Abstract
The present study aimed to identify the factors associated with the distribution of the first doses of the COVID-19 vaccine. In this study, we used 9 variables: human development index (HDI), gross domestic product (GDP per capita), Gini index, population density, extreme poverty, life expectancy, COVID cases, COVID deaths, and reproduction rate. The time period was until February 1, 2021. The variable of interest was the sum of the days after the vaccine arrived in the countries. Pearson’s correlation coefficients were calculated, and t-test was performed between the groups that received and did not receive the immunizer, and finally, a stepwise linear regression model was used. 58 (30.4%) of the 191 countries received the SARS-CoV-2 vaccine. The countries that received the most doses were the United States, China, the United Kingdom, and Israel. Vaccine access in days showed a positive Pearson correlation HDI, GDP, life expectancy, COVID-19 cases, deaths, and reproduction rate. Human development level, COVID-19 deaths, GDP per capita, and population density are able to explain almost 50% of the speed of access to immunizers. Countries with higher HDI and per capita income obtained priority access.
Keywords: SARS-CoV-2, COVID-19, vaccine, equitable distribution
What do we already know about this topic?
Several vaccines against COVID-19 have already been developed.
How does your research contribute to the field?
The distribution of the first doses was made to richer countries
What are your research’s implications toward theory, practice, or policy?
Due to the global pandemic, doses should be prioritized infections and deaths hotspots.
Introduction
Panting and distinct sounds of whooping cough, iron lungs, and orthopedic appliances designed for children paralyzed by polio, and devastating birth defects caused by rubella are infectious scourges that inspire dread and represent obscure diseases of previous years. 1 Unfortunately, in the previous era, the existence of preventive methods and therapies was not sufficient to eradicate infectious diseases such as measles, diphtheria, smallpox, and whooping cough.2,3 However, many of these devastating diseases have been identified due to the development of preventive measures, including the wide distribution of safe, effective, and accessible vaccines. 1
Although vaccination can be used as immunotherapy (post-phylaxis), its greatest use is against pathogens related to infectious diseases, being used as a means of prevention (pre-phylaxis). 4 History reveals that vaccine development has consistently involved a combination of considerable doses of ingenuity, political skills, and scientific methods. 2 Thus, vaccines are powerful medical interventions that induce biological, social, and cultural reactions. 5
There is a strong global consensus that the vaccine against the coronavirus disease (COVID-19) is an effective approach to sustainable pandemic control, 6 and therefore, the global effort for vaccine research and development in response to the COVID-19 pandemic is unprecedented in terms of scale and speed.
The new coronavirus, with its rapid transmissibility, has led to a worldwide effort to elucidate effective technical and scientific measures to contain this virus. 6 Among these measures, the ones that stand out are the investments of the scientific community, laboratories, and governments in designing treatment regimens, production of medicines, and vaccines. 7 The race for the vaccine to fight the severe acute respiratory coronavirus-2 (SARS-CoV-2) began in 2020, and almost a year later, in January 2021, many pharmaceutical companies have already crossed the final line by achieving approval for use.8,9
Currently, vaccines are being produced at an accelerated pace, which is justified by the urgency created by the pandemic. 10 Several countries have adopted immunization campaigns that determine priority access criteria depending on the vulnerability of the population. However, the criteria for global distribution remain unclear. For the World Health Organization (WHO), the challenge is to make these vaccines available to people around the globe. 11 Nevertheless, studies indicate that low-income countries may have to wait until 2024 to receive the COVID-19 vaccination. 12
Thus, there is uncertainty regarding the speed at which immunizers reach countries and what criteria are used for the distribution of the first doses. In this context, the present study is a pioneer in the search for the identification of factors associated with the distribution of vaccine doses in different countries.
Methods
Study Design and Setting
We performed descriptive research with a cross-sectional and correlational design. The data used were secondary, on an aggregate database. Therefore, there was no identification of the subjects or involvement of human beings or animals. The study used data from December 1, 2019, to February 1, 2021. The results and discussion presented follow the STROBE guidelines for observational studies.
Data Sources
The data were extracted from the database on February 1, 2021. This study used 2 databases: Our World in Data 13 and World Bank Data. 14 “Our World in Data” uses the most recent official numbers from governments and health ministries worldwide, and the data are updated daily. 13 Already, “World Bank Data” is based on primary household survey data obtained from government statistical agencies and World Bank country departments, 14 and we collect the last data update in February 1, 2021.
