Abstract
Background: Brain exposure to ionizing radiation during the radiotherapy of brain tumor or metastasis of peripheral cancer cells to the brain has resulted in cognitive dysfunction by reducing neurogenesis in hippocampus. The water extract of Lycium barbarum berry (Lyc), containing water-soluble Lycium barbarum polysaccharides and flavonoids, can protect the neuronal injury by reducing oxidative stress and suppressing neuroinflammation.
Reseach Design: To demonstrate the long-term radioprotective effect of Lyc, we evaluated the neurobehavioral alterations and the numbers of NeuN, calbindin (CB), and parvalbumin (PV) immunopositive hippocampal neurons in BALB/c mice after acute 5.5 Gy radiation with/without oral administration of Lyc at the dosage of 10 g/kg daily for 4 weeks.
Results: The results showed that Lyc could improve irradiation-induced animal weight loss, depressive behaviors, spatial memory impairment, and hippocampal neuron loss. Immunohistochemistry study demonstrated that the loss of NeuN-immunopositive neuron in the hilus of the dentate gyrus, CB-immunopositive neuron in CA1 strata radiatum, lacunosum moleculare and oriens, and PV-positive neuron in CA1 stratum pyramidum and stratum granulosum of the dentate gyrus after irradiation were significantly improved by Lyc treatment.
Conclusion: The neuroprotective effect of Lyc on those hippocampal neurons may benefit the configuration of learning related neuronal networks and then improve radiation induced neurobehavioral changes such as cognitive impairment and depression. It suggests that Lycium barbarum berry may be an alternative food supplement to prevent radiation-induced neuron loss and neuropsychological disorders.
Keywords: Lycium barbarum berry, ionizing radiation, cognitive dysfunction, calbindin, parvalbumin
Introduction
Ionizing radiation has been widely applied to brain disease diagnosis and radiotherapy to treat brain tumor and to prophylactically prevent metastasis of peripheral cancer cells to the brain. However, acute or fractional accumulative ionizing radiation may result in neurodegenerative disorders including cognitive impairment, depression, sensory disturbance, and poor motor coordination.1,2 Previous studies have shown that irradiation could lead to cognitive impairment and depression by reducing neurogenesis in hippocampus after the acute radiation exposure.3-6 Although many neuroprotective agents have been reported to have significant efficacy on radiation-induced cognitive dysfunction, amifostine is still the only approved radioprotectant during radiotherapy. 7 Our previous study has indicated that the oral administration of Chinese herbal medicine (CHM) epimedium after acute radiation exposure could significantly improve the hippocampal neurogenesis and radiation-induced depression and cognitive impairment in BALB/c mice. 5 Flavonoids, the active components of epimedium and many other CHMs, can reduce oxidative stress significantly. 8 This implicated that CHMs containing flavonoids may have great advantages as radio-neuro-protectants because of their anti-oxidative and immunoregulatory effects.
Lycium barbarum berry, also named Goji berry and wolfberry, which is rich of Lycium barbarum polysaccharides (LBPs), has been used as food supplement and herbal medicine in China for thousands of years and has been increasingly accepted as anti-oxidative and anti-aging fruit in Western countries.9,10 Its components, including flavonoids and many glycosylated derivatives of dicaffeoylspermidine, dicaffeoylspermine, and kukoamine, have been studied intensively.11,12 The mixture of those glycosylated derivatives in Lycium barbarum berry, the LBPs, can improve retinal function and reduce retinal neuron damage,13,14 reduce blood lipid and glucose,15,16 boost immune system to anti-allergy and anti-cancer, 9 protect against radiotherapy or chemotherapy induced tissue damages, 17 prevent neuron loss, and enhance neurogenesis. 18 Flavonoids can pass through the blood-brain barrier 19 to play their roles in anti-oxidation, anti-inflammation, and neuro-protection. 20 The dicaffeoylspermidine derivatives showed significant anti-oxidative and neuroprotective effects. 21 The radio-neuro-protective effects of kukoamines may have the similar anti-oxidative and anti-inflammatory pathways. 22 In addition, kukoamine A may down regulate the NMDA receptors to prevent the NMDA-induced neurotoxicity in vitro. 23 As a nutrient rich “superfood,” Lycium barbarum berry can be used as a daily diet without serious accumulative toxins or side-effect.10,24 Its potential neuroprotective effects after radiation exposure should be evaluated. In the present study, we studied the radio-neuro-protective effects of Lycium barbarum berry extract on neurobehavioral changes and hippocampal neuron loss in the mouse model of acute ionizing radiation exposure.
Materials and Methods
Lycium barbarum Berry Extraction
Five hundred grams dried Lycium barbarum berries (Zhongning, Ningxia, China) were crushed and decocted in 3 L boiled distilled water for 2 h. The decocted liquid was filtrated and the residue was decocted again in 2 L water. The 2 filtrates were combined and concentrated in rotary evaporator under 60°C to obtain 350 g thick paste which was stored under 4°C. The paste containing the water-soluble components of Lycium barbarum berry was dissolved in saline at the highest concentration of .5 g/mL (10 g/kg) before oral administration with the volume of .2 mL per 10 g body weight of mouse. Based on their efficacies on preventing animal body weight loss and behavioral changes after administration of 3 different dosages including 2.5, 5,, and 10 g/kg, the highest dosage of 10 g/kg of Lycium barbarum berry water extraction was chosen for further study.
Experimental Animal
Male specific-pathogen-free (SPF) BALB/c mice were purchased from Hubei experimental animal research center licensed with the number SCXK (Hubei) 2015-0018, and accommodated in local animal room to 8-week-old and body weight 22 ± 2 g before irradiation. Animals were divided into four groups: (1) sham X-ray exposure group (Sham) of 10 mice with oral saline administration after sham exposure; (2) experimental control group (Exp-Ctrl) of 10 mice with 5.5 Gy X-ray whole body irradiation followed by oral saline administration; (3) positive experimental control group (Ami-Exp) of 10 mice with the amifostine pretreatment (at 100 mg/kg, batch number: J0311A, Dalian Meilun Biotech. Co., China) at 30 min before 5.5 Gy X-ray whole body irradiation; and (4) experimental group (Exp-Lyc) of 10 mice with 5.5 Gy X-ray whole body irradiation followed by the oral administration of the Lycium barbarum berry extract (10 g/kg per day) 2 h after radiation exposure on the first day, and the Lycium barbarum berry extract treatment was continued for 4 weeks. The body weight was recorded after the irradiation. Behavioral tests were conducted four weeks after radiation exposure and the mice were then sacrificed for the brain sample collection.
