Abstract
Infectious bursal disease virus (IBDV) caused an acute and highly contagious infectious disease, resulting in considerable economic losses in the world poultry industry. Although this disease was well-controlled under the widely use of commercial vaccines, the novel variant IBDV strain emerged due to the highly immunized-selection pressure in the field, posting new threats to poultry industry. Here, we reported the epidemic and pathogenicity of IBDV in Hubei Province from May to August 2020. We isolated 12 IBDV strains from the broiler flocks, including 9 novel variants, 2 very virulent strains and 1 medium virulent strain. Interestingly, we identified a series of changes of amino acid sites in the VP2. Further analysis indicated that the novel variant IBDV strains caused damage to bursa of fabricius and spleen, leading to immunosuppression. Our findings underscore the importance of IBDV surveillance, and provide evidence for understanding the evolution of IBDV.
Key words: IBDV; epidemiology; novel variant, pathogenicity
Abbreviations: IBD, infectious bursal disease; IBDV, infectious bursal disease virus; HVR, hyper-variable region; vvIBDV, very virulent infectious bursal disease; BF, bursa of Fabricius; SPF, specific pathogen-free; DMEM, Dulbecco's modified Eagle's medium; RT-PCR, reverse transcription-polymerase chain reaction; dpi, days post inoculation; BBIX, bursa, body weight index; aa, amino acid; AIV, avian influenza virus; HI, Hemagglutination inhibition; H&E, hematoxylin and eosin
INTRODUCTION
Infectious bursal disease (IBD), also called Gumboro disease, is an acute and highly contagious infectious disease, causing great economic losses to the poultry industry worldwide. The causative agent of this disease is IBD virus (IBDV), which were usually divided into 2 serotypes. The serotype I viruses contain a series of pathotypic strains to chickens. Based on the molecular characteristics of the hypervariable region (HVR) of VP2, serotype I viruses are classified into 4 subtypes: classic IBDV, variant IBDV, attenuated IBDV, and very virulent IBDV (vvIBDV) (Cosgrove, 1962; Jackwood and Saif, 1987; Chettle et al., 1989; Sharma et al., 1989). Clinically, IBDV usually destroys the developing B-lymphocytes (B-lymphocyte precursors) in the bursa of Fabricius (BF) (Dey et al., 2019; Van den Berg et al., 2000). Although IBDV causing clinical manifestations lasts for only 3 to 4 d, the damage to the BF is irreversible and leads to immunosuppression. The diseased chickens usually suffer from severe immunosuppression, causing an increased susceptibility to other pathogens (Sharma et al., 2000; Rautenschlein et al., 2002; Ingrao et al., 2013). The serotype II viruses isolated from turkeys and Peking ducks are not pathogenic for chickens (Ismail et al., 1988).
IBDV belonging to the genus Avibirnavirus of the family Birnaviridae possesses 2 segments of double-stranded genomic RNAs (segment A and segment B) (Brown and Skinner, 1996). Segment A encodes 2 structural proteins (VP2 and VP3), a viral protease (VP4), and a nonstructural protein (VP5) (Raja et al., 2016). VP2 is the major structural protein, which is involved in antigenicity, cell tropism, virulence and apoptosis (Coulibaly et al., 2005; Caston et al., 2001); VP3 also participates in the formation of viral particles, and involved the serotype specificity, viral assembly, and apoptotic regulation (Tacken et al., 2002; Ye et al., 2014; Ferrero et al., 2015). VP4 plays a critical role in the interdomain proteolytic autoprocessing of the pVP2-VP4-VP3 polyprotein, and is responsible for virus-induced immune suppression (Lejal et al., 2000). VP5 inhibits apoptosis early during infection, whereas it induces apoptosis at a later stage of infection (Méndez et al., 2015). Segment B encodes an RNA-dependent RNA polymerase (VP1), which involves viral replication and genetic evolution (von Einem et al., 2004; Escaffre et al., 2013; Gao et al., 2014).
IBDV was first isolated in 1957. Since then, IBDV strains with different virulence successively emerged and spread to nearly all poultry producing countries globally, posting new challenges to the prevention and control of this disease (Withers et al., 2005; Alkie and Rautenschlein, 2016). In China, the first case of IBDV was reported in 1979, and the first vvIBDV strain was isolated in 1992 (Cao et al., 1998). Subsequently, vvIBDV became a pandemic pathogen in Chinese poultry industry. Due to the immunized procedure of commercial vaccines against IBDV and the improvement of bio-safety measures, this disease was being well-controlled. However, the widely used vaccines induced highly immunized-selection pressure in the field, contributing to the development of genetic diversity of circulating viruses.
Since 2016, the novel variant IBDV strain emerged in China, and rapidly spread to the whole country, posting a new threat to China's poultry industry (Fan et al., 2019). The novel variant IBDV was not lethal, but caused severe atrophy of bursa Fabricius and induced severe immunosuppression and the loss of production performance (Zachar et al., 2016; Fan et al., 2020; Xu et al., 2020). This study focused on the prevalence and pathogenicity of the novel variant IBDV in Hubei province of China. We reported the results of this study, including the isolation, characterization and pathogenicity of the novel variant IBDV strains. Our findings underscore the importance of IBDV surveillance, and provide evidence for understanding the evolution of IBDV.
MATERIALS AND METHODS
Ethics Statement
This study was approved by the Animal Care Committee of South China Agricultural University (approval ID: SYXK-2019-0136). All study procedures and animal care activities were conducted per the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People's Republic of China.
Animals and Eggs
The specific pathogen-free (SPF) chickens and SPF chicken embryonated eggs were purchased from the Guangdong DHN Poultry and Egg Products Co. Ltd., China.
Clinical Samples
During the period of May to August 2020, a total of 64 clinical samples of bursae were collected from 12 broiler flocks in Hubei province in southern China. These broilers had been vaccinated with 2512 or HVT vaccine at 1 or 10 d of age. All the samples labeled with collected date and area site, were transported on dry ice to the laboratory for sample processing and testing.
Virus Isolation and Identification
Virus isolation and purification were performed refer to the previous description (Wang et al., 2019). Briefly, the homogenate suspension of bursal tissues in Dulbecco's modified Eagle's medium (DMEM) containing penicillin (100 U/mL) and streptomycin (100 μg/mL) was frozen and thawed 3 times, clarified by centrifugation at 10,000 rpm for 10 min and then filtrated using a 0.22-μm filter for sterilization. The inoculum was used to inoculate 10-day-old SPF chicken embryonated eggs by the chorioallantoic membrane sac route, and blind passage till the occurrence of embryonic lesions.
The presence and identity of the IBDV were confirmed with a RT-PCR protocol targeted at the hypervariable region (HVR) of the VP2 gene. Briefly, a specific primer pairs targeting an approximately 714 bp fragment of HVRs of VP2 gene (sense primer: 5’-GCCGATGATTACCAATTCTCATC-3’ and anti-sense primer: 5’-CCGGATTATGTCTTTGAAGC-3’) were designed based on the IBDV VP2 gene using Primer Premier 5.0. RNA was extracted from the chorioallantoic membraneallantoic using a Viral DNA/RNA Miniprep Kit (Axygen, Magen Biotech Co., Ltd.) following the manufacturer's instructions, and subjected to RT-PCR for IBDV detection.
