Abstract
Background
Recently there has been increased interest in a possible association between mast cell activation (MCA) disorder and postural orthostatic tachycardia syndrome (POTS). This study examined the frequency with which symptoms and laboratory findings suggesting MCA disorder occurred in patients diagnosed with POTS.
Methods and Results
Data were obtained from patients in whom symptoms and orthostatic testing were consistent with a POTS diagnosis. Individuals with <4 months symptom duration, evident ongoing inflammatory disease, suspected volume depletion, or declined consent were excluded. All patients had typical POTS symptoms; some, however, had additional nonorthostatic complaints not usually associated with POTS. The latter patients underwent additional testing for known MCA biochemical mediators including prostaglandins, histamine, methylhistamine, and plasma tryptase. The study comprised 69 patients who met POTS diagnostic criteria. In 44 patients (44/69, 64%) additional nonorthostatic symptoms included migraine, allergic complaints, skin rash, or gastrointestinal symptoms. Of these 44 patients, 29 (66%) exhibited at least 1 laboratory abnormality suggesting MCA disorder, and 11/29 patients had 2 or more such abnormalities. Elevated prostaglandins (n=16) or plasma histamine markers (n=23) were the most frequent findings. Thus, 42% (29/69) of patients initially diagnosed with POTS exhibited both additional symptoms and at least 1 elevated biochemical marker suggesting MCA disorder.
Conclusions
Laboratory findings suggesting MCA disorder were relatively common in patients diagnosed with POTS and who present with additional nonorthostatic gastrointestinal, cutaneous, and allergic symptoms. While solitary abnormal laboratory findings are not definitive, they favor MCA disorder being considered in such cases.
Keywords: biochemical mediators, histamine, mast cell, postural orthostatic tachycardia syndrome, prostaglandins
Subject Categories: Arrhythmias, Electrophysiology
Nonstandard Abbreviations and Acronyms
- MCA
mast cell activation
- POTS
postural orthostatic tachycardia syndrome
Clinical Perspective
What Is New?
Mast cell activation (MCA) disorder may present with a postural orthostatic tachycardia syndrome–like clinical picture.
Our findings indicate that the presence of MCA as the basis for postural orthostatic tachycardia syndrome–like symptoms can be suggested by certain clinical and biochemical features.
What Are the Clinical Implications?
While no single laboratory finding provides a definitive diagnostic marker for MCA, our findings suggest that the presence of elevated circulating prostaglandin (prostaglandin D2 and/or F2 alpha), especially in conjunction with an increased second biochemical marker such as histamine or methylhistamine, indicates that MCA disorder may be associated with the postural orthostatic tachycardia syndrome–like symptoms.
Recognition of MCA in a subset of patients with postural orthostatic tachycardia syndrome–like symptoms may open an alternative beneficial therapeutic strategy (ie, antihistamines, mast cell stabilizers) for these otherwise often difficult‐to‐treat patients.
Postural orthostatic tachycardia syndrome (POTS) was initially characterized in a small group of highly selected individuals in whom palpitations and lightheadedness, triggered by upright posture, were deemed to be caused by a limited form of autonomic dysfunction. 1 , 2 Subsequently, however, the POTS diagnosis has increasingly been suspected and/or applied much more broadly, encompassing patients with often poorly understood symptoms involving multiple organ systems and that are not typically considered to be caused by POTS. 2 , 3 , 4
The cause of POTS in individual patients is usually unknown, despite a number of conditions having been implicated; these include viral illnesses, trauma, and various inflammatory diseases. Mast cell activation (MCA) disorder has also been implicated since chemical mediators released from mast cells may cause symptoms suggestive of POTS (eg, postural tachycardia, lightheadedness). On the other hand, MCA mediators may also result in systemic nonorthostatic symptoms that are not typical of POTS such as flushing, urticaria, angioedema, nasal congestion, airway reactivity, vomiting, headache, and diarrhea. 5 , 6
Recently, 2 sets of consensus criteria have been proposed for diagnosis of MCA disorder. 7 , 8 While these criteria have some differences, both define the disorder as a chronic condition affecting 2 or more systems driven by the aberrant release of mast cell biochemical mediators. Valent and colleagues 7 require that the acute symptoms be associated with documented elevations of mast cell mediators on at least 2 occasions. Afrin and colleagues 8 include elevations in mast cell mediators (either during an acute episode or at baseline) as 1 criterion, but indicate that it is possible to fulfill diagnostic criteria without elevations in mast cell mediators.
