Abstract
Background
Aortic stiffness is an independent predictor of cardiovascular events in patients with arterial hypertension. Resistant hypertension is often linked to hyperaldosteronism and associated with adverse outcomes. Spironolactone, a mineralocorticoid receptor antagonist, has been shown to reduce both the arterial blood pressure (BP) and aortic stiffness in resistant hypertension. However, the mechanism of aortic stiffness reduction by spironolactone is not well understood. We hypothesized that spironolactone reduces aortic stiffness in resistant hypertension independently of BP change.
Methods and Results
Patients with uncontrolled BP (≥140/90 mm Hg) despite use of ≥3 antihypertensive medications (including diuretics) were prospectively recruited. Participants were started on spironolactone at 25 mg/d, and increased to 50 mg/d at 4 weeks while other antihypertensive medications were withdrawn to maintain constant mean BP. Phase‐contrast cardiac magnetic resonance imaging of the ascending aorta was performed in 30 participants at baseline and after 6 months of spironolactone treatment to measure aortic pulsatility, distensibility, and pulse wave velocity. Pulse wave velocity decreased (6.3±2.3 m/s to 4.5±1.8 m/s, P<0.001) and pulsatility and distensibility increased (15.9%±5.3% to 22.1%±7.9%, P<0.001; and 0.28%±0.10%/mm Hg to 0.40%±0.14%/mm Hg, P<0.001, respectively) following 6 months of spironolactone.
Conclusions
Our results suggest that spironolactone improves aortic properties in resistant hypertension independently of BP, which may support the hypothesis of an effect of aldosterone on the arterial wall. A larger prospective study is needed to confirm our findings.
Keywords: aorta, hyperaldosteronism, resistant hypertension, spironolactone
Subject Categories: Clinical Studies, Hypertension
Nonstandard Abbreviations and Acronyms
- AD
aortic distensibility
- AP
aortic pulsatility
- AS
aortic stiffness
- PAC
plasma aldosterone concentration
- PWV
pulse wave velocity
- RHTN
resistant hypertension
Clinical Perspective
What Is New?
Data suggest that spironolactone improves aortic properties in patients with resistant hypertension independently of blood pressure change.
What Are the Clinical Implications?
Spironolactone is recommended in patients with resistant hypertension and thus results from our study are directly relevant to clinical practice.
Our finding that the improvement of arterial elastic properties in patients with hypertension undergoing aldosterone antagonist treatment can occur independently of its effect on blood pressure, if confirmed in a larger cohort, may lead to reconsideration of approaches for evaluation of the therapeutic efficacy of spironolactone in clinical practice.
Hypertension is the most prevalent risk factor for other cardiovascular disease, stroke, and renal disease, and one of the leading causes of death in the United States. 1 Using the new thresholds from the 2017 American College of Cardiology/American Heart Association guidelines, the prevalence of hypertension is 45.6% among US adults. 2 A substantial proportion of these patients do not achieve a target goal of <130/80 mm Hg. 3 Various factors account for poor blood pressure (BP) control, including lack of treatment, nonadherence to recommended treatment, and resistance to guideline‐directed medical therapy. 4 , 5 Resistant hypertension (RHTN) is defined as BP that remains above goal despite concurrent use of 3 antihypertensive agents of different classes, of which one is ideally a diuretic, all prescribed at maximum recommended or maximally tolerated dosage. 6 Among adults with treated hypertension, apparent RHTN occurs in 12% to 15% of population‐based and 15% to 18% of clinic‐based reports. 7 , 8 Patients with hypertension have a high mortality rate, and aortic stiffness (AS) is an independent predictor of all‐cause and cardiovascular mortality. 9 , 10 Moreover, the presence of AS predicts cardiovascular events in the general population, even in the absence of hypertension or cardiovascular disease. 11 , 12
Hyperaldosteronism (defined as plasma renin activity <1.0 ng/mL per hour and a urinary aldosterone level >12 μg/24 h during high urinary sodium excretion [>200 mEq/24 h]) is a common cause of RHTN. 13 , 14 Aldosterone is both a key hormone for volume homeostasis and a contributor to target organ damage. 15 Aldosterone excess increases aortic wall stiffness independent of mechanical stress. 15 Spironolactone is a mineralocorticoid receptor antagonist that has been shown to reduce mortality in patients with heart failure, and some of this benefit has been attributed to extrarenal effects on inhibiting fibrosis. 16 , 17 , 18 As spironolactone has been shown to reduce collagen synthesis and fibrosis, 16 we hypothesize that it has a beneficial effect on AS. However, any such effect cannot be easily demonstrated in the clinical setting because spironolactone is a potent antihypertensive agent often used to treat hypertension, including RHTN. 19 , 20 The antihypertensive effects of spironolactone may confound efforts to investigate vascular actions, which may be independent of BP lowering. In fact, BP reduction itself appears to improve aortic compliance in patients with hypertension. 21 Mahmud and Feely 22 have shown that, compared with thiazide diuretics, spironolactone leads to greater reduction in BP as well as improvement in arterial stiffness in patients with hypertension. As measures of AS are directly correlated with the BP profile, the study by Mahmud et al was not able to identify any BP‐independent effect of spironolactone on AS.
To test our hypothesis that spironolactone reduces AS independent of its effect on BP, we analyzed data from a prospective study in patients with RHTN. 23 Spironolactone was introduced and force‐titrated upward while other antihypertensive medications were withdrawn, to maintain the patient's original BP level. The AS indicators pulse wave velocity (PWV), aortic distensibility (AD), and aortic pulsatility (AP) were measured using cardiac magnetic resonance (CMR) imaging at baseline and after 6 months of spironolactone treatment in order to determine the spironolactone‐dependent, BP‐independent changes in aortic properties.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population and Study Design
Study participants (n=30, 54±7 years) were from a group of 45 consecutive patients referred for RHTN to the University of Alabama at Birmingham (UAB) Hypertension Clinic, who agreed to participate in 6‐month follow‐up research on spironolactone treatment (including CMR imaging) for assessment of the role of hyperaldosteronism in cardiac volume overload in RHTN. 23 Those with dedicated phase‐contrast velocity mapping CMR imaging of the ascending aorta were included in the present analysis. RHTN was defined as BP >140/90 mm Hg at 2 clinic visits in spite of use of 3 antihypertensive medications, including a diuretic, at pharmacologically effective doses. Patients with a history of heart failure, primary hyperaldosteronism before enrollment, chronic kidney disease, or chronic steroid therapy were excluded. Patients with secondary causes of hypertension other than hyperaldosteronism, such as renovascular hypertension, pheochromocytoma, or Cushing syndrome were also excluded. At the time of enrollment, all patients had been taking a stable antihypertensive regimen for ≥4 weeks. Clinical, biochemical and CMR imaging studies were performed at baseline and after 6 months of spironolactone treatment. In the present analysis, 5 participants were excluded as their baseline or 6‐month CMR imaging studies lacked the phase‐contrast images used for assessing AS (Figure 1). Another 10 patients did not complete the 6‐month follow‐up study after enrolling because of spironolactone intolerance (n=1), increased creatinine (n=1), hyperkalemia (n=1), uncontrolled BP (n=3), noncompliance to the study protocol (n=2), claustrophobia to CMR imaging study (n=1), and voluntary withdraw for adrenal venous sampling and adrenalectomy (n=1) as briefly previously described. 23 Demographic, baseline clinical characteristics, and measurements of the excluded and included participants were similar (Table S1). The study was approved by UAB's institutional review board and was conducted according to institutional guidelines. All participants provided written informed consent.
