Abstract
Background
Existing evidence indicates Black patients have higher incidence of pulmonary embolism (PE) and PE‐related mortality compared with other races/ethnicities, yet disparities in presenting severity and treatment remain incompletely understood.
Methods and Results
We retrospectively queried a multihospital healthcare system for all hospitalizations for acute PE (2012–2019). Of 10 329 hospitalizations, 8743 met inclusion criteria. Black patients (14.3%) were significantly younger (54.6±17.8 versus 63.1±16.6 years; P<0.001) and more female (56.1% versus 51.6%; P=0.003) compared with White patients. Using ordinal regression, Black race was significantly associated with higher PE severity after matching 1:3 on age and sex (1210:3264; odds ratio [OR], 1.08; 95% CI, 1.03–1.14), adjusting for clinical (OR, 1.13; 95% CI, 1.01–1.27), and socioeconomic (OR, 1.05; 95% CI, 1.05–1.35) characteristics. Among intermediate and high‐severity PE, Black race was associated with a decreased risk of intervention controlling for the competing risk of mortality and censoring on hospital discharge. This effect was modified by PE severity (P value <0.001), with a lower and higher risk of intervention for intermediate and high‐severity PE, respectively. Race was not associated with in‐hospital mortality (OR, 0.84; 95% CI, 0.69–1.02).
Conclusions
Black patients hospitalized with PE are younger with a higher severity of disease compared with White patients. Although Black patients are less likely to receive an intervention overall, this differed depending on PE severity with higher risk of intervention only for life‐threatening PE. This suggests nuanced racial disparities in management of PE and highlights the complexities of healthcare inequalities.
Keywords: healthcare disparities, outcomes, pulmonary embolism, racial disparities, venous thromboembolism
Subject Categories: Clinical Studies, Embolism, Race and Ethnicity
Clinical Perspective
What Is New?
Black race was associated with a higher pulmonary embolism severity with a decreased risk of receiving any intervention.
Among intermediate severity pulmonary embolism hospitalizations, for which current intervention guidelines are in evolution, the reduced risk persisted.
Yet, among hospitalizations for high‐severity pulmonary embolism, Black race was associated with an increased risk of intervention.
What Are the Clinical Implications?
Pulmonary embolism hospitalizations exemplified severity and management differences across Black and White races highlighting the complexities of healthcare access, biases, and treatment preferences that shape racial disparities.
Race‐related disparities in access to and quality of health care are well documented. Black Americans have shorter life expectancy, 1 experience more severe morbidity associated with chronic illness, 2 , 3 and achieve fewer quality healthcare measures compared with White Americans. 4 , 5 This disparity is especially evident in the incidence 6 and mortality 7 secondary to venous thromboembolic (VTE) disease, a serious and common process comprised of both pulmonary embolism (PE) and deep venous thrombosis, affecting more than 1 million Americans annually. 8 , 9 Although PE is the third most frequent cause of cardiovascular‐related death, 10 accounting for 2% of deaths in the United States in 2017, 11 Black patients are disproportionately affected; they are almost twice as likely to be hospitalized for PE 12 and suffer 50% higher age standardized PE‐related death compared with White patients. 11
Contributing factors to healthcare disparities are complex and can be thought of in terms of the National Institute on Minority Health and Health Disparities Research Framework, which includes biological, behavioral, physical, sociocultural, and healthcare system domains over multiple levels of influence including individual, interpersonal, community, and societal. 13 , 14 Specifically, how race may influence disease severity and management of acute PE is incompletely understood. We hypothesize that Black race is associated with higher acute PE severity on hospital presentation and lower incidence of catheter‐based or surgical intervention compared with White race. Our aim is to identify and understand racial inequalities associated with presentation and management of acute PE in order to explain previously noted differences in clinical outcomes related to PE in Black and White patients and inform treatment algorithms and resource allocation. Addressing disparities in every aspect of health care is not only key for advancing racial equality in healthcare delivery and outcomes, which is likely beneficial for a prevalent and morbid condition such as PE, it has also been shown to have a significant economic benefit. 15 , 16
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. UPMC is a large multihospital, single healthcare institution with a mix of private practice and academic facilities including over 20 hospitals. Our retrospective cohort study used data from 2012 to 2019 on medical care that are captured through structured administrative and clinical databases and an electronic health record (EHR), which are all integrated into the UPMC’s Clinical Data Warehouse, to evaluate the association between pulmonary embolism presentation severity, treatment, and outcomes for Black and White races. Patients diagnosed with an acute PE are typically evaluated by the PE response team, formally integrated in 2014, which includes pulmonary medicine, an interventionalist (ie, interventional cardiology or vascular surgery), cardiothoracic surgery, and hematology. This study was approved by the University of Pittsburgh Institutional Review Board (STUDY20120001). All data collection, analysis (Stata 15.1; Stata Corp), and presentation were in compliance with Strengthening the Reporting of Observational Studies in Epidemiology. 17
Data Source
At the time of EHR data abstraction (November 30, 2020), our regional healthcare network comprised 22 community and academic hospitals throughout southwestern Pennsylvania and the surrounding states, serving a diverse patient population that is connected digitally. Our data set provides baseline patient‐level demographics and comorbidities as well as clinical, procedural, and outcome hospital admission data. Baseline patient demographics (ie, sex, race, and ethnicity), socioeconomic factors, comorbid conditions, and medications were obtained from the outpatient medical record (Epic Systems Corporation) based on data available 90 days before admission. Socioeconomic factors included the Area of Deprivation Index, a measure of neighborhood disadvantage based on Census data incorporating information on education, employment, housing quality, and poverty. It is a validated tool that has linked residing in the top 15% to 20% of the most disadvantaged neighborhoods to limited healthcare access, care, and poor outcomes. 18 Smoking status was quantified by any (ie, former or current) or no (ie, never) prior exposure. Patients were considered to be in the postoperative period if they underwent a surgical intervention in the 90 days before hospital admission within our healthcare system. 9 Baseline comorbid conditions were quantified by the presence of validated International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) or International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) codes, as previously published. 19 Medications were identified by the presence of an e‐prescription or an active, provider‐reviewed EHR medication list. These data were linked to the in‐patient EHR (CERNER platform, Millennium) with unique patient identifiers. Hospitalization data included age, insurance status, first vital sign (heart rate, systolic blood pressure), serum laboratory value (troponin‐I, BNP [B‐type natriuretic peptide], creatinine, hemoglobin, international normalized ratio), and hospitalization events. Hospitalization events including intensive care unit admissions, relevant consultations (ie, pulmonology, cardiology, vascular surgery, and cardiothoracic surgery), echocardiograms, and treatment interventions were identified by pertinent Current Procedural Terminology codes or EHR data (Table S1). Treating hospitals were categorized by small, medium, or large in accordance with the National Inpatient Sample, which account for bed size, rurality, and teaching status. 20
Study Cohort
We identified all adult (≥18 years) acute care hospital admissions between the years 2012 and 2019 with a primary diagnosis of acute PE according to ICD‐9‐CM (415.11, 415.12, 415.13, and 415.19) and ICD‐10‐CM (I26.01, I26.02, I26.09, I26.90, I26.92, I26.94, I26.94, and I26.99) diagnosis codes. We restricted our full cohort to exclude (1) hospitals with fewer than 100 admissions for PE throughout the study duration, (2) patients missing race and/or known pertinent medical history required for classification of presentation severity, (3) patients who did not self‐identify as Black or White race, and (4) multiple, separate (ie, discharge and readmission) admissions in the healthcare network for a single PE. Intra‐healthcare network transfers were defined by the presence of 2 admissions for PE occurring within 24 hours at 2 separate hospitals. PE presentation data were combined and only the final treating (ie, tertiary care center) hospitalization was included. Multiple admissions for the same PE event were defined as 2 or more separate admissions within 30 days that were not intra‐healthcare network transfers. Missingness of these data, which may in part be representative of healthcare disparities and cannot be considered missing at random, was quantified (Table S2).
