Abstract
The ongoing global administration of vaccines for coronavirus disease 2019 (COVID-19) means that increasing numbers of patients are likely to present with post-vaccination complications. We describe the first reported case of neuralgic amyotrophy (NA) involving the lumbosacral plexus occurring after AstraZeneca COVID-19 vaccination. The patient presented with acute-onset leg paralysis following administration of the vaccine. Based on the clinical, electrodiagnostic, and radiologic findings, the patient was diagnosed with post-vaccination NA. We speculate that the COVID-19 vaccine elicited an immune-mediated inflammatory response to the injected antigen due to inflammatory immunity in a patient with predisposed susceptibility to NA.
Keywords: Coronavirus disease 2019, vaccination, lumbosacral plexus, paralysis, case report, complication
Introduction
Globally administered coronavirus disease 2019 (COVID-19) vaccinations are critical for providing protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. 1 However, the follow-up periods for monitoring vaccine-related complications have so far been short. 2 Neuralgic amyotrophy (NA) is a peripheral nerve disease that is widely underdiagnosed and/or misdiagnosed because of its heterogeneous clinical appearance. 3 Although the exact pathophysiological process of NA remains unknown, it is assumed to involve an inflammatory autoimmune pathophysiology.3,4 NA has less frequently been attributed to immunological factors, such as vaccination. 5 Here, we describe the case of a patient diagnosed with NA after developing leg paralysis following administration of a COVID-19 vaccine. There are currently no published reports of NA involving the lumbosacral plexus occurring after COVID-19 vaccination. Our patient was diagnosed with post-vaccination NA based on their clinical, electrodiagnostic, and radiologic findings.
Case Report
This case report aimed to analyze the potential nerve and muscle-related complications of COVID-19 vaccination. The study was approved by the Institutional Review Board of Keimyung University Dongsan Hospital (Institutional Review Board number: 2021-06-083). Written informed consent was obtained from the patient. A healthy 45-year-old woman presented with acute-onset left leg paralysis and paresthesia 2 days after receiving the COVID-19 AZD-1222 vaccine (AstraZeneca, Cambridge, UK) in her left deltoid muscle. Despite taking acetaminophen, the patient developed myalgia immediately after vaccination, with no signs of paralysis or paresthesia. She denied any history of previous illness, including musculoskeletal disease and recent trauma. Physical examination revealed decreased strength against resistance in her left leg (grade 4, according to the Medical Research Council grading system), during hip flexion, extension, and abduction, knee extension, ankle dorsiflexion, long-toe extension, and ankle plantar flexion. Light-touch sensation was decreased in the left lateral femoral cutaneous, saphenous, and sural nerve distributions. The deep-tendon reflex in her left leg was normal. Her laboratory results, including C-reactive protein, erythrocyte segmentation rate, creatine kinase, antinuclear antibody, rheumatoid factor, and anti-GM1 ganglioside antibodies, were negative. Magnetic resonance imaging (MRI) of the lumbar spine revealed no abnormal findings, except for a mildly protruded intervertebral disc at the L4 and L5 vertebral levels. Brain MRI revealed no abnormal lesions indicating stroke or neoplasm that might cause leg paralysis. Nerve conduction studies of her legs performed 1 week after the onset of symptoms were normal and symmetrical. Needle electromyography only showed decreased motor unit recruitment in the left leg muscles. These findings failed to clarify the cause of the patient’s symptoms. She consequently underwent a rehabilitation program involving general conditioning and the administration of nonsteroidal anti-inflammatory medication.
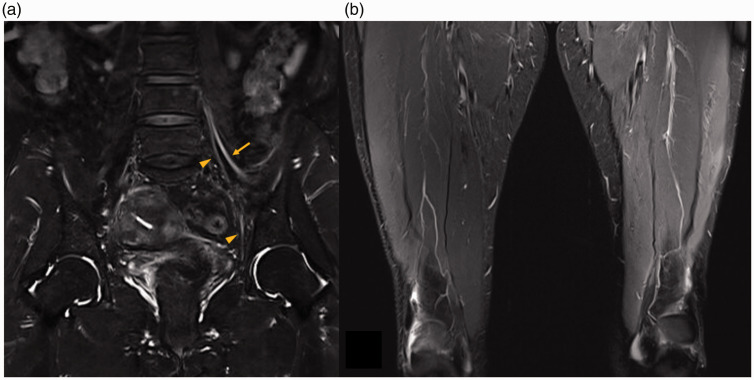
A follow-up examination 1 month after symptom onset showed persistent weakness and atrophy of the left thigh muscles. Sensory nerve conduction studies indicated decreased amplitude and prolonged latency in the left lateral femoral cutaneous and left saphenous sensory nerve action potentials, and decreased amplitude in left femoral compound motor action potential. Needle electromyography showed active denervation potentials in the left vastus medialis and iliopsoas, and decreased motor unit recruitment in the left leg muscles. This electrodiagnostic re-evaluation revealed axonal and demyelinating lumbosacral plexopathy, mainly involving the L2, L3, and L4 nerve fibers. T2-weighted contrast-enhanced lumbar MRI revealed increased intensities in the left femoral and obturator nerves at the proximal site (Figure 1a). T2-weighted thigh MRI showed increased signal intensities with atrophic changes in the vastus medialis, rectus femoris, and vastus lateralis muscles (Figure 1b). Based on the clinical, electrodiagnostic, and radiologic findings, the patient was diagnosed with NA. She received oral prednisolone for 11 days (50 mg/day for 5 days, tapered for another 6 days) to alleviate her symptoms. She subsequently showed slight motor improvement, equivalent to 0.5 grade according to the Medical Research Council grading system, in all her weakened muscles.
Figure 1.