Behavioral Tests
To test if irradiation induced depression and spatial memory impairment and if Lyc could improve those changes, tail suspension, forced swimming, open field, and Morris water maze tests were used as scheduled in Figure 1A. Behavioral tests were conducted in a quiet room with dim light and constant temperature (20-25°C) using SuperMaze animal behavior record and analysis system (Shanghai Xinruan InfoTech Co, China). The animals have been moved into the behavioral test room after the last oral administration. One day after, tail suspension and forced swimming immobility times were recorded for 4 min after 2 min environmental adaptation. The open field size of 50 cm (length) × 50 cm (width) × 30 cm (height) was divided into 25 boxes to record the total movement distance and the time spent in the central area (central 9 boxes) for 10 min. The animal was placed on the central point of open field to begin the test. The Morris water maze experiments were done as described previously. 5 The hidden platform escape latency was recorded for 5 days. On the day 6, after removing the platform, the times that the mice passed though platform area and the total resident time that the mice spent in the platform quadrant in 60 s were recorded.
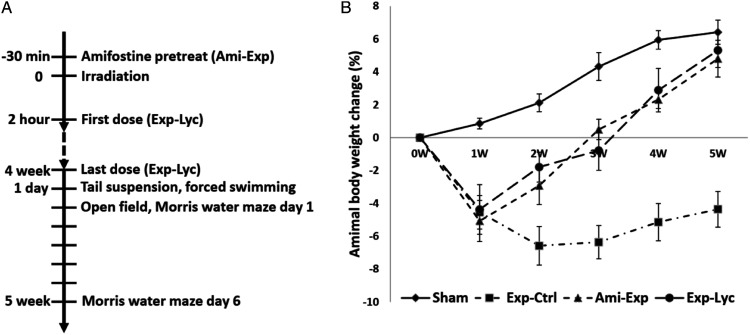
Figure 1.
The experimental time schedule (A) and the effect of Lycium barbarum berry extract on the body weight of radiation exposed BALB/c mice (B). The body weight of the mice in the sham exposure group (Sham) increases steadily, but it decreases significantly after acute X-ray irradiation with 5.5 Gy in the experimental control (Exp-Ctrl), mice treated with Lycium barbarum berries (Exp-Lyc) and amifostine (Ami-Exp) at 1 week after the exposure (F (3, 36) = 61.85, P < .0001). From the second week, amifostine pretreatment (Ami- Exp) and oral Lycium barbarum berry extract treatment after exposure (Exp-Lyc) reverse the animal weight loss (F (3, 36) = 134.0, P < .0001) and these animals have similar body weight to Sham group at the end of the fifth week (F (3, 36) = 244.0, P < .0001). However, Exp-Ctrl group has a significant weight loss from 2 to 5 week(s) after radiation exposure when compared to the other 3 groups (P < .05, n = 10 in each group).
Immunohistochemistry
The mice were anesthetized with 1% pentobarbital after behavioral tests. Animals were perfused transcardially with .9% saline to wash out the blood followed by 4% paraformaldehyde. The brain was removed, postfixed with the same fixative overnight, and then stored in 30% sucrose solution prepared with .1 M phosphate buffer (pH: 7.4). For immunohistochemistry, sagittal brain sections with 40 μm thickness were cut and a set of 3 serial sections was prepared and sections collected were placed individually in different wells of a 24 well tissue culture dish for the control, NeuN, calbindin (CB), and parvalbumin (PV) immunohistochemical reaction. The freely floating sections were treated in 4% normal goat serum for 2 h at room temperature. All sections were then washed in .1 M phosphate-buffered saline (PBS) containing NeuN (1:100) (Invitrogen, United States), CB (1: 2000), and PV (1:1000) (Swant, Switzerland). After incubation, sections were washed in PBS and placed for 1 h in biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA, United States) diluted 1:2000 in PBS/Triton X-100. After 2 washes in PBS, they were placed in avidin– biotin complex (ABC) reagent (Vector Laboratories) in PBS/Triton X-100 for 1 h, washed in PBS and reacted in a solution of .12% H2O2 and .05% 3,3-diaminobenzidine (DAB) (Sigma, United States) in Tris buffer (TB) for 15 min, then mounted, dehydrated, coverslipped, and photographed by using image analysis system.
Cell Counting and Statistical Analysis
NeuN immunopositive neurons in the hilus of the dentate gyrus, CB immunopositive interneurons in the area of CA1 strata radiatum lacunosum moleculare and stratum oriens, PV immunopositive interneurons in the CA1 stratum pyramidum, and the stratum granulosum of the dentate gyrus in the dorsal hippocampus were counted by using Leica DM4 B upright microscope and LAS X software (Leica Microsystems, Germany). The 5 sagittal brain sections from every 3 alternative sections of the dorsal hippocampus of each animal were used for immunopositive cell counting. Five animals of each group were used for cell counting. All the data sets have passed the normal distribution tests by using Prism 8. One-way ANOVA followed by the Bonferroni post-hoc test for multiple comparisons was used for statistical analysis and statistical significance was considered when the adjusted P < .05. All the data were showed as mean ± SD.
Results
Body Weight
The animal body weight changes were recorded for 5 weeks since the day of irradiation (Figure 1B). X-ray irradiation with 5.5 Gy (Exp-Ctrl) induced a significant body weight loss in the first week after exposure compared to the sham radiation exposure mice (Sham) (F (3, 36) = 61.85, P < .0001). Oral administration of Lycium barbarum berry extract at the dosage of 10 g/kg per day (350 g extract paste from 500 g dried Lycium barbarum berries) (Exp-Lyc) prevented animal weight loss from the second week after irradiation, similar to amifostine pretreatment (Ami-Exp) with intraperitoneal injection at 100 mg/kg 30 min before irradiation (F (3, 36) = 134.0, P < .0001). Both therapeutic strategies in Exp-Lyc and Ami-Exp groups effectively improved the animal weight gain to the normal level in the Sham group by the end of the fifth week after irradiation. At this time point, animals in Exp-Lyc and Ami-Exp groups were significantly heavier than those in Exp-Ctrl (F (3, 36) = 244.0, P < .0001), but had no significant weight difference compared to the Sham group (P > .05).