Sequence Alignment and Phylogenetic Analysis
Sequence alignments were performed with nucleotide sequences of VP2 gene of IBDV isolates and other IBDV strains retrieved from GenBank database using DNASTAR software (Madison, WI). Phylogenetic analysis was performed based on the nucleotide sequences of VP2 gene by neighbor-joining method by using MEGA version 7.0. The bootstrap values were determined from 1,000 replicates of the original data. All the reference IBDV strains used in this study were provided in Table 1.
Table 1.
IBDV reference strains used in this study.
| Strain | Origin | Genotype/Serotype | Accession No. |
|---|---|---|---|
| OH | Canada | Serotype 2 | U30818 |
| 23/82 | Germany | Serotype 2 | AF362773 |
| SHG358 | China | Variant | MT179721 |
| SHG352 | China | Variant | MT179720 |
| SHG19 | China | Variant | MN393076 |
| GLS | USA | Variant | AF093794 |
| 9109 | USA | Variant | AY837465 |
| Variant A | USA | Variant | M64285 |
| Variant E | USA | Variant | AF133904 |
| HLJ0504 | China | Very virulent | GQ451330 |
| HK46 | China | Very virulent | AF092943 |
| Harbin-1 | China | Very virulent | AF092171 |
| OKYM | Netherlands | Very virulent | D49706 |
| Gx | China | Very virulent | AY444873 |
| Gt | China | Attenuated | DQ403248 |
| D6948 | Netherlands | Very virulent | DQ646405 |
| B87 | China | Attenuated | DQ906921 |
| D78 | USA | Attenuated | AF499929 |
| Cu-1 | China | Attenuated | X16107 |
| CT | France | Attenuated | AJ310185 |
| CEF94 | Netherlands | Attenuated | AF133904 |
| JD1 | China | Attenuated | AF321055 |
| BD399 | Bangladesh | Attenuated | AF362776 |
| 2512 | USA | Classic | DQ355819 |
| IM | USA | Classic | AY029166 |
Challenge Study
To evaluate the pathogenicity of the novel variant IBDVs and vvIBDV, a total of twenty-four 1-day-old SPF chickens were randomly assigned into 3 groups (8 birds per group). The birds in group I and group II were inoculated with Hb06v (the novel variant strain) and Hb06t (vvIBDV strain), respectively, via intramuscular inoculation route at a dose of 104.0 EID50; the birds in group III were inoculated with an equal volume of PBS as a control. The birds in each group were maintained in independently negative pressurized isolators. Food and water were provided ad libitum. Clinical manifestations of this disease and mortality were monitored daily. At 5 d post inoculation (dpi), 3 birds of each group were weighed and then euthanized for necropsy. At 10 dpi, all the birds were euthanized for necropsy to record the gross lesions. The bursa: body weight index (BBIX) was calculated. The spleen and BF were collected for virus detection using RT-PCR.
To determine whether the variant IBDV induced immunosuppression of chickens, the influence of Hb06v infection on vaccination against avian influenza was evaluated. A total of twenty-four 10-day-old SPF chickens were randomly assigned into 3 groups (8 birds per group). The birds in group I was inoculated with Hb06v (the novel variant strain), via the ocular and intranasal routes at a dose of 104.0 EID50. At 3 dpi, the birds in group I and group II were immunized with the commercial inactivated vaccine against H9N2 avian influenza virus (AIV). The third group, without infection and vaccination, was used as the control. The chickens were observed daily for clinical symptoms for 14 d. At 7, and 14 d postvaccination, the antibody titers were examined using Hemagglutination inhibition (HI) assay.
Histopathology
The fresh affected tissues (spleen and BF) were fixed in 10% neutral-buffered formalin, routinely processed, embedded in paraffin, sectioned (4-μm thick), and stained with hematoxylin and eosin (H&E) according to standard protocols. Pathological changes were examined by light microscopy.
Statistical Analyses
A one-way ANOVA was employed to evaluate the significance of the differences among the different groups. Differences with P < 0.05 were considered significant.
RESULTS
Clinical Features of the Diseased Chickens
During May 2020 to August 2020, an epidemiological survey was performed in broiler breeders to monitor the IBDV epidemic in Hubei province. All the chickens presented typical clinical signs, such as hemorrhage in leg, atrophy occurred in thoracic gland and BF, spleen enlarge. The onset age of sick chickens mainly concentrated in 3–6 wk old. The presence of IBDV was detected using RT-PCR assay. As a result, all the 64 field strains were confirmed IBDV positive. Virus isolation and purification were performed using SPF chicken embryonated eggs. As a result, a total of 64 field IBDV strains were successfully isolated. These data indicated that IBDV is prevalent in the Chinese poultry industry.
Characterization of VP2 Genes of IBDV Isolates
In previous study, we sequenced the complete genome of IBDV isolates, Hb06v and Hb06t. We found that the nucleotide sequences of the vp1 gene are highly conserved. Therefore, in this study, we mainly focused on the molecular characteristic of the VP2 gene of IBDV isolates. Because VP2 is involved in the antigenicity, cell tropism, virulence and apoptosis, we sequenced the VP2 HVR of the IBDV field strains for diversity analysis. As a result, a total of 64 nucleotide sequences of VP2 gene were obtained, but only 12 sequences were identified (Table 2). Because many IBDV isolates from the same chicken flock had identical sequences or some of them were identified as vaccine strains. Within the VP2 genes obtained in this study, the fragments of the nucleotide sequences were 714 bp in length, and the corresponding deduced amino acid (aa) numbers were 238 aa. The nucleotide sequences of VP2 gene obtained in this study have been deposited in the GenBank database under the accession numbers MW795723-MW795734.
Table 2.
IBDV strains isolated in this study.
| Isolate | Origin | Type | Accession No. |
|---|---|---|---|
| Hb05v1 | broiler | Variant | MW795729 |
| Hb05v2 | broiler | Variant | MW795728 |
| Hb06t | broiler | Classic strain | MW795727 |
| Hb06t3 | broiler | Classic strain | MW795726 |
| Hb06v | broiler | Variant | MW795725 |
| Hb06v2 | broiler | Variant | MW795724 |
| Hb06v5 | broiler | Variant | MW795723 |
| Hb07t1 | broiler | Classic strain | MW795733 |
| Hb07v2 | broiler | Variant | MW795734 |
| Hb07v3 | broiler | Variant | MW795732 |
| Hb07v4 | broiler | Variant | MW795731 |
| Hb08v | broiler | Variant | MW795730 |
A multiple sequence alignment based on the nucleotide sequences of VP2 gene obtained in this study and other sequences retrieved from GenBank database was made using the Clustal X program. As a result, the identities of nucleotide sequence of VP2 gene between 12 IBDV isolates ranged from 90.1 to 99.0%. The nucleotide sequence identities between 12 IBDV strains and other strains retrieved from GenBank ranged from 90.3 to 99%. The highest identity (99.0%) was found between Hb06v and SHG358, whereas the lowest identity (90.3%) was found between Hb08v and Candioto-BR. Additionally, the variant IBDV strains isolated in the study had 96.3%∼99.0% identity with the novel variant IBDV reference strains, 93.2%∼94.6% identity with the early American variant strains, 90.7%∼94.9% identity with the vvIBDV reference strains, 90.3%∼94.6% identity with the attenuated strains, and 92.0%∼93.8% identity with the intermediate strains.