Figure 1. Flowchart of the study.
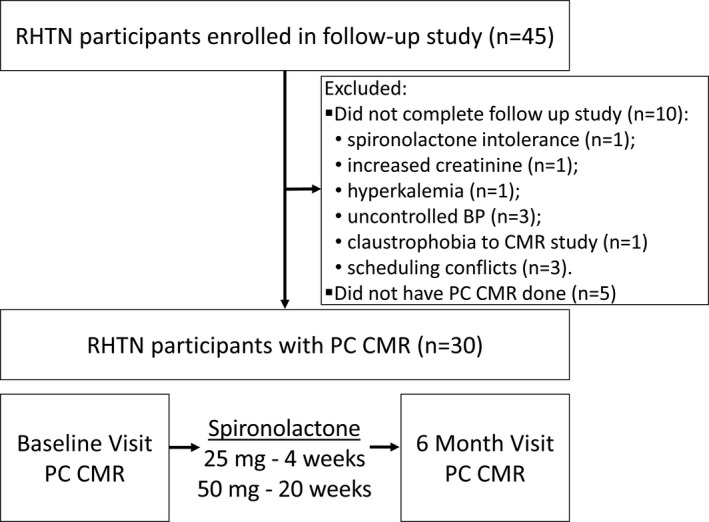
BP indicates blood pressure; CMR, cardiac magnetic resonance; PC, phase‐contrast; and RHTN, resistant hypertension. Please see Methods for details.
Spironolactone Treatment and Withdrawal of Other Antihypertensive Drugs
All participants were started on spironolactone 25 mg/d in addition to other antihypertensive medications and force‐titrated to 50 mg/d at 4 weeks. After addition of spironolactone, other antihypertensive medications were withdrawn as needed to maintain constant BP. The sequence of withdrawal was as follows: centrally acting agents or vasodilators first, followed by β‐blockers, calcium channel blockers, and renin‐angiotensin system blockers.
BP Measurements
BP was noninvasively measured using a manual brachial mercury sphygmomanometer and an appropriately sized cuff after 5 minutes of rest. During each CMR imaging study, BP measurements were performed twice, before scanning and immediately after completion of scanning; these were performed in the scan room but outside the CMR imaging instrument. The average of 2 readings was recorded and used for analysis. The average pulse pressure (PP) was calculated as the difference in average systolic and average diastolic BP.
Biochemical Testing
Plasma aldosterone concentration (PAC), plasma renin activity (PRA), brain natriuretic peptide, serum potassium, and creatinine levels were measured in the morning between 8 am and 9 am with the patient in the upright sitting position. A 24‐hour urine collection for aldosterone, cortisol, sodium, and creatinine was performed. PAC, PRA, and 24‐hour urinary aldosterone was analyzed using liquid chromatography‐tandem mass spectrometry (Mayo Medical Laboratories) with laboratory reference levels as follows: high PAC ≥16 ng/dL, high 24‐hour urinary aldosterone ≥12 μg/24‐hour, and suppressed PRA <1 ng/mL per hour.
CMR Imaging
CMR imaging was performed with a 1.5‐T scanner optimized for cardiac imaging (Signa, GE Healthcare) using a 4‐element phased‐array surface coil and prospective ECG triggering. Cine imaging for left ventricular (LV) volume and function analysis was performed using a rapid steady‐state free precession cine sequence (FIESTA; 10 lines per k‐space segment). Standard 2‐ and 4‐chamber, and short‐axis views were obtained from appropriate scout images. The following typical parameters were used: matrix size, 256×128; field of view, 40×40 cm; slice thickness, 8 mm without gaps; repetition time, 3.9 ms; echo time, 1.6 ms; flip angle 45°; bandwidth 125 Hz/pixel; and typical acquired temporal resolution, 39 ms. Cine images were reconstructed into 20 cardiac phases. Mass Analysis Plus (version 5.1; Medis) software was used to evaluate LV volumes and function.
For analysis of AS, a single end‐expiratory breath‐hold, ECG‐gated phase contrast acquisition image plane oriented perpendicularly intersecting the ascending aorta was performed with 32 cardiac phases reconstructed. Contours of the ascending aorta were automatically created during all phases of the cardiac cycle using CAAS MR Flow 1.2 (Pie Medical Imaging). Contours were then manually corrected, if needed. The maximum and minimum cross‐section areas of the ascending aorta were measured. Aortic flow‐time curves and cross‐section area‐time curves were extracted for further analysis (Figure 2).
Figure 2. Representative example of phase‐contrast cardiac magnetic resonance imaging of the ascending aorta cross‐section and measurements of aortic stiffness estimates in a patient.
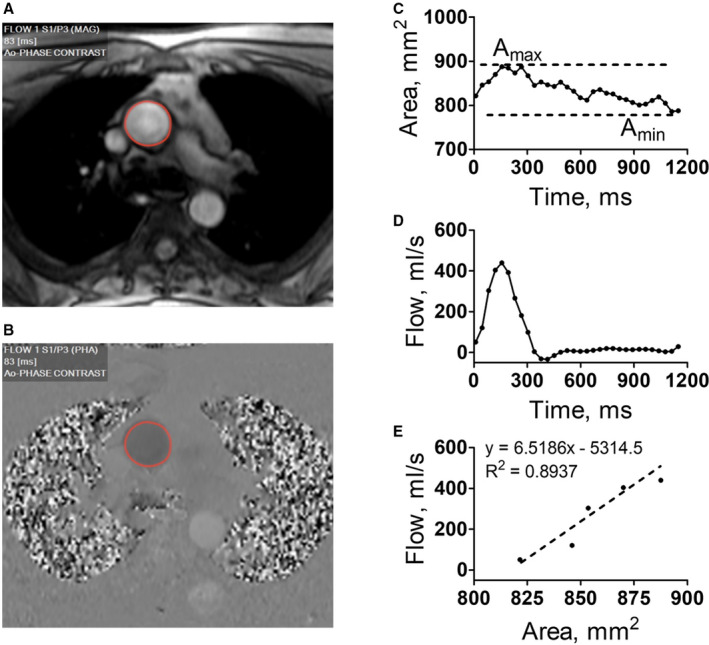
A and B, Reconstructed magnitude (MAG) and velocity‐sensitive phase (PHA) images with automatically detected contours of the ascending aorta (Ao). C, Plot depicting ascending aorta cross‐section area change over cardiac cycle. Maximum and minimum areas (Amax and Amin) are used to calculate aortic pulsatility and distensibility (see Methods). D, Plot depicting ascending aorta flow over cardiac cycle. E, Scatterplot of early systolic (acceleration) phase of flow change vs area change in ascending aorta cross‐section. The slope of best‐fit linear regression was measured as aortic pulse wave velocity (see Methods).