The possibility that MCA disorder may be associated with POTS either as a trigger or as a coexisting condition is now widely accepted, but how often is unknown. This study examined the frequency with which symptoms and laboratory findings suggesting MCA disorder occurred in patients referred for POTS evaluation and who were ultimately diagnosed with POTS based upon currently published criteria. 2 , 3 , 4
Methods
The patient population consisted of individuals referred to the Cardiac Arrhythmia and Syncope Center at University of Minnesota (Minneapolis, MN) and the Hospital of the Good Samaritan and Keck Medical Center (Los Angeles, CA) from January 2016 to January 2020 for evaluation of orthostatic intolerance symptoms consistent with POTS 9 , 10 , 11 , 12 and who were followed for a minimum of 6 months. Institutional Review Board approvals were obtained from each contributing institution. Written consent was obtained from all patients for use of deidentified data. Deidentified data used for this research will be made available in tabular form upon request by researchers in the field.
All patients were evaluated by 1 of the authors (DC, DGB). Patients who were found to have anemia, hyperthyroidism, ongoing infection, evident systemic inflammatory diseases, or suspected volume depletion that could account for POTS‐like findings were excluded. Patients with POTS symptoms present for <4 months duration were also excluded to minimize inclusion of individuals with a recent contributory acute illness. 1 , 2 Patient assessment occurred before the onset of COVID‐19 pandemic and no patient suspected of having had COVID‐19 was included.
A complete medical history was obtained along with physical examination, a 12‐lead ECG, and laboratory testing for the purpose of excluding acute inflammatory or infectious illness (ie, complete blood cell count and white blood cell count, erythrocyte sedimentation rate/C‐reactive protein). A comprehensive symptom assessment was undertaken for each patient. Furthermore, all patients underwent ambulatory ECG monitoring for ≥24 hours. Echocardiography was obtained in all cases and none evidenced underlying structural heart disease.
POTS Diagnostic Criteria
A diagnosis of POTS was established based on observations summarized by the Heart Rhythm Society and Canadian Cardiovascular Society. 2 , 3 , 4 A postural contribution to symptoms was determined by clinical history, as well as during an active standing test or tilt‐table test or both. The following findings were present:
Symptoms of orthostatic intolerance as described in the 2015 Heart Rhythm Society and 2020 Canadian Cardiovascular Society documents 3 , 4
Sustained increase in heart rate ≥30 beats per minute when moving from seated or recumbent to upright based on consensus published diagnostic criteria 2 , 3 , 4
Absence of sustained orthostatic hypotension (ie, <20 mm Hg drop of systolic pressure when moving to upright posture with prompt recovery). 1 , 2 , 3 , 4
Patient Screening
Patients included in this study were derived from all patients referred to the 2 participating centers for evaluation of “suspected” POTS. Over the study period, each of the centers averaged ≈2 referrals per week for “suspected” POTS, or ≈800 patients over 4 years. However, many of these patients did not have true POTS, and as stated in the Methods section were ultimately excluded. The majority of exclusions proved to be immediate orthostatic hypotension or sinus tachycardia that did not meet conventional POTS criteria. Approximately 10% of our referred patients proved to be true POTS. Thus, the study population incorporated ≈80 patients with verified POTS. However, some of these individuals could not be included for various logistical reasons (travel distance, insurance limitations) or because of declining participation.
In terms of potential patient selection bias, it may be assumed that patients manifesting severe symptoms were more likely to be referred for evaluation than would individuals with fewer complaints. Consequently, it is likely that the proportion of patients with MCA is higher in this study than would be expected in a broader population; this possibility precludes this study’s providing any statement of MCA prevalence.
