Abstract
Background
Younger age at final menstrual period (FMP) is associated with increased risk for cardiovascular disease events. This paper evaluated whether older age at FMP is associated with more favorable patterns of lipid changes during the menopause transition and whether these changes are associated with less subclinical carotid disease in the postmenopausal years.
Methods and Results
Lipids and lipoproteins were measured repeatedly among 1554 premenopausal women who had a natural menopause during follow‐up years (median=18.8 years); a subset of 890 women also had measures of carotid intima media thickness, adventitial diameter, and plaque. Women who had an older FMP age had less adverse changes in cholesterol from 1 to 3 years after FMP, and in triglycerides from FMP to 3 years after FMP, but they had more adverse changes in ApoB and Apo A1 from 3 years before to 1 year after the FMP. Increasing cholesterol and ApoB from 1 to 3 years after FMP were associated with greater intima media thickness and adventitial diameter, and the greater likelihood of a plaque score >2 the older the age at FMP.
Conclusions
Despite the epidemiological literature showing early age at FMP is associated with elevated risk for cardiovascular disease events, older age at FMP had inconsistent associations with less adverse lipid changes in midlife, which did not translate into less risk for subclinical carotid disease and in some cases more risk. These findings are restricted to women who experience FMP in the normative age range for the menopausal transition.
Keywords: aging, carotid disease, lipids, menopause, longitudinal cohort study
Subject Categories: Lipids and Cholesterol, Epidemiology, Risk Factors
Nonstandard Abbreviations and Acronyms
- AD
adventitial diameter
- FMP
final menstrual period
- IMT
intima media thickness
- SWAN
Study of Women's Health Across the Nation
- TC
total cholesterol
Clinical Perspective
What Is New?
Younger age at menopause is associated with greater risk for coronary heart disease but the role of lipid changes associated with age at menopause is not established.
In this longitudinal study of midlife women, those who had an older age at their final menstrual period had less adverse changes in cholesterol and triglycerides in the early postmenopausal years but more adverse changes in apolipoproteins during the menopause transition.
Unexpectedly, the magnitude of associations between adverse changes in lipids and apoB during the early postmenopausal years and later subclinical carotid disease increased with older age at menopause.
What Are the Clinical Implications?
Age at final menstrual period has little overall benefit on lipid patterns during the transition.
It is unlikely that an early age at menopause is related to elevated risk of cardiovascular disease through final menstrual period age‐related lipid patterns.
Lipid changes should be monitored frequently as women approach the menopause, regardless of their age.
Younger age at menopause is associated with greater risk for coronary heart disease (CHD) and all‐cause mortality. A meta‐analysis of 32 studies showed that women who were younger than 45 years at the onset of menopause had a higher risk of CHD, cardiovascular disease (CVD) mortality, and all‐cause mortality compared with women who were aged >45 years at onset of menopause. 1 However, the analysis also showed that women who reported menopause onset before 50 years of age with those who reported menopause at age 50–54 years showed no significant differences in risk for CVD and all‐cause mortality. A recent analysis pooling individual data from 15 observational studies found that compared with women who reported natural menopause at ages 50–51 years (median age of menopause in most studies), 2 women who had a younger age at menopause had increased risk for non‐fatal CVD, CHD, and stroke before the age of 60 years; in contrast, women who had an older age at menopause after age 52 had a lower risk of CVD events. 3 However, these relationships were attenuated for first‐time CVD events between the ages of 60 and 70 years, and not apparent for first‐time events at 70 years or later. These findings suggest that the impact of younger age at menopause may diminish with aging or time, or that risk is increased only for a window of time following menopause.
Traditional CVD risk factors may enhance our understanding of why age at menopause has an impact on women's risk for CVD events. CVD risk factors may determine both the timing of the menopause and CVD risk. 4 , 5 For example, smoking is related to an earlier age at menopause 6 and negatively affects CVD event risk, so perhaps early menopause is secondary to traditional CVD risk factors. Risk factor trajectories may also change adversely during the menopause transition, such that women who have an earlier menopause have longer cumulative exposure to elevated CVD risk factors in the postmenopausal years. This presumes that risk factor changes are not an acute response to menopause but long‐lasting, ie, continue to show adverse effects for many years. Several studies suggest that lipids increase around the time of the final menstrual period (FMP) and presumably continue to have an adverse effect thereafter. 7 , 8 , 9 However, it is not known whether the magnitude of increase differs by age at FMP, ie, whether women who have an early age at menopause are not only exposed earlier in life to adverse lipid changes but that the changes are larger compared with women who have a later age at menopause, or conversely whether women who have a later age at menopause have smaller or less adverse changes in lipids and are thereby more protected.
