Abstract
Objective
This study was carried out to explore the potential involvement of miR‐125a‐5p in the oncogenic effects of EphA2, TAZ, and TEAD2 and the activity of the Hippo signaling pathway in gastric cancer progression.
Methods
In vitro transfection of miR‐125a‐5p mimics or inhibitors, qRT‐PCR, colony formation assays, and cell invasion assays were used to assess the effect of miR‐125a‐5p on the growth and invasion in gastric cancer (GC). Male nude mice bearing tumors derived from human GC cells were used for evaluating the effects of miR‐125a‐5p on tumor growth. Luciferase reporter assay, immunofluorescence, immunohistochemistry, qRT‐PCR, and immunoblotting were performed to explore the role of miR‐125a‐5p in the epithelial‐mesenchymal transition (EMT) and association among miR‐125a‐5p, EphA2, TAZ, and TEAD2 in GC cells.
Results
MiR‐125a‐5p enhanced GC cell viability and invasion in vitro, whereas inhibition of miR‐125a‐5p using a specific inhibitor and antagomir suppressed cancer cell invasion and tumor growth. Moreover, inhibition of miR‐125a‐5p reversed EMT in vitro. miR‐125a‐5p upregulated the expression of EphA2, TAZ, and TEAD2, promoted TAZ nuclear translocation, and induced changes in the activity of the Hippo pathway by enhancing the expression of TAZ target genes. Finally, miR‐125a‐5p was overexpressed in late‐stage GCs, and positive correlations were observed with its targets EphA2, TAZ, and TEAD2.
Conclusion
miR‐125a‐5p can promote GC growth and invasion by upregulating the expression of EphA2, TAZ, and TEAD2.
Keywords: gastric cancer, Hippo pathway, miR‐125a‐5p, prognosis, TAZ
miR‐125a‐5p may function as a powerful tumor promoter, activate the expression of its target genes EphA2, TAZ, and TEAD2, and promote cell growth, invasion, and epithelial‐mesenchymal transition in certain types of gastric cancer cells through activation of the Hippo pathway. miR‐125a‐5p may thus serve as a potential therapeutic target in the treatment of advanced gastric cancer cells.
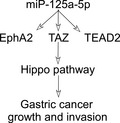
1. INTRODUCTION
Gastric cancer (GC) is the fifth most common malignancy and third most frequent cause of cancer‐related death worldwide. 1 , 2 , 3 Surgical resection is the only curative approach for resectable GCs; however, locoregional control of advanced GCs may frequently be complicated by postsurgical recurrence and metastasis. The molecular mechanism of GC recurrence and progression is unclear, and investigation of this mechanism is critical for development of targeted therapies to inhibit local recurrence and progression.
The Hippo pathway consists of a large network of proteins and controls growth of normal and malignant (carcinoma) tissues. 4 , 5 The key kinase cascade involved in the Hippo pathway is phosphorylation of upstream Yes‐associated protein (YAP) and transcriptional co‐activator PDZ‐binding motif (TAZ). YAP and TAZ promote tissue growth and cell viability by regulating the activity of transcription factor TEA domain family members (TEADs). 6 Multiple components of the Hippo pathway, including YAP1, TAZ, and TEAD1, are overexpressed in GCs and are closely correlated with lymphatic metastasis and tumor TNM staging. 7 , 8 Thus, the Hippo pathway has been recognized as a promising and important therapeutic target. 9
MicroRNAs (miRNAs) are small non‐coding RNAs that post‐transcriptionally control the translation and stability of mRNAs. miRNAs may function as oncogenes or tumor‐suppressor genes to contribute to cancer development, progression, and therapeutic responses. 10 miR‐125a‐5p has anticancer activities in breast cancer, 11 hepatocellular carcinoma, 12 lung cancer, 13 and glioblastoma. 14 However, controversial results have been reported for the function of miR‐125a‐5p in GCs in different studies. The miR‐125a‐5p expression had been reported to be significantly downregulated in GCs, and decreased miR‐125a‐5p expression was associated with gastric carcinogenesis. 15 , 16 However, miR‐125a‐5p had also been reported to be overexpressed in a GC characterized by activation of mesenchymal phenotype‐related pathways, but miR‐125a‐5p expression level itself did not associate with poor prognosis. 17 Moreover, miR‐125a, the precursor of miR‐125a‐5p (which is derived from the 5′ end of pri‐miR‐125a), was reported to be associated with invasion depth and clinical stage of GC. 18 Thus, the specific role of miR‐125a‐5p in GCs is unclear and needs additional studies to clarify.
Here, we investigated the role of miR‐125a‐5p as an oncogene in GC by analyzing its effects on the growth, invasion, and epithelial‐mesenchymal transition (EMT) in GC cells. In addition, we examined the involvement of the miR‐125a‐5p target EphA2, TAZ, and TEAD2 in these oncogenic effects and the activity of the Hippo signaling pathway in GC. Finally, we evaluated the correlations between miR‐125a‐5p expression levels and GC progression. Our results suggested that miR‐125a‐5p could be a potential therapeutic target for advanced GC.
2. MATERIALS AND METHODS
2.1. Patients and clinical specimens
This study was approved by the ethics committee of our hospital, and all patients with GCs had given their signed informed consent to participate. The GC adenocarcinoma tissues (n = 65) and corresponding noncancerous gastric tissues were obtained from samples of radical resection. No patients received neoadjuvant chemotherapy or radiotherapy before radical gastrectomy. Resected tissues were immediately frozen in liquid nitrogen and stored at –80°C until RNA extraction. The clinical stage of cancer was based on the criteria published by the 7th American Joint Committee on Cancer TNM staging classification for carcinoma of the stomach. 19
2.2. Reagents
Antibodies specific to EphA2 (ab118882), YES1 (ab109744), TAZ (ab119253), and GAPDH (ab37168) and horseradish peroxidase‐couple secondary antibodies were purchased from Abcam. Antibodies targeting TEAD2 (8870), E‐cadherin (3195), N‐cadherin (13116), and vimentin (5741) were from Cell Signaling Technology. miR‐125a‐5p mimics, inhibitors (antagomir), negative controls, 3′‐untranslated region (UTR) reporter plasmids, small hairpin RNA (shRNA) specifically targeting TAZ, and control shRNA were synthesized by Ribobio Co. The sequences are shown in Table S1.
