Abstract
Background
Readmission over the first year following hospitalization for acute myocardial infarction (AMI) is common among younger adults (≤55 years). Our aim was to develop/validate a risk prediction model that considered a broad range of factors for readmission within 1 year.
Methods and Results
We used data from the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) study, which enrolled young adults aged 18 to 55 years hospitalized with AMI across 103 US hospitals (N=2979). The primary outcome was ≥1 all‐cause readmissions within 1 year of hospital discharge. Bayesian model averaging was used to select the risk model. The mean age of participants was 47.1 years, 67.4% were women, and 23.2% were Black. Within 1 year of discharge for AMI, 905 (30.4%) of participants were readmitted and were more likely to be female, Black, and nonmarried. The final risk model consisted of 10 predictors: depressive symptoms (odds ratio [OR], 1.03; 95% CI, 1.01–1.05), better physical health (OR, 0.98; 95% CI, 0.97–0.99), in‐hospital complication of heart failure (OR, 1.44; 95% CI, 0.99–2.08), chronic obstructive pulmomary disease (OR, 1.29; 95% CI, 0.96–1.74), diabetes mellitus (OR, 1.23; 95% CI, 1.00–1.52), female sex (OR, 1.31; 95% CI, 1.05–1.65), low income (OR, 1.13; 95% CI, 0.89–1.42), prior AMI (OR, 1.47; 95% CI, 1.15–1.87), in‐hospital length of stay (OR, 1.13; 95% CI, 1.04–1.23), and being employed (OR, 0.88; 95% CI, 0.69–1.12). The model had excellent calibration and modest discrimination (C statistic=0.67 in development/validation cohorts).
Conclusions
Women and those with a prior AMI, increased depressive symptoms, longer inpatient length of stay and diabetes may be more likely to be readmitted. Notably, several predictors of readmission were psychosocial characteristics rather than markers of AMI severity. This finding may inform the development of interventions to reduce readmissions in young patients with AMI.
Keywords: acute myocardial infarction, Bayesian model averaging, psychosocial factors, risk prediction model, young adults
Subject Categories: Health Services, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- BMA
Bayesian model averaging
- VIRGO
Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients
Clinical Perspective
What Is New?
We present a new risk prediction model for all‐cause readmission within 1 year of acute myocardial infarction in younger adults (≤55 years) that considers a broad range of demographic, clinical, and psychosocial factors.
What Are the Clinical Implications?
Several predictors of readmission were psychosocial characteristics rather than markers of acute myocardial infarction severity including depressive symptoms, better physical health, low income, and being employed.
These findings may inform the development of interventions to reduce readmissions in young patients hospitalized with acute myocardial infarction.
Readmissions after an acute myocardial infarction (AMI) are common, costly, and represent a marker of suboptimal health care. 1 Each year, nearly 1 in 6 individuals hospitalized with AMI will have an unplanned readmission within 30 days of discharge. Readmissions result in over $1 billion of annual US healthcare costs, of which $365 million is spent on patients under 65 years of age. 2 , 3 , 4 , 5 Beyond the burden on the healthcare system, readmissions impose considerable physical, psychological, and financial stress on individuals. 6 , 7 , 8 Despite an overall decrease in cardiovascular disease prevalence and AMI mortality in both sexes, 9 rates of AMI hospitalization in younger adults (≤55 years) have increased over the past decade, 10 particularly for younger women. 11 , 12 Although the risk of post‐AMI readmission increases with advancing age, readmissions are also common among younger patients 5 : over 1 in 10 adults with AMI below 65 years of age are readmitted within 30 days, 5 and this risk extends over the first year after AMI. 13
To reduce rates of readmission, the Centers for Medicare and Medicaid Services publicly reports risk‐standardized readmission rates, 14 , 15 and hospitals are subject to financial penalties for excessive all‐cause 30‐day AMI readmissions under the Centers for Medicare and Medicaid Services Hospital Readmissions Reduction Program. 14 , 16 , 17 , 18 Although federal penalties have motivated efforts to develop interventions to reduce 30‐day readmissions, to date such efforts have neither been consistently successful nor addressed readmission beyond the first month after discharge in this population. 19 , 20 , 21 , 22 , 23 Tellingly, there are no available risk prediction models for 1‐year post‐AMI readmissions among younger adults. Existing risk stratification models for post‐AMI readmissions have been developed in predominantly older male patient populations 24 and have demonstrated modest predictive ability and generalizability because of methodological drawbacks including the absence of psychosocial factors. 2 , 25 , 26 , 27 The few available risk models for 1‐year post‐AMI readmissions have been intervention specific, were developed in older populations, and did not capture patient‐reported outcomes. 28 , 29 , 30 , 31 Identifying which young adults hospitalized for AMI are at the highest risk for readmissions can inform the development of interventions that more effectively prevent readmission and improve outcomes in this population.
To address this gap in knowledge, our objective was to develop and validate a global risk prediction model of 1‐year post‐AMI all‐cause readmission in younger adults that considers a broad range of demographic and clinical variables as well as patient‐reported outcomes. The purpose of the model is to use information from the in‐hospital stay to estimate each individual’s probability of readmission. We used data from the VIRGO study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients), 32 the largest prospective multicenter longitudinal study of young adults aged ≤55 years hospitalized for AMI.
Methods
All supporting data are available upon request from the corresponding author.
Participants and Study Design
Between August 21, 2008, and May 1, 2012, we enrolled patients aged 18 to 55 years old hospitalized with AMI from 103 US, 3 Australian, and 24 Spanish hospitals into the VIRGO study, called IMJOVEN (Infarto de Miocardio en la Mujer Joven) in Spain (VIRGO US grant, 5 R01 HL081153‐05; VIRGO Spanish grant, 081614) (Figure S1). This was a multicenter observational study designed to investigate factors associated with adverse clinical outcomes in young women (≤55 years) hospitalized for AMI. Patients were prospectively recruited and enrolled in the VIRGO study, which used a 2:1 female‐to‐male enrollment design to enrich the study inclusion of young women. A total of 6538 patients with AMI were screened at contributing sites, of whom 3572 were eligible and enrolled (N=2397 women; N=1175 men). For the current study only the N=2985 US patients (N=2009 women, N=976 men) hospitalized for AMI were included. 32 After excluding in‐hospital deaths (N=6), this resulted in a final cohort of 2979 participants. From this sample we randomly selected 1986 participants to serve as a development cohort with the remaining 993 as the validation cohort. This allocation of the overall sample allowed for sufficient power to both derive and validate our risk prediction model. With our development sample of 1968, an estimated sample C statistic of 0.650, and using the methods of Hanley and McNeil (1982) 33 as well as Kryzanowski and Hand (2009), 34 we are able to estimate a 2‐sided 95.0% CI from 0.62 to 0.68. With regard to validation, it has been suggested by Altman et al (2009) 35 that a validation sample should have a minimum of 100 to 200 outcome events. Our validation sample of 993 includes 300 outcome events, notably higher than the minimum suggested by Altman.
The VIRGO study has been previously described. 32 In brief, AMI was confirmed by increased cardiac biomarkers (with at least 1 cardiac biomarker above the 99th percentile of the upper reference limit) within 24 hours of admission. The study also required additional evidence of acute myocardial ischemia, including at least 1 of the following: symptoms of ischemia, ECG changes indicative of new ischemia (new ST‐T changes, new or presumably new left bundle branch block, or the development of pathological Q waves). Patients must have presented directly to the enrolling site or must have been transferred within the first 24 hours of presentation to ensure that primary clinical decision making occurred at the enrolling site. We excluded patients who were incarcerated, did not speak English or Spanish, were unable to provide informed consent or to be contacted for follow‐up, developed elevated cardiac markers because of elective coronary revascularization, or had an AMI as the result of physical trauma. Institutional review board approval was obtained at each participating institution, and patients provided informed consent for their study participation, including baseline hospitalization and follow‐up interviews.