Study Size and Variables
To analyze the time elapsed from the first dose until February 1, 2021, a variable named “Vaccine Access in Days” was created, which was set as a dependent variable.
As predictors of the model, a set of nine indicators were added, encompassing information from 191 countries. The specifications of these variables are shown in Table 1.
Table 1.
Independent Variables.
| Variable | Specification |
|---|---|
| HDI | Human development index (HDI) is a summary of dimensions of human development |
| GDP per capita | Gross domestic product per capita is gross domestic product divided by midyear population |
| Gini | Index that represents the income inequality within a nation |
| Population density | Number of people living in a country per square kilometer |
| Extreme poverty | Number of people living on less than $1.90/day |
| Life Expectancy | Average time that a person is expected to live |
| COVID Cases | Cumulative COVID-19 cases |
| COVID Deaths | Cumulative COVID-19 deaths |
| Reproduction Rate | COVID-19 Infection rate |
Statistical Analysis
For an initial analysis, the Pearson’s correlation coefficient was calculated at a significance level of 5%. The t-test for averages was performed between countries that had access to the vaccine and countries that did not. Then, a multivariate model of linear regression was structured. The regression was performed using IBM SPSS Statistics 23 software.
Multivariate linear regression models explore the relationship between a set of predictors and a dependent variable, defined by equation (1)
| (1) |
where is the independent variable (Vaccine Access in Days) of 191 countries (i), is the constant, and is a vector that includes all the explanatory variables (predictors) of the model.
The stepwise approach, which is a variation of multivariate regression, is oriented to find the best model. 15 This results in an equation containing only significant variables. 15
Stepwise regression (SWR) involves an automatic process for selecting independent variables and can be briefly described as follows. 16 We used a model containing predefined terms and set limits and an evaluation threshold for the final model. After adding or removing terms, we retested the model. Stepwise regression was halted when no further improvement in estimation occurred.
Results
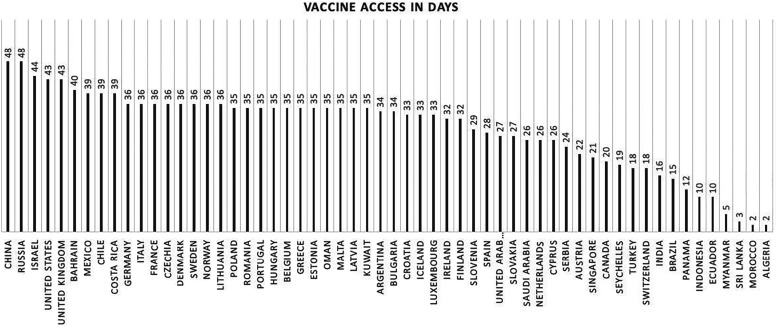
Of the analyzed 191 countries, 58 (30.4%) had already received doses of the vaccine, while 133 (69.6%) had not had access to any category of the COVID-19 immunizer. China and Russia were the countries that had access for the longest time (48 days), followed by Israel, the United Kingdom, and the United States with access to immunizers for 43 days. The country that received the most doses was the United States (32,222,402 doses of the vaccine), followed by China (24,000,000 vaccine doses), the United Kingdom (9,790,576 vaccine doses), and Israel (4,989,953 vaccine doses). The vaccine access in days is shown in Figure 1.
Figure 1.
Vaccine access in days.
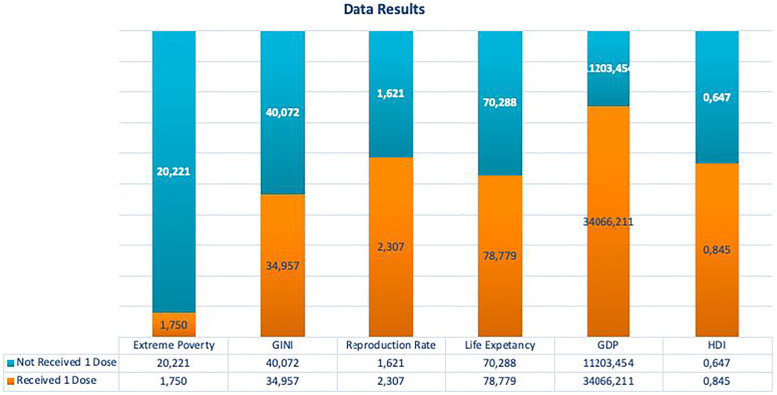
We compared the social and economic variables of the countries that received at least one batch, regardless of the time the countries that did not received. Significant results were found in the t-test for extreme poverty (P < .001), life expectancy (P < .001), HDI (P < .001), and GDP per capita (P = .004). Through this analysis, we observed that countries that have received at least one dose have better social and economic indicators, as shown in Figure 2.