Outcomes
Our primary outcome of interest was PE severity on presentation classified into (1) high (massive), (2) intermediate (submassive), or (3) low severity using recent guidelines published by the European Society of Cardiology (Table S3). 21 Briefly, high‐severity PE included evidence of hemodynamic instability, defined by vasopressor support or admission hypotension (systolic blood pressure <90 mm Hg). 22 Intermediate severity PE included patients without hypotension but with abnormal biomarkers and cardiac workup on presentation (including right heart strain, elevated troponin‐I or BNP, vital sign abnormalities, with or without admission to intensive care unit). 23 All others were considered low severity. The accuracy of the EHR PE severity definition was clinically adjudicated via 2 blinded reviewers on a random subset of included hospitalizations in each severity group with 100% agreement (Table S3).
Secondary in‐hospital outcomes included the receipt of PE specific interventions including systemic therapies (ie, systemic thrombolysis administration), targeted therapies (ie, catheter directed therapies or surgical embolectomy), and preventative therapies (ie, inferior vena cava filters), as well as in‐hospital mortality.
Statistical Analysis
Demographic, socioeconomic, and presenting hospitalization data were assessed for White and Black race patients. Continuous variables were expressed as mean (±SD) or median (±interquartile range) for normally or skewed data distributions and compared with Student t test or Kruskal–Wallis tests. Categorical variables were expressed as frequency (percentage) and compared with chi‐square tests. In the full cohort meeting all inclusion and no exclusion criteria, the percentage of PE hospitalizations per age of presentation by race was depicted with histograms overall and for each level of PE severity.
We compared the PE severity, treatments, and outcomes between the Black and White races. We matched Black and White patients 1:3 without replacement on only preexisting, unmodifiable factors including age (year integer) and sex (binary). To explore the association between outcomes and race, a series of models were used to understand how other existing risk factors may contribute to baseline disparities including (1) the age‐ and sex‐matched cohort, (2) adjusting for comorbid clinical characteristics, and (3) both comorbid clinical and socioeconomic characteristics. 24 , 25 All models were clustered on hospital size as defined by the Agency for Healthcare Research and Quality, which accounts for bed size, rurality, and teaching status. 20 Grouping in this way allowed similar hospitals (ie, large academic tertiary care hospitals) to be grouped together based on typical functionality rather than clustering by individual hospitals, which introduced an excessive amount of variability.
We evaluated the association between race and PE severity low, intermediate, or high, in the matched cohort using ordinal regression. We evaluated the effect modification on the association between race and outcomes in predefined subgroups (those in the postoperative period, body mass index ≥35 mg/kg2, sex, and a prior history of VTE) with an interaction term, in the matched cohort. A P value threshold of 0.05 was used to denote significance including among interaction terms.
To evaluate the directionality of the association between Black race and the receipt of in‐patient PE intervention in those at risk of a procedure (ie, those alive and in the hospital), we used Fine‐Gray models. Among hospitalization for intermediate and high‐severity PE, we evaluated the association between race and any PE intervention, systemic therapies, targeted therapies, and preventative therapies controlling for the competing risk of mortality and censoring for hospital discharge. Subgroup analysis was completed to evaluate the association between the receipt of an intervention and race by PE severity level. Finally, we evaluated the association between race and in‐hospital mortality using logistic regression.
Sensitivity Analysis
To understand the robustness of our results, we completed 3 sensitivity analyses. First, we evaluated our primary outcome among matched patients with 2 alternative definitions of PE severity including (1) high severity (systolic blood pressure <70 mm Hg), intermediate severity (systolic blood pressure >70 and <90 mm Hg), and low severity for all others and (2) alterations in intermediate severity only, which included removing preexisting conditions from the definition as existing and recorded diagnosis may represent either health care and access to care disparities across races. Second, we explored for potential sampling bias in our matching technique and evaluated if our outcomes were sensitive to the matching methodology by evaluating point estimates in the full, unmatched cohort with multivariable regression including (1) age and sex; (2) age, sex, and clinical characteristics; and (3) age, sex, and clinical as well as socioeconomic characteristics as covariates. Third, we considered the effect of the healthcare network‐wide implementation of a PE response team that includes multiple consulting services to streamline the management of acute PE, including pulmonary medicine, an interventionalist (ie, interventional cardiology or vascular surgery), cardiothoracic surgery, and hematology. This service has been shown to improve outcomes 26 and was formally implemented at UPMC in 2014. Thus, to explore the impact of a PE response team in our study, we restricted our analysis in the full cohort to only these years.
Results
We identified 10 329 hospitalizations with a primary diagnosis of PE, of which 8743 met all inclusion and no exclusion criteria (age, 61.9±17.0 years; 4567 [52.2%] women) including 14.3% (n=1251) who self‐identified as Black (Figure 1). Overall, 11.1% hospitalizations were for low‐, 84.0% intermediate, and 4.9% high‐severity PE presentation for which 8.7% underwent any intervention, and 2.9% suffered in‐hospital mortality. Black patients were younger (54.6±18.8 versus 63.1±16.6 years), more female (56.1% versus 51.6%), resided in the top 20% most disadvantaged neighborhoods (70.3% versus 22.9%), and had less private health insurance (19.5% versus 30.5%) when compared with White patients (Table 1).
Figure 1. Study cohort.
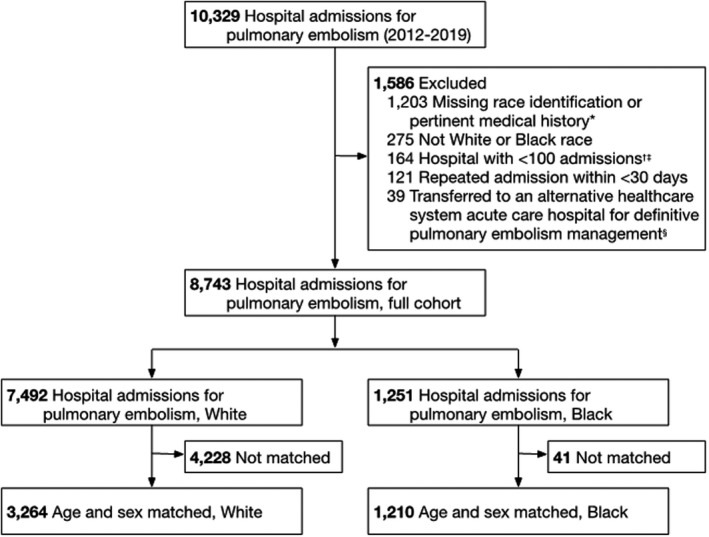
*Patients with pertinent medical history required for pulmonary embolism severity classification (ie, congestive heart failure, chronic pulmonary obstructive disease, and cancer; n=1200) and race categorization (n=3) were excluded. †Seven hospitals within the healthcare system had <100 admissions for pulmonary embolism throughout the study duration. ‡3.2% (n=6) of hospitalizations for Black patients and 14.1% (n=158) of hospitalizations for White patients occurred at a hospital with <100 admissions (P<0.001). 3.2% (n=6) of hospitalizations for Black patients and 2.9% (n=32) of hospitalizations for White patients were transferred to an alternative healthcare system (P=0.790). And 10.7% (n=20) of hospitalizations for Black patients and 9.0% (n=101) of hospitalizations for White patients had a repeat admission within <30 days (P=0.461). §For the 39 patients were admitted and diagnosed with a pulmonary embolism at a small, nonteaching hospital and transferred (<24 hours between admissions) within the healthcare network and admitted to a large, urban, tertiary‐care teaching hospital for definitive care, only the final treating hospital admission was included in the analysis. Admissions may be excluded for more than 1 indication.