Study Outcome and Readmission Data Adjudication
The primary outcome of this study was all‐cause readmission defined as any hospital or observation stay greater than 24 hours within 1 year of discharge. Readmissions were identified using a 2‐stage process. First, when a study participant’s 1‐year follow‐up window closed, the research coordinator at the local site reviewed the records within their hospital network to identify readmission records. In addition, the study participants were also asked to self‐report any readmissions during their 1‐year post‐AMI interviews, including the hospital, date and reason for admission. Second, the Yale Coordinating Center then reconciled the hospital records with the patient self‐reported events to ensure that no readmissions were missed. When necessary, the Yale Coordinating Center requested the missing records from hospitals outside of the site networks. Once a readmission had been identified, admission and discharge records were obtained. The major fields collected included number of readmissions, primary admission diagnoses, procedures completed, follow‐up visits, and discharge status. For information on principal diagnoses for readmission, emphasis was placed on discerning cardiac versus noncardiac diagnoses.
The VIRGO adjudication process was supported through the use of a custom‐developed Research Electronic Data Capture external module. 36 Adjudications were completed by 5 physicians and an advanced practice registered nurse at Yale University who received extensive training and clear guidelines. A data dictionary was created as guidance for each of the major fields, including explicit variable definitions. The data dictionary also included individual cases discussed as a team and provided guidance on future adjudication decisions. The first 253 readmissions were double adjudicated, and subsequent readmissions underwent single adjudication. Discrepancies between adjudicators were resolved by consensus including an additional physician when necessary. Adjudicators could also flag events to be reviewed and discussed by the team. Mortality events were ascertained through interviews with family members and verified with death certificates, hospital records, or obituaries.
Data Collection and Selection of Candidate Predictors
We initially selected a comprehensive list of 65 candidate variables based on our prior work and from existing AMI readmission risk models (Table S1). 2 , 13 , 37 Information was collected from medical record abstraction and standardized in‐person interviews administered by trained personnel at baseline and before discharge. Variables were classified into categories of sociodemographic factors, cardiac risk factors and medical history, presentation characteristics, in‐hospital complications, and psychosocial factors.
Variables on sociodemographics collected included age, sex, race/ethnicity, marital status, less than high school education), household primary earner status, low income (defined as personal income ≤30 000 USD), employment status, and current presence of health insurance. Baseline cardiac risk factors and medical history included diabetes mellitus, obesity (body mass index≥30 kg/m2), hypertension, dyslipidemia, current smoking, family history of cardiovascular disease, physical inactivity, prior MI, renal disease, alcohol abuse, chronic obstructive pulmonary disorder, stroke, heart failure, recreational drug use, and peripheral artery disease.
Presentation characteristics included first health service used, transfer from another institution, late presentation (>6 hours from symptom onset), aspirin at arrival, ejection fraction <40%, peak troponin, estimated glomerular filtration rate, first white blood cell count, first hematocrit, chest pain as primary symptom, Killip class, prior coronary artery bypass grafting, type of AMI, GRACE score, conservative treatment (patient did not receive percutaneous coronary intervention, thrombolysis or other standard of care procedural interventions in addition to medical therapy [e.g. aspirin, statins, beta blockers]), total length of stay (LOS) in days, discharge to other institutions, and admission to the cardiac or medical intensive care unit. In hospital complications included bleeding, re‐infarction, heart failure and cardiac arrhythmias. Discharge instructions included counselling for specific concerns (cardiac, diet, smoking), medication, and exercise. Medications at discharge included clopidogrel/thienopyridines, aspirin, statins, calcium channel blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and beta blockers.
Psychosocial factors included various items from validated patient reported outcome measures in cardiac populations. Perceived social support was measured using the ENRICHD Social Support ESSI‐7 Instrument. 38 For this study we excluded the questions on instrumental support (i.e. household chores) and marital status. The ESSI‐5 scale is highly correlated with the full length 7‐item scale, with higher scores indicating greater perceived social support.
Depression was measured using the Patient Health Questionnaire‐9. 39 This scale quantifies the frequency of depressive symptoms experienced in the prior 2 weeks based on the 9 Diagnostic and Statistical Manual of Mental Disorders (4th edition) criteria for a major depressive disorder, with higher scores indicating higher levels of depression. Perceived stress was measured using the 14‐item global Perceived Stress Scale‐14. 40 Respondents are evaluated on the degree to which they perceived their life situations over the past month to be unpredictable, uncontrollable, or overloaded, with higher scores indicating greater stress.
Health status was measured using the Seattle Angina Questionnaire and the 12‐item Short‐Form Health Survey (SF‐12). The Seattle Angina Questionnaire is a 19‐item, health‐related quality‐of‐life measure specific for patients with coronary artery disease. 41 , 42 This study used the angina frequency, physical limitation, treatment satisfaction and quality of life domains. Scores range from 0 to 100, with higher scores indicating better functioning. Lastly, the SF‐12 instrument measures overall physical and mental health status through 12 items. 43 Both the Physical Component Summary (PCS) and Mental Component Summary (MCS) scores were used for this study and range from 0 to 100, with higher scores indicating a greater level of physical or mental functioning.
Statistical Analysis
We calculated descriptive statistics for the overall population using frequencies for categorical variables and means (SDs) or medians (interquartile ranges) for continuous and count variables. Statistical differences between readmitted and non‐readmitted patients were evaluated with χ2 tests, t tests, and Wilcoxon rank‐sum tests as appropriate. From the initial list of 65 candidate variables, 20 variables were ineligible based on these criteria: (1) either very low (<0.05) or very high (>0.95) prevalence (e.g., Killip non‐reference levels); and (2) not reasonably or consistently measured or available at most hospitals (e.g., troponin). This resulted in 45 candidate variables (Figure 1) with missingness generally <3%, with perceived stress at baseline missing 6.3% and the SF‐12 physical and mental measures missing <5% and no missingness in the outcome. The missingness was assumed to be missing‐at‐random and multiple imputations were generated using fully conditional specifications as implemented in the SAS procedure.
Figure 1. Stages of selection for the final multivariable risk prediction model.