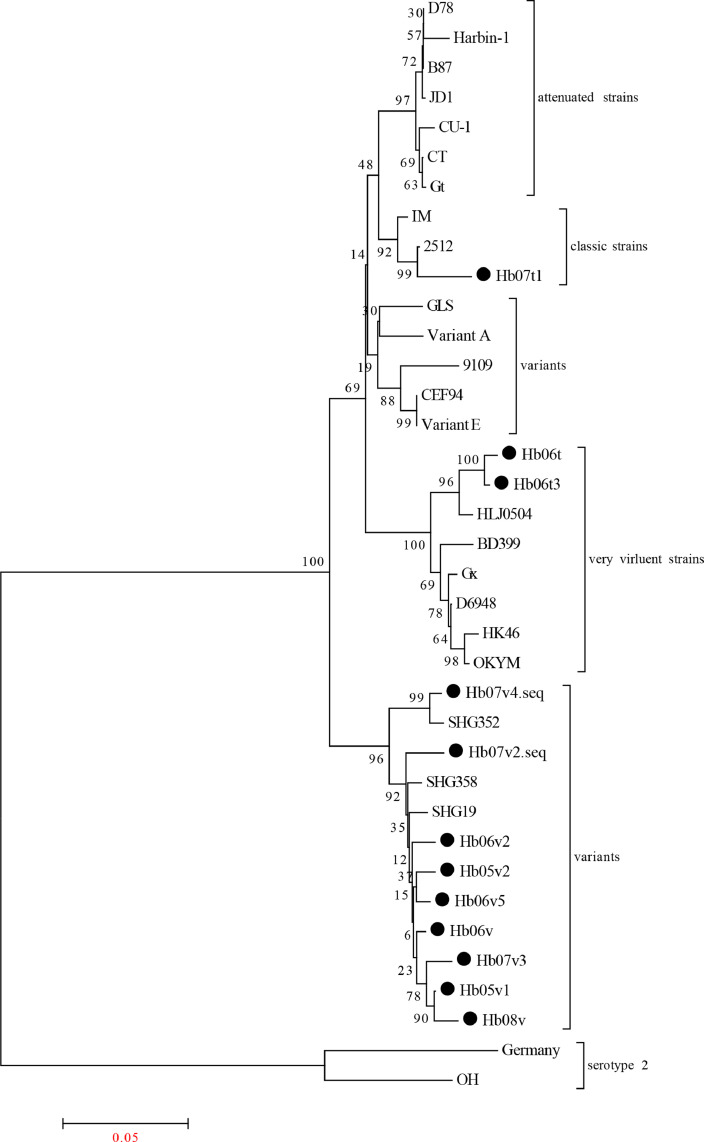
Phylogenetic analysis at the nucleotide level of VP2 gene of the IBDV isolates together with reference sequences was conducted. As a result, all the IBDV strains were divided into 5 major branches: very virulent strains, attenuated strains, classic strains, variant strains and serotype 2 (Figure 1).
Figure 1.
Phylogenic analysis based on the nucleotide sequences of VP2 gene. A phylogenic tree was constructed using the neighbor-joining method from phylogenetic distances calculated using MEGA 7.0 software. Bootstrap values obtained from 1,000 replicates are shown at the major nodes. All the VP2 gene sequences obtained in this study are indicated by solid dots. The vaccine strains are marked with solid triangles.
Molecular Characteristic of the VP2 Gene of IBDV Isolates
HVRs of the VP2 gene (206 to 350 aa) contains 4 hydrophilic regions: aa 210-225 (peak A), 247-254 (minor peak 1), 281-292 (minor peak 2), and 312-324 (peak B). In this study, we analyzed the molecular characteristic of the HVRs of VP2 gene of IBDV isolates. As a result, all the variant IBDV strains possessed similar typical amino acid residues, such as 222T, 249K, 286I, and 318D. The isolates in the subgroup 1 possessed same typical amino acid residues (222A, 242I, 256I, and 294I) with the reference very virluent strain OKYM; The isolates in the subgroup 3 possessed same typical amino acid residues (222P, 242V and 249K) with the reference classic strain 2512; The isolates in the subgroup 4 shared same typical amino acid residues (222T, 242V, 249K, 253Q, 256V, 279N, 284A, 286I, 294L, 318D, 323E and 330S) with the reference variant strain Variant E. Consistent with the previous findings (Fan et al., 2019), we also identified 4 typical amino acid residues (221K, 252I, 254N and 299S), which can distinguished subgroup 4 from the American variants (Table 3).
Table 3.
The amino acid substitutions in VP2.
| Strains | Phenotype | Amino acid site | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 221 | 222 | 242 | 249 | 252 | 253 | 254 | 256 | 279 | 284 | 286 | 294 | 299 | 318 | 323 | 330 | 359 | ||
| B87 | Attenuated strain | Q | P | V | Q | V | H | G | V | N | T | T | L | N | G | D | R | T |
| D78 | Attenuated strain | Q | P | V | Q | V | H | G | V | N | T | T | L | N | G | D | R | T |
| CT | Attenuated strain | Q | P | V | R | V | H | G | V | N | T | T | L | N | G | D | R | T |
| Variant A | Variant (USA) | Q | Q | V | K | V | Q | S | V | N | A | I | L | N | D | D | S | T |
| Variant E | Variant (USA) | Q | T | V | K | V | Q | S | V | N | A | I | L | N | D | E | S | T |
| SHG358 | Variant (China) | K | T | V | K | I | Q | N | V | N | A | I | L | S | D | E | S | T |
| SHG352 | Variant (China) | K | T | V | K | I | Q | N | V | N | A | I | L | S | D | E | S | K |
| SHG19 | Variant (China) | K | T | V | K | I | Q | N | V | D | A | I | L | S | D | E | S | T |
| HLJ0504 | Very virulent | Q | A | I | Q | V | Q | G | I | D | A | T | I | S | D | D | S | T |
| OKYM | Very virulent | Q | A | I | Q | V | Q | G | I | D | A | T | I | S | D | D | S | T |
| HK46 | Very virulent | Q | A | I | Q | V | Q | G | I | D | A | T | I | S | D | D | S | T |
| 2512 | Vaccine strain | Q | P | V | Q | V | Q | G | V | D | A | T | I | N | G | D | S | T |