Calculation of the Indices of Arterial Stiffness
Ascending AP, the relative change in lumen area during the cardiac cycle, was calculated using the equation (Figure 2) 24 : AP (%)=(Amax−Amin)/Amin×100%; where Amax and Amin are the maximal and minimal calculated ascending aorta cross‐section areas obtained during the cardiac cycle.
Ascending AD, the relative change in lumen area per unit change in pressure, was calculated using the equation 24 , 25 : AD (%/mm Hg)=AP (%)/PP.
PWV, a rate at which the systolic bolus of blood, pumped from the heart, travels through the vasculature, was calculated using the flow‐area (QA) method (Figure 2) 26 , 27 : PWV=∆Q/∆A; where ∆Q is the change of flow across a vessel and ∆A is the change in the cross‐sectional ascending aorta area during the acceleration phase of the systole. PWV was calculated from the plot of the ascending aortic flow versus cross‐section area as the slope (m/s) of the best‐fit line to the early systolic portion of the plot (acceleration phase). 26 , 27
Statistical Analysis
Baseline and follow‐up CMR imaging measurements, including aorta contouring and calculation of AS parameters, were performed blind without knowledge of other clinical data or the time point. Descriptive analyses were performed to summarize the demographics, comorbidities, and clinical and biochemical characteristics of study participants. Paired t test was used to compare values for biochemistry, BP, medications and CMR imaging findings at baseline and at 6 months of spironolactone treatment. Multivariable linear regression models were used to assess the relationship between the CMR imaging–derived indicators of AS in patients with RHTN adjusted separately for demographic factors (age, 28 sex, 29 race, 30 and hyperaldosteronism 14 ), for basic cardiac function/hemodynamic factors (LV ejection fraction, 31 LV stroke volume, 32 heart rate, 33 mean arterial pressure, 34 and PP 34 ), and for biochemical factors (serum creatinine, 35 serum potassium, 36 , 37 PRA, 38 and brain natriuretic peptide 39 ). All values are represented as mean±SD, and P<0.05 was considered statistically significant for 2‐sided tests. All analyses were performed using GraphPad Prism version 5.01 (GraphPad Software) and SPSS version 25 (IBM).
RESULTS
Baseline Characteristics
Of 45 participants enrolled, only 30 completed phase‐contrast CMR imaging at baseline and at 6‐month follow‐up (Figure 1). At baseline, the participants were aged 53.7±6.7 years, 20 of 30 (66.7%) were men, and 19 of 30 (63.3%) were of Black race. Eighteen of 30 (60.0%) were diagnosed with hyperaldosteronism, 20 (66.7%) had obstructive sleep apnea, and 9 (30.0%) had diabetes mellitus. The mean±SD values of the group were: PAC (14.1±6.4 ng/dL), PRA (1.0±0.8 ng/mL per hour), PAC/PRA ratio (21.7±19.5), 24‐hour urine aldosterone (16.0±7.4 µg), and 24‐hour urine sodium (194±75 mmol) (Tables 1 and 2).
Table 1.
Baseline Demographics, Comorbidities, and Biochemistry in Patients With Resistant Hypertension
| Demographics | |
| Age, y | 53.6±6.7 |
| Men | 20 (66.7) |
| Black race | 19 (63.3) |
| Comorbidities | |
| Hypertension duration, y | 20.9±10.7 |
| Hyperaldosteronism | 18 (60.0) |
| Obstructive sleep apnea | 20 (66.7) |
| Diabetes mellitus | 9 (30.0) |
| Coronary artery disease | 1 (3.3) |
| Measurements | |
| Body mass index, kg/m2 | 32.9±4.8 |
| Fat percentage | 33.9±8.2 |
| Neck, cm | 42.9±4.1 |
| Waist, inch | 42.8±5.0 |
| Biochemistry | |
| Plasma aldosterone, ng/dL | 14.1±6.4 |
| Plasma aldosterone—PRA ratio | 21.7±19.5 |
| 24‐h Urine aldosterone, µg | 16.0±7.4 |
| 24‐h Urine protein, mg | 346±769 |
| 24‐h Urine cortisol, µg | 151±76 |
| 24‐h Urine sodium, mmol | 194±75 |
| 24‐h Urine potassium, mmol | 73.2±26.6 |
| 24‐h Urine calculated creatinine, mg | 1622±464 |
Values are expressed as mean±SD or number (percentage). PRA indicates plasma renin activity.
Table 2.
Biochemistry, Clinic BPs, and Total Medications in Patients With Resistant Hypertension at Baseline and at 6 Months of Spironolactone Treatment
| Measurements | Baseline | Spironolactone | P value |
|---|---|---|---|
| Biochemistry | |||
| Serum creatinine, mg/dL | 1.07±0.25 | 1.15±0.29 | 0.023 |
| Serum potassium, mmol/L | 3.77±0.36 | 4.24±0.40 | <0.001 |
| PRA, ng/mL per h | 1.0±0.8 | 9.2±13.3 | 0.002 |
| Brain natriuretic peptide, pg/mL | 33.7±34.3 | 16.9±15.8 | 0.001 |
| Blood pressure | |||
| Systolic BP, mm Hg | 142±17 | 138±21 | 0.342 |
| Diastolic BP, mm Hg | 83±12 | 81±14 | 0.564 |
| Pulse pressure, mm Hg | 59.5±12.6 | 57.2±15.2 | 0.320 |
| Mean arterial pressure, mm Hg | 103±13 | 100±15 | 0.450 |
| Heart rate, beats per min | 68.5±12.2 | 69.7±13.2 | 0.544 |
| Total antihypertensive medications* | 4.4±1.2 | 2.7±1.1 | <0.001 |
BP indicates blood pressure; and PRA, plasma renin activity.
Spironolactone not included.