Table 1.
Baseline and Admission Characteristics
| Preoperative variables | Full cohort (n=8743) | Age‐ and sex‐matched cohort (n=4474) | ||||
|---|---|---|---|---|---|---|
| White (n=7492) | Black (n=1251) | P Value | White (n=3264) | Black (n=1210) | P Value | |
| Demographics | ||||||
| Age, y | 63.1 (±16.6) | 54.6 (±17.8) | <0.001 | 56.8 (±17.0) | 55.1 (±17.5) | 0.003 |
| Female sex | 3865 (51.6%) | 702 (56.1%) | 0.003 | 1769 (54.2%) | 672 (55.5%) | 0.42 |
| Hispanic ethnicity | 36 (0.5%) | 4 (0.3%) | 0.480 | 20 (0.7%) | 4 (0.4%) | 0.29 |
| Area of Deprivation Index | 58.0 (±23.5) | 80.8 (±21.3) | <0.001 | 58.8 (±23.5) | 80.6 (±21.4) | <0.001 |
| Insurance | <0.001 | <0.001 | ||||
| Commercial | 2298 (30.7%) | 238 (19.0%) | 1274 (39.0%) | 231 (19.1%) | ||
| Medicaid | 849 (11.3%) | 414 (33.1%) | 504 (15.4%) | 395 (32.6%) | ||
| Medicare | 4179 (55.8%) | 528 (42.2%) | 1395 (42.7%) | 517 (42.7%) | ||
| Self‐pay/other | 166 (2.2%) | 71 (5.7%) | 91 (2.8%) | 67 (5.5%) | ||
| Comorbid conditions | ||||||
| Cerebrovascular event* | 574 (7.7%) | 115 (9.2%) | 0.063 | 208 (6.4%) | 115 (9.5%) | <0.001 |
| Diabetes mellitus | 1267 (16.9%) | 269 (21.5%) | <0.001 | 532 (16.3%) | 265 (21.9%) | <0.001 |
| Hypertension | 3655 (48.8%) | 671 (53.6%) | 0.001 | 1389 (42.6%) | 659 (54.5%) | <0.001 |
| Heart failure | 780 (10.4%) | 180 (14.4%) | <0.001 | 298 (9.1%) | 176 (14.5%) | <0.001 |
| Chronic obstructive pulmonary disease | 1215 (16.2%) | 213 (17.0%) | 0.47 | 476 (14.6%) | 209 (17.3%) | 0.026 |
| Cancer | 2065 (27.6%) | 239 (19.1%) | <0.001 | 814 (24.9%) | 236 (19.5%) | <0.001 |
| Coronary artery disease | 1105 (14.7%) | 110 (8.8%) | <0.001 | 362 (11.1%) | 108 (8.9%) | 0.036 |
| End‐stage renal disease | 55 (0.7%) | 22 (1.8%) | <0.001 | 27 (0.8%) | 22 (1.8%) | 0.005 |
| Venous thromboembolism | 2123 (28.4%) | 464 (37.1%) | <0.001 | 979 (30.0%) | 446 (36.9%) | <0.001 |
| Smoking history | 40147 (54.4%) | 752 (61.0%) | <0.001 | 1743 (54.2%) | 726 (60.9%) | <0.001 |
| Body mass index ≥ 35 kg/m2 | 2302 (30.7%) | 471 (37.6%) | <0.001 | 1136 (34.8%) | 454 (37.5%) | 0.092 |
| Postoperative period † | 2079 (27.7%) | 313 (25.0%) | 0.045 | 910 (27.9%) | 304 (25.1%) | 0.066 |
| Medications before hospital admission | ||||||
| Aspirin | 1390 (21.3%) | 1279 (16.4%) | 0.126 | 830 (27.0%) | 284 (25.0%) | 0.19 |
| Anticoagulation ‡ | 1700 (26.0%) | 1176 (15.0%) | 0.275 | 313 (10.2%) | 179 (15.7%) | <0.001 |
| Hospital admission § | ||||||
| Vital signs | ||||||
| Heart rate, bpm | 93.9 (±20.0) | 95.6 (±20.5) | 0.006 | 94.8 (±20.1) | 95.5 (±20.5) | 0.28 |
| Systolic blood pressure, mm Hg | 135.5 (±24.6) | 137.3 (±25.1) | 0.023 | 134.8 (±23.4) | 137.6 (±25.0) | <0.001 |
| Laboratory value | ||||||
| Troponin‐I, ng/mL | 0.5 (±8.8) | 0.4 (±1.2) | 0.72 | 0.3 (±0.8) | 0.3 (±1.1) | 0.15 |
| B‐type natriuretic peptide, pg/mL | 348.9 (±510.8) | 360.6 (±616.2) | 0.75 | 277.6 (±465.0) | 354.2 (±593.3) | 0.044 |
| Creatinine, mg/dL | 1.0 (±0.6) | 1.2 (±1.2) | <0.001 | 1.0 (±0.7) | 1.2 (±1.2) | <0.001 |
| Hemoglobin, g/dL | 12.1 (±2.0) | 11.7 (±2.1) | <0.001 | 12.2 (±2.0) | 11.7 (±2.1) | <0.001 |
| International normalized ratio | 1.2 (±0.5) | 1.3 (±0.6) | 0.12 | 1.2 (±0.6) | 1.3 (±0.6) | 0.12 |
| Treating hospital characteristics | ||||||
| Intensive care admission | 1961 (26.2%) | 322 (25.7%) | 0.75 | 826 (25.3%) | 310 (25.6%) | 0.83 |
| Vasopressor exposure | 270 (3.6%) | 52 (4.2%) | 0.34 | 115 (3.5%) | 51 (4.2%) | 0.28 |
| Relevant consultation | 1007 (13.4%) | 171 (13.7%) | 0.83 | 539 (16.5%) | 167 (13.8%) | 0.027 |
| Pulmonology | 629 (8.4%) | 97 (7.8%) | 0.45 | 356 (10.9%) | 96 (7.9%) | 0.003 |
| Cardiology | 399 (5.3%) | 65 (5.2%) | 0.85 | 200 (6.1%) | 63 (5.2%) | 0.24 |
| Vascular surgery | 85 (1.1%) | 24 (1.9%) | 0.021 | 41 (1.3%) | 23 (1.9%) | 0.11 |
| Cardiothoracic surgery | 35 (0.5%) | 7 (0.6%) | 0.66 | 539 (16.5%) | 167 (13.8%) | 0.027 |
| Admission echocardiogram | 3930 (52.5%) | 595 (47.6%) | 0.001 | 1658 (50.8%) | 578 (47.8%) | 0.072 |
| Right heart strain | 1072 (34.5%) | 165 (31.5%) | 0.18 | 443 (33.8%) | 156 (30.8%) | 0.22 |
| Treating hospital bed size || | <0.001 | <0.001 | ||||
| Large | 5533 (73.9%) | 1062 (84.9%) | 2444 (74.9%) | 1028 (85.0%) | ||
| Medium | 1206 (16.1%) | 132 (10.6%) | 518 (15.9%) | 125 (10.3%) | ||
| Small | 753 (10.1%) | 57 (4.6%) | 302 (9.3%) | 57 (4.7%) | ||
| Admission year | 0.081 | 0.41 | ||||
| 2012 | 465 (6.2%) | 105 (8.4%) | 220 (6.7%) | 101 (8.3%) | ||
| 2013 | 612 (8.2%) | 116 (9.3%) | 276 (8.5%) | 111 (9.2%) | ||
| 2014 | 895 (11.9%) | 144 (11.5%) | 406 (12.4%) | 138 (11.4%) | ||
| 2015 | 1001 (13.4%) | 161 (12.9%) | 440 (13.5%) | 159 (13.1%) | ||
| 2016 | 1140 (15.2%) | 167 (13.3%) | 497 (15.2%) | 162 (13.4%) | ||
| 2017 | 1181 (15.8%) | 200 (16.0%) | 500 (15.3%) | 194 (16.0%) | ||
| 2018 | 1154 (15.4%) | 184 (14.7%) | 495 (15.2%) | 179 (14.8%) | ||
| 2019 | 1044 (13.9%) | 174 (13.9%) | 430 (13.2%) | 166 (13.7%) | ||
Includes a prehospitalization stroke or transient ischemic attack, as defined by International Classification of Diseases, Clinical Modification of the Ninth or Tenth Revisions.