2.3. Cell culture and transfection
The gastric epithelial cell line GES‐1, the GC cell lines AGS, SGC‐7901, BGC‐823, MGC‐803, MKN‐45, N87, SNU‐5, and KATO‐III, and HEK293T cells were purchased from the Cell Resource Center of Xiangya Medical School, Central South University. GC cells were cultured in RPMI 1640 medium, whereas GES‐1 and HEK293T cells were cultured in Dulbecco’s modified Eagle medium (Gibco) with 10% fetal bovine serum (Hyclone) and incubated in 5% CO2 at 37°C. Cells were seeded into six‐well plates, incubated overnight, and then transfected with miRNA mimics, inhibitors, or shRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
2.4. RNA quantification
Total RNA was extracted from tissues and cells with TRIzol reagent (Invitrogen). Reverse transcription reactions were conducted using a RevertAid H Minus First Strand cDNA Synthesis Kit (Fermentas). Quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) was conducted using a qSYBR‐Green‐containing PCR kit (Qiagen) on an ABI 7500 System (Applied Biosystems). RNU6 and β‐actin were used as internal controls for miRNA and mRNA, respectively. Relative expression levels were determined using the method. The primers for qRT‐PCR detection were synthesized by GenePharma Co., and the sequences are presented in Table S2.
2.5. Colony formation assay
Gastric cancer cells were seeded in 3.5‐cm dishes at 1 × 103 cells per dish and incubated at 37°C for 2 weeks. Colonies were fixed with methanol and stained with 0.1% crystal violet for 10 min. The number of colonies was counted using an inverted microscope (Olympus). The colony formation rate was calculated according to the following formula: colony formation rate = number of colonies/number of seeded cells.
2.6. Cell invasion assays
Briefly, cells transfected with miR‐125a‐5p mimics or inhibitors and their negative normal controls were plated onto the basement membrane matrix in the inserts of 24‐well culture plates (8 μm; BD Biosciences) at a density of 1 × 105 cells per insert. The cells were incubated at 37°C for 48 h, and noninvasive cells and matrix were then gently removed with a cotton swab. The invaded cells on the lower membrane surface were fixed with methanol for 10 min, stained with crystal violet, counted, and imaged.
2.7. Luciferase reporter assay
The 3′‐UTRs of EphA2, YES1, TAZ, and TEAD2 mRNA containing the intact miR‐125a‐5p recognition sequence or mutant sequences were amplified by PCR and subcloned into the pmiR‐RB‐REPORT vector (Ribobio Co.). The miR‐125a‐5p mimics were cotransfected with luciferase reporter plasmids into cultured HEK293T cells. At 48 h after transfection, the cells were assayed for luciferase activity using the Dual‐Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions. The relative luciferase activity was normalized to the renilla luciferase activity.
2.8. Immunoblotting
After transfection and culture, cells were harvested and lysed in RIPA buffer (Promega). Total proteins were separated by 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and then transferred onto polyvinylidene fluoride membranes (Millipore). After blocking of nonspecific binding, the transferred membranes were subsequently incubated overnight at 4°C with primary and secondary antibodies for 1 h. Protein bands were visualized using enhanced chemiluminescence detection (Millipore).
2.9. Immunohistochemistry
Immunohistochemistry was performed as previously described. 20 Briefly, peroxidase activity within the sections was blocked with 2.5% hydrogen peroxide in methanol for 30 min at room temperature, and the slides were then incubated with primary antibodies overnight at 4°C. After washing with phosphate‐buffered saline, the sections were incubated with peroxidase‐labeled polymer and substrate chromogen. Finally, the slides were visualized in diaminobenzidine solution at room temperature.
2.10. Immunofluorescence
Cells grown on glass coverslips were fixed in 4% paraformaldehyde and permeabilized using 0.5% Triton X‐100. The primary antibodies were applied for 1 h at 37°C and visualized using Alexa‐Fluor 488‐conjugated secondary antibodies (Invitrogen). Cells were counterstained with 4′,6‐diamidino‐2‐phenylindole and then examined by confocal microscopy (LSM5 PASCAL, Carl Zeiss).
2.11. In vivo assay
Male nude mice bearing tumors derived from human GC cells were used for evaluating the effects of miR‐125a‐5p on tumor growth. For preparation of the subcutaneous xenograft model, 1 × 106 N87 cells were subcutaneously injected into the flanks of mice. Two weeks after subcutaneous injection, 10 nmol antagomir in 0.1 ml saline buffer was locally injected into the tumor mass once every 3 days for 2 weeks. 21 The length (a) and width (b) of the tumor were measured every 3 days. The tumor volume (V) was calculated according to the formula V = (ab)2/2. 22 All mouse experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, with the approval of the Animal Ethics Committee of Central South University.
2.12. Statistical analyses
Continuous variables were presented as means ± standard deviations (SDs). Comparisons between groups were analyzed using the t test and chi‐squared test. Correlations between the expression levels of two mRNAs were evaluated by Pearson’s coefficient correlation analyses. Differences with p values of <0.05 were considered significant. Statistical analyses were conducted using GraphPad Prism5 software (GraphPad Software, Inc.).
3. RESULTS
3.1. miR‐125a‐5p promoted GC growth and invasion
To select suitable GC cells for use, the expression levels of miR‐125a‐5p were tested in a series of GC cell lines and a normal gastric epithelial cell line (GES‐1 cells). The highest expression of miR‐125a‐5p was observed in N87 cells, whereas the lowest level was demonstrated in the KATO‐III cells (Figure 1A). Therefore, the N87 and KATO‐III cells were selected for functional deficient and acquired studies, respectively.