| Hb06v5 | Variant | K | T | V | K | I | Q | D | V | N | A | I | L | S | D | E | S | S |
| Hb06v2 | Variant | K | T | V | K | I | Q | N | V | N | A | I | L | S | D | E | S | T |
| Hb06v | Variant | K | T | V | K | I | Q | N | V | N | A | I | L | S | D | E | S | T |
| Hb05v2 | Variant | K | T | V | K | I | Q | N | V | N | A | I | L | S | D | E | S | T |
| Hb05v1 | Variant | K | T | V | K | I | Q | N | V | N | A | I | L | S | D | E | S | T |
| Hb08v | Variant | K | T | V | K | I | Q | N | V | N | A | I | V | S | D | E | S | T |
| Hb07v4 | Variant | K | T | V | K | I | Q | N | V | N | A | I | L | S | D | E | S | K |
| Hb07v3 | Variant | K | T | V | K | I | Q | N | V | N | A | I | L | S | D | E | S | T |
| Hb07v2 | Variant | K | T | V | K | I | Q | N | V | N | A | I | L | S | D | E | S | T |
| Hb06t3 | Very virulent | Q | A | I | Q | V | Q | G | V | D | A | T | L | S | G | D | S | T |
| Hb06t | Very virulent | Q | A | I | Q | V | Q | G | V | D | A | T | L | S | G | D | S | T |
| Hb07t1 | Moderate virulent | Q | P | V | Q | V | Q | G | V | D | A | T | L | N | G | D | S | T |
Clinical Symptoms and Gross Lesions of Hb06v- and Hb06t-Infected Chickens
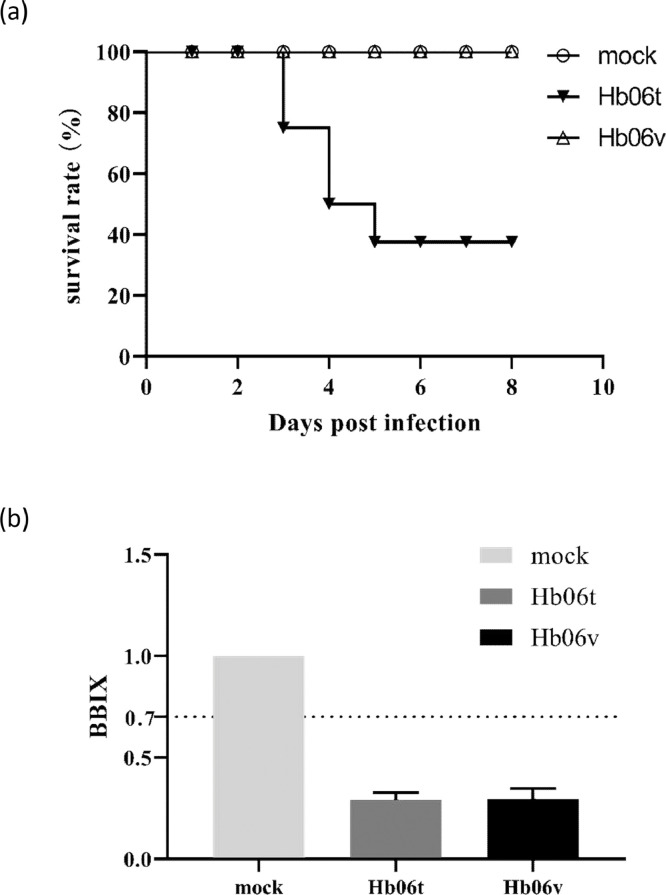
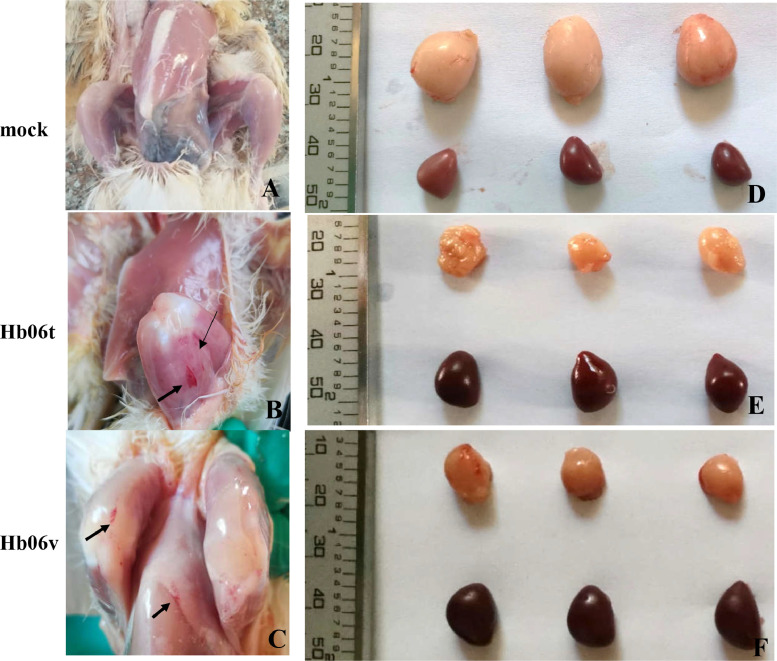
To assess the pathogenicity of IBDV strains, animal experiment was performed using novel variant strain Hb06v and vvIBDV strain Hb06t. As a result, compared to the chickens in the control group, all the Hb06v-infected chickens presented slight listlessness but no death occurred during the study, while Hb06t-infected chickens began to die at 3 dpi, and the mortality reached to 62.5% during the experiment period (Figure 2A). BBIX values for both the Hb06v and Hb06v groups were < 0.7 (Figure 2B), indicating severe lesions of the bursa of Fabricius of Hb06v- and Hb06t-infected chickens. At necropsy, compared to the chickens in the control group, both Hb06v- and Hb06t-infected chickens showed similar lesions, including the atrophy of the BF, severe hemorrhaging in the muscles of leg and chest, and the swelling of spleen (Figure 3).
Figure 2.
Clinical manifestations of SPF chickens infected with IBDV. A total of twenty-four 1-day-old SPF chickens were randomly assigned into 3 groups (8 birds per group), and inoculated with Hb06v (novel IBDV variant strain), Hb06t (vvIBDV strain), or PBS as a control. Clinical manifestations of this disease and mortality were monitored daily. (A) Survival curves for each group. (B) The bursa: body weight index (BBIX) at 7 d postinfection.
Figure 3.
Gross pathologic analysis of the diseased chickens. The SPF chickens were inoculated with Hb06t and Hb06v isolate at a dose of 104.5 EID50 and PBS as controls respectively. All the chickens were euthanized at 7 dpi. Necropsies were performed and the gross lesions were recorded at the time of necropsy. (A-C) Pathogenic lesions of the leg musal: The microscopic lesions were haemorrhage of leg.of Hb06t-inoculated and Hb06v-inoculated chickens. (D-F) Pathogenic changes in the spleen and bursal: Compared to those of controls, the Hb06t-inoculated and Hb06v-inoculated chickens presented swollen spleens and atrophic bursa of Fabricius. The lesions were marked with arrows.