Any surgical intervention in the 90 d before pulmonary embolism hospitalization.
Anticoagulation therapies include the presence of warfarin, dabigatran, rivaroxaban, edoxaban, apixiaban before admission.
Maximal initially recorded vital sign or resulted laboratory value that first resulted upon admission to the transferring or treating hospital.
Hospital bed size is based upon the admission capacity (ie, hospital beds), rural or urban location, and teaching status. 20
Age at hospitalization for Black patients occurred at a younger age when compared with White patients overall (Figure 2A), and among those in the low‐, intermediate, and high‐severity PE groups (Figure 2B). Matching on age and sex in a 1:3 ratio of Black to White patients resulted 1210 Black and 3264 White patients. In our matched cohort, the age differences decreased from nearly 10 years to 1 year. Statistically significant differences between groups, such as cardiopulmonary comorbidities and the prevalence of cancer, remained (Table 1; Figure S1). Unmatched Black patients (n=41) were younger, more female, with a higher body mass index and more likely to have a history of VTE, whereas unmatched White patients (n=4228) were older, more male, with a lower body mass index, and less likely to have a history of VTE (Table S4).
Figure 2. Age of hospitalization for pulmonary embolism by age, per classification for severity in the full cohort.
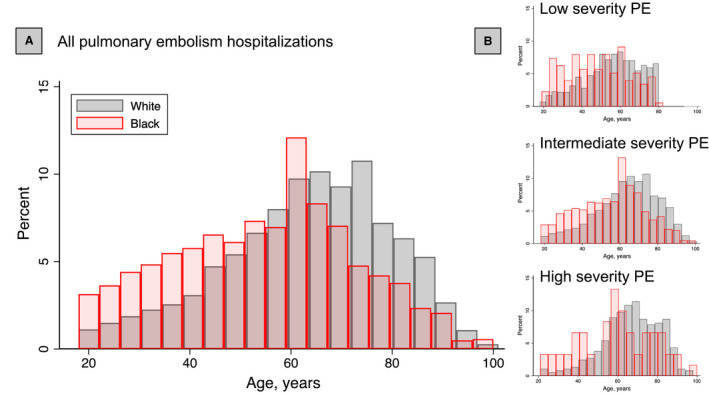
A, Overall, Black patients (red) are hospitalized for pulmonary embolism younger than White (gray) patients. B, These patterns are consistently observed for hospitalizations for low (top), intermediate (middle), and high (bottom) severity pulmonary embolism. PE indicates pulmonary embolism.
In the matched cohort, 12.6% of hospitalizations were for low‐, 82.9% intermediate, and 4.5% high‐severity PE (Table S5). Observed trends were consistent among severity groups. Clinical severity on presentation was associated with race after accounting for matching characteristics (OR, 1.08; 95% CI, 1.03–1.14), and after adjusting for both clinical (OR, 1.13; 95% CI, 1.01–1.27) as well as clinical and socioeconomic variables (OR, 1.05; 95% CI, 1.05–1.35) with Black patients having a higher risk of presenting with a higher severity PE when compared with White patients (Table 2; Table S6). 27 On subgroup analysis, postoperative status, sex, and history of VTE were found to modify the association of race with PE severity (Figure 3), with a higher risk of PE severity appreciated in patients who were in a postoperative state (OR, 1.34; 95% CI, 1.11–1.60) compared with a non‐postoperative state (OR, 1.04; 95% CI, 0.99–1.08), female (OR, 1.24; 95% CI, 1.01–1.53) compared with male (OR, 0.93; 95% CI, 0.86–1.00) sex, and those without a history of VTE (OR, 1.15; 95% CI, 1.09–1.22) compared with those with a history of VTE (OR, 0.97; 95% CI, 0.86–1.10).
Table 2.