Laboratory Measurement of MC Mediators and Metabolites
Patients who, apart from experiencing typical POTS symptoms, also reported symptoms less often associated with POTS (eg, migraine, “allergy,” skin rash/urticaria, gastrointestinal symptoms), underwent laboratory measurements of common mast cell mediators and metabolites, including the following: plasma tryptase, either plasma prostaglandin D2 or 24‐hour urine prostaglandin 11‐β‐PGF 2‐alpha or both, plasma histamine, and urine methylhistamine. Timing of laboratory measurements was based on patient convenience and was not specifically associated with symptom “flares.” In certain cases, insurance coverage limitations precluded acquiring complete laboratory studies.
Statistical Analysis
Data are expressed as mean±SD for continuous variables and percentages for categorical variables. Normality of data was assessed by the Kolmogorov Smirnov test. All comparisons were assessed with the Student t test, and ANOVA for continuous variables, and by Fisher exact test for categorical variables. A P value <0.05 was considered statistically significant. Analyses were performed using the software JMP 14 (SAS Institute Inc., Cary, NC).
Results
Patient Population
The study population comprised 69 patients (66/69 female) in whom 1 or more symptoms/signs aggravated by upright posture suggested POTS (eg, orthostatic palpitations, fatigue, lightheadedness, absence of orthostatic hypotension) (Table 1), and in whom other potentially contributory medical conditions had been excluded as discussed above. Among these individuals, 25/69 presented only typical POTS symptoms (ie, postural tachycardia/palpitations, lightheadedness/brain fog) (Figure 1). The remaining 44/69 individuals reported both typical POTS symptoms as well as additional complaints as noted above that are not typically considered to be caused by POTS. These 44 individuals underwent laboratory studies summarized below for MCA disorder (Figure 1).
Table 1.
Clinical Comparison: MCA Laboratory Abnormality Versus POTS* Alone
|
Atypical Symptoms + Abnormal Laboratory Tests (n=29) |
% |
Normal Laboratory Evaluation or Typical Symptoms Only (n=40) |
% | P Values | |
|---|---|---|---|---|---|
| Age, y | 33 ± 10.4 | 28 ± 10.3 | <0.02 | ||
| Female sex | 28 | 93 | 38 | 95 | 0.739 |
| Palpitation | 23 | 79 | 37 | 93 | 0.108 |
| Syncope | 8 | 27 | 13 | 33 | 0.297 |
| Fatigue | 22 | 76 | 22 | 55 | 0.075 |
|
Lightheadedness /dizzy/ “brain fog” |
26 | 90 | 35 | 88 | 0.078 |
| Migraines | 12 | 41 | 5 | 13 | 0.05 |
| Depression/anxiety | 11 | 38 | 9 | 23 | 0.163 |
| Fibromyalgia | 5 | 17 | 3 | 8 | 0.212 |
| Allergy | 15 | 52 | 1 | 3 | <0.0 |
| Skin rash | 13 | 52 | 2 | 5 | <0.0 |
| Gastrointestinal symptoms | 28 | 76 | 12 | 30 | <0.0 |
MCA indicates mast cell activation; and POTS, postural orthostatic tachycardia syndrome.
*Includes patients with typical POTS symptoms only (n=15) and those with typical and atypical symptoms but with normal laboratory studies (n=25).
Figure 1. Patient flow.
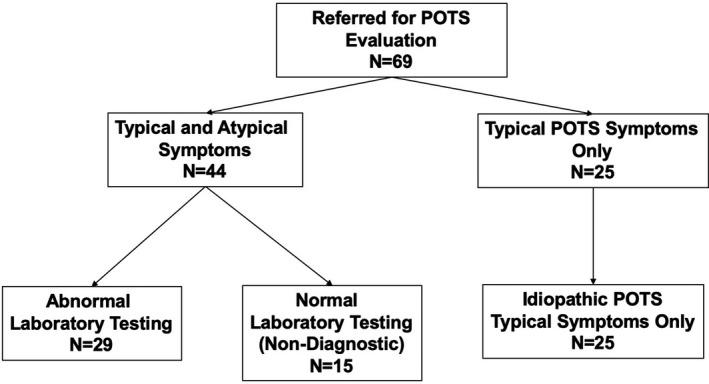
MCA indicates mast cell ; and POTS, postural orthostatic tachycardia syndrome.