Behavioral Tests
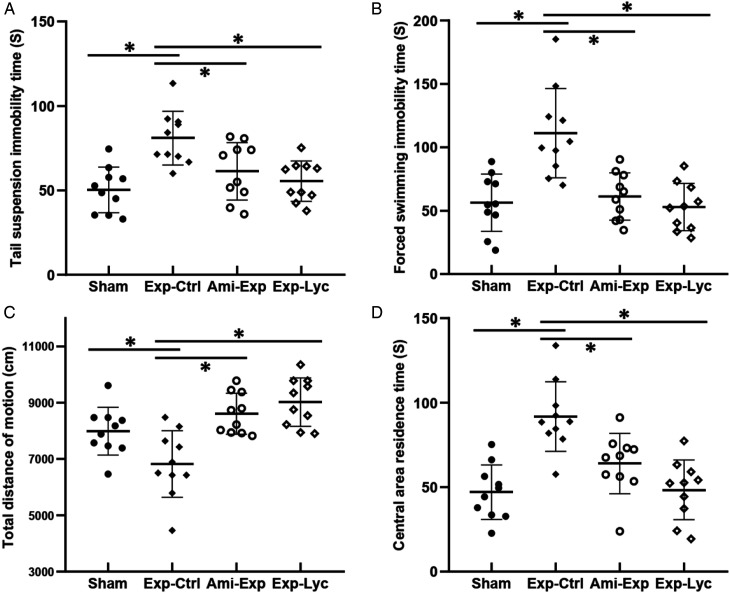
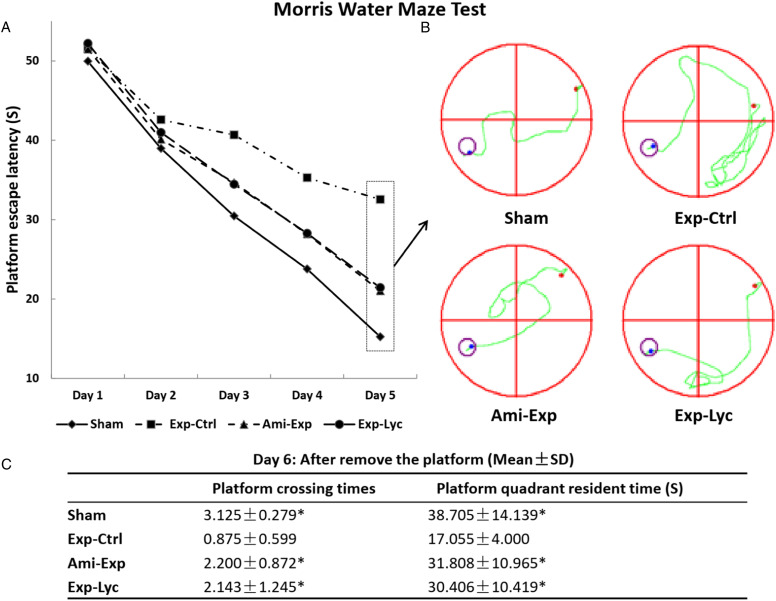
The average tail suspension (Figure 2A, F (3, 36) = 8.267, P = .0003) and forced swimming (Figure 2B, F (3, 36) = 12.27, P < .0001) immobility time in 4 min of those radiation exposed mice with saline treatment were significantly longer than the sham exposed mice. Amifostine or Lycium barbarum berry treatment significantly reduced immobility time when compared to irradiated animals treated with saline (Figures 2A, B, P < .05). No significant difference in immobility time was observed among those exposed animals treated with amifostine or Lycium barbarum berry and the Sham group (P > .05). Open field experiment showed that the total travel distance in the Exp-Ctrl group within 10 min was less than the Sham group, amifostine, or Lycium barbarum treated mice, indicating both treatments significantly improved animal locomotor activity (Figure 2C, F (3, 36) = 10.85, P < .0001). Similarly, Lycium barbarum berry also reduced the time animals stayed in the central area of test field when compared to radiation exposed control mice (Figure 2D, F (3, 36) = 13.14, P < .0001). Morris water maze experiments showed that the average platform escape latency time of radiation exposure mice (Exp-Ctrl) was significantly longer than the Sham, Ami-Exp, or Exp-Lyc group on the day 5 (Figures 3A, B, F (3, 36) = 35.53, P < .0001). In addition, the average platform crossing time and platform quadrant resident time of the Exp-Ctrl group on day 6 were less than the Sham, Ami-Exp, or Exp-Lyc group (Figure 3C, F (3, 36) = 29.24, P < .0001). Amifostine or Lycium barbarum berry treatment reduced the escape latency and increased the platform crossing time and platform quadrant resident time significantly, indicating that both treatments could improve the spatial memory of mice with radiation exposure. These data of behavioral tests demonstrated that the oral administration of Lycium barbarum berry extract could effectively prevent or treat the radiation damages in the motor activity and cognitive function of mice.
Figure 2.
Tail suspension, forced swimming, and open field tests. Similar to the amifostine pretreated (Ami-Exp) group, Lycium barbarum berry extract (Exp-Lyc) significantly reduces the immobility times in tail suspension (A, F (3, 36) = 8.267, P = .0003) and forced swimming (B, F (3, 36) = 12.27, P < .0001) tests. Open field test shows the average total travelling distance of the mice in radiation exposure control group (Exp-Ctrl) is significantly less than those of the mice in the other 3 groups (C, F (3, 36) = 10.85, P < .0001). However, the average central area staying time of Exp-Ctrl mice is significantly longer (D, F (3, 36) = 13.14, P < .0001). Asterisks * indicate P < .05. The values are the mean ± SD calculated from the data of 10 mice in each group.
Figure 3.
Morris water maze test. Morris water maze test shows that the average escape latency of the Exp-Ctrl group is significantly longer than those of the Sham group, the Ami-Exp or Exp-Lyc group in the training trials on the day 5 (A, B, F (3, 36) = 35.53, P < .0001). The average time spent in the platform quadrant and the times of animal crossing platform area of the Exp-Ctrl group are significantly lower than those of other 3 groups on day 6 (C, F (3, 36) = 29.24, P < .0001). Asterisks * in panel C indicate P < .05 when compared with the Exp-Ctrl group. The values are the mean ± SD calculated from the data of 10 mice in each group.