Changes in Biochemistry
Serum creatinine, serum potassium, and PRA significantly increased after 6 months of spironolactone treatment, with a reduction of brain natriuretic peptide (Table 2).
Changes in Hemodynamic Parameters
There was no significant change in systolic or diastolic BP, PP, or heart rate from baseline to 6 months of spironolactone treatment (Table 2).
Changes in Antihypertensive Medications
There was a significant reduction in number of other antihypertensive medications needed to maintain baseline BP from 4.4±1.2 at baseline to 2.7±1.1 at 6 months after addition of spironolactone (P<0.001, Table 2).
Changes in LV Function
No significant change in LV ejection fraction occurred after 6 months of spironolactone intervention (65.9±6.5% at baseline versus 66.9±6.7% at 6 months, P=0.360). There was a reduction of LV volumes from baseline to 6 months after spironolactone intervention (end‐diastolic volume: 165±38 mL versus 153±39 mL, P=0.020; end‐systolic volume: 55±17 mL versus 51±23 mL, P=0.331; stroke volume: 110±25 mL versus 101±21 mL, P=0.041). Cardiac output was not changed (7.4±1.6 L/min versus 7.1±1.3 L/min, P=0.290).
Changes in AS
Reference individual phase‐contrast CMR imaging–derived measurements of ascending aorta maximum and minimum cross‐sectional area, PWV, systolic and diastolic BP, and basic patient characteristics are presented in Table S2. Overall, there was a significant decrease in ascending aorta PWV (6.3±2.3 m/s to 4.5±1.8 m/s, P<0.001 [unadjusted]) and significant increases in ascending AP (15.9%±5.3% to 22.1%±7.9%, P<0.001 [unadjusted]) and distensibility (0.28%±0.10%/mm Hg to 0.40%±0.14%/mm Hg, P<0.001 [unadjusted]) following 6 months of spironolactone treatment (Figure 3A through 3C). These values of estimates of ascending aorta stiffness at baseline and after spironolactone treatment were similar to the corresponding values measured in patients who had either only baseline measurements or only follow‐up phase‐contrast CMR imaging measurements, and thus were excluded from the primary study analysis (Table S3).
Figure 3. Effect of spironolactone on the ascending aorta pulsatility, distensibility, and pulse wave velocity (PWV).
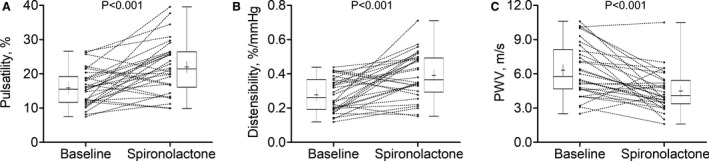
Ascending aorta pulsatility (A), distensibility (B), and PWV (C) at baseline and after spironolactone intervention in individual patients (connected lines) and the group (box and whiskers; whiskers represent maximum and minimum; box edges represent 25th and 75th percentiles; center line the median, and cross the mean).
Multivariable Regression
A multivariable linear regression model adjusted for effects of age, sex, race, and hyperaldosteronism shows that the difference in ascending aorta pulsatility and distensibility between baseline and 6 months was significantly associated with sex (Figure S1). The increase in ascending AP and AD in men was less than that in women (pulsatility: 3.5%±6.5% in men versus 11.5%±8.3% in women, P=0.008; distensibility: 0.08%±0.12%/mm Hg in men versus 0.20%±0.16%/mm Hg in women, P=0.027). The change in PWV was not sex dependent (Figure S1). In this model, race, hyperaldosteronism, and age (within the ranges studied) did not significantly affect spironolactone‐related changes of the AS estimates (Figure S1). In separate multivariable linear models adjusted for effects of LV ejection fraction, stroke volume, heart rate, mean arterial pressure, and PP, and for effects of serum creatinine, serum potassium, PRA, and brain natriuretic peptide, none of these factors had significant effects on differences in the AS estimates (Figures S2 and S3, respectively).
DISCUSSION
This study is the first to show an effect of spironolactone on AS without a change in systemic BP in patients with RHTN, with increases in AP and AD, accompanied by a decrease in PWV. These changes in noninvasive estimates of AS suggest an improvement in elastic properties of the aorta with spironolactone administration.
Aldosterone, the primary endogenous ligand for the mineralocorticoid receptor, causes BP elevation as a result of changes in arteriolar vasoactive tone and sodium homeostasis, and has been shown to play an important role in the pathogenesis of RHTN. 40 Aldosterone excess leads to collagen accumulation and fibrosis in the left ventricle and aortic wall, and immunohistochemical evidence suggests that aldosterone receptors are present in the aorta. 38 , 41 Aldosterone also increases arterial stiffness and PP in salt‐fed rats through alteration in the elastin and collagen densities, an effect that was prevented by treatment with a mineralocorticoid receptor antagonist. 42 However, data regarding the impact of aldosterone on vascular changes in humans are scant. 22 , 43 Aldosterone levels are elevated in 10% of patients with essential hypertension and up to 15% to 20% of patients with RHTN. 13 Aldosterone exacerbates oxidative stress and inflammation in vascular tissue, with adverse effects on endothelial function that lead to increased vascular stiffness, atherosclerosis, and ultimately to worsening of cardiovascular disease outcomes. 44
AS is recognized as a major cardiovascular risk factor in individuals with hypertension. 9 , 10 Population studies, including the Rotterdam study, the Framingham Heart Study, and the Health ABC study all arrived at a common finding of increased AS (measured by Doppler flow–derived carotid‐femoral PWV) associated with increased cardiovascular morbidity and mortality after adjusting for traditional risk factors. 11 , 45 , 46 The most extensively studied marker of AS, the carotid‐femoral PWV, has proven to be a robust predictive marker for assessing future cardiovascular events and all‐cause mortality beyond classical risk predictors such as the Framingham Risk Score and BP. 47 A recent post hoc analysis of SPRINT (Systolic Blood Pressure Intervention Trial) data by Vlachopoulos et al 48 that utilized estimated PWV, calculated based on patient's age and mean BP, found better survival in individuals whose estimated PWV responded to antihypertensive treatment independently of systolic BP reduction. This finding suggests a role for markers of AS as surrogate treatment targets in patients with hypertension. 48 Here, we utilized several CMR‐derived measures of arterial stiffness, including aortic PWV, pulsatility, and distensibility. Since the first reported assessment of PWV by CMR imaging in 1989, 49 segmental and single‐point methodology has been validated against tonometry 50 , 51 , 52 , 53 and used extensively in multiple clinical studies. 25 , 26 , 27 These indices of arterial stiffness measured by CMR imaging have emerged as reliable measures of vascular function with useful prognostic information. 28 , 54
Increasing AS reduces AD, AP, and aortic compliance, and increases PWV. Aldosterone antagonism could ameliorate this increase in stiffness by either reducing sodium ion reabsorption, 55 increasing potassium levels, 56 or inhibiting fibrosis. 16 Mahmud and Feely 22 have shown that administration of spironolactone to untreated patients with essential hypertension leads to reduction in radial artery stiffness. However, because of a significant decrease in BP in those study participants, it was difficult to differentiate a possible effect of spironolactone on AS from its antihypertensive effect. 22 It has been shown that BP affects indices of AS. 21 Therefore, in the present study where BP was constrained to not change with therapy, we were able to assess the effect of spironolactone on AS independently of its antihypertensive effects.