Additional selected laboratory testing was also undertaken in the 69 patients at provider discretion based on individual medical history. All patients had normal hemoglobin, hematocrit, and white blood cell counts. None of the patients exhibited abnormal thyroid function. Antinuclear antibody titers were evaluated in a minority of cases and were identified to be abnormal or borderline abnormal at approximately the same frequency in the patients with POTS alone, and those who also had abnormal laboratory findings suggesting MCA disorder.
Patient Population: Laboratory Findings Suggesting MCA Disorder
Findings in the 44 patients who underwent laboratory evaluation for common MCA mediators are summarized in Table 2. Twenty‐three patients (52%) exhibited either an elevated plasma histamine or 24‐hour urine n‐methylhistamine. Sixteen (36%) patients had an elevation either in plasma prostaglandin D2 or 24‐hour urine prostaglandin 11‐β‐PGF 2‐alpha. Only 2/23 (9%) patients who underwent testing had an elevated plasma tryptase. Eleven patients had elevations in 2 or more MCA mediators or metabolites.
Table 2.
Comparison of Symptoms in 44 Patients Who Underwent Laboratory Testing
| Abnormal Values |
POTS‐like With Atypical Symptoms (n=29) |
POTS Alone (n=15) |
P Value |
|---|---|---|---|
|
ESR or CRP abnormal |
6/28* (21%) |
3/14* (21%) |
>.99 |
|
Tryptase |
2/23* (9%) |
0/9* (0% |
>.99 |
|
Prostaglandin |
16/28* (57%) |
0/15 (0%) |
0.0002 |
|
Histamine |
17/29 (59%) |
0/15 (0%) |
0.0001 |
|
Histamine or methylhistamine abnormal |
23/29 (79%) |
0/15 (0%) |
0.0001 |
|
≥2 Abnormal: Tryptase Prostaglandin Histamine |
12/29 (41%) |
0/15 (0%) |
0.0034 |
CRP indicates C‐reactive protein; ESR, erythrocyte sedimentation rate; and POTS, postural orthostatic tachycardia syndrome.
*See Methods, not measured in all patients.
All laboratory tests for MCA were normal in the subgroup of 15 patients deemed to have POTS alone who underwent laboratory testing (Table 2) (Figure 1).
Patient Population: Symptoms
Typical POTS symptoms (ie, palpitations, fatigue, lightheadedness/brain fog) did not differ between the patients with or without abnormal laboratory testing suggesting MCA (Table 1). However, patients with laboratory abnormalities more often reported migraine, allergy, skin rash, and chronic gastrointestinal disturbances.
Table 3 summarizes symptom profiles in the 44 individuals who underwent both clinical and laboratory evaluation. Again, the frequency of symptoms expected with typical POTS (ie, palpitations, fatigue, and lightheadedness/brain fog) did not differ significantly between patients with and without abnormal laboratory findings suggesting MCA (Table 3). However, allergy, gastrointestinal complaints, and to a lesser extent skin rash, tended to be more frequent in those patients in whom laboratory findings suggested MCA.
Table 3.
Comparison of Symptoms in 44 Patients Who Underwent Laboratory Testing
| Abnormal Laboratory Tests (n=29) | % | Normal Laboratory Tests (n=15) | % | P Value | |
|---|---|---|---|---|---|
| Age, y, mean±SD | 34±9.5 | 33 ± 12.2 | NS | ||
| % Female | 29/29 | 13/15 | 0.111 | ||
| Palpitation | 25/29 | 86 | 13/15 | 87 | 1.0 |
| Syncope | 11/29 | 38 | 4/15 | 27 | 0.524 |
| Fatigue | 21/29 | 72 | 8/15 | 53 | 0.317 |
|
Lightheadedness /dizzy/brain fog |
23/29 | 79 | 11/15 | 73 | 0.714 |
| Migraines | 11/29 | 38 | 3/15 | 20 | 0.314 |
| Depression/anxiety | 6/29 | 21 | 6/15 | 40 | 0.284 |
| Fibromyalgia | 4/29 | 14 | 1/15 | 7 | 0.647 |
| Allergy | 13/29 | 45 | 2/15 | 13 | 0.048 |
| Skin rash | 10/29 | 34 | 1/15 | 13 | 0.067 |
| Gastrointestinal symptoms | 18/29 | 62 | 3/15 | 23 | 0.001 |
NS indicates not significant.