Immunohistochemistry
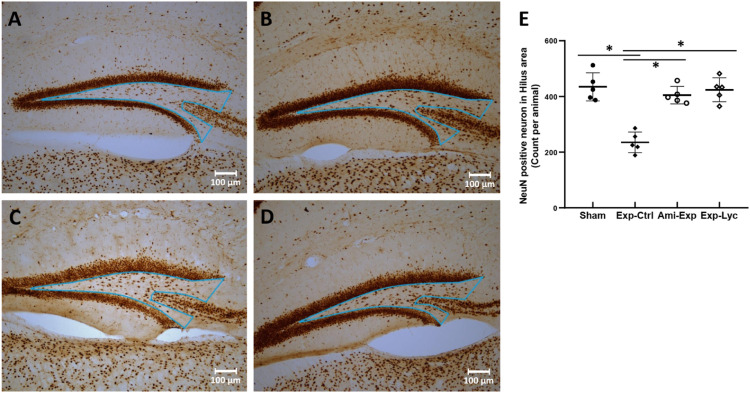
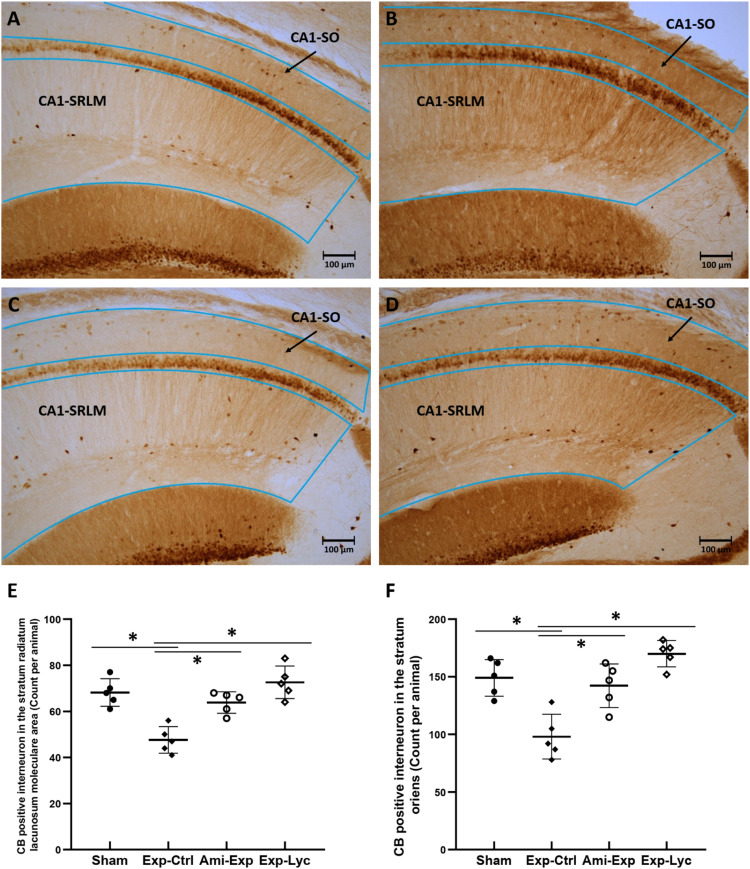
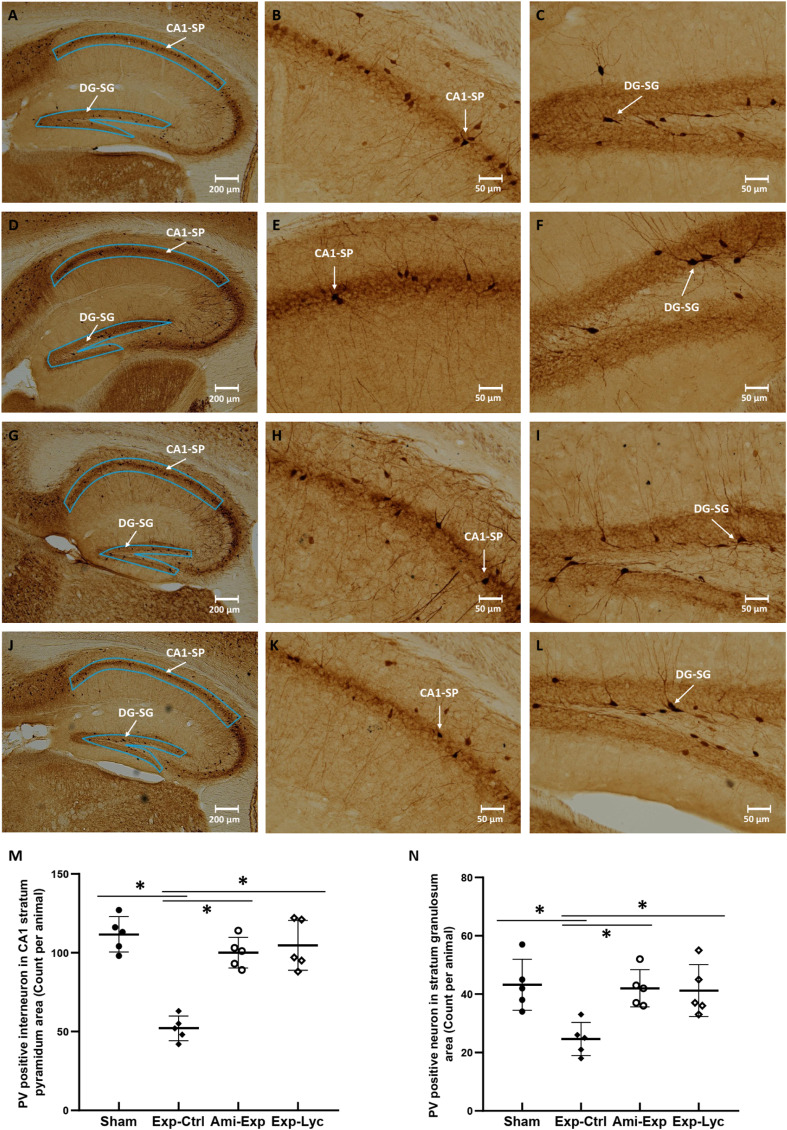
NeuN, CB, and PV immunopositive neurons in the target areas of dorsal hippocampus were counted from 5 slices of each mouse brain. The number of NeuN immunopositive neurons in the hilus of the dentate gyrus was significantly reduced after irradiation with 5.5 Gy (Figure 4, F (3, 36) = 26.04, P < .0001, Exp-Ctrl compared to Sham, P < .05). Both amifostine pretreatment and Lycium barbarum berry extract treatment prevented hilar neuronal loss when compared to the Exp-Ctrl (Figure 4, Ami-Exp or Exp-Lyc compared to Exp-Ctrl, P < .05). Similarly, the number of CB positive neurons in the strata radiatum lacunosum moleculare (Figure 5E, F (3, 36) = 16.88, P < .0001) and stratum oriens (Figure 5F, F (3, 36) = 16.47, P < .0001), and the number of PV positive interneurons in the CA1 stratum pyramidum (Figure 6M, F (3, 36) = 27.75, P < .0001) and the stratum granulosum of the dentate gyrus (Figure 6N, F (3, 36) = 6.78, P = .0037) were also reduced by radiation exposure, indicating the radiation induced neuron loss in these areas. Oral administration of Lycium barbarum berry effectively increased the numbers of CB and PV immunopositive cells in the corresponding areas (Figures 5, 6, Exp-Lyc compared to Exp-Ctrl, P < .05). These immunochemistry results demonstrated that the Lycium barbarum berry could effectively prevent the loss of NeuN, CB, and PV immunopositive cells in the hilus and stratum granulosum of the dentate gyrus, strata radiatum lacunosum moleculare, stratum oriens, and pyramidum of CA1 area of the mouse hippocampus.
Figure 4.