Figure 2.
Comparison between countries that receive and have not received the vaccine.
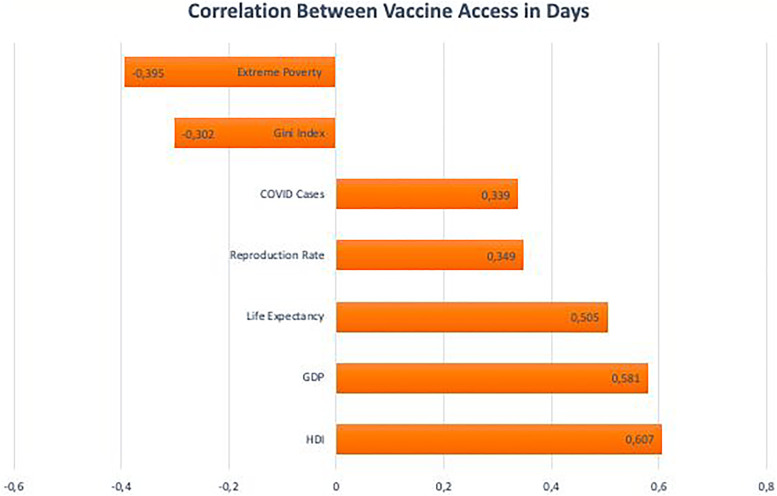
The variable of interest (Vaccine Access in Days) showed positive and moderate Pearson’s correlation with human development level (r = 0.607, P < .001). Vaccine Access in Day A was also positively associated with GDP per capita (r = 0.581, P < .001), Life Expectancy (r = 0.505, P < .001), COVID-19 cases (r = 0.300, P < .001), COVID-19 Deaths (r = 0.339, P < .001), and Reproduction Rate (r = 0.349, P < .001).
Two negative correlations were observed between Vaccine Access in Days and the GINI index (r = −0.302, P < .001) and Extreme Poverty (r = −0.395, P <0.001). Thus, unequal countries with high indicators of extreme poverty were also among those who received doses of the vaccine late. The Pearson correlation between indexes and vaccine access is shown in Figure 3.
Figure 3.
Pearson correlation between vaccine access in days.
Stepwise regression proposed 4 models, the first including only the Human Development Level variable. In the second step, the COVID-19 deaths variable was added. Third, the GDP per capita variable was added. In the fourth and last stages, Population Density was added. Four variables were not included in the final model due to their level of significance (GINI index, Extreme Poverty, Life Expectancy, COVID-19 Cases, and Reproduction Rate).
The R squared is the percentage of variation in Vaccine Access in Days, which can be explained by a model composed of multiple independent variables. In the first model, containing only the Human Development Level variable, the . When adding the variable COVID-19 Deaths, there was an increase of 0.048 in the explanatory power of the model, resulting in an . The third step, with the addition of GDP per capita, resulted in an . The full composition (4 models), with all the previous variables, together with population density, obtained an . The Durbin–Watson test statistic was 1.935, indicating that there is no autocorrelation between the residues.
The R-squared analysis shows that the set of the 4 variables (human development level, COVID-19 Deaths, GDP per capita, and Population Density) can explain almost 50% of the variation in days when a country receives the vaccine.
The ANOVA presented a significance level of P < .001 in all 4 scenarios. Thus, is rejected in favor of , indicating that the model with the 4 independent variables can explain part of the variation of Vaccine Access in Days.
The model coefficients provide information about the direction of influence that the predictors have on the independent variable (Vaccine Access in Days). Thus, for each increase of 0.1 in the Human Development Level, there is an increase of 34.4 days in the time that a country had access to the immunizer. Demographic density, on the other hand, had a negative influence on access to the vaccine. Countries with lower demographic densities took a longer time in terms of the number of days to receive the vaccine than those in densely populated countries. Thus, the increase of 1 person in the population density level can reduce the immunization time by 0.02 days.
It is important to emphasize that the interpretation of the coefficients must be restricted to the direction (positive or negative). The dimensional evaluation of the coefficients must start from the premise of the existence of causality between the variables, something that was not ensured in our model. The regression coefficients are shown in Table 2.
Table 2.