Migraine frequency was lower in the overall cohort of patients with presumed typical POTS than was the case in patients with MCA (Table 1: POTS 13% vs MCA 41%, P=0.05). However, the difference between the 2 groups was no longer statistically significant when only those patients with POTS with negative tests were included in the comparison (Table 3: POTS 20% vs MCA 38%).
Discussion
Main Findings
This study provides 4 main findings regarding differences between patients in whom the clinical presentation included symptoms that are not typically considered to be caused by POTS compared with patients with a typical POTS presentation. First, the frequency with which MCA laboratory abnormalities were detected in the former population was relatively high. While solitary laboratory abnormalities cannot provide a definitive MCA diagnosis, the possibility of a coexisting MCA disorder merits consideration given its potential impact on treatment strategy. 2 , 5 , 6 , 7 , 8 Second, patients with abnormal laboratory findings manifested a more diverse symptom landscape, particularly including allergy complaints and gastrointestinal issues (most often gastroparesis), and to a lesser extent skin rash/flushing, than did those individuals who had normal laboratory findings (Table 3). Third, while elevated plasma tryptase level is a valuable biochemical marker for mastocytosis or mast cell leukemia, and may be useful in confirming MCA as suggested in Consensus‐1, 7 abnormal tryptase levels were uncommon (9%) among the laboratory findings in our study. Finally, elevated prostaglandins in conjunction with an abnormally elevated histamine marker (ie, plasma histamine or urine methyl‐histamine) was the most frequent diagnostic combination supporting a possible MCA diagnosis in our patients.
Laboratory Recognition of MCA and Its Limitations
Many conditions, including MCA, have been implicated in producing symptoms suggestive of POTS. MCA is particularly important in this regard because its presence markedly alters therapeutic strategy. 2 , 5 , 6 , 7 , 8 Specifically, patients with MCA may benefit from histamine receptor (H1 and H2) blockers used in conjunction with mast cell stabilizer agents and possibly vitamin D. 5 , 6 , 7 , 8 These agents are not usually part of a typical POTS treatment regimen.
Mast cells are estimated to contain several hundred chemical mediators that are either stored in situ or manufactured de novo as requirement demands. 13 , 14 While many such chemicals may be released by other cells, particularly basophils, the mast cell appears to be the most important.
Chemical mediators released from mast cells may affect multiple systems resulting in diverse symptoms (eg, allergic reactions, cutaneous disturbances, and gastrointestinal complaints) that may wax and wane in severity over time. 3 The result is a potentially very heterogeneous clinical picture, with only some affected individuals having symptoms suggesting POTS. It is in the latter cases that the presence of complaints beyond the typical expected with POTS (ie, palpitations, “lightheadedness,” and “brain fog”) should raise the possibility of an underlying or coexisting MCA disorder. 6 , 7
As noted above, 2 relatively recent consensus reports provide diagnostic criteria for identification of MCA‐related syndromes. 7 , 8 Both reports advocate identifying clinical and laboratory markers in order to establish an MCA diagnosis. Specifically, Afrin et al 6 recommend relying on (1) symptoms that are consistent with MCA, (2) signs/symptoms involving at least 2 organ systems, and (3) absence of other explanatory disease state. In terms of laboratory findings, Valent et al 7 emphasize the important diagnostic specificity associated with elevated tryptase levels; however, tryptase was an insensitive marker in our patients. In any event, both consensus reports offer elements of flexibility in establishing an MCA diagnosis. In particular, Valent et al acknowledge that prostaglandin D2 is acceptable when tryptase is not substantially elevated. 6 The approach offered by Afrin et al. 8 acknowledges the validity of histamine markers. Consequently, our diagnostic algorithm combined criteria from both consensus documents as a reasonable compromise for MCA diagnosis.