In the SWAN (Study of Women's Health Across the Nation) study, women's total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), and ApoB increased within the period from 1 year before and 1 year after the FMP, relative to the years before or after this 2‐year interval. 10 Similarly, high‐density lipoprotein cholesterol (HDL‐C) and Apo A1 also increased in the years approaching the FMP, relative to after the FMP. The magnitude of changes in LDL‐C within 1 year of FMP was clinically relevant. Greater increases in LDL‐C were related to greater likelihood of carotid plaque within the follow‐up period. 11 In the Tromso Study, retrospectively assessed earlier age at menopause was associated with increased prevalence of carotid atherosclerosis in postmenopausal women. 12 The Pittsburgh Healthy Women Study also reported premenopausal levels of LDL‐C, HDL‐C, and triglycerides were strong predictors of postmenopausal carotid intima media thickness (IMT) and plaque, with change in LDL‐C from premenopause to first year postmenopause tending to be larger among women with elevated postmenopausal plaque scores. 13 Leveraging longitudinal SWAN data, the current paper used prospective measures of the menopause transition and lipids, followed by carotid assessment later in life, to address 2 inter‐related questions: (1) Do women who are older at FMP have less adverse lipid changes around the FMP and thereafter into the postmenopausal years? (2) Do the hypothesized less adverse changes associated with older age at FMP predict less carotid plaque, IMT, and adventitial diameter measures later in life?
METHODS
Participants
SWAN is a multi‐site observational study of women who were recruited when premenopausal and between the ages of 42 and 52 years, not pregnant or breastfeeding, not on hormone therapy (HT) including birth control medications, had at least 1 menstrual period in the 3 months before study entry, and identified with a site's designated race/ethnic groups. 14 All sites enrolled White women and 1 of the 4 ethnic/racial groups: Black (Boston, MA; Detroit area MI, Chicago, IL; and Pittsburgh, PA); Chinese (Oakland, CA); Japanese (Los Angeles, CA), and Hispanic (Newark, NJ). A total of 3302 participants were recruited between 1995 to 1997 and followed approximately annually thereafter with longer between evaluation intervals in the later follow‐up years. Each site's Institutional Review Board approved the study protocols, and all women gave written informed consent before participation.
All supporting data are available within the article (and its Data Supplement). SWAN provides access to public use data sets that include data from SWAN screening, baseline, and follow‐up visits (https://agingresearchbiobank.nia.nih.gov/ and http://www.swanstudy.org/swan‐research/data‐access/). To preserve participant confidentiality, some, but not all, of the data are contained in the public use data sets. Investigators who require assistance accessing the public use data set may contact the SWAN Coordinating Center (swanaccess@edc.pitt.edu).
The present analyses are based on 2 analytic samples selected from the 3299 women who had at least 1 lipid value during the SWAN protocol out of the total 3302 SWAN women. The first analytic sample addressed the association of age at FMP on lipid changes from the baseline visit (1995–1997) to visit 15 (2015–2017) among 1554 women who had natural menopause with an observed FMP date (defined below). Of the women who had no observed FMP or lipid values, 541 had dropped out of the study when still menstruating and 262 had a hysterectomy or bilateral oophorectomy. The second analytic sample was based on a subset of the 1554 women in the first sample and addressed the association of age at FMP on the relationships between lipid changes and carotid measures. This sample included 890 who had carotid ultrasound measurement available at visit 12 (n=862) or 13 (n=28) (2009–2013).
Menopausal Status and Date of FMP
Menopausal status was assessed at each evaluation based on bleeding patterns and medical history. Natural menopause was defined as no bleeding for at least 12 months not attributable to hysterectomy or oophorectomy; FMP was identified as the date of the last menstrual period reported in the visit immediately before the first visit that a woman was classified as postmenopausal.