FIGURE 1.
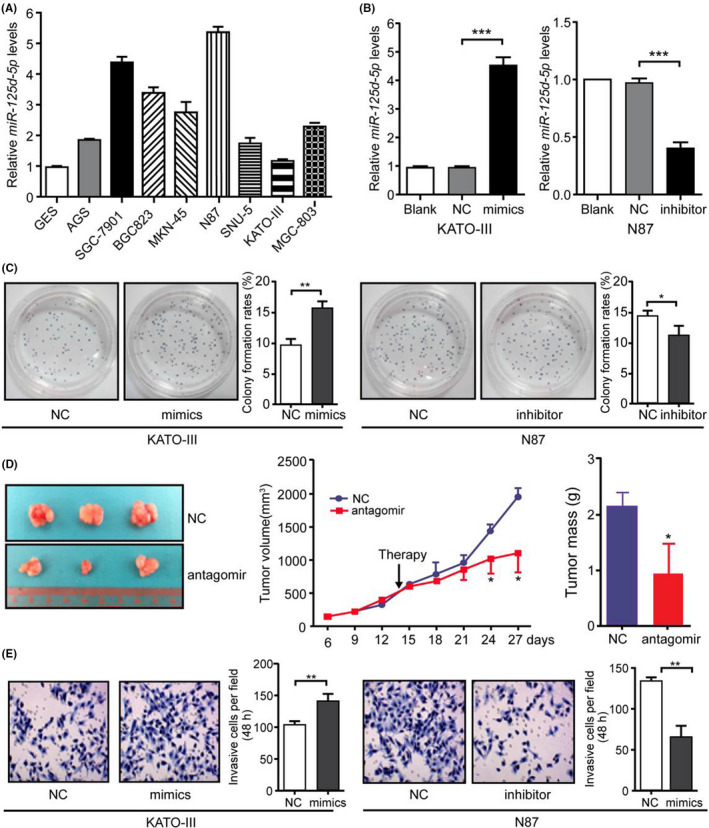
miR‐125a‐5p promoted gastric cancer (GC) growth and invasion. (A) miR‐125a‐5p expression in the gastric epithelial cell line GES‐1 and the GC cell lines AGS, SGC‐7901, BGC‐823, MGC‐803, MKN‐45, N87, SNU‐5, and KATO‐III was detected using real‐time qRT‐PCR. (B) KATO‐III and N87 cells were transfected with miR‐125a‐5p mimics and inhibitors at a final concentration of 50 nM. miR‐125a‐5p expression was detected using real‐time qRT‐PCR at 48 h after transfection. (C) Cell viability was evaluated using colony formation assays at the indicated times after transfection. (D) The effects of miR‐125a‐5p antagomir on N87 tumor growth were evaluated using the growth curve and tumor weights. (E) Invasive capacity was evaluated in cells treated with miR‐125a‐5p mimics and inhibitors as described in B. Blank, blank control; NC, negative control; *p < 0.05; **p < 0.01; ***p < 0.001
Next, the effects of miR‐125a‐5p were examined on GC growth and invasion. miR‐125a‐5p mimics significantly increased miR‐125a‐5p expression in KATO‐III cells, whereas antisense oligonucleotides significantly decreased miR‐125a‐5p expression in N87 cells (Figure 1B). Increased expression of miR‐125a‐5p enhanced the viability of KATO‐III cells, whereas loss of miR‐125a‐5p significantly attenuated N87 cell viability (Figure 1C). To identify the effects of miR‐125a‐5p on cell growth, subcutaneous xenografts were established from N87 cells in nude mice. Two weeks after implantation, intratumoral injection of miR‐125a‐5p antagomir was performed. The miR‐125a‐5p antagomir significantly inhibited the growth of subcutaneous tumors after 10 days of antagomir treatment (Figure 1D). Moreover, ectopic expression of miR‐125a‐5p in KATO‐III cells significantly increased cell invasion, whereas inhibition of miR‐125a‐5p by antisense oligonucleotides inhibited the invasion of N87 cells (Figure 1E). Thus, these results demonstrated that miR‐125a‐5p promoted GC growth and invasion. (*, p < 0.05; **, p < 0.01; ***, p < 0.001.)
3.2. Inhibition of miR‐125a‐5p suppressed the EMT
Because overexpression of miR‐125a‐5p has been shown to activate mesenchymal phenotype‐related pathways, including the EMT, and regulate mesenchymal cell proliferation in GC tissues, 17 the effects of miR‐125a‐5p on the EMT were subsequently tested. Loss of miR‐125a‐5p resulted in a decrease in the number of spindle‐like N87 cells (Figure 2A). Moreover, in cells treated with a miR‐125a‐5p inhibitor, E‐cadherin protein levels were increased significantly compared with that in the control. Inversely, the expression levels of N‐cadherin and vimentin were significantly decreased in cells treated with miR‐125a‐5p inhibitors (Figure 2B). The same phenomenon was observed using immunofluorescence (Figure 2C).
FIGURE 2.