NeuN immunohistochemistry. A, B, C, and D show the NeuN immunopositive neurons in the hilus of the dentate gyrus (the triangle area indicated in each slice) of the representative slices from the Sham, Exp-Ctrl, Ami-Exp, and Exp-Lyc groups, respectively. E, Statistical analysis shows the number of NeuN immunopositive neurons in the hilus of the Exp-Ctrl group (pyramidal neurons in CA3c were excluded) is significantly fewer than the Sham group (F (3, 36) = 26.04, P < .0001). The Ami-Exp or Exp-Lyc group has more NeuN immunopositive neurons in the hilus than the Exp-Ctrl group. Asterisks * indicate P < .05 when compared with the Exp-Ctrl group (n = 5 in each group, cell number is counted in 5 sagittal brain sections from every 3 alternative sections of the dorsal hippocampus of each mouse, and indicated as the mean ± SD).
Figure 5.
Calbindin (CB) immunohistochemistry. A, B, C, and D show the CB immunopositive interneurons in the strata radiatum, lacunosum moleculare (SRLM) and oriens (SO) in CA1 areas (the areas indicated in the slice) of the slices from the Sham, Exp-Ctrl, Ami-Exp, and Exp-Lyc groups, respectively. E and F show the numbers of CB immunopositive interneurons in the CA1-SRLM (F (3, 36) = 16.88, P < .0001) and CA1-SO (Panel F, F (3, 36) = 16.47, P < .0001) areas of the Exp-Ctrl group are statistical significantly fewer than the Sham group. Both Ami-Exp and Exp-Lyc groups have more CB immunopositive interneurons in these areas than the Exp-Ctrl group. Asterisks * indicate P < .05 when compared with the Exp-Ctrl group (n=5 in each group, cell number is counted in 5 sagittal brain sections from every 3 alternative sections of the dorsal hippocampus of each mouse, and indicated as the mean ± SD).
Figure 6.
Parvalbumin (PV) immunohistochemistry. The areas indicated in the slices A, D, G, and J show the PV immunostaining interneurons in the CA1 stratum pyramidum (CA1-SP, B, E, H, and K) and the stratum granulosum of the dentate gyrus (DG-SG, C, F, I, and L) from the Sham, Exp-Ctrl, Ami-Exp, and Exp-Lyc groups, respectively. The picture D shows significant reduced numbers of PV immunopositive interneurons in the CA1-SP (E and M, F (3, 36) = 27.75, P < .0001) and the DG-SG (F and N, F (3, 36) = 6.78, P = .0037) of the Exp-Ctrl group when compared to the Sham group. The numbers of PV immunopositive interneurons in the CA1-SP (M) and the DG-SG (N) of the Ami-Exp (G, H, I) or Exp-Lyc group (J, K, and L) are significantly more than the Exp-Ctrl group. Asterisks * indicate P < .05 when compared with Exp-Ctrl group (n = 5 in each group, cell number is counted in 5 sagittal brain sections from every 3 alternative sections of the dorsal hippocampus of each mouse, and indicated as the mean ± SD).
Discussion
Lyc Improves Radiation-Induced Body Weight Loss
Radiotherapy with ionizing radiation may lead to body weight loss in brain or neck cancer patients 25 and animals exposed to acute high dose radiation. 5 Lycium barbarum berry has been increasingly accepted as an anti-oxidative, immune-boosting, and neuroprotective food supplement which may strengthen the body resistance to damages, pathogens, and even ageing. 10 In the present study, oral administration of Lyc effectively prevented weight loss of the mice X-ray-irradiated with 5.5 Gy, indicating that this traditional herbal medicine should have a role in controlling radiation damages. To further study the radioprotective role of Lyc, we investigated its effect on the neurobehavioral performance and neuroprotection on the hippocampal interneurons and hilar cells in the dentate gyrus.
Lyc Improves Radiation-Induced Behavioral Impairments
Our data from open field, forced swimming, and tail suspension tests have shown that mice irradiated with 5.5 Gy suffered less locomotor activity and depression. These results confirmed that the radiation dosage we applied was sufficient to induce the neurobehavioral impairments of the exposed mice. 5 Morris water maze test has suggested the radiation induced spatial cognitive dysfunction. 26 Four-week oral administration of Lyc could effectively prevent those radiation induced behavioral and cognitive impairments. It has been reported that LBP could significantly improve the dextromethorphan-induced depression in the rat model. 27 In the APP/PS1 double transgenic mice, a widely used animal model of Alzheimer’s disease, 2-week oral administration of Lyc significantly improved their spatial memory in Morris water maze test. 28 This radioprotective effect of Lyc should be related to the previously suggested effects of Lyc or LBP, including anti-oxidation, anti-inflammation, preventing neuron loss, and promoting neuronal regeneration. 29 The anti-oxidative effect of Lyc or LBP should be due to their activities in scavenging reactive oxygen species (ROS) and inhibiting oxidases.30-32 By using primary cultured neonatal rat hippocampal neurons, LBP significantly decreased the overexpression of inflammatory cytokines and apoptotic biomarkers induced by oxygen–glucose deprivation/reperfusion. 33 These anti-oxidative and anti-inflammatory effects of Lyc should contribute to its neuroprotection and subsequent improvement of spatial memory and depression.
Lyc Improves Radiation-Induced Hippocampal Neuron Loss
Our previous study has demonstrated that the oral administration of epimedium 5 or intraperitoneal injection of amitriptyline, an inhibitor of acid sphingomyelinase (ASMase), 6 significantly prevent radiation-induced loss of Ki67, doublecortin (DCX), and PV immunopositive cells in the subgranular zone (SGZ) of the dentate gyrus, indicating the improvement of neurogenesis in SGZ. In the pilocarpine induced mouse model of status epilepticus, dramatic reduction of NeuN, CB, and PV immunopositive neurons in different hippocampal areas implicated that these biomarkers should help to present the status of neuron loss and neuron regeneration in hippocampus.34,35 A recent study has reported that LBP treatment could increase the number of NeuN positive neurons in the dentate gyrus of APP/PS1 mice. 18 In this study, our behavioral tests have demonstrated obvious cognitive impairment and depression in those irradiated mice. Therefore, we focused on the changes of those hippocampal neurons and interneurons which should related to the cognitive dysfunction and depression.