Primary and secondary outcomes
| Age and sex matched* | Matched and adjusted for clinical characteristics † | Matched and adjusted for clinical and socioeconomic characteristics ‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| Primary outcome | Risk ratio (95% CIs) § | P value | Risk ratio (95% CIs) | P value | Risk ratio (95% CIs) | P value | ||
| Pulmonary embolism severity | NA | NA | 1.08 (1.03–1.14) | 0.003 | 1.13 (1.01–1.27) | 0.003 | 1.05 (1.05–1.35) | 0.002 |
| Secondary outcome (in‐hospital) || | White (N=3264), No. events (%) | Black (N=1210), No. events (%) | Risk ratio (95% CIs) § | P value | Risk ratio (95% CIs) | P value | Risk ratio (95% CIs) | P value |
| Any intervention | 354 (10.9%) | 94 (7.8%) | 0.77 (0.66–0.89) | 0.001 | 0.73 (0.64–0.84) | <0.001 | 0.72 (0.63–0.83) | <0.001 |
| Severity subgroup | <0.001 | <0.001 | <0.001 | |||||
| Intermediate risk | 306 (9.4%) | 71 (5.9%) | 0.68 (0.57–0.80) | <0.001 | 0.65 (0.56–0.75) | <0.001 | 0.63 (0.54–0.75) | <0.001 |
| High risk | 48 (1.5%) | 23 (1.9%) | 1.51 (1.29–1.77) | <0.001 | 1.45 (1.36–1.55) | <0.001 | 1.45 (1.37–1.55) | <0.001 |
| Systemic therapeutic intervention | 0.47 (0.18–1.22) | 0.120 | 0.46 (0.15–1.34) | <0.001 | 0.43 (0.21–0.91) | 0.030 | ||
| Severity subgroup | 0.050 | <0.001 | <0.001 | |||||
| Intermediate risk | 28 (1.0%) | 2 (0.2%) | 0.29 (0.09–1.02) | 0.050 | 0.27 (0.09–0.88) | 0.030 | 0.24 (0.13–0.45) | <0.001 |
| High risk | 12 (8.5%) | 6 (10.2%) | 1.14 (0.94–1.38) | 0.180 | 1.41 (0.91–2.19) | 0.120 | 1.58 (0.8–3.13) | 0.190 |
| Targeted therapeutic interventions # | 0.67 (0.44–1.03) | 0.070 | 0.65 (0.43–0.98) | 0.040 | 0.65 (0.41–10.5) | 0.080 | ||
| Severity subgroup | <0.001 | <0.001 | <0.001 | |||||
| Intermediate risk | 150 (5.6%) | 25 (2.5%) | 0.52 (0.29–0.93) | 0.030 | 0.48 (0.27–0.86) | 0.010 | 0.48 (0.25–0.94) | 0.030 |
| High risk | 19 (13.4%) | 10 (17.0%) | 3.03 (2.57–3.56) | <0.001 | 3.76 (3.05–4.63) | <0.001 | 3.51 (2.5–4.94) | <0.001 |
| Preventative intervention | 0.9 (0.82–0.99) | 0.040 | 0.81 (0.79–0.82) | <0.001 | 0.78 (0.75–0.82) | <0.001 | ||
| Severity subgroup | 0.470 | 0.960 | 0.920 | |||||
| Intermediate risk | 140 (5.2%) | 45 (4.5%) | 0.81 (0.71–0.92) | 0.001 | 0.80 (0.72–0.9) | <0.001 | 0.78 (0.73–0.84) | <0.001 |
| High risk | 32 (23.5%) | 14 (23.7%) | 1.00 (0.63–1.57) | 0.990 | 0.82 (0.48–1.39) | 0.460 | 0.8 (0.49–1.32) | 0.390 |
| Mortality** | 0.83 (0.69–1.01) | 0.060 | 0.81 (0.61–1.07) | 0.150 | 0.76 (0.54–1.06) | 0.100 | ||
| Severity subgroup | 0.390 | 0.900 | 0.500 | |||||
| Intermediate risk | 33 (1.2%) | 8 (0.8%) | 0.65 (0.39–1.08) | 0.090 | 0.80 (0.46–1.40) | 0.430 | 0.85 (0.48–1.50) | 0.570 |
| High risk | 50 (35.5%) | 18 (30.5%) | 0.81 (0.63–1.03) | 0.090 | 0.83 (0.67–1.02) | 0.090 | 0.69 (0.52–0.92) | 0.010 |
Matched 1:3 (Black:White) on age and sex without replacement (89% 1:3 pairs; 9% 1:2 pairs; 2% 1:1 pairs). Regression analysis of secondary outcomes include pulmonary embolism severity covariates.
Adjusted clinical characteristics included in the ordinal regression (severity) and Fine‐Gray models (interventions), and logistic regression (in‐hospital mortality) include race, age, sex, body mass index ≥35 mg/kg, recent surgery in the last 90 d, prior venous thromboembolism, and aspirin use.
Adjusted clinical and socioeconomic characteristics included in the ordinal regression (severity) and Fine‐Gray models (interventions), and logistic regression (in‐hospital mortality) are expanded to include the Area of Deprivation Index, and insurance status.
Ordinal (low‐, intermediate, and high‐risk pulmonary embolism) logistic regression evaluating the risk of interest, clustered on hospital size (Tables S6 through S7). Risk ratios corresponding to odds ratios for the primary and mortality outcomes, subdistribution hazard ratios for intervention related secondary outcomes. Of note, the reported subdistribution hazard ratios are reported to demonstrate the direction of the effect, their quantification of the magnitude of this effect on the cumulative incidence must be considered an approximation. 27
Interventions as defined by Current Procedural Terminology codes (Table S1).
Only among hospitalizations for intermediate and high‐risk pulmonary embolisms
The average time to death was 6.42±7.37 d for Black patients (n=24), and 4.24±5.91 d for White patients (n=83). This was not different between groups (P=0.150).
Figure 3. Risk of pulmonary embolism severity risk among subgroups in the matched cohort.
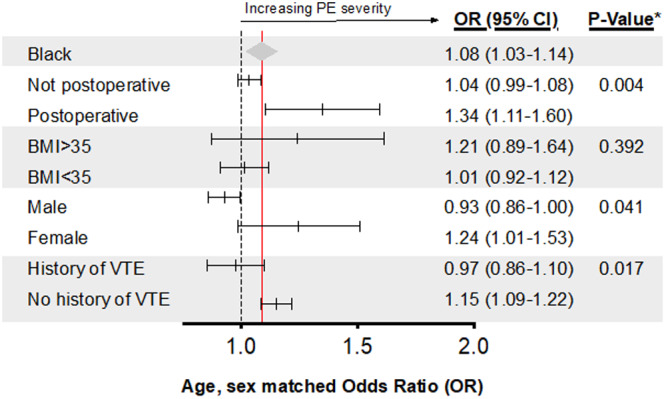
*P value of the interaction term. The dashed line corresponds to an OR of 1. The red line and the gray triangle corresponding to the overall OR and 95% CI for Black race in the matched cohort. Postoperative within 90 days of admission date. BMI indicates body mass index; OR, odds ratio; and PE, pulmonary embolism.
There was a total of 462 hospitalizations with PE interventions in the matched cohort, and >95% occurred in those with intermediate and high‐severity PE hospitalizations. Of these, 49 (1.3%) received systemic, 209 (5.7%) targeted, and 232 (6.3%) preventative interventions. Black patients had a lower risk of receipt of any intervention (including systemic, targeted, and preventative therapies) compared with White patients across the sequential models (Table 2, Figure 4A; Table S7). This association was modified by PE severity with the risk of receipt of interventions lower in intermediate severity and higher in high‐severity PE hospitalizations (Table 2; Figure 4B and 4C).
Figure 4. Cumulative hazard of the risk of in‐hospital procedures overall among intermediate and high‐severity pulmonary embolisms in the matched cohort together (A) and separately (B and C).
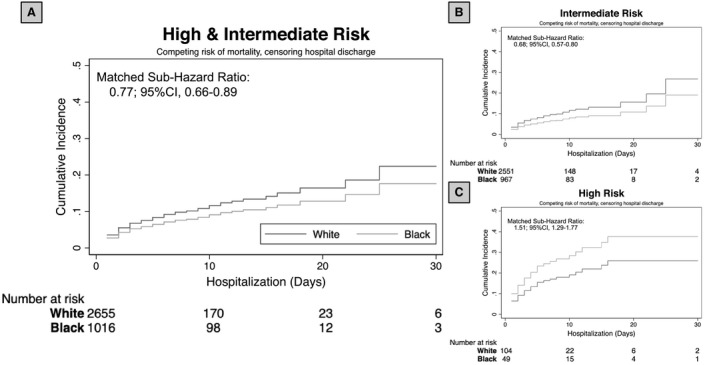
Cumulative hazard curves demonstrate the risk of any intervention in the combined high‐ and intermediate severity pulmonary embolisms (A), intermediate severity only (B), and high severity only (C) adjusting for the competing risk of mortality, clustering by hospital size, and censoring for hospital discharge in the matched cohort (risk tables). A, Black (light gray) patients hospitalized with intermediate or high‐severity pulmonary embolism have a lower relative risk of undergoing any interventions when compared with White (dark gray) patients. The association between receipt of therapy and race differed between intermediate and high‐severity subgroups (P value of interaction, <0.001; B, Risk of intervention for intermediate severity pulmonary embolism. C, Risk of intervention for high‐severity pulmonary embolism).