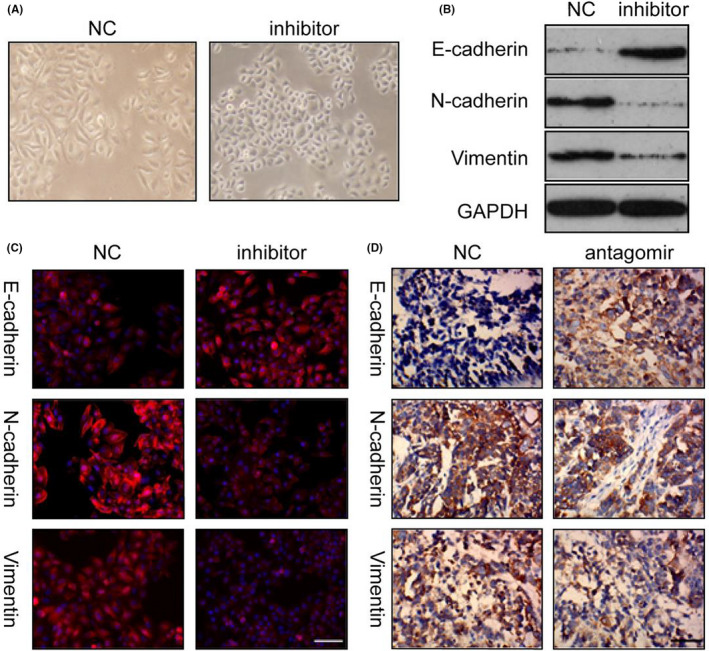
miR‐125a‐5p antagomir inhibited the epithelial‐mesenchymal transition (EMT). (A) N87 cells treated with miR‐125a‐5p inhibitor lost mesenchymal characteristics (200×). (B) Expression of epithelial and mesenchymal markers in N87 cells transfected with miR‐125a‐5p inhibitors. (C) EMT markers were detected using immunofluorescence. (D) E‐cadherin, N‐cadherin, and vimentin were detected in subcutaneous tumors using immunohistochemistry. NC, negative control. Bar in C, 100 μm; bar in D, 50 μm
To further investigate the role of miR‐125a‐5p in EMT, the epithelial marker E‐cadherin and the mesenchymal markers N‐cadherin and vimentin were detected in subcutaneous tumors using immunohistochemistry. As shown in Figure 2D, the expression of E‐cadherin was increased, whereas those of N‐cadherin and vimentin were decreased in tumors treated with the miR‐125a‐5p antagomir. Thus, inhibition of miR‐125a‐5p reversed the EMT.
3.3. miR‐125a‐5p regulated EphA2 and multiple components of the Hippo pathway
Different miRNA target‐predicting algorithms such as TargetScan, Pictar, and miRDB were used to identify potential effectors of miR‐125a‐5p, and conserved miR‐125a‐5p sites were found at the 3′‐UTR of three genes related to the Hippo pathway (YES1, TAZ, and TEAD2) and of EphA2 (Figure S1A), an Eph family member that is overexpressed in GCs and has been shown to promote the proliferation, invasion, and EMT in GC cells through the Wnt/β‐catenin pathway. 20 To further validate targets of miR‐125a‐5p, the 3′‐UTRs of EphA2, YES1, TAZ, and TEAD2 (containing the putative miR‐125a‐5p target sites) were cloned into luciferase reporter constructs. The luciferase activity of plasmids containing the wild‐type 3′‐UTRs of EphA2, YES1, TAZ, and TEAD2 was significantly reduced in the presence of miR‐125a‐5p but not in the mutant 3′‐UTR (Figure S1B). However, the expression of EphA2, TAZ, and TEAD2 mRNA and protein was significantly increased by transfection with miR‐125a‐5p mimics and decreased by transfection with miR‐125a‐5p inhibitors, and no significant changes were present in the YES1 expression (Figure 3A and Figure S2). Moreover, transfection with the miR‐125a‐5p antagomir significantly downregulated the expression of EphA2, TAZ, and TEAD2 in subcutaneous tumors (Figure 3B).
FIGURE 3.
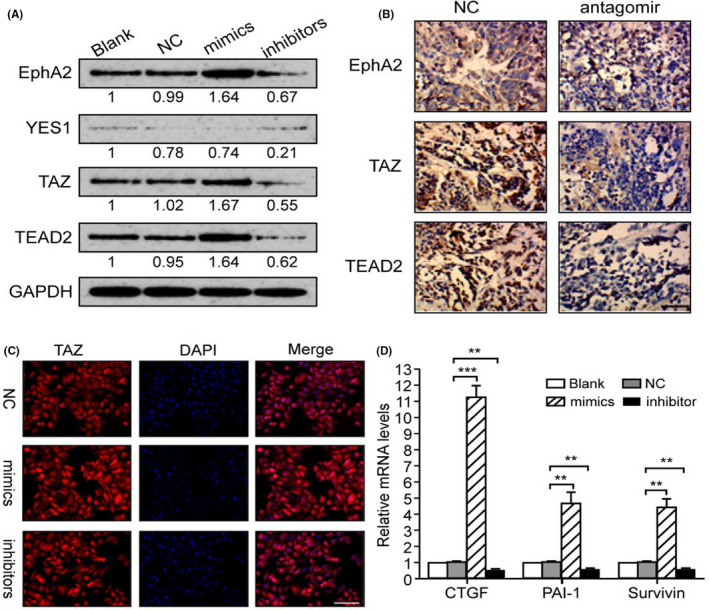
Targeting of EphA2 and the Hippo pathway by miR‐125a‐5p. (A) Proteins levels of EphA2, YES1, TAZ, and TEAD2 were detected after transfection with miR‐125a‐5p mimics or inhibitors. (B) EphA2, TAZ, and TEAD2 were detected in subcutaneous tumors using immunohistochemistry. (C) Effects of miR‐125a‐5p on TAZ nuclear translocation were detected with immunofluorescence. (D) Expression levels of TAZ target genes were tested using real‐time qRT‐PCR. Blank, blank control; NC, negative control; miR, miR‐125a‐5p mimics; WT, wild‐type 3′‐UTR plasmid; Mut, mutant 3′‐UTR plasmid; *p < 0.05; **p < 0.01; ***p < 0.001. Bar in B, 50 μm; Bar in C, 100 μm
Because TAZ is a key co‐activator of the Hippo pathway, the subcellular distribution of TAZ was tested by immunofluorescence, and the expression of TAZ target genes, including connective tissue growth factor, plasminogen activator inhibitor 1, and surviving, was detected by qPCR. 23 It was found that miR‐125a‐5p promoted the nuclear translocation of TAZ and activated the transcription of TAZ target genes (Figure 3C,D). Taken together, these findings suggested that miR‐125a‐5p contributed to activation of EphA2, TAZ, and TEAD2. (*p < 0.05; **,p < 0.01; ***, p < 0.001.)