The results of our study extend the information from previous studies in animals 57 and humans 58 showing that spironolactone has beneficial effects beyond BP lowering alone. Our data suggest that these unique properties of aldosterone, rather than its hypertensive effect, cause (or at least contribute) to physiology of the aortic vascular smooth muscle, leading to increased stiffness. The presence of aldosterone receptors in the aorta and other vessels also suggests a local action of mineralocorticoid receptor antagonism in the vasculature. 59 Potentially supporting this conjecture, in a study using female mice, low‐dose spironolactone was shown to prevent the pathological aortic stiffening induced by a Western diet caused by blockade of vascular endothelial mineralocorticoid receptors. 60 , 61
Increased AS has been reported to have different prognostic implications in men and women older than 55 years, with a 2‐fold stronger association with mortality in women than in men. 62 Proximal AS is greater in women than men, 29 which may contribute to the greater risk of heart failure with preserved LV ejection fraction in women. 63 Recent CMR imaging and echocardiographic studies also reported a much faster decline in AD and arterial compliance in aging women than in men, despite no sex‐related difference in PWV increase. 64 , 65 , 66 We undertook a multivariate analysis exploring the possible effects of multiple demographic and physiologic features on the stiffness parameters, and this suggested that the response to spironolactone treatment may be influenced by sex. However, the small study size, including a low number of women, limits our ability to infer an actual sex effect. In this respect, our potential finding of a greater effect of spironolactone in improving proximal AD and AP (although not PWV) in women warrants further research and confirmation. We are aware that a sex‐specific relationship of aldosterone to cardiac structure and cardiovascular disease and a role of female steroid sex hormones in effects of aldosterone have been reported. 67 , 68 Furthermore, in the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial, spironolactone therapy in patients with heart failure with preserved LV ejection fraction was associated with reduced all‐cause mortality in women but not in men, 69 which also calls for more research in sex‐specific clinical care in cardiovascular disease.
Study Limitations
Because of our relatively small sample size and other limitations, our results should be cautiously interpreted. Inclusion of an additional control group in which the medications are not changed or adjusted during spironolactone treatment could potentially shed additional light on the effect size and enable discernment of how much of an effect BP lowering could have on AS, in addition to any spironolactone effect on the vascular wall. We did not pursue this question, as these effects have already been partially explored. 22 , 70 However, we were able to explore the effects of possible demographic, hemodynamic, and biochemical interactions on our results, using multivariate regression analysis. Although none of the hemodynamic and biochemical factors we tested showed a significant role in the model, we cannot exclude potential additive contributing effects of some of the tested or other (untested here) factors affecting the changes in AS induced by spironolactone treatment. Nevertheless, our results are consistent with previously published effects of antihypertensive drugs in long‐term trials. 71 , 72 , 73 The majority of our study cohort was composed of Black participants. Although the prevalence of RHTN in Black individuals is higher than in other races, 74 , 75 the generalizability of our findings to other patient populations with a different racial makeup might be limited. There are reports suggesting variances in some responses to spironolactone in different racial groups, especially in respect to patients with heart failure. 76 , 77 , 78 , 79 However, in the patients with RHTN, race was not significantly associated with BP response to spironolactone or electrolyte changes. 20 , 80 Also, the observed sex differences of spironolactone effects on aortic properties may be a chance finding, attributable to the lower female prevalence in the study cohort. In addition, the complex effect of aortic wave reflections, which are significant determinants of central aortic pressure and are typically different in men versus women (because of different height‐related aortic arch length), 81 were not accounted for in this study. Accuracy of CMR imaging–measured AS estimates is subject to several systematic limitations, including relatively limited temporal resolution and effects of through‐plane motion caused by ventricular contraction. In addition, BP was not simultaneously measured with CMR imaging, but instead was assessed at the beginning and end of the CMR imaging examination. These limitations are potential sources of random error effects. Also (although similar to other clinical studies), we used brachial rather than central BP for calculation of AD. 25 , 64 Potentially, this could be mitigated by employing validated, commercially available, noninvasive methods for assessment of aortic pressure waveforms. 82 These limitations exist, but because they are unbiased with respect to comparison of baseline and follow‐up measurements, their impact is somewhat mitigated.
Conclusions
The results of our study suggest that the arterial stiffening in patients with RHTN may be, at least in part, caused by an effect of aldosterone on the vascular wall, independent of the elevation in BP, and is reversible with spironolactone treatment, independent of spironolactone's effects on BP reduction. Because of the exploratory study design and other limitations, our results should be considered hypothesis generating and cannot be generalized to a larger cohort without validation.
Sources of Funding
This study was supported by The National Heart, Lung, and Blood Institute Specialized Centers of Clinically Oriented Research program P50 HL077100, National Institutes of Health (NIH) RO1‐HL79040, and General Clinical Research Centers grant M01RR00032. Drs Gaddam and Lloyd were supported by NIH T32 HL007457 and the American College of Cardiology Foundation/GE Healthcare Career Development Award, respectively.
Disclosures
Dr Oparil reports research grants from Bayer, George Medicines Pty Limited, and Idorsia Pharmaceuticals, reports ad hoc lecture honoraria from Ascension, reports personal fees from Preventric Diagnostics, Inc as Chief Medical Officer and from Cincor Pharma Inc as Scientific Advisory Board Member, and reports annual stipend as Editor‐in‐Chief for Current Hypertension Reports (Springer Science Business Media LLC), all outside of the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figures S1–S3
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019434
For Sources of Funding and Disclosures, see page 9.