Race did not significantly associate with in‐hospital mortality (Table 2).
Sensitivity Analysis
The robustness of our results was apparent in multiple sensitivity analyses. In the matched cohort, the association between race and the primary outcome was consistent throughout alternative definitions of PE severity with an OR of 1.10 (95% CI, 1.02–1.17) after vital sign adjustment and 1.10 (95% CI, 1.02–1.17) after exclusion of comorbidities from the classification scheme.
In the full cohort (n=8743), the association between race and the primary outcome was consistent in our multivariate model (Table S8) after multivariable adjustment for age and sex (OR, 1.10; 95% CI, 1.01–1.19), clinical characteristics (OR, 1.12; 95% CI, 1.01–1.25), and clinical characteristics in combination with socioeconomic status (OR, 1.12; 95% CI, 0.98–1.27).
When restricting the full cohort to include only hospitalizations after implementation of a network‐wide PE response team service (n=7445), race continued to be associated with PE severity on presentation (OR, 1.14; 95% CI, 1.08–1.20).
Discussion
Existing data demonstrate race‐based disparities in the incidence and clinical sequelae associated with PE. 6 , 7 , 9 , 11 , 12 , 28 In this large study including over 20 hospitals caring for patients, we observed that Black race was associated with higher severity of PE on presentation and lower risk of receiving catheter‐based or surgical intervention including catheter‐directed thrombolysis, suction thrombectomy, surgical embolectomy, or inferior vena cava filters. No difference in in‐hospital mortality between races was detected.
Although our study was designed to investigate the influence of race on PE severity and mortality, we found interesting patterns related to age and sex. We demonstrate that Black patients present nearly 10 years younger (55 versus 63 years), with women accounting for a higher percentage of Black (56%) compared with White (52%) patients. The observed age differences persisted across severity groups. Differences in age of disease onset among races has been seen previously in PE 12 and is well known in other cardiovascular disease processes such as hypertension, heart failure, peripheral arterial disease, and stroke. 29 Earlier age of disease onset has been shown to increase the likelihood of long‐term disability and disease‐related dysfunction in other disease processes 30 and is thus likely to be the case with acute PE. The reason for this younger age at PE presentation is unclear. Although the incidence of certain inherited thrombophilias (ie, factor V Leiden and prothrombin gene mutations) has been shown to be lower in people of African descent compared with those of European descent, 31 , 32 Black patients have been known to have higher levels of a number of hemostatic factors and endothelial markers such as factor VIII, von Willebrand factor, plasmin antiplasmin complex, and D‐dimer. 33 The mechanistic differences in thrombophilias among races remains to be investigated; however, varying biologic etiologies may contribute to age differences on presentation. In addition, our findings should heighten the alertness for VTE in younger Black patients and focus resources on preventive measures and more aggressive VTE prophylaxis in this susceptible group.
Our observation that Black patients present with a higher clinical severity PE compared with age‐ and sex‐matched White patients is consistent with data from other clinical events and conditions, 34 and some have suggested this may be related to disparities in access to care. 35 We recognize, however, that difference in the PE severity on presentation is multifactorial and may also be related to a more aggressive disease process with biologic differences between races, 32 hereditary predisposition to hypercoagulability, 36 and underlying conditions such as malignancy. 37 In our cohort, we observed that more White patients had a diagnosis of cancers; however, we have little information regarding genetic mutations, thrombophilias, or family history of PE.
In our analysis, the relative risk increase in presentation severity ranged from 5% to 13% in our sequential adjustment for potential confounders and was consistent and robust throughout our analysis. Although large measurable racial inequalities are often the target of scientific inquiry, it is likely that the culmination of multiple small differences affect care. These findings highlight the need for targeted interventions to include improved access to care and identification of populations at high risk for VTE.
Our data add to existing evidence that racial disparities exist in cardiovascular health care. 38 , 39 Across all severity groups, Black patients were less likely to get any intervention compared with age‐ and sex‐matched White patients after controlling for clinical and socioeconomic characteristics. When stratified by severity class and intervention type, Black patients presenting with intermediate severity PE less frequently received a catheter‐based or surgical intervention compared with White patients. It is possible that overt and/or implicit bias contributes to differences in intervention. Although explicit racial bias has declined over time, many people still harbor implicit bias and negative attitudes toward Black people, 40 often manifesting in miscommunications and subtle unintentional forms of discrimination. This in addition to overt historical mistreatment of Black patients contributes to racial distrust in the medical community. Therefore, given that the utility of catheter‐directed thrombolysis is still controversial in intermediate severity PE, it is possible that Black patients are less likely to consent to a procedure that is still under investigation and is not universally endorsed by the current guidelines. 41 , 42 It is also possible that there are differences in available resources at different hospitals where Black patients present. However, our analysis took into account variations between hospitals including rurality, teaching status, and size where care was received, and many Black patients were managed at tertiary care facilities that do not experience restrictions in resource allocation or subspecialists that may be encountered at more rural hospitals. Alternatively, as mentioned previously, catheter‐directed thrombolosis is still controversial in intermediate severity PE. 21 It is possible that this finding is indicative of unjustified procedures being performed in our control group, and further examination in this area is warranted.
In contrast to what we observed in the intermediate severity group, Black patients with high‐severity PE were more likely to receive a surgical or catheter‐directed intervention compared with White patients. Although this may be in part due to racial differences in preferences for end‐of‐life treatment, 43 it may also be due to an exaggerated tendency of physicians to avoid discussing limiting end‐of‐life care in Black and minority patients. 44
Despite race‐related differences in severity and intervention, we did not observe a difference in mortality. This may have been due, in part, to the low rate (<5%) of in‐hospital mortality associated with acute PE. 9 , 45 Although some retrospective studies have suggested that patients with intermediate severity PE who receive catheter‐directed therapy compared with medical therapy alone (systemic anticoagulation or systemic thrombolysis) have a lower 30‐day and 1‐year mortality 23 and improved ventricular functional recovery, 46 data from multiple randomized trials have not supported this notion, 47 , 48 nor has there been evidence that in‐hospital mortality is improved. Therefore, thoroughly investigating the association between severity, management, and pertinent clinical outcomes would require analysis of long‐term cardiopulmonary function, PE‐related disability (ie, chronic thromboembolic pulmonary hypertension), and mortality.