3.4. Silencing of TAZ reversed the effects of miR‐125a‐5p on GC cells
The above findings indicated that TAZ was a candidate effector of miR‐125a‐5p. TAZ is a nuclear effector of Hippo‐related pathways that regulate EMT. 24 Moreover, inhibition of TAZ by miRNA has been shown to inhibit tumor growth and metastasis in GCs. 25 Therefore, it was hypothesized that miR‐125a‐5p promoted EMT in GC cells through modulation of TAZ. To test this hypothesis, TAZ was silenced using specific shRNA in GC cells overexpressing miR‐125a‐5p (Figure 4A). It was demonstrated that knockdown of TAZ inhibited the effects of miR‐125a‐5p on promotion of EMT, cell viability, and invasion in GC cells (Figure 4B,C). These data implied that miR‐125a‐5p contributed to cell growth, invasion, and EMT in GCs through the key Hippo pathway component TAZ.
FIGURE 4.
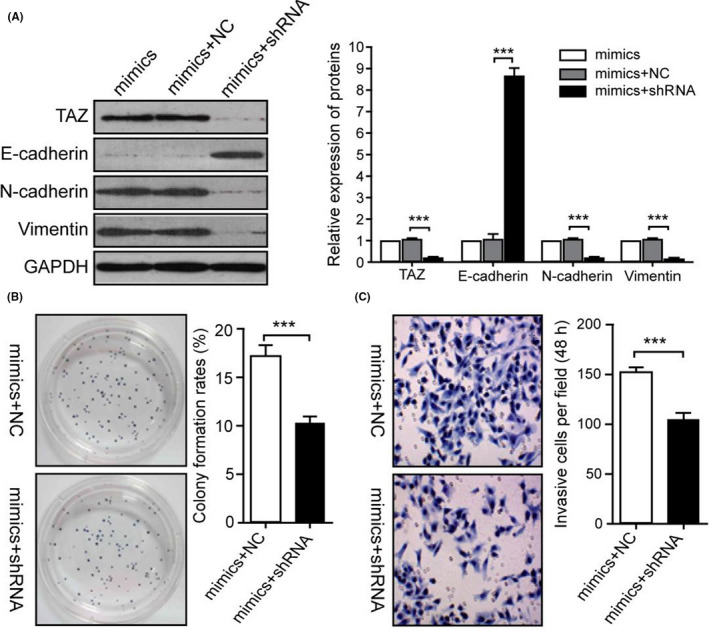
Silencing of TAZ inhibited the effects of miR‐125a‐5p in GC cells. (A) Proteins levels of E‐cadherin, N‐cadherin, and vimentin were detected in KATO‐III cells transfected with miR‐125a‐5p mimics in the presence of TAZ shRNA. (B) Cell viability was evaluated using colony formation assays. (C) The invasive capacity of cells was determined using transwell assays. NC, negative control; shRNA, TAZ‐shRNA; ***p < 0.001
3.5. Clinical correlation between miR‐125a‐5p and its targets in GCs
To further elucidate the potential biological significance of miR‐125a‐5p expression in GC progression, the expression levels of miR‐125a‐5p were detected in tumor specimens and corresponding noncancerous gastric tissues. The results showed that the expression levels of miR‐125a‐5p in GC tissues were significantly higher than those in noncancerous gastric tissues (Figure 5A). Next, the expression of miR‐125a‐5p was analyzed in patients with GCs of different grades. miR‐125a‐5p expression was significantly higher in stage III and IV GCs than in stage I and II GCs. No significant differences were detected among noncancerous gastric tissues, stage I GCs, and stage II GCs (Figure 5B).
FIGURE 5.
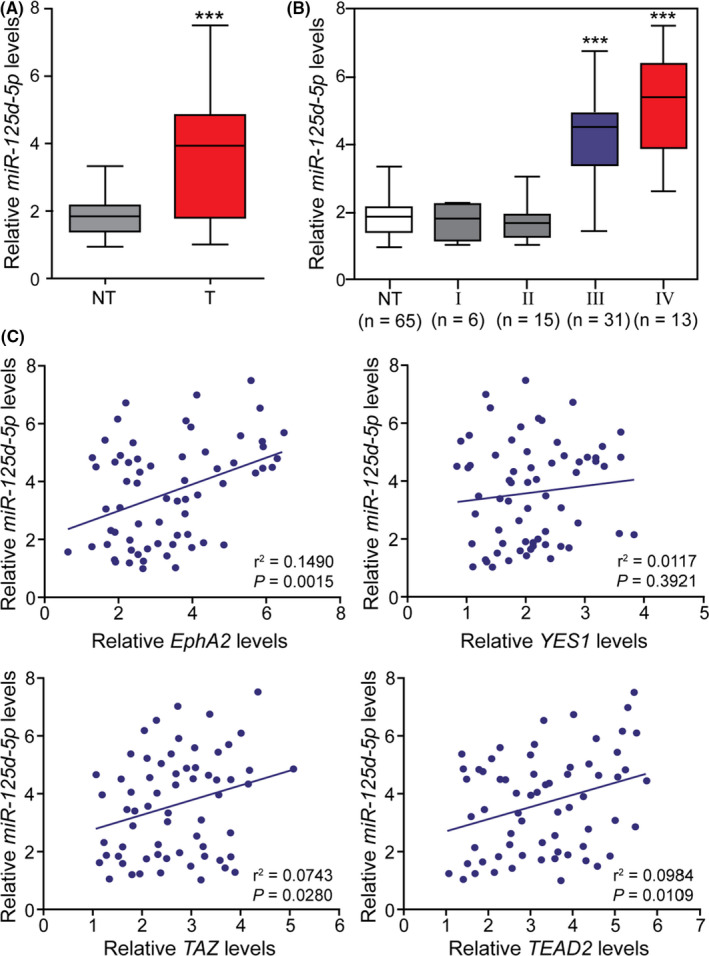
Clinical correlations between miR‐125a‐5p and its targets in gastric cancers (GCs). (A) The expression of miR‐125a‐5p was detected in 65 GC tissues and noncancerous gastric tissues. (B) Expression levels of miR‐125a‐5p in GCs of different stages. (C) Correlations between miR‐125a‐5p levels and the expression levels of putative targets in GCs were demonstrated. NC, noncancerous gastric tissues; ***p < 0.001 compared with NC, stage I GCs, and stage II GCs
The expression of EphA2, YES1, TAZ, and TEAD2 was tested in 65 pairs of gastric adenocarcinoma tissues by qRT‐PCR. Pearson's correlation coefficient analysis revealed significant positive correlations in miR‐125a‐5p expression with EphA2, TAZ, and TEAD2 mRNA levels. However, no correlation was detected between miR‐125a‐5p and YES1 mRNA expression (Figure 5C). These results indicated that miR‐125a‐5p upregulation was correlated with tumor staging and may play a significant role in the progression of advanced GCs.