Laboratory findings have important limitations in the recognition of MCA. 6 , 7 , 8 , 15 , 16 , 17 , 18 Specifically, although mast cells produce many mediators, at present, most can be measured only in research laboratories. Of the minority that can be measured in clinical laboratories, many may be derived from cell types other than mast cells. Nevertheless, there are some mediators that are relatively mast cell specific. Examples include tryptase, histamine, prostaglandin D2, 2,3‐dinor‐11beta‐prostaglandin F2α (a metabolite of prostaglandin D2), and heparin.
Tryptase is a protein derived primarily from mast cells and would be expected to be increased in MCA disorder as it is in systemic mastocytosis. However, in our population tryptase was only infrequently abnormal. Potentially, elevated tryptase levels may be transient, and only elevated during or shortly after an MCA flare. Thus, Kacar et al. 15 reported that serum tryptase levels were periodically elevated, ranging from 10 ng/mL to 20 ng/mL between attacks, but increasing to >100 ng/mL following episodes of anaphylaxis. 5 Similarly, Akin et al. reported a level of 8 ng/mL or greater detected within 4 hours of a suspected anaphylactic event, a finding that confirms that MCA has occurred in that patient. Consequently, to ascertain a potential role for tryptase in MCA, levels are best measured within 4 hours after a suspected MCA event. 16
Regarding methylhistamine, Shibao et al. 17 noted that urinary methylhistamine is usually normal between flares in patients with MCA. Patients should be instructed to collect urine for a 4‐hour period immediately after a severe spontaneous flushing episode. Urine histamine is often measured in the evaluation of flushing but has been considered to be less specific than methylhistamine and less useful in the diagnosis of MCA.
Clinical Differences: Typical POTS Presentation Versus That Associated With Suspected MCA
In its original description, POTS was primarily characterized by symptoms that were provoked on standing, and that were relieved by recumbence. 1 Subsequently, nonpostural symptoms have become accepted as part of the clinical picture. 1 , 2 , 3 , 4 , 10 The principal resulting orthostatic symptoms are palpitations, fatigue, lightheadedness (including “brain fog”), and near‐syncope. Common nonorthostatic symptoms include nausea, diminished concentration, and tremulousness. 3 , 4 , 8 , 10 , 11 However, patients exhibiting MCA disorder may manifest similar complaints.
In our study, palpitations were observed in almost all patients, and the combination of lightheadedness/dizziness/brain fog was the next most common symptom. Furthermore, the frequency of these symptoms did not differ between patients with suspected MCA and patients with POTS. Consequently, one cannot distinguish between these 2 patient groups by conventional symptoms alone. On the other hand, certain symptoms (particularly allergy, gastrointestinal issues, and skin rash/flushing) tended to be more frequent in patients with MCA; these may be helpful diagnostically but must be actively sought for during the history‐taking. 17 , 18 , 19 , 20
Initially, greater migraine frequency appeared to distinguish the MCA group from the typical patients with POTS (Table 1). Furthermore, this difference persisted when patients with MCA were compared only with the smaller group of typical patients with POTS in whom laboratory testing results were normal (Table 3); however, the difference was no longer statistically significant. Thus, although release of multiple neurohormones from mast cells (eg, adenylate cycles activating pituitary polypeptide, histamine, and others) provides biologic plausibility for more frequent migraine in MCA, the role of migraine as a clinically differentiating feature cannot be relied upon; further study is needed.