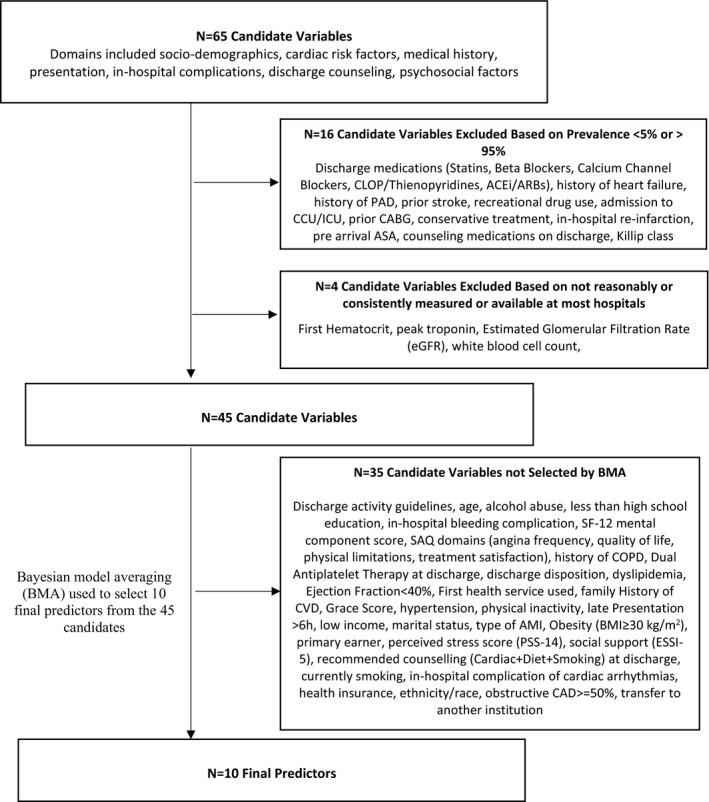
ACEi indicates angiotensin‐converting enzyme inhibitors; AMI, acute myocardial infarction; ARBs, angiotensin receptor blockers; ASA, aspirin; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CCU/ICU, cardiac or medical intensive care unit; CLOP, clopidegrel; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; ESSI‐7, ENRICHD Social Support Instrument; GRACE, Global Registry of Acute Coronary Events; PAD, peripheral arterial disease; PSS‐14, Perceived Stress Scale‐14; and SAQ, Seattle Angina Score.
Our development and validation processes followed the practices outlined in the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement. 44 Selection for the multivariable model used Bayesian model averaging (BMA), a selection approach used in the SILVER‐AMI study and described elsewhere. 45 , 46 , 47 A detailed description of the BMA methodology is provided in Data S1. Per our practice in prior studies, 48 , 49 , 50 the final predictors were those exhibiting a positive posterior probability in at least half of the imputations. Because BMA was used for selection rather than the corresponding P values, some model terms may not exhibit P‐values below 0.05.
Finally, we fit logistic regressions of readmission separately to each of the imputations, with each imputation‐specific model using Firth penalized maximum likelihood to estimate the associations. The coefficients from the imputation‐specific models were subsequently combined using Rubin’s rules. 50 , 51 The development model was evaluated by assessing area under the curve (AUC) and calibration of the predicted risk. We deemed good fit in each imputation as an AUC ≥65% and good calibration as plots of the mean observed probabilities with CIs that overlap with the diagonal line representing perfect agreement between predicted and observed values, as illustrated in the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis materials.
The global model coefficients from the development model then were directly applied to the values of the final predictors for all eligible participants in the validation data, with discrimination and calibration evaluated using the previously mentioned criteria. With the exception of BMA, as implemented in the R package “BMA,” 51 , 52 all analyses were conducted using SAS Version 9.4 with SAS/STAT 14.3 (SAS Institute Inc, Cary, NC, 2014). 52 , 53 Statistical significance was defined as a 2 sided P value <0.05.
RESULTS
Baseline Characteristics
Baseline characteristics for the overall sample (N=2979) and for strata by readmission status are presented in Table 1. The mean age of the study population was 47.1±6.2 years, 67.4% were women, and 23.2% were Black. In terms of socio‐demographics, patients readmitted within 1 year post AMI were more likely to be female, Black, not married or living with a spouse, and of lower income. They were also less likely to be primary household earners or to be employed, and were also more likely to have diabetes mellitus, hypertension and sedentary lifestyles. In terms of comorbidities and disease severity, patients readmitted within 1‐year were more likely to have a prior AMI, history of renal disease, chronic obstructive pulmomary disease, non–ST‐segment–elevation myocardial infarction, longer hospitalizations, and in‐hospital complications of heart failure. Readmitted patients were more likely to have a higher burden of psychosocial stressors, including higher rates of depression and stress, and poorer physical and mental health. Lastly, readmitted patients reported lower disease specific quality of life as per physical limitations, frequency of angina, and quality of life and treatment satisfaction.
Table 1.
Baseline Characteristics of Young Adults With AMI Who Were Readmitted Versus Not Readmitted at 1 Year (44 Candidate Variables)
|
All patients (N=2979) |
All patients (Missing) |
No readmission (N=2074) |
No readmission (Missing) |
Readmission within 1 year (N=905) |
Readmission within 1 year (Missing) |
P value | |
|---|---|---|---|---|---|---|---|
| Sociodemographics/socioeconomic status | |||||||
| Age, mean (SD), y | 47.1 (6.18) | 0 (0.0%) | 47.2 (6.10) | 0 (0.0%) | 46.9 (6.36) | 0 (0.0%) | 0.1755 |
| Age, median (interquartile range), y | 48.0 (44.0–52.0) | 0 (0.0%) | 48.0 (44.0–52.0) | 0 (0.0%) | 48.0 (44.0–52.0) | 0 (0.0%) | 0.2628 |
| Sex | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | <0.0001 | |||
| Female | 2007 (67.4%) | 1323 (63.8%) | 684 (75.6%) | ||||
| Male | 972 (32.6%) | 751 (36.2%) | 221 (24.4%) | ||||
| Race | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0001 | |||
| White | 2289 (76.8%) | 1631 (78.6%) | 658 (72.7%) | ||||
| Black | 533 (17.9%) | 323 (15.6%) | 210 (23.2%) | ||||
| Married or living with spouse | 1658 (55.7%) | 0 (0.0%) | 1207 (58.2%) | 0 (0.0%) | 451 (49.8%) | 0 (0.0%) | <0.0001 |
| Primary earner | 2214 (74.3%) | 0 (0.0%) | 1578 (76.1%) | 0 (0.0%) | 636 (70.3%) | 0 (0.0%) | 0.0008 |
| Low income | 1262 (42.4%) | 0 (0.0%) | 793 (38.2%) | 0 (0.0%) | 469 (51.8%) | 0 (0.0%) | <0.0001 |
| Less than high school education | 1280 (43.0%) | 0 (0.0%) | 864 (41.7%) | 0 (0.0%) | 416 (46.0%) | 0 (0.0%) | 0.0319 |
| Currently employed | 1828 (61.4%) | 0 (0.0%) | 1367 (65.9%) | 0 (0.0%) | 461 (50.9%) | 0 (0.0%) | <0.0001 |
