the state is also a limitation of our study which assumed women were healthy and not lost to follow‐up unless an event occurred or they were seen in the ED or hospital. Unfortunately, we were unable to include Hispanic women in racial‐ and ethnic‐specific analyses due to small numbers of events. Finally, power may be low for women in the pre‐pregnancy hypertension group, who had few events. There are many strengths of our statewide study, which included a diverse cohort of women with live, singleton births over a 13‐year period (2004–2016) and self‐reported race and ethnicity. NHB women, who made up a large proportion of the cohort, are a critical population that is currently understudied given their high maternal morbidity and mortality. HF was measured in the short term (within 1 year of delivery) and a longer term, within 5 years of delivery. This is critical because the data support starting screening for CVD in high‐risk women soon after delivery. The index pregnancy was defined as the first exposed (HDP and/or pre‐pregnancy hypertension) or unexposed (neither condition) pregnancy in our data set. While diagnoses prior to the index pregnancy and pregnancy history were unknown, birth certificate data included past birth information (e.g., previous Cesarean section), diagnoses at delivery (e.g., gestational hypertension, pre‐pregnancy hypertension), and covariates (e.g., pre‐pregnancy BMI, smoking during pregnancy). Another strength is adjustment of the receipt of prenatal care based on the R‐GINDEX, although the R‐GINDEX could not be calculated due to missing information for 28.4% of women.
Conclusions
In summary, our findings demonstrate strong associations between HDP and/or pre‐pregnancy hypertension exposure with incident HF up to 5 years following delivery, and the highest risk was found in the group with superimposed preeclampsia. Differences in incident HF risk were also observed by racial/ethnic group. Our study provides further evidence related to long‐term maternal effects of HDP and pre‐pregnancy hypertension, specifically for maternal HF including fatal and nonfatal events. These findings fill an important gap in the literature on racial/ethnic health differences in HDP and related‐maternal, long‐term cardiovascular outcomes. Future studies are needed to further examine racial/ethnic differences in maternal incident HF subsequent to delivery with consideration of pre‐pregnancy hypertension and/or HDP, and in particular the individual components of HDP as well as severity. Differentiation between HF with preserved versus reduced ejection fraction should also be studied. In addition, development of a prediction model to predict the risk of adverse maternal outcomes subsequent to delivery in women with and without HDP or pre‐pregnancy hypertension is of interest and could help guide intensified follow‐up.
Clinical and public health implications of this research could include motivation for screening for adverse maternal outcomes in women identified as high risk and prevention of maternal morbidity and mortality through changes to clinical practice including the reduction of modifiable cardiovascular risk factors.
Sources of Funding
This work was supported by a grant from the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) under Award Number K01HL138273. This project was also supported in part by the National Center for Advancing Translational Sciences (NCATS) of the NIH/under grant number UL1 TR001450. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NCATS.
Disclosures
None.
Supporting information
Table S1–S4
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021616
For Sources of Funding and Disclosures, see page 11.