Regression Coefficients.
| Model/Variables | Unstandardized Coefficient | Standardized Coefficient | t | Sig | |
|---|---|---|---|---|---|
| B | Standard Error | ||||
| 1 (Constant) | −32.531 | 5.151 | −6.315 | .000 | |
| HDI | 58.360 | 7.098 | .607 | 8.222 | .000 |
| 2 (Constant) | −30.358 | 5.024 | −6.043 | .000 | |
| HDI | 53.932 | 7.004 | .561 | 7.700 | .000 |
| COVID Deaths | 7.743 × 10−5 | .000 | .223 | 3.069 | .003 |
| 3 (Constant) | −21.189 | 6.420 | −3.300 | .001 | |
| HDI | 36.244 | 10.490 | .377 | 3.455 | .001 |
| COVID Deaths | 7.824 × 10−5 | .000 | .226 | 3.154 | .002 |
| GDP per capita | .000 | .000 | .241 | 2.235 | .027 |
| 4 (Constant) | −20.248 | 6.309 | −3.209 | .002 | |
| HDI | 34.403 | 10.317 | .358 | 3.335 | .001 |
| COVID Deaths | 7.394 × 10−5 | .000 | .213 | 3.031 | .003 |
| GDP per capita | .000 | .000 | .310 | 2.821 | .006 |
| Population Density | -.002 | .001 | -.171 | −2.352 | .020 |
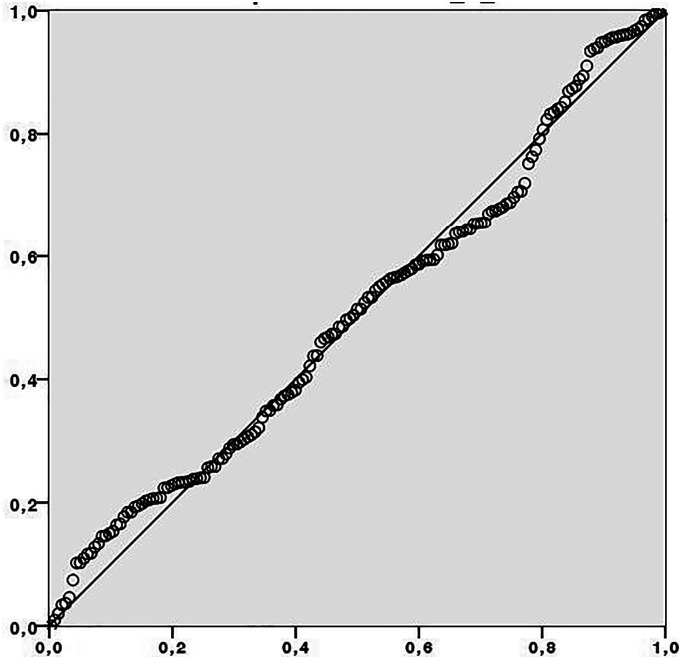
The analysis of the residues did not demonstrate the presence of outliers, one of the requirements for performing multivariate regression. The Durbin–Watson test showed a value of 1.935, which indicates the absence of autocorrelation between the residues. The errors also showed a normal distribution, as shown in Figure 4.
Figure 4.
Distribution of errors.
Discussion
In our study, we assessed the number of days it had taken for a country to receive the first dose of a COVID-19 vaccine. Thus, the higher the value of this variable, the sooner the immunizer was available in the country. We observed that a large number of countries are yet to get access to the vaccine. Most of these belong to the most unequal groups of countries that have the highest proportion of the population in extreme poverty.
Our study also showed a positive correlation between life expectancy and access to the vaccine. One of the possible causes is the fact that the countries that invested the most in the development of immunizers were also those that have a longer life expectancy, such as Canada, the United States, and the European Union.
Vaccination is widely recognized as one of the most successful and economic public health interventions in the world. 17 It is a simple, effective, and safe way to protect against potentially serious infectious diseases, allowing the population to acquire group immunity or indirect immunity. 18
The international race for immunization against SARS-CoV-2 has encouraged a large number of vaccines to be developed. By the end of September 2020, more than 200 studies had started preclinical development. 8
Despite the growing number of vaccine options, there remain several questions concerning the safety and efficacy of the vaccine that are being outlined and discussed by regulators and the scientific community. 19
Although the WHO advocates that all individuals should have access to the COVID-19 vaccine, global and equitable access is a major challenge. 20 According to the Duke Global Health Innovation estimates, several low-income countries may have to wait until 2024 for vaccination. 12
Anticipating the difficulties for future access of the vaccine, the COVAX Facility (COVAX) was created. The initiative seeks to promote fair and equitable access to immunizers against COVID-19. It consists of a voluntary arrangement that allows countries to develop the necessary political and logistical infrastructure for the distribution of the vaccine. 21 Even when the government already has access to vaccines, COVAX acts as an insurance policy through economies of scale. 22
Formally, none of the institutes or laboratories used economic or social criteria for dose distribution. However, as was the case with immunization against other diseases,17,23 priority access to doses of the vaccine against COVID-19 was associated with factors such as income per capita and human development. Thus, richer nations, that is, countries with higher HDI and higher GDP per capita, received the immunizer earlier and obtained priority access over the others.