| Has health insurance | 2294 (77.0%) | 0 (0.0%) | 1588 (76.6%) | 0 (0.0%) | 706 (78.0%) | 0 (0.0%) | 0.4427 |
| Cardiac risk factors | |||||||
| Diabetes mellitus | 1058 (35.5%) | 0 (0.0%) | 657 (31.7%) | 0 (0.0%) | 401 (44.3%) | 0 (0.0%) | <0.0001 |
| Obesity (body mass index≥30 kg/m2) | 1571 (52.7%) | 0 (0.0%) | 1069 (51.5%) | 0 (0.0%) | 502 (55.5%) | 0 (0.0%) | 0.0528 |
| Hypertension | 1974 (66.3%) | 0 (0.0%) | 1321 (63.7%) | 0 (0.0%) | 653 (72.2%) | 0 (0.0%) | <0.0001 |
| Dyslipidemia | 2582 (86.7%) | 0 (0.0%) | 1781 (85.9%) | 0 (0.0%) | 801 (88.5%) | 0 (0.0%) | 0.0516 |
| Current Smoking | 891 (29.9%) | 0 (0.0%) | 635 (30.6%) | 0 (0.0%) | 256 (28.3%) | 0 (0.0%) | 0.2015 |
| Family history of cardiovascular disease | 2004 (67.3%) | 0 (0.0%) | 1373 (66.2%) | 0 (0.0%) | 631 (69.7%) | 0 (0.0%) | 0.0833 |
| Inactivity | 1054 (35.4%) | 0 (0.0%) | 683 (32.9%) | 0 (0.0%) | 371 (41.0%) | 0 (0.0%) | <0.0001 |
| Medical history | |||||||
| Prior myocardial infarction | 635 (21.3%) | 0 (0.0%) | 379 (18.3%) | 0 (0.0%) | 256 (28.3%) | 0 (0.0%) | <0.0001 |
| History of renal disease | 337 (11.3%) | 0 (0.0%) | 204 (9.8%) | 0 (0.0%) | 133 (14.7%) | 0 (0.0%) | 0.0001 |
| Alcohol abuse | 1011 (33.9%) | 0 (0.0%) | 743 (35.8%) | 0 (0.0%) | 268 (29.6%) | 0 (0.0%) | 0.0010 |
| History of chronic obsructive pulmonary disease | 346 (11.6%) | 0 (0.0%) | 198 (9.5%) | 0 (0.0%) | 148 (16.4%) | 0 (0.0%) | <0.0001 |
| History of depression | 1212 (40.7%) | 0 (0.0%) | 766 (36.9%) | 0 (0.0%) | 446 (49.3%) | 0 (0.0%) | <0.0001 |
| Presentation characteristics | |||||||
| First health service used | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.3395 | |||
| Directly ER from home | 2654 (89.1%) | 1852 (89.3%) | 802 (88.6%) | ||||
| Before ER, Dr office | 162 (5.4%) | 105 (5.1%) | 57 (6.3%) | ||||
| Before ER, other health services | 163 (5.5%) | 117 (5.6%) | 46 (5.1%) | ||||
| Late presentation >6 h | 1319 (44.3%) | 0 (0.0%) | 894 (43.1%) | 0 (0.0%) | 425 (47.0%) | 0 (0.0%) | 0.0538 |
| Ejection fraction >40% | 319 (10.7%) | 0 (0.0%) | 209 (10.1%) | 0 (0.0%) | 110 (12.2%) | 0 (0.0%) | 0.0849 |
| Chest pain as primary symptom | 2600 (87.3%) | 0 (0.0%) | 1830 (88.2%) | 0 (0.0%) | 770 (85.1%) | 0 (0.0%) | 0.0176 |
| Angiogram | 317 (10.6%) | 208 (10.0%) | 109 (12.0%) | 0.0333 | |||
| Nonobstructive CAD <50% | 257 (8.6%) | 195 (9.4%) | 62 (6.9%) | ||||
| Obstructive coronary artery disease ≥50% | 2405 (80.7%) | 1671 (80.6%) | 734 (81.1%) | ||||
| Intravenous (cardiogenic shock) | 13 (0.4%) | 8 (0.4%) | 5 (0.6%) | ||||
| Type of myocardial infarction | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.0096 | |||
| ST‐segment–elevation myocardial infarction | 1483 (49.8%) | 1065 (51.4%) | 418 (46.2%) | ||||
| Non–ST‐segment–elevation myocardial infarction | 1496 (50.2%) | 1009 (48.6%) | 487 (53.8%) | ||||
| Global Registry of Acute Coronary Events score, mean (SD) | 75.2 (19.05) | 49 (1.6%) | 74.6 (18.00) | 23 (1.1%) | 76.6 (21.26) | 26 (2.9%) | 0.0145 |
| Total length of stay, d, mean (SD) | 4.2 (3.93) | 13 (0.4%) | 3.9 (3.41) | 8 (0.4%) | 4.9 (4.85) | 5 (0.6%) | <0.0001 |
| Disposition to other institutions at discharge | 2806 (94.2%) | 0 (0.0%) | 1962 (94.6%) | 0 (0.0%) | 844 (93.3%) | 0 (0.0%) | 0.0071 |
| Discharge counseling | |||||||
| Recommended counseling (cardiac+diet+smoking) | 951 (31.9%) | 0 (0.0%) | 674 (32.5%) | 0 (0.0%) | 277 (30.6%) | 0 (0.0%) | 0.3089 |
| Exercise counseling | 2751 (92.3%) | 0 (0.0%) | 1913 (92.2%) | 0 (0.0%) | 838 (92.6%) | 0 (0.0%) | 0.7343 |
| Dual antiplatelet therapy | 1964 (65.9%) | 0 (0.0%) | 1389 (67.0%) | 0 (0.0%) | 575 (63.5%) | 0 (0.0%) | 0.0688 |
| In‐hospital complications | |||||||
| Bleeding | 197 (6.6%) | 0 (0.0%) | 133 (6.4%) | 0 (0.0%) | 64 (7.1%) | 0 (0.0%) | 0.5056 |
| Heart failure | 215 (7.2%) | 0 (0.0%) | 118 (5.7%) | 0 (0.0%) | 97 (10.7%) | 0 (0.0%) | <0.0001 |
| Cardiac arrhythmias | 205 (6.9%) | 0 (0.0%) | 132 (6.4%) | 0 (0.0%) | 73 (8.1%) | 0 (0.0%) | 0.0923 |
| Psychosocial factors, mean (SD) | |||||||
| Social support (ENRICHD Social Support Instrument‐7) | 21.3 (4.56) | 57 (1.9%) | 21.5 (4.34) | 33 (1.6%) | 20.9 (5.01) | 24 (2.7%) | 0.0058 |
| Depression (Patient Health Questionnaire‐9) | 7.8 (6.45) | 117 (3.9%) | 7.2 (6.21) | 71 (3.4%) | 9.4 (6.73) | 46 (5.1%) | <0.0001 |
| Stress (Perceived Stress Scale‐14) | 26.0 (9.78) | 185 (6.2%) | 25.3 (9.83) | 117 (5.6%) | 27.6 (9.48) | 68 (7.5%) | <0.0001 |
| Physical limitations (SAQ) | 80.6 (25.79) | 74 (2.5%) | 83.5 (23.83) | 48 (2.3%) | 73.9 (28.75) | 26 (2.9%) | <0.0001 |
| Angina frequency (SAQ) | 83.2 (20.77) | 9 (0.3%) | 84.8 (19.12) | 7 (0.3%) | 79.4 (23.71) | 2 (0.2%) | <0.0001 |
| Treatment satisfaction (SAQ) | 91.8 (13.02) | 25 (0.8%) | 92.4 (12.12) | 18 (0.9%) | 90.2 (14.76) | 7 (0.8%) | <0.0001 |
| Quality of life (SAQ) | 57.4 (24.95) | 18 (0.6%) | 59.6 (24.35) | 12 (0.6%) | 52.6 (25.63) | 6 (0.7%) | <0.0001 |
| General health, SF‐12 (physical component score) | 43.0 (12.09) | 142 (4.8%) | 44.6 (11.53) | 101 (4.9%) | 39.3 (12.53) | 41 (4.5%) | <0.0001 |
| General health, SF‐12 (mental component score) | 45.5 (12.41) | 142 (4.8%) | 46.2 (12.12) | 101 (4.9%) | 43.9 (12.94) | 41 (4.5%) | <0.0001 |
ER indicates emergency room; SAQ, Seattle Angina Questionnaire; and SF‐12, Short Form‐12.