4. DISCUSSION
In this study investigating the role of miR‐125a‐5p in the progression of GCs, it was found that miR‐125a‐5p played a substantial role in the growth, invasion, and EMT in GC cells in vitro and in an animal model through the Hippo pathway. Additionally, miR‐125a‐5p expression was significantly associated with clinical stages in patients with GCs. This comprehensive study provided in vitro, in vivo, and clinical evidence supporting the significant role of miR‐125a‐5p in the progression of GCs.
MiR‐125a‐5p expression was significantly higher in stage III and IV GCs than in stage I and II GCs, but no significant differences were detected in miR‐125a‐5p levels between noncancerous tissues, stage I and II GCs. These data indicated that miR‐125a‐5p played a significant role in the progression of GCs of advanced rather than early stages. It has been found that miR‐125a‐5p expression did not associate with poor prognosis in patients with the mesenchymal subtype GC. 17 However, controversies also existed regarding the expression and role of miR‐125a‐5p in GCs. Nishida et al 26 reported that miR‐125a‐5p was downregulated in GC tissues and that miR‐125a‐5p potently suppressed the proliferation of GC cells by directly targeting ERBB2. The exact reason for this is unknown, but may be related to intratumor heterogeneity 27 and racial variations. However, other reasons may also account for this difference. The use of different GC cell lines may contribute to the difference. In our study, the GC cell lines used were the N87 cells with the highest miR‐125a‐5p expression and the KATO‐III cells with the lowest miR‐125a‐5p expression. Other studies used different cell lines including MGC‐803 and SGC‐7901 in the study by Song et al., 17 NUGC4 by Nishida et al., 26 OE19 and OE33 by Fassan et al., 28 and AGS and MGC‐803 by Xu et al. 16 In one study investigating somatic mutations in human cancers, 29 the MKN45 GC cell lines which encode a p53 heterozygous mutation did not have either miR‐125a‐5p overexpression or Dies1 promoter methylation in regulating GC pathogenesis. However, in other cell lines of MKN28, MKN45, SNU1, GP202, and AGS, the Dies1 expression existed to adjust the GC pathogenesis. 30 Moreover, combinatory mechanisms may exist to play a role in the initiation and development of GCs. For example, p53 mutations were present in the MKN 28 GC cell lines and might play a combinatory effect with Dies1 in regulating GC pathogenesis. 29 , 30 The initiation, development, aggravation, or regression of GCs are caused by the total effect of all factors. In our study, the Hippo pathway and its regulator miR‐125a‐5p were investigated, whereas other studies explored varied pathways like the HER2‐miR125a‐5p/miR125b loop in the study by Fassan et al., 28 the miR‐125a‐5p expression and the targeting E2F3 by Xu et al., 16 and miR125a‐5p and GC prognosis by Nishida et al. 26 Every study focused only on one point or one series of issues related to the pathogenesis of GCs. Different pathways and targeting points exist to play combining effects in the same GC cells and may result in different overall outcomes in the initiation, development, aggravation, regression, or relief.
miR‐125a‐5p is a tumor‐suppressive miRNA in breast cancer, 11 hepatocellular carcinoma 12 lung cancer, 13 and glioblastoma. 14 However, the study by Song et al. did not find the miR‐125a‐5p expression to be significantly associated with poor prognosis in patients with the mesenchymal subtype GC, 17 and our results indicated that miR‐125a‐5p may serve as an oncogenic miRNA in GCs. These findings strongly suggest that the function of miR‐125a‐5p as a tumor suppressor or oncogenic promoter is tissue specific, consistent with findings of miR‐146 and miR‐29, which have opposing roles in tumorigenesis depending on the cellular context. 31 Interestingly, the same miRNA may have opposing roles in the same type of cancer. For example, miR‐125a‐5p expression is lower in non‐small cell lung cancer tissues than in adjacent normal lung tissues, and an inverse relationship between miR‐125a‐5p expression and pathological stage or lymph node metastasis is observed. In contrast, miR‐125a‐5p has been shown to enhance lung cancer cell migration and invasion. 32 Therefore, more refined analyses of miR‐125a‐5p function in GCs are needed.