References
- 1. Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon‐Moran D. Hypertension prevalence and control among adults: United states, 2015‐2016. NCHS Data Brief. 2017;289:1–8. [PubMed] [Google Scholar]
- 2. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137:109–118. DOI: 10.1161/CIRCULATIONAHA.117.032582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2018;138:e484–e594. DOI: 10.1161/CIR.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 4. Pierdomenico SD, Lapenna D, Bucci A, Di Tommaso R, Di Mascio R, Manente BM, Caldarella MP, Neri M, Cuccurullo F, Mezzetti A. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18:1422–1428. DOI: 10.1016/j.amjhyper.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 5. Sarafidis PA, Georgianos P, Bakris GL. Resistant hypertension–its identification and epidemiology. Nat Rev Nephrol. 2013;9:51–58. DOI: 10.1038/nrneph.2012.260. [DOI] [PubMed] [Google Scholar]
- 6. Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison‐Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–e90. DOI: 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–1080. DOI: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 8. Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–1058. DOI: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. DOI: 10.1161/01.HYP.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 10. Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. DOI: 10.1161/01.HYP.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 11. Sutton‐Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well‐functioning older adults. Circulation. 2005;111:3384–3390. DOI: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 12. Willum‐Hansen T, Staessen JA, Torp‐Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. DOI: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 13. Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. DOI: 10.1161/01.HYP.0000040261.30455.B6. [DOI] [PubMed] [Google Scholar]
- 14. Martins LC, Figueiredo VN, Quinaglia T, Boer‐Martins L, Yugar‐Toledo JC, Martin JF, Demacq C, Pimenta E, Calhoun DA, Moreno H Jr. Characteristics of resistant hypertension: ageing, body mass index, hyperaldosteronism, cardiac hypertrophy and vascular stiffness. J Hum Hypertens. 2011;25:532–538. DOI: 10.1038/jhh.2010.95. [DOI] [PubMed] [Google Scholar]
- 15. Stewart PM. Mineralocorticoid hypertension. Lancet. 1999;353:1341–1347. DOI: 10.1016/S0140-6736(98)06102-9. [DOI] [PubMed] [Google Scholar]
- 16. Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102:2700–2706. DOI: 10.1161/01.CIR.102.22.2700. [DOI] [PubMed] [Google Scholar]
- 17. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. DOI: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 18. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA. 2013;309:781–791. DOI: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 19. Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, Poulter NR; Anglo‐Scandinavian Cardiac Outcomes Trial I . Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845. DOI: 10.1161/01.HYP.0000259805.18468.8c. [DOI] [PubMed] [Google Scholar]
- 20. Nishizaka MK, Zaman MA, Calhoun DA. Efficacy of low‐dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–930. DOI: 10.1016/S0895-7061(03)01032-X. [DOI] [PubMed] [Google Scholar]
- 21. Ripley DP, Negrou K, Oliver JJ, Worthy G, Struthers AD, Plein S, Greenwood JP. Aortic remodelling following the treatment and regression of hypertensive left ventricular hypertrophy: a cardiovascular magnetic resonance study. Clin Exp Hypertens. 2015;37:308–316. DOI: 10.3109/10641963.2014.960974. [DOI] [PubMed] [Google Scholar]
- 22. Mahmud A, Feely J. Aldosterone‐to‐renin ratio, arterial stiffness, and the response to aldosterone antagonism in essential hypertension. Am J Hypertens. 2005;18:50–55. DOI: 10.1016/j.amjhyper.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 23. Gaddam K, Corros C, Pimenta E, Ahmed M, Denney T, Aban I, Inusah S, Gupta H, Lloyd SG, Oparil S, et al. Rapid reversal of left ventricular hypertrophy and intracardiac volume overload in patients with resistant hypertension and hyperaldosteronism: a prospective clinical study. Hypertension. 2010;55:1137–1142. DOI: 10.1161/HYPERTENSIONAHA.109.141531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanz J, Kariisa M, Dellegrottaglie S, Prat‐Gonzalez S, Garcia MJ, Fuster V, Rajagopalan S. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging. 2009;2:286–295. DOI: 10.1016/j.jcmg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 25. van der Meer RW, Diamant M, Westenberg JJ, Doornbos J, Bax JJ, de Roos A, Lamb HJ. Magnetic resonance assessment of aortic pulse wave velocity, aortic distensibility, and cardiac function in uncomplicated type 2 diabetes mellitus. J Cardiovasc Magn Reson. 2007;9:645–651. DOI: 10.1080/10976640601093703. [DOI] [PubMed] [Google Scholar]
- 26. Ibrahim el SH, Johnson KR, Miller AB, Shaffer JM, White RD. Measuring aortic pulse wave velocity using high‐field cardiovascular magnetic resonance: comparison of techniques. J Cardiovasc Magn Reson. 2010;12:26. DOI: 10.1186/1532-429X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vulliemoz S, Stergiopulos N, Meuli R. Estimation of local aortic elastic properties with MRI. Magn Reson Med. 2002;47:649–654. DOI: 10.1002/mrm.10100. [DOI] [PubMed] [Google Scholar]
- 28. Resnick LM, Militianu D, Cunnings AJ, Pipe JG, Evelhoch JL, Soulen RL. Direct magnetic resonance determination of aortic distensibility in essential hypertension: relation to age, abdominal visceral fat, and in situ intracellular free magnesium. Hypertension. 1997;30:654–659. DOI: 10.1161/01.HYP.30.3.654. [DOI] [PubMed] [Google Scholar]
- 29. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61:96–103. DOI: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morris AA, Patel RS, Binongo JNG, Poole J, Mheid IA, Ahmed Y, Stoyanova N, Vaccarino V, Din‐Dzietham R, Gibbons GH, et al. Racial differences in arterial stiffness and microcirculatory function between black and white Americans. J Am Heart Assoc. 2013;2:e002154. DOI: 10.1161/JAHA.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohyama Y, Ambale‐Venkatesh B, Noda C, Chugh AR, Teixido‐Tura G, Kim J‐Y, Donekal S, Yoneyama K, Gjesdal O, Redheuil A, et al. Association of aortic stiffness with left ventricular remodeling and reduced left ventricular function measured by magnetic resonance imaging: the Multi‐Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2016;9:e004426. DOI: 10.1161/CIRCIMAGING.115.004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Obata Y, Mizogami M, Singh S, Nyhan D, Berkowitz DE, Steppan J, Barodka V. The effects of hemodynamic changes on pulse wave velocity in cardiothoracic surgical patients. Biomed Res Int. 2016;2016:9640457. DOI: 10.1155/2016/9640457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–1087. DOI: 10.1161/01.HYP.0000019132.41066.95. [DOI] [PubMed] [Google Scholar]