Readmission at 1 Year Post AMI
Within the first year of discharge for AMI, 905 (30.4%) of patients experienced at least 1 all‐cause readmission. Overall there were 1658 readmissions: 563 (18.9%) patients were readmitted once, 167 (5.6%) patients were readmitted twice, and 175 (5.9%) patients were readmitted 3 or more than 3 times. Notably, some patients had up to 17 readmissions within this time period. Patients who were readmitted 3 or more times were younger (46.7 years), were mostly female (78.8%) and Black (65.7%), and presented with predominately cardiac complaints. The majority of readmissions were for cardiac related reasons, the most common being either stable or unstable angina (34.08%). Among cardiac readmissions, there were 133 (8.02%) readmissions for AMI recurrence (Table 2). The rate of readmission was relatively constant over the first year with median time to first readmission being 70 days (Figure S2), with 68 deaths (2.3% of sample population).
Table 2.
Causes of 1‐Year Readmission Among Younger Adults Hospitalized for AMI
| Total number of readmissions 1 year post AMI (N=1658) | Percentage of total readmissions at 1 year post AMI | |
|---|---|---|
| Cardiac readmission | 994* | 59.95%* |
| Acute myocardial infarction | 133 | 8.02% |
| Heart failure | 126 | 7.6% |
| Stable/unstable angina | 565 | 34.08% |
| Stroke | 10 | 0.6% |
| Other cardiac | 160 | 9.65% |
| Noncardiac readmission | 658* | 39.69%* |
| Unknown | 6* | 0.36%* |
AMI indicates acute myocardial infarction.
<0.0001.
Multivariable Results: Risk Model for 1‐Year Readmission Post AMI
Bayesian model averaging chose 10 predictors in the development cohort (Figure 2): higher level of depression at admission (measured using the Patient Health Questionnaire‐9) (OR=1.03, 95% CI 1.01–1.05), better baseline physical health (per the SF‐12) (OR=0.98, 95% CI 0.97–0.99), in‐hospital complications of heart failure (OR=1.44, 95% CI 0.99–2.08), chronic obstructive pulmomary disease (OR=1.29, 95% CI 0.96–1.74), diabetes mellitus (OR=1.23, 95% CI 1.00–1.52), female sex (OR=1.31, 95% CI 1.05–1.65), low income (OR=1.13, 95% CI 0.89–1.42), prior AMI (OR=1.47, 95% CI 1.15–1.87), greater in‐hospital length of stay (OR=1.13, 95% CI 1.04–1.23), and being employed (OR=0.88, 95% CI 0.69–1.12). The strongest predictor was history of a prior AMI, followed by female sex. Of the 10 predictors, only 2 were protective: better physical health and being employed. Variables not selected included medical risk factors and comorbidities, disease severity, discharge counseling, and other in‐hospital complications (Figure 1). The model had excellent calibration (calibration plots) and modest discrimination (C statistic=0.67 derivation cohort [AUC (95% CI)=0.671 (0.646–0.697)]), (C statistic=0.67 validation cohort [AUC (95% CI)=0.673 (0.656–0.689)]) across all multiply imputed data sets. The calibration plots for the development and validation cohorts, as shown in Figure 3A and 3B, exhibit strong overlap of the CIs of the observed probabilities with the diagonal line that represents perfect agreement. Baseline characteristics of young patients with AMI stratified by sex who were readmitted versus not readmitted at 1‐year for the 10 final candidate variables are presented in Table S2.
Figure 2. Forest plot showing predictors of 1‐year readmission post AMI (odds ratio for readmitted vs not readmitted).
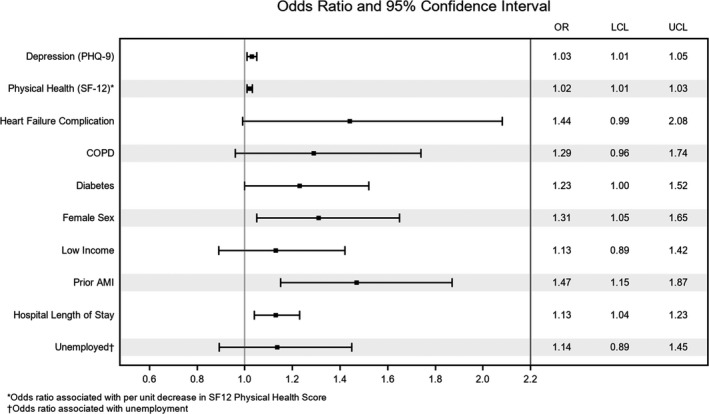
Note that for the purposes of interpretability we have inverted 2 predictors so they align better in the figure (physical health [SF‐12], unemployment status). AMI indicates acute myocardial infarction; COPD, chronic obstructive pulmonary disease; LCL, lower control limit; OR, odds ratio; PHQ‐9, Patient Health Questionnaire‐9; SF‐12, Short Form‐12; and UCL, upper control limit.
Figure 3. Calibration plots of observed vs predicted risk from the 10‐predictor risk model of all‐cause readmission within 1‐year of hospitalization for AMI among younger adults.
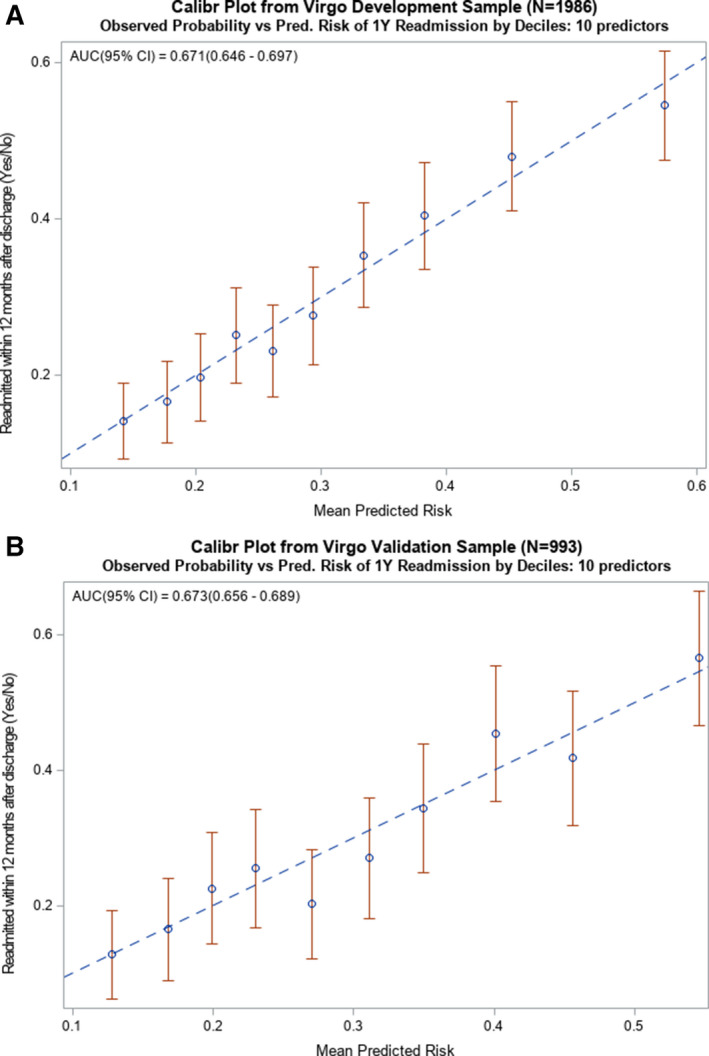
A, Calibration plot from the development sample (N=1986) used to create the 10‐predictor risk model that demonstrates how well the deciles of observed and predicted probabilities of 1‐year readmission agree over the entire range of predicted risk, where the diagonal line represents perfect agreement. B, Calibration plot from the validation sample (N=993) used to exhibit successful application of the 10‐predictor model by demonstrating how well the deciles of observed and predicted probabilities of 1‐year readmission agree over the entire range of predicted risk, where the diagonal line represents perfect agreement. AMI indicates acute myocardial infarction; and AUC, area under the curve.