The number of NeuN-immunopositive neurons in the dentate hilus could be reduced by ionizing irradiation. 36 In the chronic mouse model of temporal lobe epilepsy (TLE), reduced number of the hilar mossy cells was found to be the major reason of spatial memory deficit. 37 As the major excitatory neurons in dentate hilus, mossy cells are responsible for receiving inputs from local interneurons, granule cells and the CA3 input and protruding axons into different layers of the dentate gyrus.38,39 They play essential roles in maintaining cognitive neuronal network and even in the regeneration and/or the reorganization of neuronal network after neuronal damage.34,40,41 Therefore, the decreased number of hilar NeuN immunopositive cells after irradiation in this study may implicate the mossy cell loss and the consequent cognitive deficit and depression. 41
Several previous studies have demonstrated that the loss of hippocampal interneurons should lead to cognitive dysfunction and behavior alteration.42-44 As a calcium binding protein expressed in some hippocampal interneurons, CB plays a critical role in hippocampal learning function. 45 Down-regulated hippocampal CB expression and/or CB positive neurons were observed in various neurological disorders and were related to cognitive deficits,46-48 while the increased number of CB positive hippocampal neurons implicated the generation of new neurons and the establishment of functional neuronal network. 49 The CB positive interneurons located in the strata radiatum, lacunosum moleculare (CA1-SRLM) and oriens (CA1-SO) of CA1 area are mostly GABAergic interneurons which mainly form synapses with CA1 pyramidal cells and are significantly reduced during ageing.50,51 In the present study, we found that the radiation exposure reduced the number of CB positive interneurons in the CA1-SRLM, which are involved in the regulation of the inputs from the entorhinal cortex to hippocampal CA1 apical dendrites of pyramidal neurons or spatial information. 45 And the reduced number of CB expressing interneurons in the CA1-SO may affect the interaction between the CA1 pyramidal cells and CA1-projecting subiculum neurons which should play a role in the spatial memory.52,53
PV positive cells in hippocampal area are inhibitory GABAergic interneurons which function to configure learning related neuronal network. 54 The PV positive interneurons in CA1 area provide inhibitory synapses onto CA1 pyramidal neurons to regulate their activities in cognitive network.55,56 Promotion of PV immunopositive interneurons in CA1 area could rescue cognitive dysfunction of adult mouse model of schizophrenia. 57 In addition, the activity of PV positive interneurons in dentate gyrus should have an essential role in cognitive function.58,59 The irradiation-induced PV immunopositive neuron loss in the stratum pyramidum of CA1 (CA1-SP) and in the stratum granulosum of the dentate gyrus (DG-SG) was observed in current study, which should be responsible for the cognitive deficits of those irradiated mice.
The Lyc has been reported as an anti-depressive drug previously. 60 Our behavioral tests indicated the spatial memory impairment and depression of those radiation exposure mice. Given these, we proposed that the radiation exposure at the dosage of 5.5 Gy induced the depressive behavior and the spatial memory impairment of mice by promoting the hippocampal NeuN-positive neuron loss in the hilus, CB-positive interneuron loss in the CA1-SO and CA1-SRLM, and the PV-positive interneuron loss in the CA1-SP and DG-SG areas. Oral administration of Lyc significantly prevented the neuron loss in these areas and then maintained the hippocampal neuronal network which consequently improved the radiation induced cognitive deficit and depression.
Conclusion
Ionizing radiation at the dosage of 5.5 Gy induced the depressive behavior and spatial memory impairment in BALB/c mice 4 weeks after irradiation. Significant NeuN-positive neuron loss in the hilus of the dentate gyrus, CB-positive interneuron loss in the CA1-SO and CA1-SRLM, and PV-positive interneuron loss in the CA1-SP and DG-SG areas 6 weeks after irradiation were demonstrated by immunohistochemistry study. Oral administration of Lyc prevented the loss of those hippocampal neurons and then improved the radiation-induced cognitive dysfunction and depression. While further study is still needed to elucidate the molecular mechanism of Lyc, current study strongly suggests that this “superfood” may be a promising radio-neuro-protective supplement to prevent radiotherapy or other ionizing radiation induced cognitive impairment.
Footnotes
Author Contributions: LG, Q-QD, and P-QC: Investigation. T-TY, C-QX, and X-ZL: Formal analysis. X-CP: Resources, Methodology. FQ: Conceptualization, Data Curation, Writing—Original Draft. J-RH: Project administration, F-RT: Conceptualization, Methodology, Writing—Review and Editing.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the College Students Innovative Entrepreneurial Training Program of Hubei province (S20191049071) to GL, the Scientific Research Foundation of Hubei Health Commission (WJ2016-Y-01), the Jingzhou Science and Technology Department (2019EC61-17) and the Yangtze Fund for Youth Teams of Science and Technology Innovation (2016cqt04) to QF, the nature science foundation of Hubei province (2017CFB786) and the Jingzhou Science and Technology Department (2017-93) to PXC, the Research Project of Traditional Chinese Medicine (WJ2017-15) and the Scientific Research Foundation of Hubei Health Commission (WJ2019-02) to HJR, and National Research Foundation of Singapore to Singapore Nuclear Research and Safety Initiative (TFR).
ORCID iDs
Feng Qian https://orcid.org/0000-0001-5766-4623
Feng-Ru Tang https://orcid.org/0000-0003-2462-1787
References
- 1.Jacob J, Durand T, Feuvret L, et al. Cognitive impairment and morphological changes after radiation therapy in brain tumors: A review. Radiother Oncol. 2018;128(2):221-228. doi: 10.1016/j.radonc.2018.05.027 [DOI] [PubMed] [Google Scholar]
- 2.Segaran RC, Chan LY, Wang H, Sethi G, Tang FR. Neuronal development-related miRNAs as biomarkers for Alzheimer’s disease, depression, schizophrenia and ionizing radiation exposure. Curr Med Chem. 2021;28(1):19-52. doi: 10.2174/0929867327666200121122910 [DOI] [PubMed] [Google Scholar]
- 3.Hladik D, Tapio S. Effects of ionizing radiation on the mammalian brain. Mutat Res. 2016;770(Pt B):219-230. doi: 10.1016/j.mrrev.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 4.Tang FR, Loke WK, Khoo BC. Postnatal irradiation-induced hippocampal neuropathology, cognitive impairment and aging. Brain Dev. 2017;39(4):277-293. doi: 10.1016/j.braindev.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 5.Wang SW, Ren BX, Qian F, et al. Radioprotective effect of epimedium on neurogenesis and cognition after acute radiation exposure. Neuroscience Research. 2019;145:46-53. [DOI] [PubMed] [Google Scholar]