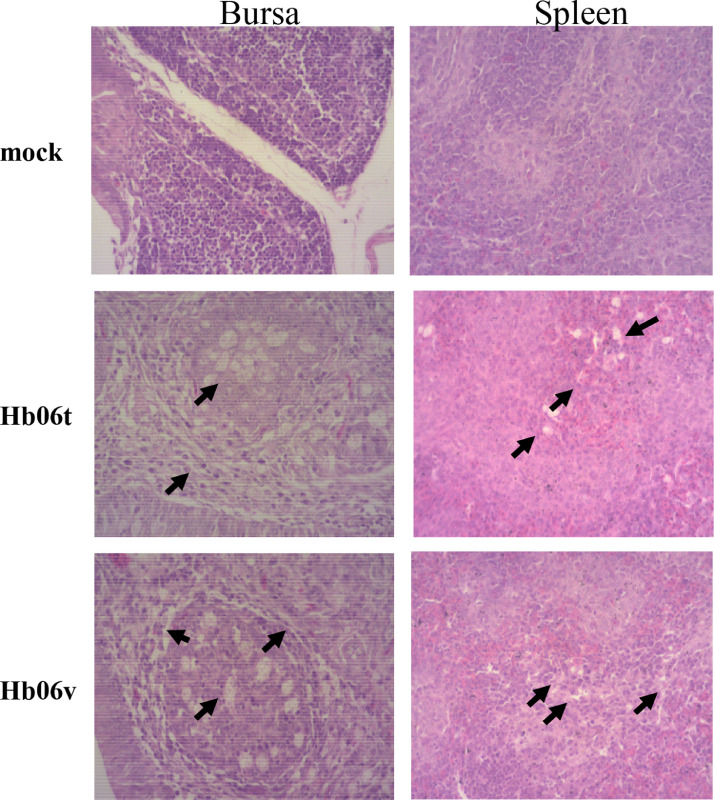
To further analyze the pathogenicity of IBDV field isolates, the histopathological lesions in the bursae and spleen were detected at 7 dpi (Figure 4). Severe lesions were observed in bursa and spleen from both the Hb06t and Hb06v groups, including a reduction in lymphocyte numbers, endothelial cell exposure and degeneration or necrosis in the bursa and spleen. No pathological lesions were observed in the control group.
Figure 4.
Histopathological appearance of bursal and spleen from IBDV-inoculated SPF chickens. The bursal and spleen samples were fixed by immersion in 10% neutral-buffered formalin, routinely processed, embedded in parafn, sectioned (4 μm thick), and stained with hematoxylin and eosin. Histological changes were examined by light microscopy. The left side is bursa and the right side are spleen
Evaluation of Immunosuppression of the Variant IBDV
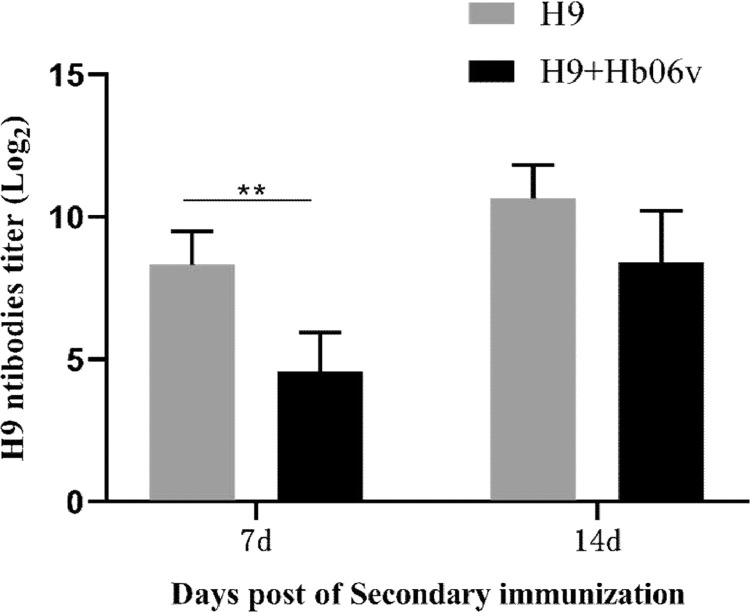
To evaluate the effect of the novel variant IBDV on host immune response, we immunized SPF chickens with commercial inactivated vaccine against H9N2 avian influenza virus (AIV), following the infection of the novel variant IBDV, and examined the antibody titers against H9N2 AIV using Hemagglutination inhibition (HI) assay. As a result, compared with the control group, the HI titer of antibodies against H9N2 AIV was effectively lowered in the Hb06v-infected group (Figure 5).
Figure 5.
Evaluation of immunosuppression of IBDV variant strain Hb06v using SPF chickens. Antibody against the H9N2 AIV vaccine was determined by a HI assay. The average titers and standard deviations (error bars) from ten independent samples are shown.
DISCUSSION
With the first emergence of classic IBDV in America in 1957, variant IBDV was reported in America in the late 1980s, which evaded the immune protection of classic IBDV (Jackwood and Saif, 1987). In China, there were only sporadic reports of IBDV variants in the early 1990s (Chen et al., 2012; He et al., 2012; Cui et al., 2013). While the variant IBDV was inadvertently neglected because of the sporadic distribution of vvIBDV (Jackwood et al., 2006; Letzel et al., 2007). Thanks to the rational use of vaccine and the improvement of feeding level, the infection of vvIBDV has been gradually controlled in China (Xu et al., 2015). However, the novel variant IBDV emerged in recent years in China. Although the novel variant IBDV was not lethal, the causative agent always mainly damages the bursa of Fabricius, the central immune organ of chickens for the development and maturation of B lymphocytes, directly causing a rapid, progressive loss of B lymphocytes in the bursa, spleen, and peripheral blood by apoptosis, leading to immunosuppression in survived chickens. Previous study reported that 76 broiler flocks immunized with IBD vaccine in 6 provinces in China had detected novel variant strains for the first time (Fan et al., 2019). Moreover, the novel IBDV variants had spread to other countries, such as Japan, South Korea and Malaysia (Aliyu et al., 2021; Myint et al., 2021; Thai et al., 2021). The commercial vaccines against IBDV cannot provide complete protection to birds (Fan et al., 2020). Considering the rapid spread of the novel variants, the continuing surveillance is significantly important to control this disease.
Molecular biology research of IBDV mainly focused on the HVRs of VP2 gene, which is beneficial for tracking the genetic evolution of IBDV (Jackwood and Sommer-Wanger, 2005). The changes in some regions of VP2 gene lead to changes of viral antigenicity (Durairaj et al, 2011). The mutation of amino acid site 222 can change the monoclonal antibody reactivity and induce the immune escape (Jackwood and Sommer-Wanger, 2011). Therefore, the study on the key amino acid sites of VP2 is of great significance for the prevention and control of IBDV.