Study Limitations
The findings reported here are subject to important limitations. First, since both true POTS and MCA disorder are infrequent conditions, our study population is small, limiting the power to detect differences among clinical and laboratory findings. Second, there remain differences of opinion regarding the optimal definition of MCA, 6 , 7 with some arguing that the condition is inadequately defined. 20 , 21 , 22 Consequently, despite the 2 consensus statements upon which we based our observations, the definition of which if any patients actually exhibit MCA disorder may be disputed. In any case, a solitary laboratory abnormality is insufficient to make a definitive diagnosis of MCA disorder; confirmatory laboratory abnormalities should be sought. In this regard, it is widely acknowledged that prostaglandin D2 and F2alpha are known markers of MCA. 6 , 7 , 21 , 22 However, it has been previously cautioned that an increased prostaglandin D2 or F2alpha level should not be relied upon solely as a marker of MCA disorder. 16 In our patients, we found that >50% of patients with elevated prostaglandin D2 also exhibited an increased histamine marker, thereby strengthening the MCA diagnosis. Third, the blood tests in our studies were not necessarily obtained in conjunction with an acute symptom ”flare,” but more often at the time of a convenient outpatient visit. In each instance, however, every effort was made to optimize the handling of specimens including appropriate refrigerated centrifugation and storage. Outside laboratories were not used. Nonetheless, false‐negative laboratory findings may have occurred. The sensitivity of testing is currently unknown. Optimal sampling would be in temporal proximity to an acute symptomatic event. Finally, laboratory costs precluded biochemical testing in many of the patients with typical POTS. Consequently, whether such testing may have been positive in some of those patients is unknown. However, among those who were tested, the principal MCA markers were normal (Table 2, Figure 1).
Conclusions
Patients with MCA disorder may have symptoms suggestive of POTS. However, in these cases one may anticipate that the clinical presentation will be characterized by a broader symptom profile than is the case with typical POTS. In particular, the presence of allergic reactions, gastrointestinal complaints, and to a lesser extent skin disturbances should lead to considering MCA. While no single laboratory finding provides an assured diagnostic marker, the presence of an elevated prostaglandin (prostaglandin D2 and/or F2 alpha), especially in conjunction with an increased second marker such as histamine or less often tryptase, suggests that MCA disorder may be responsible for the POTS‐like symptoms.
Sources of Funding
Dr. Benditt was supported in part by a grant from the Dr. Earl E Bakken Family in support of Heart‐Brain research.
Disclosures
Dr Benditt reports consulting fees from Medtronic Inc and St Jude Medical. Dr Fedorowski reports consulting fees from Medtronic Inc. and Biotronik Inc. Dr Olshansky reports consulting fees from Amarin, Boehringer Ingelheim, Sanofi Aventis, Respicardia, and Lundbeck Inc.
(J Am Heart Assoc. 2021;10:e021002. DOI: 10.1161/JAHA.121.021002.)
This manuscript was sent to Marwan M. Refaat, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 7.
References
- 1. Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. DOI: 10.1212/WNL.43.1_Part_1.132. [DOI] [PubMed] [Google Scholar]
- 2. Olshansky B, Cannom D, Fedorowski A, Stewart J, Gibbons C, Sutton R, Shen W‐K, Muldowney J, Chung TH, Feigofsky S, et al. Postural orthostatic tachycardia syndrome (POTS): a critical assessment. Prog CV Disease. 2020;63:263–270. DOI: 10.1016/j.pcad.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheldon RS, Grubb BP, Olshansky B, Shen W‐K, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, et al. Heart Rhythm Society Expert Consensus Statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia and vasovagal syncope. Heart Rhythm. 2015;12:e41–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raj SR, Guzman JC, Harvey P, Richer L, Schondorf R, Seifer C, Thibodeau‐Jarry N, Sheldon RS. Canadian Cardiovascular Society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol. 2020;36:357–372. DOI: 10.1016/j.cjca.