- 6.Guo YR, Liu ZW, Peng S, et al. The neuroprotective effect of amitriptyline on radiation-induced impairment of hippocampal neurogenesis. Dose Response. 2019;17(4):1559325819895912. doi: 10.1177/1559325819895912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen BD, Acharya MM, Lu C, et al. Remediation of radiation-induced cognitive dysfunction through oral administration of the neuroprotective compound NSI-189. Radiat Res. 2018;189(4):345-353. doi: 10.1667/RR14879.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang XH, Li L, Xue YB, Zhou XX, Tang JH. Flavonoids from epimedium pubescens: Extraction and mechanism, antioxidant capacity and effects on CAT and GSH-Px of drosophila melanogaster. PeerJ. 2020;8:e8361. doi: 10.7717/peerj.8361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulbricht C, Bryan JK, Costa D, et al. An evidence-based systematic review of goji (Lycium spp.) by the natural standard research collaboration. J Diet Suppl. 2014;12(2):184-240. doi: 10.3109/19390211.2014.904128 [DOI] [PubMed] [Google Scholar]
- 10.Ma ZF, Zhang H, Teh SS, et al. Goji berries as a potential natural antioxidant medicine: An insight into their molecular mechanisms of action. Oxid Med Cell Longev. 2019;2019:2437397. doi: 10.1155/2019/2437397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mocan A, Moldovan C, Zengin G, et al. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem Toxicol. 2018;115:414-424. doi: 10.1016/j.fct.2018.01.054 [DOI] [PubMed] [Google Scholar]
- 12.Xiao X, Ren W, Zhang N, et al. Comparative study of the chemical constituents and bioactivities of the extracts from fruits, leaves and root barks of lycium barbarum. Molecules. 2019;24(8):1585. doi: 10.3390/molecules24081585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang D, So KF, Lo AC. Lycium barbarum polysaccharide extracts preserve retinal function and attenuate inner retinal neuronal damage in a mouse model of transient retinal ischaemia. Clin Exp Ophthalmol. 2017;45(7):717-729. doi: 10.1111/ceo.12950 [DOI] [PubMed] [Google Scholar]
- 14.Li HY, Huang M, Luo QY, Hong X, Ramakrishna S, So KF. Lycium barbarum (Wolfberry) increases retinal ganglion cell survival and affects both microglia/macrophage polarization and autophagy after rat partial optic nerve transection. Cell Transplant. 2019;28(5):607-618. doi: 10.1177/0963689719835181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Souza Zanchet MZ, Nardi GM, de Oliveira Souza Bratti L, Filippin-Monteiro FB, Locatelli C. Lycium barbarum reduces abdominal fat and improves lipid profile and antioxidant status in patients with metabolic syndrome. Oxid Med Cell Longev. 2017;2017:9763210. doi: 10.1155/2017/9763210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Yao Y, Liu X, Wang K, Zhou Q, Tang Y. Protective effects of lycium barbarum polysaccharides on blood-retinal barrier via ROCK1 pathway in diabetic rats. Am J Transl Res. 2019;11(10):6304-6315. [PMC free article] [PubMed] [Google Scholar]
- 17.Tian X, Liang T, Liu Y, Ding G, Zhang F, Ma Z. Extraction, structural characterization, and biological functions of lycium barbarum polysaccharides: A review. Biomolecules. 2019;9(9):389. doi: 10.3390/biom9090389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Duan Y, Huang S, et al. Polysaccharides from Lycium barbarum ameliorate amyloid pathology and cognitive functions in APP/PS1 transgenic mice. Int J Biol Macromol. 2020;144:1004-1012. doi: 10.1016/j.ijbiomac.2019.09.177 [DOI] [PubMed] [Google Scholar]
- 19.Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J Neurochem. 2003;85(1):180-192. doi: 10.1046/j.1471-4159.2003.01652.x [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Xie C, Xi S, et al. Radioprotective effect of flavonoids on ionizing radiation-induced brain damage. Molecules. 2020;25(23):5719. doi: 10.3390/molecules25235719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou ZQ, Fan HX, He RR, et al. New dicaffeoylspermidine derivatives from wolfberry, with activities against Alzheimer’s disease and oxidation. J Agric Food Chem. 2016;64(11):2223-2237. doi: 10.1021/acs.jafc.5b05274 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Gao L, Cheng Z, et al. Kukoamine a prevents radiation-induced neuroinflammation and preserves hippocampal neurogenesis in rats by inhibiting activation of NF-κB and AP-1. Neurotox Res. 2017;31(2):259-268. doi: 10.1007/s12640-016-9679-4 [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Gao L, Niu Y, et al. Kukoamine a protects against NMDA-induced neurotoxicity accompanied with down-regulation of GluN2B-containing NMDA receptors and phosphorylation of PI3K/Akt/GSK-3β signaling pathway in cultured primary cortical neurons. Neurochem Res. 2020;45(11):2703-2711. doi: 10.1007/s11064-020-03114-y [DOI] [PubMed] [Google Scholar]
- 24.Wenli S, Shahrajabian MH, Qi C. Health benefits of wolfberry (Gou Qi Zi, Fructus barbarum L.) on the basis of ancient Chineseherbalism and Western modern medicine. Avicenna J Phytomed. 2021;11(2):109-119. [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SC, Wang TJ, Chu PY. Predictors of weight loss during and after radiotherapy in patients with head and neck cancer: A longitudinal study. Eur J Oncol Nurs. 2019;39:98-104. [DOI] [PubMed] [Google Scholar]
- 26.Schoenfeld R, Schiffelholz T, Beyer C, Leplow B, Foreman N. Variants of the Morris water maze task to comparatively assess human and rodent place navigation. Neurobiol Learn Mem. 2017;139:117-127. doi: 10.1016/j.nlm.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 27.Po KKT, Leung JWH, Chan JNM, et al. Protective effect of Lycium barbarum polysaccharides on dextromethorphan-induced mood impairment and neurogenesis suppression. Brain Research Bulletin. 2017;134:10-17. [DOI] [PubMed] [Google Scholar]
- 28.Zhang QL, Du XP, Xu YP, Dang L, Xiang L, Zhang JW. The effects of Gouqi extracts on Morris maze learning in the APP/PS1 double transgenic mouse model of Alzheimer’s disease. Exp Ther Med. 2013;5(5):1528-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xing XW, Liu FY, Xiao J, So KF. Neuro-protective mechanisms of Lycium barbarum. Neuromolecular Med. 2016;18(3):253-263. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh FC, Hung CT, Cheng KC, et al. Protective effects of Lycium barbarism extracts on UVB-induced damage in human retinal pigment epithelial cells accompanied by attenuating ROS and DNA damage. Oxid Med Cell Longev. 2018;2018:4814928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, Sha XY, Wu YN, Chen MT, Zhong JX. Lycium barbarum polysaccharides protects retinal ganglion cells against oxidative stress injury. Neural Regen Res. 2020;15(8):1526-1531. doi: 10.4103/1673-5374.274349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YY, Ding L, Li YM, Guan CR, Guo J. Lycium barbarum polysaccharides can reduce the oxidative damage of the retinal nerve cells in diabetic rats. Int J Clin Exp Med. 2017;10(3):5168-5174. [Google Scholar]