From May to August in 2020, IBDV broke out frequently in many broiler farms in Hubei Province. We isolated 12 field strains of IBDV from 64 samples. The outbreak time of IBDV was from May to August, and the weather was hot, so it was easier for IBDV to survive and spread in hot environment (Ling, 2021). Exposure to heat stress suppresses poultry immune responses, which can increase susceptibility to infectious diseases (Mashaly et al., 2004; Monson et al., 2018). VP2 is the capsid protein of IBDV and the main protective antigen of IBDV. The hypervariable region of VP2 is often used for genetic variation analysis of IBDV (Jackwood et al., 2018). According to the phylogenetic tree of partial VP2 gene sequences and the key amino acid sites, we identified 9 novel variants, 2 vvIBDV and 1 Classic strain. The homology between the 9 variants and other novel variants (SHG19, etc.) isolated previously was high, while the sequence homology with the early American variant was low. Therefore, the pathogen of atypical infectious bursal disease might belong to IBDV variants, it is significantly different from the early American variant. Interestingly, we found that different types of IBDV strains have their own unique amino acids, which is consistent with the early research findings (Ren et al., 2009; Jackwood, 2012). Similar to the previous reports, the HVRs of VP2 gene of 9 strains isolated in this study contain the characteristic amino acids: 221K, 252I and 299S. Interestingly, 299S is not only the unique amino acid of IBDV novel variant, but also the characteristic amino acid of vvIBDV. However, the biological significance of these amino acid sites is unclear. More effort will be required reveal the function of these sites in the future. The amino acid site 222T, 249K, 286I and 318D have been proved to be closely related to IBDV antigen variation (Vakharia et al., 1994; Jackwood et al., 2006; Letzel et al., 2007). The mutation at amino acid site 222 involved viral replication and virulence because this residue is located at the tip of the PBC loop, which contains neutralizing epitopes (Letzel et al., 2007; Qi et al., 2016). Previous reports indicated that the virulent IBDV strains could be weakened when the mutation of Q249R emerged, providing evidence for the association of amino acid site 249 with virulence (Brandt et al., 2001; Qi et al., 2013). Both amino acid sites 286 and 318 may be associated with the binding of virus and cell receptor, or with the cytotropism and virulence of IBDV (Boot et al., 2000).
It has been reported that the novel variant IBDV was not lethal, but induced severe damage to the BF (Fan et al., 2020; Xu et al., 2020). Our results also proved this appearance. Although the chickens infected with the novel variant strain survived, they showed slight listlessness and the chickens were weak. Consistent with the results from necropsy, histopathology analysis also indicated the severe lesions of the bursa of chickens infected with the novel variant IBDV. Many studies have reported that the diseased chickens usually suffer from severe immunosuppression (Spackman et al., 2018; Tomás et al., 2019). The novel variants significantly suppressed the host immune response to the NDV live vaccine (La Sota) (Fan et al., 2019). In this study, we also evaluated the effect of the novel variant on the host response in SPF chickens. Compared to that of controls, the lower titers of antibodies against H9N2 AIV in IBDV-infected chickens might be associated with the damage of the BF. Although chickens survived, the immune deficiency caused by IBDV increases the risk of infection by other viruses, bacteria and parasites (Van den Berg et al., 2000; Zachar et al., 2014; Jackwood, 2017). The commercial vaccines against IBDV widely used in poultry industry cannot provide complete protection to the novel variant IBDV strains (Jackwood, 2011; Qi et al., 2015). More effort will be required to develop vaccines against the novel variant IBDV strains. The surveillance of IBDV will provide meaningful information, which is beneficial for vaccine development.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (31802206, 32072842), the Natural Science Foundation of Guangdong Province (2021A1515011147), and the Construction Project of Modern Agricultural Science and Technology Innovation Alliance in Guangdong Province (2021KJ128), Guangdong Basic and Applied Basic Research Foundation (2019A1515012006).
Ethical Approval and Consent to Participate: The animal experiments were approved by the Animal Care Committee of the South China Agricultural University, Guangzhou, China (approval ID: SYXK-2019-0136). All study procedures and animal care activities were conducted in accordance with the national and institutional guidelines for the care and use of laboratory animals. The birds were maintained in isolators with negative pressure and food and water were provided ad libitum.
Availability of Data and Materials: The nucleotide sequences of 12 IBDV isolated strains have been deposited in GenBank under the accession no.MW795723∼MW795734. The authors declare that all data supporting the findings of this study are available within the article.
DISCLOSURES
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
REFERENCES
- Aliyu H.B., Hair-Bejo M., Omar A.R., Ideris A. Genetic diversity of recent infectious bursal disease viruses isolated from vaccinated poultry flocks in Malaysia. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.643976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkie T.N., Rautenschlein S. Infectious bursal disease virus in poultry: current status and future prospects. Vet. Med. (Auckl) 2016;7:9–18. doi: 10.2147/VMRR.S68905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boot H.J., ter Huurne A.A., Hoekman A.J., Peeters B.P., Gielkens A.L. Rescue of very virulent and mosaic infectious bursal disease virus from cloned cDNA: VP2 is not the sole determinant of the very virulent phenotype. J. Virol. 2000;74:6701–6711. doi: 10.1128/jvi.74.15.6701-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M., Yao K., Liu M., Heckert R.A., Vakharia V.N. Molecular determinants of virulence, cell tropism, and pathogenic phenotype of infectious bursal disease virus. J. Virol. 2001;75:11974–11982. doi: 10.1128/JVI.75.24.11974-11982.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.D., Skinner M.A. Coding sequences of both genome segments of a European’ very virulent’ infectious bursal disease virus. Virus Res. 1996;40:1–15. doi: 10.1016/0168-1702(95)01253-2. [DOI] [PubMed] [Google Scholar]
- Cao Y.C., Yeung W.S., Law M., Bi Y.Z., Leung F.C., Lim B.L. Molecular characterization of seven Chinese isolates of infectious bursal disease virus: classical, very virulent, and variant strains. Avian Dis. 1998;42:340–351. [PubMed] [Google Scholar]