As a sensitivity analysis, we used a single imputation to develop a separate model to examine the predictors of readmission after a first AMI event. The BMA approach chose 9 variables, most of which are also in the model for the full cohort (Table S3). Baseline higher scores for physical health (as per the SF‐12), the Global Registry of Acute Coronary Events score, and marital status (ie, being married/living with partner) were protective whereas all other variables such as depression (as per the Patient Health Questionnaire‐9), obstructive coronary artery disease (ie, coronary stenosis >=50%), diabetes mellitus, female sex, low income, and length of stay were positively associated with higher likelihood of readmission within 1 year of discharge. As shown in Figure S3, calibration was good whereas discrimination, with a C statistic of 66%, was modest.
Discussion
This study demonstrates that one third of young adults with AMI experience readmission in the first year after their initial hospitalization, with a substantial subset enduring multiple readmissions. Women, individuals with longer hospitalization, a history of prior AMI, and with depression or diabetes mellitus were more likely to be readmitted. Individuals with better physical health and those who were employed were less likely to be readmitted. Unlike traditional cardiac prediction models, several predictors (better physical health, more frequent depressive symptoms, low income, and employment) were psychosocial characteristics rather than markers of cardiac disease severity. Our study is robust in its generalizability, representing data from 103 hospitals across the United States, with adjudicated readmissions confirmed with retroactive chart review in lieu of the more commonly used patient self‐reported readmissions. 54 These results can inform the development of psychosocial interventions, particularly those which are sex specific, to reduce readmissions in young patients with AMI.
Our study extends the literature in several important ways. Foremost, this is the first study to develop a risk prediction model for 1‐year readmission post AMI among young adults aged 18 to 55 years, while incorporating psychosocial parameters. Prior risk stratification models examining post‐AMI readmission have been developed in older populations (aged ≥50 years) 2 : with the few studies that included younger patients having a mean patient age in the 60s. These prior models also did not conduct specific subgroup analyses by age. 2 Beyond the age limitation, prior models, including the Centers for Medicare and Medicaid Services administrative model examining post‐AMI readmission, demonstrated modest discrimination (median C statistic, 0.65; range 0.53–0.79), and exhibited methodological limitations. 2 , 25 , 26 , 27 Overall, there has been a lack of validation and significant reliance on single‐center study designs, limiting generalizability, with data obtained exclusively from administrative records, electronic medical records, and clinical databases rather than from patient‐reported outcome measures. Lastly, there has been a focus on the 30‐day time point, thereby failing to quantify the high risk of readmission in young patients over the entirety of the first year after hospitalization for AMI.
Second, addressing a key draw back in prior models, our risk prediction model included data from the in‐hospital stay instead of relying solely on postdischarge variables. This allowed for consideration of predictors that may inform interventions during the acute care episode. Third, our work builds on prior studies by drawing from novel domains such as patient‐reported outcome measures and psychosocial factors. In prior models, between 7 and 37 predictors were typically included, among which demographics, comorbidities, and usage metrics were the most frequently included domains, 2 with only 2 models including psychosocial factors. 2 Of note, our model showed that physical health, mental health, and employment status were predictors of readmission, contrasting with findings from prior models largely built around disease severity. Interestingly, the type of myocardial ischemia (ie, obstructive versus nonobstructive coronary artery disease) was considered in our study but was not associated with the outcome or selected for the final model. Furthermore, our final model had fewer clinical factors than previous models, implying that in the young adult population, psychosocial and gender‐based variables are potent predictors of readmission in the first year post AMI.
We found that women and individuals with a history of prior AMI, depression, or longer hospital stays were at greater risk for readmission, whereas better physical health and employment were protective. Young women being at higher risk is in line with our previous studies that showed women were more likely to be readmitted at both 30 days and 1 year post AMI. 13 , 37 There are a host of psychosocial factors, such as poorer health status, more depression and stress, and less social support, contributing to this difference. 55 Also, relative to men with similar cardiac risk, women are less likely to receive preventive treatment such as management of risk factors. 56 Indeed, suboptimal medical management post AMI increases the risk of future events. Women have also been found to be more prone to complications during hospitalization (eg, bleeding events), contributing to longer lengths of stay. 56 These results inform our hypothesis that women may experience more stressful and difficult hospitalizations, in turn creating a higher allostatic load that leads to greater vulnerability to readmission. 37
In addition, longer length of stay is considered a proxy for poorer overall health. Prior studies have shown that extended hospitalizations are associated with a history of medical comorbidities such as diabetes mellitus and stroke. 57 Depression has also been shown to be associated with readmission, though the mechanism is less clear. 58 Depression itself is a known risk factor for worse cardiac morbidity and mortality, which could explain its positive association with readmission. 59 Other proposed mechanisms include its impact on patients’ help‐seeking behavior, health behavior, medication adherence, and perception of chest pain. 58
Lastly, better self‐reported physical health status and employment at baseline hospitalization were the only protective factors against readmission. It has been shown that AMI confers significant risk for decline in physical function and that those with worse physical health include the uninsured and those not referred to cardiac rehabilitation. 60 Prior research has also shown that better self‐reported physical health status is correlated with less perceived limitations in self‐care, improved disease‐specific self‐care behaviors, and higher levels of health literacy in patients with coronary heart disease. It can also be inferred that these patients benefit from the social determinants of health that contribute to higher levels of health literacy. All of these factors likely enable patients with better self‐reported physical health status to engage in protective health behaviors that decrease the likelihood of readmission. 61 , 62
Clinical Implications
Our study has several important clinical implications to improve in‐hospital and post‐AMI care for young adults with AMI. Based on our findings, a practical intervention at discharge could include solutions to reduce health inequities associated with low income and that are mitigated by reliable employment. For example, social work involvement for coordination of childcare and return to work interventions that support employment may include policy‐based interventions promoting more flexible return to work policies to lessen the frequency of joblessness and disability. Such interventions designed to support employment for those at higher risk could also focus on self‐management strategies that allow individuals to return to work despite high‐risk behaviors. 63 Other interventions at discharge could include digital health applications and wearables that not only track activity but also focus on supporting the psychosocial aspects of care (eg, depression, social support) and promote adherence to secondary prevention targets. Finally, our findings reaffirm the importance of cardiac rehabilitation counseling at discharge and promoting physical health as a primary prevention strategy to lower risk of readmission among young adults hospitalized for AMI.
Limitations
This study should be interpreted in the context of several limitations. First, some key variables were excluded from the analysis owing to either very high or low prevalence such as the nonreference levels of Killip. Troponin was excluded because of the inconsistency in how it is measured and reported at disparate hospitals. Second, our findings may not be generalizable to other minority groups (ie, American Indian, Alaska Native, Asian, Pacific Islander, East Indian, other race) and Hispanic individuals because of the smaller proportion of these individuals enrolled in our study. Despite this limitation it is important to note that to date this is the largest subset of young patients with AMI in the United States. Future studies need to ensure adequate representation of these ethnic/racial groups. Third, noncardiac causes of readmission could not be obtained owing to time and resource limitations. Finally, although the median C statistic of our study at 0.67 is modest, it lies within the upper part of the range of previously published models for readmission. 2 Of note, readmission, being a complex interaction between the patient, community, environment, and the healthcare system, is a much more difficult outcome to predict than mortality, which is largely driven by disease. 2
Conclusions
Among young adults hospitalized for AMI, women and those with a prior AMI, as well as those who had diabetes mellitus, longer hospitalization, or more severe depressive symptoms, were more likely to be readmitted. Only 2 predictors, better physical health at admission and being employed, were protective for readmission in our model. Several predictors were found to be psychosocial characteristics (such as employment, depressive symptoms and self‐reported personal health), rather than markers of cardiac disease severity. These results may inform the development of psychosocial interventions to prevent readmission among younger adults hospitalized for AMI.