- 33.Zhao P, Ma NT, Chang RY, et al. Mechanism of Lycium barbarum polysaccharides on primary cultured rat hippocampal neurons. Cell Tissue Res. 2017;369(3):455-465. doi: 10.1007/s00441-017-2648-2 [DOI] [PubMed] [Google Scholar]
- 34.Zhang S, Khanna S, Tang FR. Patterns of hippocampal neuronal loss and axon reorganization of the dentate gyrus in the mouse pilocarpine model of temporal lobe epilepsy. J Neurosci Res. 2009;87(5):1135-1149. [DOI] [PubMed] [Google Scholar]
- 35.Xu JH, Tang FR. Voltage-dependent calcium channels, calcium binding proteins, and their interaction in the pathological process of epilepsy. Int J Mol Sci. 2018;19(9):2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pipová Kokošová N, Kisková T, Vilhanová K, et al. Melatonin mitigates hippocampal and cognitive impairments caused by prenatal irradiation. Eur J Neurosci. 2020;52(6):3575-3594. doi: 10.1111/ejn.14687 [DOI] [PubMed] [Google Scholar]
- 37.Bui AD, Nguyen TM, Limouse C, et al. Dentate gyrus mossy cells control spontaneous convulsive seizures and spatial memory. Science. 2018;359(6377):787-790. doi: 10.1126/science.aan4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Houser CR, Peng Z, Wei X, Huang CS, Mody I. Mossy cells in the dorsal and ventral dentate gyrus differ in their patterns of axonal projections. J Neurosci. 2021;41(5):991-1004. doi: 10.1523/jneurosci.2455-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.GoodSmith D, Chen X, Wang C, et al. Spatial representations of granule cells and mossy cells of the dentate gyrus. Neuron. 2017;93(3):677-690.e5. doi: 10.1016/j.neuron.2016.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marqués-Marí AI, Nacher J, Crespo C, Gutièrrez-Mecinas M, Martínez-Guijarro FJ, Blasco-Ibáñez JM. Loss of input from the mossy cells blocks maturation of newly generated granule cells. Hippocampus. 2007;17(7):510-524. doi: 10.1002/hipo.20290 [DOI] [PubMed] [Google Scholar]
- 41.Oh SJ, Cheng J, Jang JH, et al. Hippocampal mossy cell involvement in behavioral and neurogenic responses to chronic antidepressant treatment. Mol Psychiatry. 2020;25(6):1215-1228. doi: 10.1038/s41380-019-0384-6 [DOI] [PubMed] [Google Scholar]
- 42.Ma K, McLaurin J. Alpha-melanocyte stimulating hormone prevents GABAergic neuronal loss and improves cognitive function in Alzheimer’s disease. J Neurosci. 2014;34(20):6736-6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adotevi NK, Leitch B. Synaptic changes in AMPA receptor subunit expression in cortical parvalbumin interneurons in the stargazer model of absence epilepsy. Front Mol Neurosci. 2017;10:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi H, Brasnjevic I, Rutten BPF, et al. Hippocampal interneuron loss in an APP/PS1 double mutant mouse and in Alzheimer’s disease. Brain Struct Funct. 2010;214(2-3):145-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li YD, Xu JM, Liu YF, et al. A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat Neurosci. 2017;20(4):559-570. [DOI] [PubMed] [Google Scholar]
- 46.You JC, Muralidharan K, Park JW, et al. Epigenetic suppression of hippocampal calbindin-D28k by delta FosB drives seizure-related cognitive deficits. Nat Med. 2017;23(11):1377-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li JT, Xie XM, Yu JY, et al. Suppressed calbindin levels in hippocampal excitatory neurons mediate stress-induced memory loss. Cell Rep. 2017;21(4):891-900. [DOI] [PubMed] [Google Scholar]
- 48.Goffigan-Holmes J, Sanabria D, Diaz J, Flock D, Chavez-Valdez R. Calbindin-1 expression in the hippocampus following neonatal hypoxia-ischemia and therapeutic hypothermia and deficits in spatial memory. Dev Neurosci. 2019;12:1-15. doi: 10.1159/000497056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramirez-Rodriguez GB, Olvera-Hernandez S, Vega-Rivera NM, Ortiz-Lopez L. Melatonin influences structural plasticity in the axons of granule cells in the dentate gyrus of balb/C mice. Int J Mol Sci. 2019;20(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gulyás AI, Freund TF. Pyramidal cell dendrites are the primary targets of calbindin D28k-immunoreactive interneurons in the hippocampus. Hippocampus. 1996;6(5):525-534. [DOI] [PubMed] [Google Scholar]
- 51.Potier B, Krzywkowski P, Lamour Y, Dutar P. Loss of calbindin-immunoreactivity in CA1 hippocampal stratum radiatum and stratum lacunosum-moleculare interneurons in the aged rat. Brain Res. 1994;661(1-2):181-188. doi: 10.1016/0006-8993(94)91195-9 [DOI] [PubMed] [Google Scholar]
- 52.Sun Y, Jin S, Lin X, et al. CA1-projecting subiculum neurons facilitate object-place learning. Nat Neurosci. 2019;22(11):1857-1870. doi: 10.1038/s41593-019-0496-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blasco-Ibáñez JM, Freund TF. Synaptic input of horizontal interneurons in stratum oriens of the hippocampal CA1 subfield: Structural basis of feed-back activation. Eur J Neurosci. 1995;7(10):2170-2180. doi: 10.1111/j.1460-9568.1995.tb00638.x [DOI] [PubMed] [Google Scholar]
- 54.Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504(7479):272-276. [DOI] [PubMed] [Google Scholar]
- 55.Udakis M, Pedrosa V, Chamberlain SEL, Clopath C, Mellor JR. Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. Nat Commun. 2020;11(1):4395. doi: 10.1038/s41467-020-18074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi F, Ball J, Stoll KE, et al. Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: Roles in cellular excitability, inhibitory transmission and cognition. J Physiol. 2014;592(16):3463-3494. doi: 10.1113/jphysiol.2014.275453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mukherjee A, Carvalho F, Eliez S, Caroni P. Long-lasting rescue of network and cognitive dysfunction in a genetic schizophrenia model. Cell. 2019;178(6):1387-1402.e14. [DOI] [PubMed] [Google Scholar]
- 58.Elgueta C, Bartos M. Dendritic inhibition differentially regulates excitability of dentate gyrus parvalbumin-expressing interneurons and granule cells. Nat Commun. 2019;10(1):5561. doi: 10.1038/s41467-019-13533-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medrihan L, Umschweif G, Sinha A, et al. Reduced Kv3.1 activity in dentate gyrus parvalbumin cells induces vulnerability to depression. Biol Psychiatr. 2020;88(5):405-414. doi: 10.1016/j.biopsych.2020.02.1179 [DOI] [PubMed] [Google Scholar]
- 60.Zhang E, Yau SY, Lau BW, et al. Synaptic plasticity, but not hippocampal neurogenesis, mediated the counteractive effect of wolfberry on depression in rats(1). Cell Transplant. 2012;21(12):2635-2649. doi: 10.3727/096368912x655181 [DOI] [PubMed] [Google Scholar]