Limitations
This study has several limitations. Our database is generated from a large, multihospital institution with a wide catchment area with a racial and ethnic profile similar to other large regional and national databases used in the study of acute PE 49 , 50 , 51 and recently reported national percentages. 52 Although the most recent US census data indicate that 12.5% of the population is Hispanic, less than 1% of our total cohort identified as such. 52 Therefore, the generalizability of our data may be limited in other regions. Next, we observed that more White patients had a baseline diagnosis of cancer and more Black patients were prescribed anticoagulation; however, we have little information regarding genetic mutations, thrombophilias, or family history of PE. In addition, our data were limited by lack of knowledge of any contraindication to systemic thrombolysis. Our data were also limited by our matching strategy, which left 14.8% (n=179) of Black patients and 9.6% (n=313) of White patients to be matched to less than 1:3 Black to White, thus limiting the variation in White participants for these specific age and sex combinations. And finally, PE severity classification was based on recent guidelines 21 combining preexisting comorbidities and patient characteristics along with laboratory values and diagnostic test results. Although this is an accepted measure of PE classification, 41 our data are limited by absent variables in the data set including arterial oxygenation level. Given this, it was possible that a proportion of our cohort were misclassified on the basis of severity.
Conclusions
Despite the limitations, this large database offers enough granularity and clinical details that allow for meaningful observations and potential targets for intervention to reduce racial disparities in the care of patients with PE. We demonstrated that Black patients hospitalized with PE are younger with a higher severity compared with White patients. Although Black patients are less likely to receive an intervention overall, this interestingly differed depending on PE severity with higher risk of intervention only for life‐threatening PE. This suggests nuanced racial disparities in being offered or accepting an intervention and highlights the complexities of healthcare inequalities. No difference in the overall low rates of in‐hospital mortality was observed between races, and further studies are needed to elucidate racial disparities in mortality and PE‐related disability. Investigating the long‐term benefit of procedural intervention, regardless of race, and resource allocation to heighten PE prevention in young Black patients is needed and may result in improved outcomes for patients with acute PE and lessen the societal socioeconomic burden of VTE.
Sources of Funding
This research was supported in part by the grant 5T32HL0098036 from the National Heart, Lung, and Blood Institute (Phillips, Reitz, Andraska), L30 AG064730 National Institute on Aging (Reitz), and the University of Pittsburgh holds a Physician‐Scientist Institutional Award from the Burroughs Wellcome Fund (Andraska). These funding sources had no role in the design and conduct of the study; data collection, management, analysis, and/or interpretation; preparation, review, and/or approval of the article; or decision to submit for publication.
Disclosures
None.
Supporting information
Table S1–S8
Figure S1
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021818.
For Sources of Funding and Disclosures, see page 11.
References
- 1. National Center for Health Statistics (US) . Health, United States, 2018. National Center for Health Statistics (US) 2019. [PubMed]
- 2. Laiteerapong N, Fairchild P, Chou C, Chin M, Huang E. Revisiting disparities in quality of care among US adults with diabetes in the era of individualized care, NHANES 2007–2010. Med Care. 2015;53:25–31. DOI: 10.1097/MLR.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casagrande S, Fradkin J, Saydah S, Rust K, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Am Diabetes Assoc. 2013;36:2271–2279. DOI: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiscella K, Sanders MR. Racial and ethnic disparities in the quality of health care. Annu Rev Public Health. 2016;37:375–394. DOI: 10.1146/annurev-publhealth-032315-021439. [DOI] [PubMed] [Google Scholar]
- 5. 2018 National Healthcare Quality and Disparities Report. Content last reviewed April 2020. Agency for Healthcare Research and Quality, Rockville, MD. n.d. https://WwwAhrqGov/Research/Findings/Nhqrdr/Nhqdr18/IndexHtml [PubMed]
- 6. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I‐4–I‐8. DOI: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 7. Horlander K, Mannino D, Leeper K. Pulmonary embolism mortality in the United States, 1979–1998: an analysis using multiple‐cause mortality data. Arch Intern Med. 2003;163:1711–1717. DOI: 10.1001/archinte.163.14.1711. [DOI] [PubMed] [Google Scholar]
- 8. Arshad N, Isaksen T, Hansen JB, Brækkan SK. Time trends in incidence rates of venous thromboembolism in a large cohort recruited from the general population. Eur J Epidemiol. 2017;32:299–305. DOI: 10.1007/s10654-017-0238-y. [DOI] [PubMed] [Google Scholar]
- 9. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain A, Chang A, Cheng S, Delling F, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. DOI: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 10. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–1846. DOI: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 11. Barco S, Valerio L, Ageno W, Cohen AT, Goldhaber SZ, Hunt BJ, Iorio A, Jimenez D, Klok FA, Kucher N, et al. Age‐sex specific pulmonary embolism‐related mortality in the USA and Canada, 2000–18: an analysis of the WHO Mortality Database and of the CDC Multiple Cause of Death database. Lancet Respir Med. 2021;9:33–42. DOI: 10.1016/S2213-2600(20)30417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin KA, McCabe ME, Feinglass J, Khan SS. Racial disparities exist across age groups in Illinois for pulmonary embolism hospitalizations. Arterioscler Thromb Vasc Biol. 2020;40:2338–2340. DOI: 10.1161/ATVBAHA.120.314573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu Y, Siegal DM, Anand SS. Ethnoracial variations in venous thrombosis: implications for management, and a call to action. J Thromb Haemost. 2020;19:1–11. DOI: 10.1111/jth.15140. [DOI] [PubMed] [Google Scholar]
- 14. National Institute on Minority Health and Health Disparities . NIMHD Research Framework. 2017. Available at: https://nimhd.nih.gov/researchFramework. Accessed June 7, 2021.
- 15. Laveist T, Gaskin D, Richard P. Estimating the economic burden of racial health inequalities in the United States. Int J Health Serv. 2011;41:231–238. DOI: 10.2190/HS.41.2.c. [DOI] [PubMed] [Google Scholar]
- 16. Nanney MS, Myers SL, Xu M, Kent K, Durfee T, Allen ML. The economic benefits of reducing racial disparities in health: the case of Minnesota. Int J Environ Res Public Health. 2019;16:742. DOI: 10.3390/ijerph16050742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. DOI: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 18. Kind AJH, Buckingham WR. Making neighborhood‐disadvantage metrics accessible — the neighborhood atlas. N Engl J Med. 2018;378:2456–2458. DOI: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reitz KM, Marroquin OC, Zenati MS, Kennedy J, Korytkowski M, Tzeng E, Koscum S, Newhouse D, Garcia R, Vates J, et al. Association between preoperative metformin exposure and postoperative outcomes in adults with type 2 diabetes. JAMA Surg. 2020;155:e200416. DOI: 10.1001/jamasurg.2020.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Healthcare Cost and Utilization Project (HCUP) NIS Notes. n.d. Available at: https://www.hcup‐us.ahrq.gov/db/vars/hosp_bedsize/nisnote.jsp. Accessed January 1, 2021.