- 34. Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross‐sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. DOI: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 35. Gosse P, Safar ME. Arterial stiffness and plasma creatinine in untreated hypertensive patients. Am J Hypertens. 2005;18:1140–1145. DOI: 10.1016/j.amjhyper.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 36. Chang YY, Chen A, Chen YH, Hung CS, Wu VC, Wu XM, Lin YH, Ho YL, Wu KD. Hypokalemia correlated with arterial stiffness but not microvascular endothelial function in patients with primary aldosteronism. J Renin Angiotensin Aldosterone Syst. 2015;16:353–359. DOI: 10.1177/1470320314524996. [DOI] [PubMed] [Google Scholar]
- 37. Lennon‐Edwards S, Allman BR, Schellhardt TA, Ferreira CR, Farquhar WB, Edwards DG. Lower potassium intake is associated with increased wave reflection in young healthy adults. Nutr J. 2014;13:39. DOI: 10.1186/1475-2891-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tzamou V, Kyvelou SM, Karpanou E, Petras D, Vyssoulis G. Aldosterone levels, aortic stiffness, and wave reflection in essential hypertensive patients. Am J Hypertens. 2015;28:852–857. DOI: 10.1093/ajh/hpu244. [DOI] [PubMed] [Google Scholar]
- 39. Levy D, Hwang S‐J, Kayalar A, Benjamin EJ, Vasan RS, Parise H, Larson MG, Wang TJ, Selhub J, Jacques PF, et al. Associations of plasma natriuretic peptide, adrenomedullin, and homocysteine levels with alterations in arterial stiffness: the Framingham Heart Study. Circulation. 2007;115:3079–3085. DOI: 10.1161/CIRCULATIONAHA.106.652842. [DOI] [PubMed] [Google Scholar]
- 40. Acelajado MC, Calhoun DA. Aldosteronism and resistant hypertension. Int J Hypertens. 2011;2011:837817. DOI: 10.4061/2011/837817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmidt BM, Schmieder RE. Aldosterone‐induced cardiac damage: focus on blood pressure independent effects. Am J Hypertens. 2003;16:80–86. DOI: 10.1016/S0895-7061(02)03199-0. [DOI] [PubMed] [Google Scholar]
- 42. Lacolley P, Labat C, Pujol A, Delcayre C, Benetos A, Safar M. Increased carotid wall elastic modulus and fibronectin in aldosterone‐salt‐treated rats: effects of eplerenone. Circulation. 2002;106:2848–2853. DOI: 10.1161/01.CIR.0000039328.33137.6C. [DOI] [PubMed] [Google Scholar]
- 43. Joffe HV, Adler GK. Effect of aldosterone and mineralocorticoid receptor blockade on vascular inflammation. Heart Fail Rev. 2005;10:31–37. DOI: 10.1007/s10741-005-2346-0. [DOI] [PubMed] [Google Scholar]
- 44. McCurley A, Jaffe IZ. Mineralocorticoid receptors in vascular function and disease. Mol Cell Endocrinol. 2012;350:256–265. DOI: 10.1016/j.mce.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mattace‐Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. DOI: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 46. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. DOI: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen C‐H, Cruickshank JK, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. DOI: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vlachopoulos C, Terentes‐Printzios D, Laurent S, Nilsson PM, Protogerou AD, Aznaouridis K, Xaplanteris P, Koutagiar I, Tomiyama H, Yamashina A, et al. Association of estimated pulse wave velocity with survival: a secondary analysis of SPRINT. JAMA Netw Open. 2019;2:e1912831. DOI: 10.1001/jamanetworkopen.2019.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mohiaddin RH, Longmore DB. MRI studies of atherosclerotic vascular disease: structural evaluation and physiological measurements. Br Med Bull. 1989;45:968–990. DOI: 10.1093/oxfordjournals.bmb.a072377. [DOI] [PubMed] [Google Scholar]
- 50. Rogers WJ, Hu YL, Coast D, Vido DA, Kramer CM, Pyeritz RE, Reichek N. Age‐associated changes in regional aortic pulse wave velocity. J Am Coll Cardiol. 2001;38:1123–1129. DOI: 10.1016/S0735-1097(01)01504-2. [DOI] [PubMed] [Google Scholar]
- 51. Suever JD, Oshinski J, Rojas‐Campos E, Huneycutt D, Cardarelli F, Stillman AE, Raggi P. Reproducibility of pulse wave velocity measurements with phase contrast magnetic resonance and applanation tonometry. Int J Cardiovasc Imaging. 2012;28:1141–1146. DOI: 10.1007/s10554-011-9929-8. [DOI] [PubMed] [Google Scholar]
- 52. Dogui A, Kachenoura N, Frouin F, Lefort M, De Cesare A, Mousseaux E, Herment A. Consistency of aortic distensibility and pulse wave velocity estimates with respect to the Bramwell‐Hill theoretical model: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2011;13:11. DOI: 10.1186/1532-429X-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Westenberg JJ, van Poelgeest EP, Steendijk P, Grotenhuis HB, Jukema JW, de Roos A. Bramwell‐hill modeling for local aortic pulse wave velocity estimation: a validation study with velocity‐encoded cardiovascular magnetic resonance and invasive pressure assessment. J Cardiovasc Magn Reson. 2012;14:2. DOI: 10.1186/1532-429X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zimmerli LU, Mark PB, Steedman T, Foster JE, Berg GA, Dargie HJ, Jardine AG, Delles C, Dominiczak AF. Vascular function in patients with end‐stage renal disease and/or coronary artery disease: a cardiac magnetic resonance imaging study. Kidney Int. 2007;71:68–73. DOI: 10.1038/sj.ki.5002024. [DOI] [PubMed] [Google Scholar]
- 55. Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA. 2007;104:16281–16286. DOI: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oberleithner H, Callies C, Kusche‐Vihrog K, Schillers H, Shahin V, Riethmuller C, Macgregor GA, de Wardener HE. Potassium softens vascular endothelium and increases nitric oxide release. Proc Natl Acad Sci USA. 2009;106:2829–2834. DOI: 10.1073/pnas.0813069106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lacolley P, Safar ME, Lucet B, Ledudal K, Labat C, Benetos A. Prevention of aortic and cardiac fibrosis by spironolactone in old normotensive rats. J Am Coll Cardiol. 2001;37:662–667. DOI: 10.1016/S0735-1097(00)01129-3. [DOI] [PubMed] [Google Scholar]
- 58. Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early‐stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54:505–512. DOI: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 59. Lombes M, Oblin ME, Gasc JM, Baulieu EE, Farman N, Bonvalet JP. Immunohistochemical and biochemical evidence for a cardiovascular mineralocorticoid receptor. Circ Res. 1992;71:503–510. DOI: 10.1161/01.RES.71.3.503. [DOI] [PubMed] [Google Scholar]