References
- 1. Cunningham FG, Leveno KJ, Bloom SL. Williams Obstetrics. Toronto: McGraw Hill Medical; 2010. [Google Scholar]
- 2. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. DOI: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 3. Panaitescu AM, Syngelaki A, Prodan N, Akolekar R, Nicolaides KH. Chronic hypertension and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2017;50:228–235. DOI: 10.1002/uog.17493. [DOI] [PubMed] [Google Scholar]
- 4. Bateman BT, Bansil P, Hernandez‐Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206:134.e1–134.e8. DOI: 10.1016/j.ajog.2011.10.878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banhidy F, Acs N, Puho EH, Czeizel AE. The efficacy of antihypertensive treatment in pregnant women with chronic and gestational hypertension: a population‐based study. Hypertens Res. 2010;33:460–466. DOI: 10.1038/hr.2010.17. [DOI] [PubMed] [Google Scholar]
- 6. Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc. 2018;7:e009382. DOI: 10.1161/JAHA.118.009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, Smith GN, Gore GC, Ray JG, Nerenberg K, et al. Cardiovascular disease‐related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139:1069–1079. DOI: 10.1161/CIRCULATIONAHA.118.036748. [DOI] [PubMed] [Google Scholar]
- 8. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, Zaman A, Fryer AA, Kadam U, Chew‐Graham CA, et al. Preeclampsia and future cardiovascular health: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2017;10:e003497. DOI: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 9. Malek AM, Wilson DA, Turan TN, Mateus J, Lackland DT, Hunt KJ. Maternal coronary heart disease, stroke, and mortality within 1, 3, and 5 years of delivery among women with hypertensive disorders of pregnancy and pre‐pregnancy hypertension. J Am Heart Assoc. 2021;10:e018155. DOI: 10.1161/JAHA.120.018155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haug EB, Horn J, Markovitz AR, Fraser A, Klykken B, Dalen H, Vatten LJ, Romundstad PR, Rich‐Edwards JW, Asvold BO. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: analysis of the nord‐trondelag health study. JAMA Cardiol. 2019;4:628–635. DOI: 10.1001/jamacardio.2019.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott NS, Peloso GM, Natarajan P. Long‐term cardiovascular risk in women with hypertension during pregnancy. J Am Coll Cardiol. 2019;74:2743–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre‐eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta‐analysis. BMJ. 2007;335:974. DOI: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James‐Todd TM, Rich‐Edwards JW. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: an observational cohort study. Ann Intern Med. 2018;169:224–232. DOI: 10.7326/M17-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jonsdottir LS, Arngrimsson R, Geirsson RT, Sigvaldason H, Sigfusson N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74:772–776. DOI: 10.3109/00016349509021195. [DOI] [PubMed] [Google Scholar]
- 15. Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357:2002–2006. DOI: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 16. Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. DOI: 10.1136/bmj.326.7394.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wikstrom AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;112:1486–1491. DOI: 10.1111/j.1471-0528.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 18. Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre‐eclampsia: population based cohort study. BMJ. 2001;323:1213–1217. DOI: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. DOI: 10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daubert MA, Douglas PS. Primary prevention of heart failure in women. JACC Heart Fail. 2019;7:181–191. DOI: 10.1016/j.jchf.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 21. Honigberg MC, Riise HKR, Daltveit AK, Tell GS, Sulo G, Igland J, Klungsøyr K, Scott NS, Wood MJ, Natarajan P, et al. Heart failure in women with hypertensive disorders of pregnancy: insights from the cardiovascular disease in Norway project. Hypertension. 2020;76:1506–1513. DOI: 10.1161/HYPERTENSIONAHA.120.15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alexander GR, Kotelchuck M. Quantifying the adequacy of prenatal care: a comparison of indices. Public Health Rep. 1996;111:408–418; discussion 419. [PMC free article] [PubMed] [Google Scholar]
- 23. Tayebi T, Hamzehgardeshi Z, Ahmad Shirvani M, Dayhimi M, Danesh M. Relationship between revised graduated index (r‐gindex) of prenatal care utilization & preterm labor and low birth weight. Glob J Health Sci. 2014;6:131–137. DOI: 10.5539/gjhs.v6n3p131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. SAS Institute . Statistical analytical software. 2012.
- 25. StataCorp . Stata statistical software. 2013.