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 5. Molderings GJ, Brettner S, Homann J, Afrin LB. Mast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol. 2011;22(4):10. DOI: 10.1186/1756-8722-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Afrin LB, Self S, Menk J, Lazarchick J. Characterization of mast cell activation syndrome. Am J Med Sci. 2017;353:207–215. DOI: 10.1016/j.amjms.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Valent P, Akin C, Bonadonna P, Hartmann K, Brocjow K, Niedoszytko M, Nedoszytko B, Siebenhaar F, Sperr WR, Elberink JNGO, et al. Proposed diagnostic algorithm for patients with suspected Mast Cell activation syndrome. J Allergy Clin Immunol Pract. 2019;7:1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Afrin LB, Ackerley MB, Bluestein LS, Brewer JH, Brook JB, Buchanan AD, Cuni JR, Davey WP, Dempsey TT, Dorff SR, et al. Diagnosis of mast cell activation syndrome: a global “consensus‐2”. Diagnosis. 2020;8:137–152. DOI: 10.1515/dx-2020-0005. [DOI] [PubMed] [Google Scholar]
- 9. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, Cheshire WP, Chalimsky T, Cortelli P, Gibbon CH, et al. Consensus statement on definition of orthostatic hypotension, neutrally mediated syncope and the postural tachycardia syndrome. Autonomic Neuroscience‐Basic & Clinical 2011;161:46–8 and Clinical Autonomic. Research. 2011;21:69–72. DOI: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 10. Low PA, Sandroni P, Joyner M, Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol. 2009;20:352–358. DOI: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome – Diagnosis, physiology, and prognosis. Auton Neurosci. 2018;215:3–11. DOI: 10.1016/j.autneu.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dahan S, Tomljenovic L, Shoenfeld Y. Postural orthostatic tachycardia syndrome (POTS). A novel member of the autoimmune family. Lupus. 2016;25:339–342. DOI: 10.1177/0961203316629558. [DOI] [PubMed] [Google Scholar]
- 13. Gilfillan AM, Tkaczyk C. Integrated signaling pathways for mast cell activation. Nat Rev Immunol. 2006;6:218–230. [DOI] [PubMed] [Google Scholar]
- 14. Draber P, Halova I, Polakovicova I, Kawakami T. Signal transduction and chemotaxis in mast cells. Eur J Pharmacol. 2016;778:11–23. DOI: 10.1016/j.ejphar.2015.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kacar M, Denman S, Savic S. Selective response to omalizumab in a patient with concomitant ncMCAS and POTS: what does it teach us about the underlying disease? J Investig Allerg Clin Immunol. 2018;28:261–263. DOI: 10.18176/jiaci.0251. [DOI] [PubMed] [Google Scholar]
- 16. Akin C. Mast cell activation syndromes. J Allergy Clin Immunol. 2017;140:349–355. DOI: 10.1016/j.jaci.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 17. Shibao C, Arzubiaga C, Roberts LJ 2nd, Raj S, Black B, Harris P, Biaggioni I. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45:385–390. DOI: 10.1161/01.HYP.0000158259.68614.40. [DOI] [PubMed] [Google Scholar]
- 18. Beck SC, Wilding T, Buka RJ, Baretto RL, Krishna MT. Biomarkers in human anaphylaxis: A critical appraisal of current evidence and perspectives. Front Immunol. 2019;10:494. DOI: 10.1186/1756-8722-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weinstock LB, Brook JB, Myers TL, Goodman B. Successful treatment of postural orthostatic tachycardia and mast cell activation syndromes using naltrexone, immunoglobulin and antibiotic treatment. BMJ Case Rep. 2018;10:2017–221405. DOI: 10.1136/bcr-2017-221405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kohn A, Chang C. The relationship between hypermobile Ehlers‐Danlos syndrome (hEDS), postural orthostatic tachycardia syndrome (POTS), and mast cell activation syndrome (MCAS). Clin Rev Allergy Immunol. 2020;58:273–297. DOI: 10.1007/s12016-019-08755-8. [DOI] [PubMed] [Google Scholar]
- 21. Metcalfe DD, Pawankar R, Ackerman SJ, Akin C, Clayton F, Falcone FH, Gleich GJ, Irani A‐M, Johansson MW, Klion AD, et al. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ J. 2016;9:7. DOI: 10.1186/s40413-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fanning LB, Boyce JA. Lipid mediators and allergic diseases. Ann Allergy Asthma Immunol. 2013;111:155–162. DOI: 10.1016/j.anai.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]