- 60. Jia G, Habibi J, Aroor AR, Martinez‐Lemus LA, DeMarco VG, Ramirez‐Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, et al. Endothelial mineralocorticoid receptor mediates diet‐induced aortic stiffness in females. Circ Res. 2016;118:935–943. DOI: 10.1161/CIRCRESAHA.115.308269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez‐Perez FI, Martinez‐Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, et al. Low‐dose mineralocorticoid receptor blockade prevents Western diet‐induced arterial stiffening in female mice. Hypertension. 2015;66:99–107. DOI: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Regnault V, Thomas F, Safar ME, Osborne‐Pellegrin M, Khalil RA, Pannier B, Lacolley P. Sex difference in cardiovascular risk: role of pulse pressure amplification. J Am Coll Cardiol. 2012;59:1771–1777. DOI: 10.1016/j.jacc.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Masoudi FA, Havranek EP, Smith G, Fish RH, Steiner JF, Ordin DL, Krumholz HM. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. DOI: 10.1016/S0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 64. Nethononda RM, Lewandowski AJ, Stewart R, Kylinterias I, Whitworth P, Francis J, Leeson P, Watkins H, Neubauer S, Rider OJ. Gender specific patterns of age‐related decline in aortic stiffness: a cardiovascular magnetic resonance study including normal ranges. J Cardiovasc Magn Reson. 2015;17:20. DOI: 10.1186/s12968-015-0126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Łoboz‐Rudnicka M, Jaroch J, Kruszyńska E, Bociąga Z, Rzyczkowska B, Dudek K, Szuba A, Łoboz‐Grudzień K. Gender‐related differences in the progression of carotid stiffness with age and in the influence of risk factors on carotid stiffness. Clin Interv Aging. 2018;13:1183–1191. DOI: 10.2147/CIA.S161711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle‐aged men and women. Hypertension. 2007;49:1248–1255. DOI: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 67. Vasan RS, Evans JC, Benjamin EJ, Levy D, Larson MG, Sundstrom J, Murabito JM, Sam F, Colucci WS, Wilson PW. Relations of serum aldosterone to cardiac structure: gender‐related differences in the Framingham Heart Study. Hypertension. 2004;43:957–962. DOI: 10.1161/01.HYP.0000124251.06056.8e. [DOI] [PubMed] [Google Scholar]
- 68. Mihailidou AS, Ashton AW. Cardiac effects of aldosterone: does gender matter? Steroids. 2014;91:32–37. DOI: 10.1016/j.steroids.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 69. Merrill M, Sweitzer NK, Lindenfeld J, Kao DP. Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT trial. JACC Heart Fail. 2019;7:228–238. DOI: 10.1016/j.jchf.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roderjan CN, Cardoso CR, Ferreira MT, Muxfeldt ES, Salles GF. Correlates of aortic stiffness progression in patients with resistant hypertension: importance of clinic and ambulatory blood pressure changes. J Hypertens. 2015;33:827–834; discussion 834–825. DOI: 10.1097/HJH.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 71. Ong KT, Delerme S, Pannier B, Safar ME, Benetos A, Laurent S, Boutouyrie P. Aortic stiffness is reduced beyond blood pressure lowering by short‐term and long‐term antihypertensive treatment: a meta‐analysis of individual data in 294 patients. J Hypertens. 2011;29:1034–1042. DOI: 10.1097/HJH.0b013e328346a583. [DOI] [PubMed] [Google Scholar]
- 72. Honda T, Hamada M, Shigematsu Y, Matsumoto Y, Matsuoka H, Hiwada K. Effect of antihypertensive therapy on aortic distensibility in patients with essential hypertension: comparison with trichlormethiazide, nicardipine and alacepril. Cardiovasc Drugs Ther. 1999;13:339–346. DOI: 10.1023/a:1007711617112. [DOI] [PubMed] [Google Scholar]
- 73. Lacourcière Y, Béliveau R, Conter HS, Burgess ED, Lepage S, Pesant Y, Spence JD, Asmar R, Carrière S, Plante GE. Effects of perindopril on elastic and structural properties of large arteries in essential hypertension. Can J Cardiol. 2004;20:795–799. [PubMed] [Google Scholar]
- 74. Smith SM, Gurka MJ, Winterstein AG, Pepine CJ, Cooper‐DeHoff RM. Incidence, prevalence, and predictors of treatment‐resistant hypertension with intensive blood pressure lowering. J Clin Hypertens (Greenwich). 2019;21:825–834. DOI: 10.1111/jch.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Al Kibria GM. Racial/ethnic disparities in prevalence, treatment, and control of hypertension among US adults following application of the 2017 American College of Cardiology/American Heart Association guideline. Prev Med Rep. 2019;14:100850. DOI: 10.1016/j.pmedr.2019.100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Taylor JS, Ellis GR. Racial differences in responses to drug treatment: implications for pharmacotherapy of heart failure. Am J Cardiovasc Drugs. 2002;2:389–399. DOI: 10.2165/00129784-200202060-00004. [DOI] [PubMed] [Google Scholar]
- 77. Cavallari LH, Groo VL, Momary KM, Fontana D, Viana MA, Vaitkus P. Racial differences in potassium response to spironolactone in heart failure. Congest Heart Fail. 2006;12:200–205. DOI: 10.1111/j.1527-5299.2006.05502.x. [DOI] [PubMed] [Google Scholar]
- 78. Vardeny O, Cavallari LH, Claggett B, Desai AS, Anand I, Rossignol P, Zannad F, Pitt B, Solomon SD. Race influences the safety and efficacy of spironolactone in severe heart failure. Circ Heart Fail. 2013;6:970–976. DOI: 10.1161/CIRCHEARTFAILURE.113.000530. [DOI] [PubMed] [Google Scholar]
- 79. Biolo A, Chao T, Duhaney TA, Kotlyar E, Allensworth‐Davies D, Loscalzo J, Sam F. Usefulness of the aldosterone synthase gene polymorphism C‐344‐T to predict cardiac remodeling in African‐Americans versus non‐African‐Americans with chronic systolic heart failure. Am J Cardiol. 2007;100:285–290. DOI: 10.1016/j.amjcard.2007.02.097. [DOI] [PubMed] [Google Scholar]
- 80. Shuey M, Perkins B, Nian H, Yu C, Luther JM, Brown N. Retrospective cohort study to characterise the blood pressure response to spironolactone in patients with apparent therapy‐resistant hypertension using electronic medical record data. BMJ Open. 2020;10:e033100. DOI: 10.1136/bmjopen-2019-033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Boudoulas KD, Vlachopoulos C, Raman SV, Sparks EA, Triposciadis F, Stefanadis C, Boudoulas H. Aortic function: from the research laboratory to the clinic. Cardiology. 2012;121:31–42. DOI: 10.1159/000336147. [DOI] [PubMed] [Google Scholar]
- 82. Ott C, Haetinger S, Schneider MP, Pauschinger M, Schmieder RE. Comparison of two noninvasive devices for measurement of central systolic blood pressure with invasive measurement during cardiac catheterization. J Clin Hypertens (Greenwich). 2012;14:575–579. DOI: 10.1111/j.1751-7176.2012.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S3