Sources of Funding
The VIRGO study was supported by a 4‐year National Heart, Lung, and Blood Institute grant (No. 5R01HL081153). Dr Dreyer is supported by an American Heart Association Transformational Project Award (#19TPA34830013). Dr Murphy is supported by the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342). This project was additionally supported by a Canadian Institutes of Health Research project grant (PJT‐159508).
Disclosures
None.
Supporting information
Data S1
Tables S1–S3
Figures S1–S3
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021047
For Sources of Funding and Disclosures, see page 11.
References
- 1. Desai NR, Ross JS, Kwon JY, Herrin J, Dharmarajan K, Bernheim SM, Krumholz HM, Horwitz LI. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316:2647–2656. doi: 10.1001/jama.2016.18533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smith LN, Makam AN, Darden D, Mayo H, Das SR, Halm EA, Nguyen OK. Acute myocardial infarction readmission risk prediction models: a systematic review of model performance. Circ Cardiovasc Qual Outcomes. 2018;11:e003885. doi: 10.1161/CIRCOUTCOMES.117.003885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fingar K (Truven Health Analytics), Washington R (AHRQ) . Trends in Hospital Readmissions for Four High‐Volume Conditions, 2009‐2013. HCUP Statistical Brief #196. November 2015. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup‐us.ahrq.gov/reports/statbriefs/sb196‐Readmissions‐Trends‐High‐Volume‐Conditions.pdf. [PubMed]
- 4. Yale New Haven Health Services Corporation Center for Outcomes Research and Evaluation. Medicare hospital quality chartbook: variation in 30‐day readmission rates across hospitals following hospitalization for acute myocardial infarction. centers for medicare and medicaid services. 2015. Washington, D.C, United States.
- 5. Khera R, Jain S, Pandey A, Agusala V, Kumbhani DJ, Das SR, Berry JD, de Lemos JA, Girotra S. Comparison of readmission rates after acute myocardial infarction in 3 patient age groups (18 to 44, 45 to 64, and >/=65 Years) in the United States. Am J Cardiol. 2017;120:1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruiz B, Garcia M, Aguirre U, Aguirre C. Factors predicting hospital readmissions related to adverse drug reactions. Eur J Clin Pharmacol. 2008;64:715–722. doi: 10.1007/s00228-008-0473-y [DOI] [PubMed] [Google Scholar]
- 7. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563 [DOI] [PubMed] [Google Scholar]
- 8. Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow‐up visit and 30‐day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010;170:1664–1670. doi: 10.1001/archinternmed.2010.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spatz ES, Beckman AL, Wang Y, Desai NR, Krumholz HM. Geographic variation in trends and disparities in acute myocardial infarction hospitalization and mortality by income levels, 1999–2013. JAMA Cardiol. 2016;1:255–265. doi: 10.1001/jamacardio.2016.0382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde‐Price C, D'Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–345. doi: 10.1016/j.jacc.2014.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilmot KA, O'Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arora S, Stouffer GA, Kucharska‐Newton AM, Qamar A, Vaduganathan M, Pandey A, Porterfield D, Blankstein R, Rosamond WD, Bhatt DL, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139:1047–1056. doi: 10.1161/CIRCULATIONAHA.118.037137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dreyer RP, Dharmarajan K, Kennedy KF, Jones PG, Vaccarino V, Murugiah K, Nuti SV, Smolderen KG, Buchanan DM, Spertus JA, et al. Sex differences in 1‐year all‐cause rehospitalization in patients after acute myocardial infarction: a prospective observational study. Circulation. 2017;135:521–531. doi: 10.1161/CIRCULATIONAHA.116.024993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kocher RP, Adashi EY. Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA. 2011;306:1794–1795. DOI: 10.1001/jama.2011.1561. [DOI] [PubMed] [Google Scholar]
- 15. U.S. Department of Health and Human Services . Hospital Compare. [Online]. 2013. Available at: https://www.hospitalcompare.hhs.gov/. Accessed 1August 17, 2020.
- 16. Kripalani S, Theobald CN, Anctil B, Vasilevskis EE. Reducing hospital readmission rates: current strategies and future directions. Annu Rev Med. 2014;65:471–485. doi: 10.1146/annurev-med-022613-090415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patient Protection and Affordable Care Act . Hospital Readmissions Reduction Program. Pub l No. 111‐148, 124 Stat 408, S3025.
- 18. McIlvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;131:1796–1803. doi: 10.1161/CIRCULATIONAHA.114.010270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008 [DOI] [PubMed] [Google Scholar]
- 20. Parker SG, Peet SM, McPherson A, Cannaby AM, Abrams K, Baker R, Wilson A, Lindesay J, Parker G, Jones DR. A systematic review of discharge arrangements for older people. Health Technol Assess. 2002;6:1–183. doi: 10.3310/hta6040 [DOI] [PubMed] [Google Scholar]
- 21. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital‐initiated transitional care interventions as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:433–440. doi: 10.7326/0003-4819-158-5-201303051-00011 [DOI] [PubMed] [Google Scholar]
- 22. Goldman LE, Sarkar U, Kessell E, Guzman D, Schneidermann M, Pierluissi E, Walter B, Vittinghoff E, Critchfield J, Kushel M. Support from hospital to home for elders: a randomized trial. Ann Intern Med. 2014;161:472–481. doi: 10.7326/M14-0094 [DOI] [PubMed] [Google Scholar]
- 23. Hansen LO, Greenwald JL, Budnitz T, Howell E, Halasyamani L, Maynard G, Vidyarthi A, Coleman EA, Williams MV. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427. doi: 10.1002/jhm.2054 [DOI] [PubMed] [Google Scholar]
- 24. Dodson JA, Hajduk AM, Murphy TE, Geda M, Krumholz HM, Tsang S, Nanna MG, Tinetti ME, Ouellet G, Sybrant D, et al. 180‐day readmission risk model for older adults with acute myocardial infarction: the SILVER‐AMI study. Open Heart. 2021;8:e001442. doi: 10.1136/openhrt-2020-001442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson JL, Greaves L, Repta R. Better Science with Sex and Gender: a Primer for Health Research. Vancouver: Women's Health Research Network; 2007. [Google Scholar]
- 26. Ristvedt SL. The evolution of gender. JAMA Psychiatry. 2014;71:13–14. doi: 10.1001/jamapsychiatry.2013.3199 [DOI] [PubMed] [Google Scholar]
- 27. Unger RK. Toward a redefinition of sex and gender. Am Psychol. 1979;34:1085–1094. doi: 10.1037/0003-066X.34.11.1085 [DOI] [Google Scholar]
- 28. Rodriguez‐Padial L, Elola FJ, Fernandez‐Perez C, Bernal JL, Iniguez A, Segura JV, Bertomeu V. Patterns of inpatient care for acute myocardial infarction and 30‐day, 3‐month and 1‐year cardiac diseases readmission rates in Spain. Int J Cardiol. 2017;230:14–20. doi: 10.1016/j.ijcard.2016.12.121 [DOI] [PubMed] [Google Scholar]