Major research on the COVID-19 vaccine is taking place in 19 countries, which together represent more than three-quarters of the global population. 24 The proportion of contribution of North America toward developmental research is 46%, while that of China, Australia, and Europe is 18% each. 24 Finally, there is little involvement and resources allocated to conducting vaccine research in Africa and Latin America. 25
Another aspect that should be highlighted regarding the distribution of vaccines is its developers. More than 70% of the vaccines are being developed by private companies, while only 28% are developed by universities, the public sector, and other non-profit organizations. 24 Although most of these developers are multinational companies with extensive experience in vaccines, large-scale production poses a challenge, since many producers of the COVID-19 vaccine are inexperienced in the manufacturing of large-scale immunobiological products. 24
According to the WHO, 26 there is an effort to develop and disseminate vaccine techniques, models, and implementation planning that aims to help countries in the logistics, application, monitoring, demand, and acceptance of vaccines and, consequently, aims to guarantee access to the vaccine.
Each country must develop and apply a National Plan for COVID-19 vaccines, which will be crucial for carrying out coordinated efforts for its execution. 26 This could ensure that vaccines can be manufactured in sufficient quantities and provided equitably to all affected areas, particularly in poor countries, which requires strong international coordination and cooperation between researchers, regulators, policymakers, financiers, public health agencies, and governments. 25
Thus, the inequalities identified in this study regarding access to vaccines in poorer countries highlight the urgent need to target additional efforts and efficient distribution strategies that can be applied more equitably to the world population. Therefore, health equity remains a major challenge for policymakers and academics in this area. Even if socioeconomic issues are eventually overcome, the difficulties of the poorest countries in terms of organization and planning of vaccination logistics for their storage, distribution, and application with the population still stand out, which can delay the beginning and continuity of the process.
Our findings assess the importance of a more equitable distribution due to reducing the pandemic. Despite this, we recognize that the model could support more economical and social variables. A second limitation is the absence of variables that would indicate whether the country was a vaccine developer or sponsor.
Conclusions
The global demand for vaccines and the wide geographic diversity of the pandemic called for unprecedented efforts by the biotechnology and pharmaceutical industries, governments, and academia.
The actions in research and development for the provision of an effective immunizer brought results in an extremely short time, given that the time horizon for vaccine development has historically been in the order of decades. In about 10 months, Pfizer and BioNTech had their vaccines approved for emergency use in the United Kingdom and were nominated by the United States regulatory agency, Food and Drug Administration (FDA).
However, there is a lack of information associated with the distribution of the immunizer. The present study identified that, thus far, countries that have unfavorable socioeconomic rates, especially HDI and GPD per capita, have reduced access to vaccines. Many developed countries have funded or participated in vaccine development. This certainly influences the future distribution of the immunizer, highlighting the difficulty of equity in health.
The limitations of this study are the fact that the set of variables used did not encompass all social variables that could improve the adherence of the model, such as, for example, participation in the development of vaccines and types of vaccines. In addition, no statistical test was performed to show a causal relationship between the variables of interest.