- 26. Theilen LH, Meeks H, Fraser A, Esplin MS, Smith KR, Varner MW. Long‐term mortality risk and life expectancy following recurrent hypertensive disease of pregnancy. Am J Obstet Gynecol. 2018;219:107.e1–107.e6. DOI: 10.1016/j.ajog.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lykke JA, Langhoff‐Roos J, Lockwood CJ, Triche EW, Paidas MJ. Mortality of mothers from cardiovascular and non‐cardiovascular causes following pregnancy complications in first delivery. Paediatr Perinat Epidemiol. 2010;24:323–330. DOI: 10.1111/j.1365-3016.2010.01120.x. [DOI] [PubMed] [Google Scholar]
- 28. Brown DW, Dueker N, Jamieson DJ, Cole JW, Wozniak MA, Stern BJ, Giles WH, Kittner SJ. Preeclampsia and the risk of ischemic stroke among young women: results from the stroke prevention in young women study. Stroke. 2006;37:1055–1059. DOI: 10.1161/01.STR.0000206284.96739.ee. [DOI] [PubMed] [Google Scholar]
- 29. Bhattacharya S, Prescott GJ, Iversen L, Campbell DM, Smith WC, Hannaford PC. Hypertensive disorders of pregnancy and future health and mortality: a record linkage study. Pregnancy Hypertens. 2012;2:1–7. DOI: 10.1016/j.preghy.2011.08.116. [DOI] [PubMed] [Google Scholar]
- 30. Arnaout R, Nah G, Marcus G, Tseng Z, Foster E, Harris IS, Divanji P, Klein L, Gonzalez J, Parikh N. Pregnancy complications and premature cardiovascular events among 1.6 million California pregnancies. Open Heart. 2019;6:e000927. DOI: 10.1136/openhrt-2018-000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kestenbaum B, Seliger SL, Easterling TR, Gillen DL, Critchlow CW, Stehman‐Breen CO, Schwartz SM. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am J Kidney Dis. 2003;42:982–989. DOI: 10.1016/j.ajkd.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 32. Garovic VD, White WM, Vaughan L, Saiki M, Parashuram S, Garcia‐Valencia O, Weissgerber TL, Milic N, Weaver A, Mielke MM. Incidence and long‐term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75:2323–2334. DOI: 10.1016/j.jacc.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen SN, Cheng CC, Tsui KH, Tang PL, Chern CU, Huang WC, Lin LT. Hypertensive disorders of pregnancy and future heart failure risk: a nationwide population‐based retrospective cohort study. Pregnancy Hypertens. 2018;13:110–115. DOI: 10.1016/j.preghy.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 34. Redman CWG, Staff AC, Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol. 2020;S0002‐9378(0020)31115‐31117. DOI: 10.1016/j.ajog.2020.09.047. [DOI] [PubMed] [Google Scholar]
- 35. Redman CW, Sargent IL, Staff AC. IFPA senior award lecture: making sense of pre‐eclampsia ‐ two placental causes of preeclampsia? Placenta. 2014;35(Suppl):S20–S25. DOI: 10.1016/j.placenta.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 36. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. DOI: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre‐eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63:1815–1822. DOI: 10.1016/j.jacc.2014.02.529. [DOI] [PubMed] [Google Scholar]
- 38. Hilmert CJ, Dominguez TP, Schetter CD, Srinivas SK, Glynn LM, Hobel CJ, Sandman CA. Lifetime racism and blood pressure changes during pregnancy: implications for fetal growth. Health Psychol. 2014;33:43–51. DOI: 10.1037/a0031160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stancil TR, Hertz‐Picciotto I, Schramm M, Watt‐Morse M. Stress and pregnancy among African‐American women. Paediatr Perinat Epidemiol. 2000;14:127–135. DOI: 10.1046/j.1365-3016.2000.00257.x. [DOI] [PubMed] [Google Scholar]
- 40. Gaillard R, Steegers EA, Hofman A, Jaddoe VW. Associations of maternal obesity with blood pressure and the risks of gestational hypertensive disorders. The Generation R Study. J Hypertens. 2011;29:937–944. DOI: 10.1097/HJH.0b013e328345500c. [DOI] [PubMed] [Google Scholar]
- 41. Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87:4231–4237. DOI: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 42. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults. United states, 2017–2018. Nchs data brief, no 360. 2020. [PubMed]
- 43. Fisher SC, Kim SY, Sharma AJ, Rochat R, Morrow B. Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev Med. 2013;56:372–378. DOI: 10.1016/j.ypmed.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre‐pregnancy obesity in nine states, 1993–2003. Obesity. 2007;15:986–993. DOI: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 45. Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy‐induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. 2014;180:41–44. DOI: 10.1093/aje/kwu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Geller SE, Ahmed S, Brown ML, Cox SM, Rosenberg D, Kilpatrick SJ. International classification of diseases‐9th revision coding for preeclampsia: how accurate is it? Am J Obstet Gynecol. 2004;190:1629–1633. DOI: 10.1016/j.ajog.2004.03.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S4