- Caston J.R., Martinez-Torrecuadrada J.L., Maraver A., Lombardo E., Rodriguez J.F., Casal J.I., Carrascosa J.L. C terminus of infectious bursal disease virus major capsid protein VP2 is involved in definition of the T number for capsid assembly. J. Virol. 2001;75:10815–10828. doi: 10.1128/JVI.75.22.10815-10828.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Liu J.J., Yan Z.Q., Liu D., Ji J., Qin J.P., Li H.Y., Ma J.Y., Bi Y.Z., Xie Q.M. Complete genome sequence analysis of a natural reassortant infectious bursal disease virus in China. J. Virol. 2012;86:11942–11943. doi: 10.1128/JVI.02043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chettle N., Stuart J.C., Wyeth P.J. Outbreak of virulent infectious bursal disease in East Anglia. Vet. Rec. 1989;125:271–272. doi: 10.1136/vr.125.10.271. [DOI] [PubMed] [Google Scholar]
- Cosgrove A.S. An apparently new disease of chickens: avian nephrosis. Avian Dis. 1962;6:385–389. [Google Scholar]
- Coulibaly F., Chevalier C., Gutsche I., Pous J., Navaza J., Bressanelli S., Delmas B., Rey F.A. The birnavirus crystal structure reveals structural relationships among icosahedral viruses. Cell. 2005;120:761–772. doi: 10.1016/j.cell.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Cui P., Ma S.J., Zhang Y.G., Li X.S., Gao X.Y., Cui B.A., Chen H.Y. Genomic sequence analysis of a new reassortant infectious bursal disease virus from commercial broiler flocks in Central China. Arch. Virol. 2013;158:1973–1978. doi: 10.1007/s00705-013-1682-y. [DOI] [PubMed] [Google Scholar]
- Dey S., Pathak D.C., Ramamurthy N., Maity H.K., Chellappa M.M. Infectious bursal disease virus in chickens: prevalence, impact, and management strategies. Vet. Med. (Auckl.). 2019;10:85–97. doi: 10.2147/VMRR.S185159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durairaj V., Sellers H.S., Linnemann E.G., Icard A.H., Mundt E. Investigation of the antigenic evolution of field isolates using the reverse genetics system of infectious bursal disease virus (IBDV) Arch. Virol. 2011;156:1717–1728. doi: 10.1007/s00705-011-1040-x. [DOI] [PubMed] [Google Scholar]
- Escaffre O., Le Nouen C., Amelot M., Ambroggio X., Ogden K.M., Guionie O., Toquin D., Muller H., Islam M.R., Eterradossi N. Both genome segments contribute to the pathogenicity of very virulent infectious bursal disease virus. J. Virol. 2013;87:2767–2780. doi: 10.1128/JVI.02360-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L., Wang Y., Jiang N., Chen M., Gao L., Li K., Gao Y., Cui H., Pan Q., Liu C., Zhang Y., Wang X., Qi X. Novel variant infectious bursal disease virus suppresses Newcastle disease vaccination in broiler and layer chickens. Poult Sci. 2020;99:6542–6548. doi: 10.1016/j.psj.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L.J., Wu T.T., Hussain A., Gao Y.L., Zeng X.Y., Wang Y.L., Gao L., Li K., Wang Y.Q., Liu C.J., Cui H.Y., Pan Q., Zhang Y.P., Liu Y.F., He H.J., Wang X.M., Qi X.L. Novel variant strains of infectious bursal disease virus isolated in China. Vet. Microbiol. 2019;230:212–220. doi: 10.1016/j.vetmic.2019.01.023. [DOI] [PubMed] [Google Scholar]
- Ferrero D., Garriga D., Navarro A., Rodríguez J.F., Verdaguer N. Infectious bursal disease virus VP3 upregulates VP1-mediated RNA-dependent RNA replication. J. Virol. 2015;89:11165–11168. doi: 10.1128/JVI.00218-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Li K., Qi X., Gao H., Gao Y., Qin L., Wang Y., Shen N., Kong X., Wang X. Triplet amino acids located at positions 145/146/147 of the RNA polymerase of very virulent infectious bursal disease virus contribute to viral virulence. J Gen Virol. 2014;95:888–897. doi: 10.1099/vir.0.060194-0. [DOI] [PubMed] [Google Scholar]
- He X., Wei P., Yang X., Guan D., Wang G., Qin A. Molecular epidemiology of infectious bursal disease viruses isolated from Southern China during the years 2000-2010. Virus Genes. 2012;45:246–255. doi: 10.1007/s11262-012-0764-3. [DOI] [PubMed] [Google Scholar]
- Ingrao F., Rauw F., Lambrecht B., van den Berg T. Infectious bursal disease: a complex host-pathogen interaction. Dev Comp Immunol. 2013;41:429–438. doi: 10.1016/j.dci.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Ismail N.M., Saif Y.M., Moorhead P.D. Lack of pathogenicity of five serotype 2 infectious bursal disease viruses in chickens. Avian Dis. 1988;32:757–759. [PubMed] [Google Scholar]
- Jackwood D.J. Molecular epidemiologic evidence of homologous recombination in infectious bursal disease viruses. Avian Dis. 2012;56:574–577. doi: 10.1637/10053-010912-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J. Advances in vaccine research against economically important viral diseases of food animals: infectious bursal disease virus. Vet. Microbiol. 2017;206:121–125. doi: 10.1016/j.vetmic.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J. Viral competition and maternal immunity influence the clinical disease caused by very virulent infectious bursal disease virus. Avian Dis. 2011;55:398–406. doi: 10.1637/9671-012811-Reg.1. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J., Cookson K.C., Sommer-Wagner S.E., Le Galludec H., de Wit J.J. Molecular characteristics of infectious bursal disease viruses from asymptomatic broiler flocks in Europe. Avian Dis. 2006;50:532–536. doi: 10.1637/7528-032006R1.1. [DOI] [PubMed] [Google Scholar]
- Jackwood D.H., Saif Y.M. Antigenic diversity of infectious bursal disease viruses. Avian Dis. 1987;31:766–770. [PubMed] [Google Scholar]
- Jackwood D.J., Schat K.A., Michel L.., de Wit S. A proposed nomenclature for infectious bursal disease virus isolates. Avian Pathol. 2018;47:576–584. doi: 10.1080/03079457.2018.1506092. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J., Sommer-Wagner S.E. Molecular epidemiology of infectious bursaldisease viruses: distribution and genetic analysis of newly emerging viruses in the United States. Avian Dis. 2005;49:220–226. doi: 10.1637/7289-101404R. [DOI] [PubMed] [Google Scholar]
- Jackwood D.J., Sommer-Wagner S.E. Amino acids contributing to antigenic drift in the infectious bursal disease Birnavirus (IBDV) Virology. 2011;409:33–37. doi: 10.1016/j.virol.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Lejal N., Da Costa B., Huet J.-C., Delmas B. Role of Ser-652 and Lys-692 in the protease activity of infectious bursal disease virus VP4 and identification of its substrate cleavage sites. J. Gen. Virol. 2000;81:983–992. doi: 10.1099/0022-1317-81-4-983. [DOI] [PubMed] [Google Scholar]
- Letzel T., Coulibaly F., Rey F.A., Delmas B., Jagt E., van Loon A.A., Mundt E. Molecular and structural bases for the antigenicity of VP2 of infectious bursal disease virus. J. Virol. 2007;81:12827–12835. doi: 10.1128/JVI.01501-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z. Control measures of infectious bursal disease in chickens. China Livest. Poult. Seed Ind. 2021;17:191–192. [Google Scholar]
- Mashaly M.M., Hendricks G.L., 3rd, Kalama M.A., Gehad A.E., Abbas A.O., Patterson P.H. Effect of heat stress on production parameters and immune responses of commercial laying hens. Poult. Sci. 2004;83:889–894. doi: 10.1093/ps/83.6.889. [DOI] [PubMed] [Google Scholar]