Future research must be conducted to broaden the discussion on access to the vaccine, the formation of public policies that prioritize democratic and equal access in the face of a global emergency, such as that currently experienced during this ongoing pandemic. The present study reflects the initial period of the worldwide immunization process and there is a long way to go before the population definitively overcomes COVID-19 and its health, economic, and social consequences through the immunization process.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Marcos Felipe Falcão Sobral https://orcid.org/0000-0002-4768-2622
References
- 1.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865-872. https://www.nature.com/articles/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson T. Vaccines: History, Science, and Issues. Santa Barbara, CA: ABC-CLIO; 2017. [Google Scholar]
- 3.Saad-Roy CM, Wagner CE, Baker RE, et al. Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science. 2020;370(6518):811-818. doi: 10.1126/science.abd7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plotkin SA. Vaccines: past, present and future. Nat Med. 2005;11(4):S5-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigues CMC, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Front Microbiol. 2020;11. doi: 10.3389/fmicb.2020.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koirala A, Joo YJ, Khatami A, Chiu C, Britton PN. Vaccines for COVID-19: the current state of play. Paediatr Respir Rev. 2020;35(35):43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotez P. The physician-scientist: defending vaccines and combating antiscience. J Clin Invest. 2019;129(6):2169-2171. doi: 10.1172/JCI129121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tregoning JS, Brown ES, Cheeseman HM, et al. Vaccines for COVID-19. Clin Exp Immunol. 2021;202(2):162-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur SP, Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288(15):198114. DOI: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennet BM, Wolf J, Laureano R, Sellers RS. Review of Current Vaccine Development Strategies to Prevent Coronavirus Disease 2019 (COVID-19) (Vol. 48). Thousand Oaks, CA: Toxicologic pathology, Sage publications; 2020. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Coronavirus Disease (COVID-19) Pandemic. Geneva, Switzerland: World Health Organization; 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Google Scholar]
- 12.Mullard A. How COVID vaccines are being divided up around the world. Nature. 2020. DOI: 10.1038/d41586-020-03370-6. [DOI] [PubMed] [Google Scholar]
- 13.Our World in Data . Coronavirus (COVID-19) Vaccinations. Wales, UK: Our World in Data; 2021. https://ourworldindata.org/covid-vaccinations. [Google Scholar]
- 14.World Bank . Gini Index (World Bank Estimate). Washington, DC: The World Bank; 2018. https://data.worldbank.org/indicator/SI.POV.GINI. [Google Scholar]
- 15.Fritz M, Berger PD. Improving the User Experience through Practical Data Analytics: Gain Meaningful Insight and Increase Your Bottom Line. 1st ed. Burlington, MA: Morgan Kaufmann; 2015. 10.1016/B978-0-12-800635-1.00010-0. [DOI] [Google Scholar]
- 16.Bata T. Analysis and selection of a regression model for the use case points method using a stepwise approach. J Syst Software. 2017;125:1-14. DOI: 10.1016/j.jss.2016.11.029. [DOI] [Google Scholar]
- 17.Turner HC, Thwaites GE, Clapham HE. Vaccine-preventable diseases in lower-middle-income countries. Lancet Infect Dis. 2018;18(9):937-939. [DOI] [PubMed] [Google Scholar]
- 18.Prada L, Ferreira J. COVID-19, diabetes e vacinas. Revista Portuguesa De Diabetes. 2020;15(4):131-138. [Google Scholar]
- 19.Krause PR, Gruber MF. Emergency use authorization of Covid vaccines - safety and efficacy follow-up considerations. N Engl J Med. 2020;383(19):e107. [DOI] [PubMed] [Google Scholar]
- 20.Bollyky TJ, Gostin LO, Hamburg MA. The equitable distribution of COVID-19 therapeutics and vaccines. Jama. 2020;323(24):2462-2463. [DOI] [PubMed] [Google Scholar]
- 21.Herzog LM, Norheim OF, Emanuel EJ, McCoy MS. Ovax must go beyond proportional allocation of Covid vaccines to ensure fair and equitable access. BMJ. 2020;372:4853. [DOI] [PubMed] [Google Scholar]
- 22.The COVAX facility . The COVAX Facility. Geneva, Switzerland: World Health Organisation; 2020. https://www.who.int/docs/default-source/coronaviruse/act-accelerator/covax/covax-facility-background.pdf?sfvrsn=810d3c22_2. [Google Scholar]
- 23.Bocquier A, Ward J, Raude J, Peretti-Watel P, Verger P. Socioeconomic differences in childhood vaccination in developed countries: a systematic review of quantitative studies. Expet Rev Vaccine. 2017;16(11):1107-1118. [DOI] [PubMed] [Google Scholar]
- 24.Thanh Le T, Andreadakis Z, Kumar A, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305-306. [DOI] [PubMed] [Google Scholar]
- 25.Lima E, Almeida A, Kfouri R, da Fonseca Lima E. Vacinas para COVID-19: perspectivas e desafios. Residência Pediátrica. 2020;10. doi: 10.25060/residpediatr-2020.v10n2-04. [DOI] [Google Scholar]
- 26.WHO . Coronavirus Disease (COVID-19): Vaccine Access and Allocation. Geneva, Switzerland: World Health Organisation; 2020. https://www.who.int/news-room/q-a-detail/coronavirus-disease-(covid-19)-vaccine-access-and-allocation. [Google Scholar]