- 21. Konstantinides SV, Meyer G, Galié N, Simon R Gibbs J, Aboyans V, Ageno W, Agewall S, Almeida A, Andreotti F, Barbato E, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543–603. [DOI] [PubMed] [Google Scholar]
- 22. Wiske CP, Shen C, Amoroso N, Brosnahan SB, Goldenberg R, Horowitz J, Jamin C, Sista A, Smith D, Maldonado T. Evaluating time to treatment and in‐hospital outcomes of pulmonary embolism response teams. J Vasc Surg. 2020;8:717–724. DOI: 10.1016/j.jvsv.2019.12.077. [DOI] [PubMed] [Google Scholar]
- 23. D’Auria S, Sezer A, Thoma F, Sharbaugh M, McKibben J, Maholic R, Avgerinos E, Rivera‐Lebron B, Toma C. Outcomes of catheter‐directed thrombolysis vs. standard medical therapy in patients with acute submassive pulmonary embolism. Pulm Circ. 2020;10:1–8. DOI: 10.1177/2045894019898368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care (with CD). National Academies Press; 2003. [PubMed] [Google Scholar]
- 25. VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology. 2014;25:473–484. DOI: 10.1097/EDE.0000000000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaudhury P, Gadre S, Schneider E, Renapurkar R, Gomes M, Haddadin I, Heresi G, Tong M, Bartholomew J. Impact of multidisciplinary pulmonary embolism response team availability on management and outcomes. Am J Cardiol. 2019;124:1465–1469. DOI: 10.1016/j.amjcard.2019.07.043. [DOI] [PubMed] [Google Scholar]
- 27. Austin PC, Fine JP. Practical recommendations for reporting Fine‐Gray model analyses for competing risk data. Stat Med. 2017;36. DOI: 10.1002/sim.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ibrahim SA, Stone RA, Obrosky S, Sartorius J, Fine MJ, Aujesky D. Racial differences in 30‐day mortality for pulmonary embolism. Am J Public Health. 2006;96:2161–2164. DOI: 10.2105/AJPH.2005.078618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA, Willis M, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. DOI: 10.1161/CIR.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 30. Ellis C. Stroke in young adults. Disabil Health J. 2010;3:222–224. DOI: 10.1016/j.dhjo.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 31. Kujovich JL. Factor v Leiden thrombophilia. Genet Med. 2011;13:1–6. DOI: 10.1097/GIM.0b013e3181faa0f2 [DOI] [PubMed] [Google Scholar]
- 32. Morange PE, Suchon P, Trégouët DA. Genetics of venous thrombosis: update in 2015. Thromb Haemost. 2015;114:910–919. DOI: 10.1160/TH15-05-0410. [DOI] [PubMed] [Google Scholar]
- 33. Lutsey PL, Cushman M, Steffen LM, Green D, Barr RG, Herrington D, Ouyang P, Folsom AR, et al. Plasma hemostatic factors and endothelial markers in four racial/ethnic groups: the MESA study. J Thromb Haemost. 2006;4:2629–2635. DOI: 10.1111/j.1538-7836.2006.02237.x. [DOI] [PubMed] [Google Scholar]
- 34. Mielck A, Reitmeir P, Wjst M. Severity of childhood asthma by socioeconomic status. Int J Epidemiol. 1996;25:388–393. DOI: 10.1093/ije/25.2.388. [DOI] [PubMed] [Google Scholar]
- 35. Duerson W, Lafer M, Ahmed O, Bandler I, Wang B, Lieberman S, Lebowitz R. Health care disparities in patients undergoing endoscopic sinus surgery for chronic rhinosinusitis: differences in disease presentation and access to care. Ann Otol Rhinol Laryngol. 2019;128:608–613. DOI: 10.1177/0003489419834947. [DOI] [PubMed] [Google Scholar]
- 36. Zöller B, Li X, Sundquist J, Sundquist K. A nationwide family study of pulmonary embolism: identification of high risk families with increased risk of hospitalized and fatal pulmonary embolism. Thromb Res. 2012;130:178–182. DOI: 10.1016/j.thromres.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 37. Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real‐world population: the Q‐VTE study cohort. Am J Med. 2013;126:832.e13–832.e21. DOI: 10.1016/j.amjmed.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 38. Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, Zhao X, Peterson E, Fonarow G. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–1501. DOI: 10.1161/CIRCULATIONAHA.109.881490. [DOI] [PubMed] [Google Scholar]
- 39. Dong L, Fakeye OA, Graham G, Gaskin DJ. Racial/ethnic disparities in quality of care for cardiovascular disease in ambulatory settings: a review. Med Care Res Rev. 2018;75:263–291. DOI: 10.1177/1077558717725884. [DOI] [PubMed] [Google Scholar]
- 40. Dovidio JF, Penner LA, Albrecht TL, Norton WE, Gaertner SL, Shelton JN. Disparities and distrust: the implications of psychological processes for understanding racial disparities in health and health care. Soc Sci Med. 2008;67:478–486. DOI: 10.1016/j.socscimed.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 41. Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani DJ, Desai ND, Piazza G, Gladwin M, Chatterjee S, Kobayashi T, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence. Circulation. 2019;140:E774–E801. [DOI] [PubMed] [Google Scholar]
- 42. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. DOI: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 43. Barnato AE, Anthony DL, Skinner J, Gallagher PM, Fisher ES. Racial and ethnic differences in preferences for end‐of‐life treatment. J Gen Intern Med. 2009;24:695–701. DOI: 10.1007/s11606-009-0952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. de Souza J, Gillett K, Froggatt K, Walshe C. Perspectives of elders and their adult children of Black and minority ethnic heritage on end‐of‐life conversations: a meta‐ethnography. Palliat Med. 2020;34:195–208. DOI: 10.1177/0269216319887070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agarwal S, Menon V, Jaber WA. Residential zip code influences outcomes following hospitalization for acute pulmonary embolism in the United States. Vasc Med. 2015;20:439–446. DOI: 10.1177/1358863X15592486. [DOI] [PubMed] [Google Scholar]
- 46. Avgerinos ED, Abou Ali AN, Liang NL, Rivera‐Lebron B, Toma C, Maholic R, Makaroun M, Chaer R. Catheter‐directed interventions compared with systemic thrombolysis achieve improved ventricular function recovery at a potentially lower complication rate for acute pulmonary embolism. J Vasc Surg. 2018;6:425–432. DOI: 10.1016/j.jvsv.2017.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Konstantinides SV, Vicaut E, Danays T, Becattini C, Bertoletti L, Beyer‐Westendorf J, Bouvaist H, Couturaud F, Dellas C, Duerschmied D, et al. Impact of thrombolytic therapy on the long‐term outcome of intermediate‐risk pulmonary embolism. J Am Coll Cardiol. 2017;69:1536–1544. DOI: 10.1016/j.jacc.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 48. Avgerinos ED, Saadeddin Z, Abou Ali AN, Fish L, Toma C, Chaer M, Rivera‐Lebron B, Chaer R. A meta‐analysis of outcomes of catheter‐directed thrombolysis for high‐ and intermediate‐risk pulmonary embolism. J Vasc Surg. 2018;6:530–540. DOI: 10.1016/j.jvsv.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 49. The ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 50. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1:263–276. DOI: 10.1016/1047-2797(91)90005-W. [DOI] [PubMed] [Google Scholar]
- 51. Agarwal S, Clark D, Sud K, Jaber WA, Cho L, Menon V. Gender disparities in outcomes and resource utilization for acute pulmonary embolism hospitalizations in the United States. Am J Cardiol. 2015;116:1270–1276. DOI: 10.1016/j.amjcard.2015.07.048. [DOI] [PubMed] [Google Scholar]
- 52. 2019 US census data. https://WwwCensusGov/Quickfacts/Fact/Table/US/IPE120219. n.d.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S8
Figure S1