One unique characteristic of the relationship between miRNAs and their targets is that a single miRNA sequence may be able to target numerous genes that are simultaneously involved in the same functional event. 33 Our experimental findings showed that miR‐125a‐5p targeted EphA2, TAZ, and TEAD2 mRNAs at the same time. Although dual‐luciferase reporter assays indicated that the 3′‐UTR of YES1 was a target of miR‐125a‐5p, cellular and clinical validation studies did not support this result. Even though miR‐125a‐5p mimics reduced the fluorescent signal, the mRNA and protein levels of EphA2, TAZ, and TEAD2 were elevated in the presence of miR‐125a‐5p mimics. This result demonstrated that miR‐125a‐5p activated rather than inhibited the translation of these targets.
miRNAs primarily recognize complementary sequences in the 3′‐UTRs of their target mRNAs, resulting in inhibition of the expression of target genes by translational repression or mRNA degradation. 34 However, recent studies have reported that miRNAs can also bind to the 5′‐UTR of promoter and upregulate the translation of target genes. 35 Moreover, complementarity of seven or more bases with the 5′ end of miRNA is sufficient to confer regulation, even if the target 3′‐UTR contains only a single site. 36 Based on bioinformatics analysis from GenBank, in addition to the 3′‐UTR, sequences that are complementary with the miR‐125a‐5p sequence were also found in the promoter regions of EphA2, TAZ, and TEAD2. Although the effects of miR‐125a‐5p were not investigated on the promotion of luciferase reporter activity, it was speculated that miR‐125a‐5p might directly target the promoters of EphA2, TAZ, and TEAD2, thereby activating gene expression.
Epithelial‐mesenchymal transition is a biological process that allows polarized epithelial cancer cells to undergo multiple biochemical changes, enabling the cells to assume a mesenchymal cell phenotype. 37 Various miRNAs can regulate EMT through distinct mechanisms. 38 GCs exhibiting miR‐125a‐5p overexpression also show activation of EMT‐related pathways. 17 Thus, miR‐125a‐5p plays a significant role in EMT. Our findings showed that inhibition of miR‐125a‐5p suppressed EMT by upregulating E‐cadherin but downregulating N‐cadherin and vimentin. Induction of TAZ expression has been shown to trigger EMT by stimulating E‐cadherin transcriptional inhibitors Snail and Slug. 24 , 39 Moreover, as a critical component in the Hippo pathway, TAZ is a target of miR‐125a‐5p. Our study showed that silencing of TAZ blocked the function of miR‐125a‐5p in promoting GC growth, invasion, and EMT. Collectively, these data supported the idea that miR‐125a‐5p may promote cell growth, invasion, and EMT in GCs by activation of the Hippo pathway through TAZ.
Recently, studies have also explored the role of miRNAs in GCs including miR‐148a‐3p 40 and miR‐212. 41 Bao et al. investigated the diagnostic potential and biological function of miR‐148a‐3p in GC progression in the GC tissues and plasma of patients and found that miR‐148a‐3p was significantly downregulated in both the gastric tissue and plasma of GC patients, suggesting that miR‐148a‐3p may inhibit cancer progression and is a novel diagnostic biomarker for GC. 40 Shao et al. 41 analyzed the relationship of serum level of miR‐212 with GC through detecting the serum level of miR‐212 in 100 healthy people and 110 GC patients, and these authors found that miR‐212 was epigenetically downregulated in GC and could directly regulate the 3′UTR of SOX4 mRNA to suppress p53 and Bax, suggesting that low level of miR‐212 can be a potential circulation biomarker and poor prognosis predicator of GC. Novel findings in our study and in other studies 40 , 41 will ultimately accumulate to clarify the mechanism of GC and clear the way for better treatment of this cancer.
In conclusion, miR‐125a‐5p can promote GC growth and invasion by upregulating the expression of EphA2, TAZ, and TEAD2. miR‐125a‐5p may thus serve as a potential therapeutic target in the treatment of advanced GCs.
CONFLICT OF INTEREST
None.
AUTHORS’ CONTRIBUTIONS
Study design: Ruixin Li, Guojun Wang. Data collection: Ruixin Li, Zhihao Hu, Zhuoyin Wang, Tianyu Zhu, Bulang Gao, Jingtao Wang, Xiumei Deng. Data analysis: Ruixin Li, Zhihao Hu. Supervision: Ruixin Li. Writing of the original version: Ruixin Li. Revision: Bulang Gao.
CODE AVAILABILITY
None.
CONSENT TO PARTICIPATE
All subjects agreed to participate in the study.
CONSENT FOR PUBLICATION
All subjects agreed to the publication of the data in the study.
Supporting information
Supplementary Material
Li R, Hu Z, Wang Z, et al. miR‐125a‐5p promotes gastric cancer growth and invasion by regulating the Hippo pathway. J Clin Lab Anal. 2021;35:e24078. 10.1002/jcla.24078
Ruixin Li and Zhihao Hu contributed equally to this work.
Funding information
This study was funded by the National Natural Scientific Foundation of China (grant nos.: 81172297 and 81472692) and the Natural Scientific Foundation of Hunan province (grant no.: 2015JJ4049)
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author on reasonable account.