- 29. Kwok CS, Shah B, Al‐Suwaidi J, Fischman DL, Holmvang L, Alraies C, Bagur R, Nagaraja V, Rashid M, Mohamed M, et al. Timing and causes of unplanned readmissions after percutaneous coronary intervention: insights from the nationwide readmission database. JACC Cardiovasc Interv. 2019;12:734–748. doi: 10.1016/j.jcin.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Y, Li J, Zheng X, Jiang Z, Hu S, Wadhera RK, Bai X, Lu J, Wang Q, Li Y, et al. Risk factors associated with major cardiovascular events 1 year after acute myocardial infarction. JAMA Network Open. 2018;1:e181079. doi: 10.1001/jamanetworkopen.2018.1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shah IT, Keeley EC. Unplanned readmissions after acute myocardial infarction: 1‐year trajectory following discharge from a safety net hospital. Crit Pathw Cardiol. 2019;18:72–74. doi: 10.1097/HPC.0000000000000170 [DOI] [PubMed] [Google Scholar]
- 32. Lichtman JH, Lorenze NP, D'Onofrio G, Spertus JA, Lindau ST, Morgan TM, Herrin J, Bueno H, Mattera JA, Ridker PM, et al. Variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–693. doi: 10.1161/CIRCOUTCOMES.109.928713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747 [DOI] [PubMed] [Google Scholar]
- 34. Krzanowski WJ, Hand DJ. ROC Curves for Continuous Data. 1st ed. Boca Raton, FL: Chapman and Hall/CRC; 2009. [Google Scholar]
- 35. Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. doi: 10.1136/bmj.b605 [DOI] [PubMed] [Google Scholar]
- 36. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dreyer RP, Ranasinghe I, Wang Y, Dharmarajan K, Murugiah K, Nuti SV, Hsieh AF, Spertus JA, Krumholz HM. Sex differences in the rate, timing, and principal diagnoses of 30‐day readmissions in younger patients with acute myocardial infarction. Circulation. 2015;132:158–166. doi: 10.1161/CIRCULATIONAHA.114.014776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. ENRICHD Investigators . Enhancing recovery in coronary heart disease patients (ENRICHD): study design and methods Psychosocial intervention. Psychosom Med. 2001;63:747–755. [PubMed] [Google Scholar]
- 39. Kroenke K, Spitzer RL, Williams JBW. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 41. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonnell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9 [DOI] [PubMed] [Google Scholar]
- 42. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. doi: 10.1016/0002-9149(94)90555-X [DOI] [PubMed] [Google Scholar]
- 43. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 44. Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–W73. doi: 10.7326/M14-0698 [DOI] [PubMed] [Google Scholar]
- 45. Murphy TE, Tsang SW, Leo‐Summers LS, Geda M, Kim DH, Oh E, Allore HG, Dodson J, Hajduk AM, Gill TM, et al. Bayesian model averaging for selection of a risk prediction model for death within thirty days of discharge: the SILVER‐AMI study. Int J Stat Med Res. 2019;8:1–7. doi: 10.6000/1929-6029.2019.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoeting JA, Madigan D, Raftery AE, Volinsky CT. Bayesian model averaging: a tutorial. Stat Sci. 1999;14:382–417. [Google Scholar]
- 47. Raftery AE, Richardson S. Model selection for generalized linear models via GLIB, with application to epidemiology; 1996.
- 48. Madigan M, Raftery AE. Model selection and accounting for model uncertainty in graphical models using occam's window. J Am Stat Assoc. 1994;89:1535–1546. doi: 10.1080/01621459.1994.10476894 [DOI] [Google Scholar]
- 49. Dodson JA, Hajduk AM, Murphy TE, Geda M, Krumholz HM, Tsang S, Nanna MG, Tinetti ME, Goldstein D, Forman DE, et al. Thirty‐day readmission risk model for older adults hospitalized with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2019;12:e005320. doi: 10.1161/CIRCOUTCOMES.118.005320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dodson JA, Hajduk AM, Geda M, Krumholz HM, Murphy TE, Tsang S, Tinetti ME, Nanna MG, McNamara R, Gill TM, et al. Predicting 6‐month mortality for older adults hospitalized with acute myocardial infarction: a cohort study. Ann Intern Med. 2020;172:12–21. doi: 10.7326/M19-0974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 2004. [Google Scholar]
- 52. Raftery A, Hoeting J, Volinsky CT, Painter I, Yeung KY. R package “BMA,” version 3.18.11. 2019.
- 53. SAS Institute Inc . Base SAS® 9.4 Procedures Guide. Cary, NC: SAS Institute Inc; 2013. [Google Scholar]
- 54. Krishnamoorthy A, Peterson ED, Knight JD, Anstrom KJ, Effron MB, Zettler ME, Davidson‐Ray L, Baker BA, McCollam PL, Mark DB, et al. How reliable are patient‐reported rehospitalizations? Implications for the design of future practical clinical studies. J Am Heart Assoc. 2016;5:e002695. doi: 10.1161/JAHA.115.002695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dreyer RP, Sciria C, Spatz ES, Safdar B, D’Onofrio G, Krumholz HM. Young women with acute myocardial infarction. Circulation. 2017;10:e003480. doi: 10.1161/CIRCOUTCOMES.116.003480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, et al.; American Heart Association Cardiovascular Disease in W, Special Populations Committee of the Council on Clinical Cardiology CoE, Prevention CoC, Stroke N, Council on Quality of C and Outcomes R . Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 57. Saczynski JS, Lessard D, Spencer FA, Gurwitz JH, Gore JM, Yarzebski J, Goldberg RJ. Declining length of stay for patients hospitalized with AMI: impact on mortality and readmissions. Am J Med. 2010;123:1007–1015. doi: 10.1016/j.amjmed.2010.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reese RL, Freedland KE, Steinmeyer BC, Rich MW, Rackley JW, Carney RM. Depression and rehospitalization following acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4:626–633. doi: 10.1161/CIRCOUTCOMES.111.961896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lett HS, Blumenthal JA, Babyak MA, Sherwood A, Strauman T, Robins C, Newman MF. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66:305–315. [DOI] [PubMed] [Google Scholar]
- 60. Dodson JA, Arnold SV, Reid KJ, Gill TM, Rich MW, Masoudi FA, Spertus JA, Krumholz HM, Alexander KP. Physical function and independence 1 year after myocardial infarction: observations from the Translational Research Investigating Underlying disparities in recovery from acute Myocardial infarction: Patients' Health status registry. Am Heart J. 2012;163:790–796. doi: 10.1016/j.ahj.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Piotrowicz K, Noyes K, Lyness JM, McNitt S, Andrews ML, Dick A, Hall WJ, Moss AJ, Zareba W. Physical functioning and mental well‐being in association with health outcome in patients enrolled in the Multicenter Automatic Defibrillator Implantation Trial II. Eur Heart J. 2007;28:601–607. doi: 10.1093/eurheartj/ehl485 [DOI] [PubMed] [Google Scholar]
- 62. Aaby A, Friis K, Christensen B, Rowlands G, Maindal HT. Health literacy is associated with health behaviour and self‐reported health: a large population‐based study in individuals with cardiovascular disease. Eur J Prev Cardiol. 2017;24:1880–1888. doi: 10.1177/2047487317729538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. White‐Williams C, Rossi LP, Bittner VA, Driscoll A, Durant RW, Granger BB, Graven LJ, Kitko L, Newlin K, Shirey M. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141:e841–e863. doi: 10.1161/CIR.0000000000000767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S3
Figures S1–S3