- Méndez F., de Garay T., Rodríguez D., Rodríguez J.F. Infectious bursal disease virus VP5 polypeptide: a phosphoinositide-binding protein required for efficient cell-to-cell virus dissemination. PLoS One. 2015;17 doi: 10.1371/journal.pone.0123470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson M.S., Van Goor A.G., Ashwell C.M., Persia M.E., Rothschild M.F., Schmidt C.J., Lamont S.J. Immunomodulatory effects of heat stress and lipopolysaccharide on the bursal transcriptome in two distinct chicken lines. BMC Genomics. 2018;19:643. doi: 10.1186/s12864-018-5033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint O., Suwanruengsri M., Araki K., Izzati U.Z., Pornthummawat A., Nueangphuet P., Fuke N., Hirai T., Jackwood D.J., Yamaguchi R. Bursa atrophy at 28 days old caused by variant infectious bursal disease virus has a negative economic impact on broiler farms in Japan. Avian Pathol. 2021;50:6–17. doi: 10.1080/03079457.2020.1822989. [DOI] [PubMed] [Google Scholar]
- Qi X., Gao X., Lu Z., Zhang L., Wang Y., Gao L., Gao Y., Li K., Gao H., Liu C., Cui H., Zhang Y., Wang X. A single mutation in the PBC loop of VP2 is involved in the in vitro replication of infectious bursal disease virus. Sci. China Life Sci. 2016;59 doi: 10.1007/s11427-016-5054-1. 717–7. [DOI] [PubMed] [Google Scholar]
- Qi X., Qin L., Gao Y., Gao H., Li Y., Li G., Lu Z., Wang N., Chen Y., Zhang L., Li K., Wang Y., Wang X. Genetic analysis of the VP2 hypervariable region of thirty-six infectious bursal disease virus isolates in China during 2009-2012. Agric. Sci. Technol. 2015;16:1565–1569. [Google Scholar]
- Qi X., Zhang L., Chen Y., Gao L., Wu G., Qin L., Wang Y., Ren X., Gao Y., Gao H., Wang X. Mutations of residues 249 and 256 in VP2 are involved in the replication and virulence of infectious Bursal disease virus. PLoS One. 2013;8:e70982. doi: 10.1371/journal.pone.0070982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja P., Senthilkumar T.M., Parthiban M., Thangavelu A., Gowri A.M., Palanisammi A., Kumanan K. Complete genome sequence analysis of a naturally reasserted infectious bursal disease virus from India. Genome Announc. 2016;4:e00709–e00716. doi: 10.1128/genomeA.00709-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautenschlein S., Yeh H.Y., Njenga M.K., Sharma J.M. Role of intrabursal T cells in infectious bursal disease virus (IBDV) infection: T cells promote viral clearance but delay follicular recovery. Arch. Virol. 2002;147:285–304. doi: 10.1007/s705-002-8320-2. [DOI] [PubMed] [Google Scholar]
- Ren X., Xue C., Zhang Y., Chen F., Cao Y. Genomic analysis of one Chinese strain YS07 of infectious bursal disease virus reveals unique genetic diversity. Virus Genes. 2009;39:246–248. doi: 10.1007/s11262-009-0379-5. [DOI] [PubMed] [Google Scholar]
- Sharma J.M., Dohms J.E., Metz A.L. Comparative pathogenesis of serotype 1 and variant serotype 1 isolates of infectious bursal disease virus and their effect on humoral and cellular immune competence of specific-pathogen-free chickens. Avian Dis. 1989;33:112–124. [PubMed] [Google Scholar]
- Sharma J.M., Kim I.J., Rautenschlein S., Yeh H.Y. Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Dev. Comp. Immunol. 2000;24:223–235. doi: 10.1016/s0145-305x(99)00074-9. [DOI] [PubMed] [Google Scholar]
- Spackman E., Stephens C.B., Pantin-Jackwood M.J. The effect of infectious bursal disease virus-induced immunosuppression on vaccination against highly pathogenic avian influenza virus. Avian Dis. 2018;62:36–44. doi: 10.1637/11769-110717-Reg.1. [DOI] [PubMed] [Google Scholar]
- Tacken M.G., Peeters B.P., Thomas A.A., Rottier P.J., Boot H.J. Infectious bursal disease virus capsid protein VP3 interacts both with VP1, the RNA-dependent RNA polymerase, and with viral double-stranded RNA. J. Virol. 2002;76:11301–11311. doi: 10.1128/JVI.76.22.11301-11311.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai T.N., Jang I., Kim H.A., Kim H.S., Kwon Y.K., Kim H.R. Characterization of antigenic variant infectious bursal disease virus strains identified in South Korea. Avian Pathol. 2021;50:174–181. doi: 10.1080/03079457.2020.1869698. [DOI] [PubMed] [Google Scholar]
- Tomás G., Marandino A., Courtillon C., Amelot M., Keita A., Pikula A., Hernández M., Hernández D., Vagnozzi A., Panzera Y., Domańska-Blicharz K., Eterradossi N., Pérez R., Soubies S.M. Antigenicity, pathogenicity and immunosuppressive effect caused by a South American isolate of infectious bursal disease virus belonging to the “distinct” genetic lineage. Avian Path. 2019;48:245–254. doi: 10.1080/03079457.2019.1572867. [DOI] [PubMed] [Google Scholar]
- Vakharia V.N., He J., Ahamed B., Snyder D.B. Molecular basis of antigenic variation in infectious bursal disease virus. Virus Res. 1994;31:265–273. doi: 10.1016/0168-1702(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Van den Berg T.P., Eterradossi N., Toquin D., Meulemans G. Infectious bursal disease (Gumboro disease) Rev. Sci. Tech. 2000;19:509–554. [PubMed] [Google Scholar]
- von Einem U.I., Gorbalenya A.E., Schirrmeier H., Behrens S.E., Letzel T., Mundt E. VP1 of infectious bursal disease virus is an RNA-dependent RNA polymerase. J. Gen. Virol. 2004;85:2221–2229. doi: 10.1099/vir.0.19772-0. [DOI] [PubMed] [Google Scholar]
- Wang Q., Hu H., Chen G., Liu H., Wang S., Xia D., Yu Y., Zhang Y., Jiang J., Ma J., Xu Y., Xu Z., Ou C., Liu X. Identification and assessment of pathogenicity of a naturally reassorted infectious bursal disease virus from Henan, China. Poult. Sci. 2019;98:6433–6444. doi: 10.3382/ps/pez498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers D.R., Young J.R., Davison T.F. Infectious bursal disease virus-induced immunosuppression in the chick is associated with the presence of undifferentiated follicles in the recovering bursa. Viral Immunol. 2005;18:127–137. doi: 10.1089/vim.2005.18.127. [DOI] [PubMed] [Google Scholar]
- Xu M.Y., Lin S.Y., Zhao Y., Jin J.H., Tang N., Zhang G.Z. Characteristics of very virulent infectious bursal disease viruses isolated from Chinese broiler chickens (2012-2013) Acta Trop. 2015;141:128–134. doi: 10.1016/j.actatropica.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Xu A., Pei Y., Zhang K., Xue J., Ruan S., Zhang G. Phylogenetic analyses and pathogenicity of a variant infectious bursal disease virus strain isolated in China. Virus Res. 2020;15:276. doi: 10.1016/j.virusres.2019.197833. 197833. [DOI] [PubMed] [Google Scholar]
- Ye C., Jia L., Sun Y., Hu B., Wang L., Lu X., Zhou J. Inhibition of antiviral innate immunity by birnavirus VP3 protein via blockage of viral double-stranded RNA binding to the host cytoplasmic RNA detector MDA5. J. Virol. 2014;88:11154–11165. doi: 10.1128/JVI.01115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachar T., Popowich S., Goodhope B., Knezacek T., Ojkic D., Willson P., Ahmed K.A., Gomis S. A 5-year study of the incidence and economic impact of variant infectious bursal disease viruses on broiler production in Saskatchewan, Canada. Can. J. Vet Res. = Revue canadienne de recherche veterinaire. 2016;80:255–261. [PMC free article] [PubMed] [Google Scholar]
- Zachar T., Popowich S., Goodhope B., Knezacek T., Ojkic D., Willson P., Ahmed Ye.C., Jia L., Sun Y., Hu B., Wang L., Lu X., Zhou J. Inhibition of antiviral innate immunity by birnavirus VP3 protein via blockage of viral double-stranded RNA binding to the host cytoplasmic RNA detector MDA5. J. Virol. 2014;88:11154–11165. doi: 10.1128/JVI.01115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]