REFERENCES
- 1. Chen D, Liu Z, Liu W, et al. Predicting postoperative peritoneal metastasis in gastric cancer with serosal invasion using a collagen nomogram. Nat Commun. 2021;12:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gojayev A, Ersen O, Mercan U, et al. Evaluation of peroperative and oncological results in laparoscopic surgery of gastric cancer in elderly patients: single‐center study. J Laparoendosc Adv Surg Tech A. 2021;31:657‐664. [DOI] [PubMed] [Google Scholar]
- 3. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635‐648. [DOI] [PubMed] [Google Scholar]
- 4. Gao Y, Li J, Xi H, et al. Stearoyl‐coA‐desaturase‐1 regulates gastric cancer stem‐like properties and promotes tumour metastasis via Hippo/YAP pathway. Br J Cancer. 2020;122:1837‐1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qiao Y, Li T, Zheng S, Wang H. The hippo pathway as a drug target in gastric cancer. Cancer Lett. 2018;420:14‐25. [DOI] [PubMed] [Google Scholar]
- 6. Harvey KF, Zhang X, Thomas DM. The hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246‐257. [DOI] [PubMed] [Google Scholar]
- 7. Song M, Cheong JH, Kim H, Noh SH, Kim H. Nuclear expression of yes‐associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res. 2012;32:3827‐3834. [PubMed] [Google Scholar]
- 8. Zhou GX, Li XY, Zhang Q, et al. Effects of the Hippo signaling pathway in human gastric cancer. Asian Pac J Cancer Prev. 2013;14:5199‐5205. [DOI] [PubMed] [Google Scholar]
- 9. Jiao S, Wang H, Shi Z, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166‐180. [DOI] [PubMed] [Google Scholar]
- 10. Santos RM, Moreno C, Zhang WC. Non‐coding RNAs in lung tumor initiation and progression. Int J Mol Sci. 2020;21:1‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hsieh TH, Hsu CY, Tsai CF, et al. HDAC inhibitors target HDAC5, upregulate microRNA‐125a‐5p, and induce apoptosis in breast cancer cells. Mol Ther. 2015;23:656‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim JK, Noh JH, Jung KH, et al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors miR‐125a‐5p and miR‐125b. Hepatology. 2013;57:1055‐1067. [DOI] [PubMed] [Google Scholar]
- 13. Wang G, Mao W, Zheng S, Ye J. Epidermal growth factor receptor‐regulated miR‐125a‐5p – a metastatic inhibitor of lung cancer. FEBS J. 2009;276:5571‐5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuan J, Xiao G, Peng G, et al. MiRNA‐125a‐5p inhibits glioblastoma cell proliferation and promotes cell differentiation by targeting TAZ. Biochem Biophys Res Commun. 2015;457:171‐176. [DOI] [PubMed] [Google Scholar]
- 15. Wang S, Ran L, Zhang W, et al. FOXS1 is regulated by GLI1 and miR‐125a‐5p and promotes cell proliferation and EMT in gastric cancer. Sci Rep. 2019;9:1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu Y, Huang Z, Liu Y. Reduced miR‐125a‐5p expression is associated with gastric carcinogenesis through the targeting of E2F3. Mol Med Rep. 2014;10:2601‐2608. [DOI] [PubMed] [Google Scholar]
- 17. Song F, Yang D, Liu B, et al. Integrated microRNA network analyses identify a poor‐prognosis subtype of gastric cancer characterized by the miR‐200 family. Clin Cancer Res. 2014;20:878‐889. [DOI] [PubMed] [Google Scholar]
- 18. Ueda T, Volinia S, Okumura H, et al. Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis. Lancet Oncol. 2010;11:136‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dikken JL, van de Velde CJ, Gönen M, Verheij M, Brennan MF, Coit DG The New American Joint Committee on Cancer/International Union Against Cancer staging system for adenocarcinoma of the stomach: increased complexity without clear improvement in predictive accuracy. Ann Surg Oncol. 2012;19:2443‐2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang J, Xiao D, Li G, et al. EphA2 promotes epithelial‐mesenchymal transition through the Wnt/beta‐catenin pathway in gastric cancer cells. Oncogene. 2014;33:2737‐2747. [DOI] [PubMed] [Google Scholar]
- 21. Hou J, Lin L, Zhou W, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR‐199a/b‐3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232‐243. [DOI] [PubMed] [Google Scholar]
- 22. Tu Y, Gao X, Li G, et al. MicroRNA‐218 inhibits glioma invasion, migration, proliferation, and cancer stem‐like cell self‐renewal by targeting the polycomb group gene Bmi1. Cancer Res. 2013;73:6046‐6055. [DOI] [PubMed] [Google Scholar]
- 23. Xiang L, Gilkes DM, Hu H, et al. Hypoxia‐inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget. 2014;5:12509‐12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiao H, Jiang N, Zhou B, Liu Q, Du C. TAZ regulates cell proliferation and epithelial‐mesenchymal transition of human hepatocellular carcinoma. Cancer Sci. 2015;106:151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zuo QF, Zhang R, Li BS, et al. MicroRNA‐141 inhibits tumor growth and metastasis in gastric cancer by directly targeting transcriptional co‐activator with PDZ‐binding motif, TAZ. Cell Death Dis. 2015;6:e1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishida N, Mimori K, Fabbri M, et al. MicroRNA‐125a‐5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17:2725‐2733. [DOI] [PubMed] [Google Scholar]
- 27. Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fassan M, Pizzi M, Realdon S, et al. The HER2‐miR125a5p/miR125b loop in gastric and esophageal carcinogenesis. Hum Pathol. 2013;44:1804‐1810. [DOI] [PubMed] [Google Scholar]
- 29. Forbes SA, Beare D, Gunasekaran P, et al. Cosmic: exploring the world's knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015;43:D805‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oliveira P, Carvalho J, Rocha S, et al. Dies1/VISTA expression loss is a recurrent event in gastric cancer due to epigenetic regulation. Sci Rep. 2016;6:34860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849‐864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jiang L, Huang Q, Zhang S, et al. Hsa‐miR‐125a‐3p and hsa‐miR‐125a‐5p are downregulated in non‐small cell lung cancer and have inverse effects on invasion and migration of lung cancer cells. BMC Cancer. 2010;10:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin CW, Chang YL, Chang YC, et al. MicroRNA‐135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat Commun. 2013;4:1877. [DOI] [PubMed] [Google Scholar]
- 34. Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2017;9:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fang Y, Zhu X, Wang J, et al. MiR‐744 functions as a proto‐oncogene in nasopharyngeal carcinoma progression and metastasis via transcriptional control of ARHGAP5. Oncotarget. 2015;6:13164‐13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brennecke J, Stark A, Russell RB, Cohen SM. Principles of microRNA‐target recognition. PLoS Biol. 2005;3:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Weinberg RA. Epithelial‐to‐mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12:361‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ding XM. MicroRNAs: regulators of cancer metastasis and epithelial‐mesenchymal transition (EMT). Chin J Cancer. 2014;33:140‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bao C, Guo L. MicroRNA‐148a‐3p inhibits cancer progression and is a novel screening biomarker for gastric cancer. J Clin Lab Anal. 2020;34:e23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shao JP, Su F, Zhang SP, et al. miR‐212 as potential biomarker suppresses the proliferation of gastric cancer via targeting SOX4. J Clin Lab Anal. 2020;34:e23511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data are available from the corresponding author on reasonable account.