Magnetic resonance imaging of the lumbar spine and thigh. (a) Coronal T2-weighted image with gadolinium-enhancement showing increased intensities in the femoral and obturator nerves at the left proximal site (arrow: femoral nerve, arrowhead: obturator nerve). (b) Coronal T2-weighted image with fat suppression showing increased signal intensities with atrophic changes in the vastus medialis, rectus femoris, and vastus lateralis muscles of the left thigh.
Discussion
NA is characterized by the sudden onset of pain, followed by patchy flaccid paralysis, muscle atrophy, and sensory deficits.5,6 Most patients with NA typically present with paralysis preceded by pain; however, atypical cases may suffer painless attacks.6,7 Most cases of NA involve the brachial plexus and, less frequently, the lumbosacral plexus, peripheral nerves, and nerve roots.7,8 The present patient presented with NA in her left leg.
The exact pathophysiology of NA remains unclear, and it may be associated with multiple etiologies, including immunological, mechanical, and genetic factors. 5 Rapid inflammation, also known as plexitis, is considered as an important cause of NA. 5 Over 50% of patients with NA have an inciting event, such as infection, vaccination, surgery, pregnancy, or stress, which triggers the immune system. 7 However, post-vaccination-related NA, as in the current patient, is thought to be very rare, although its precise incidence is unknown. 9 Before establishing a diagnosis of NA, clinicians should rule out other disorders such as radiculopathy, entrapment neuropathy, mononeuritis multiplex, multifocal motor neuropathy, and hereditary neuropathy. 5 In the present case, we also excluded infectious neuritis, including human immunodeficiency virus infection and Lyme disease.
The COVID-19 vaccine, AZD-1222 (AstraZeneca), prepares the immune system by inducing antibodies to attack the SARS-CoV-2 virus. 2 AZD-1222 is a recombinant, replication-deficient adenoviral vector vaccine containing a surface glycoprotein antigen gene. 2 Most reported adverse effects following COVID-19 vaccination have been systemic, local, or allergic reactions. 1 Although rare, there is still concern over neurological vaccine-related side effects 10 ; however, only two patients to date have reportedly developed NA involving the brachial plexus after receiving the BNT-162b2 (Pfizer, New York, NY, USA) vaccination antigen.6,9 The vaccination is suspected to elicit an immune-mediated inflammatory response to the injected antigen,6,9 and this vaccine-triggered immune reaction may result in an NA attack involving the lumbosacral plexus.
In the current case, we performed an initial electrodiagnostic study 1 week after the NA attack. Electrodiagnostic findings of nerve lesions can generally be observed 7 days post-injury, depending on the severity of damage. 11 However, our initial study revealed no acute symptoms, and corticosteroid treatment was thus delayed. We therefore failed to obtain any information that would have led to the patient’s early treatment and indicated her prognosis after therapy, which was a limitation of this case report.
To the best of our knowledge, this is the first report of NA involving the lumbosacral plexus following administration of the AstraZeneca COVID-19 vaccine. The continuing global administration of COVID-19 vaccines means that more cases of post-vaccination NA are likely to occur. This study highlights the importance of assessing the clinical, electrodiagnostic, and radiologic findings to make an accurate diagnosis of NA after COVID-19 vaccination. Clinicians should closely monitor patients for possible side effects, including vaccine-related neurological complications. Their timely recognition, proper diagnosis, and management are essential to minimize the impacts of this condition.
Acknowledgement
The authors would like to thank Myeong Geun Jeong for assistance in preparing the references.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This research was funded by the Korea Institute of Machinery & Materials with a grant for the Basic Research Program [grant number: NK232D].
ORCID iD: Jang Hyuk Cho https://orcid.org/0000-0001-7916-8428
References
- 1.Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 2021; 21: 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andalib S, Biller J, Di Napoli M, et al. Peripheral nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep 2021; 21: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Eijk JJ, Groothuis JT, Van Alfen N. Neuralgic amyotrophy: An update on diagnosis, pathophysiology, and treatment. Muscle Nerve 2016; 53: 337–350. [DOI] [PubMed] [Google Scholar]
- 4.Seror P. Neuralgic amyotrophy. An update. Joint Bone Spine 2017; 84: 153–158. [DOI] [PubMed] [Google Scholar]
- 5.Kim TU, Chang MC. Neuralgic amyotrophy: an underrecognized entity. J Int Med Res 2021; 49: 3000605211006542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diaz-Segarra N, Edmond A, Gilbert C, et al. Painless idiopathic neuralgic amyotrophy after COVID-19 vaccination: A case report. PM R. Epub ahead of print 22 April 2021. DOI: 10.1002/pmrj.12619. [DOI] [PMC free article] [PubMed]
- 7.Van Alfen N, Van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain 2006; 129: 438–450. [DOI] [PubMed] [Google Scholar]
- 8.Chang MC. Neuralgic amyotrophy in the lower extremity diagnosed with gadolinium-enhanced lumbar magnetic resonance imaging: A case report. Neurol Asia 2017; 22: 377–379. [Google Scholar]
- 9.Mahajan S, Zhang F, Mahajan A, et al . Parsonage Turner syndrome after COVID-19 vaccination. Muscle Nerve 2021; 64: E3–E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samudralwar RD. Commentary: The spectrum of neurological manifestations related to COVID-19 and vaccinations. J Neuroimmunol. Epub ahead of print 10 July 2021. DOI: 10.1016/j.jneuroim.2021.577660. [DOI] [PMC free article] [PubMed]
- 11.Kamble N, Shukla D, Bhat D. Peripheral Nerve Injuries: Electrophysiology for the neurosurgeon. Neurol India 2019; 67: 1419–1422. [DOI] [PubMed] [Google Scholar]