Carotid Ultrasound Measures
Carotid ultrasound measures were obtained at 6 of the 7 SWAN sites using a Terason t3000 Ultrasound System (Teratech Corp, Burlington, MA) equipped with a variable frequency 5–12 MHz linear array transducer. Two digitized images for later reading were obtained of the near and far wall from each of the left and right distal common carotid artery, 1 cm proximal to the carotid bulb. IMT measures were obtained by electronically tracing the lumen‐intima interface and the media‐adventitia interface across a 1‐cm segment for each of these 4 segments; 1 measurement was generated for each pixel over the area, for a total of ≈140 measures for each segment. The average values for these measures were recorded for all 4 locations, with the mean of the average at all 4 locations used in analyses. Presence and extent of plaque were evaluated in each of 5 segments of the left and right carotid artery (distal and proximal common carotid artery, carotid bulb, and proximal internal and external carotid arteries). Consistent with the Mannheim definition of plaque, 15 plaque was defined as a distinct area protruding into the vessel lumen that was at least 50% thicker than the adjacent IMT and summarized as the presence or absence of any plaque. 16 For each segment, the degree of plaque was graded as follows: 0 (no observable plaque), 1 (1 small plaque, <30% vessel diameter); 2 (1 medium plaque between 30%–50% of vessel diameter or multiple small plaques); and 3 (plaque covering ≥50% of the vessel diameter). The grades from all segments of the combined left and right carotid artery were summed to create the carotid plaque index. Technicians at each of the 6 study sites were trained by the University of Pittsburgh Ultrasound Research laboratory and monitored during the study period for reliability. Carotid scan images were read centrally at the SWAN Ultrasound Reading Center using the AMS software developed by Dr. Thomas Gustavsson that has an edge detection algorithm. 17 Reproducibility of IMT and plaque measures was excellent. 18
Sample Characteristics and Lipid Measures
Self‐identified race/ethnicity and education attainment (college degree, some college, and high school or less) were determined from the SWAN screening interview. Age, smoking status (current, past, or never), chronic health conditions and major surgeries, medications, physical activity, and menopausal status were derived from questionnaires and interviews administered during the clinic visits, including the visit concurrent with the carotid measures. Self‐reported cardiovascular events recorded were myocardial infarction, stroke, and angina. Women were defined as ever user of HT if they reported use of HT at any time point in the study up to visit 15. Height and weight were measured at all visits and body mass index was calculated in kg/m2. After women were seated for 5 minutes with feet flat on the ground and legs uncrossed, their blood pressure was measured twice by trained and certified staff using an appropriately sized arm cuff. Blood pressure was the average of 2 measures. Current medications were coded into the following categories: antihypertensive, anti‐diabetic, and lipid‐lowering.
Phlebotomy was performed in the morning following overnight (min 10‐hour) fast. Blood was separated, frozen (−80 °C), and sent on dry ice to Medical Research Laboratory, Cincinnati for clinical visit 0–7, and visits 9, 12, 13 and 15 to University of Michigan Pathology Laboratory, both are CLIA‐certified and accredited by the College of American Pathologists. The exception to the above was that Apo A1 and ApoB for visits 12 and 13 were measured at the University of Pittsburgh Heinz Laboratory, which is also CLIA‐certified. Lipid fractions were determined from EDTA‐treated plasma. 19 , 20 TC and triglycerides concentrations were determined by coupled enzymatic methods. HDL‐C was isolated based upon the method of Izawa et al. 21 LDL‐C was calculated based on the Friedewald equation for values with triglycerides <400 mg/dL. 22 Apo A1 and ApoB were measured by immunonephelometry calibrated with a World Health Organization traceable standard. Because of fiscal issues, lipid values were not available at visit 2, 8, and 10; visits 11 and 14 did not include a blood draw.
Calibration analyses were conducted because several different laboratories performed the assays over time. In brief, a random sample was drawn across the full range of values, with checks for the distribution of the selected sample by menopausal status, race/ethnicity, and study visit to assure adequate representation of the full cohort. For TC, LDL‐C, and triglycerides, 340 samples were drawn, and for Apo A1 and B, 100 samples were drawn. Based on these analyses, correction factors were applied to convert the University of Michigan and University of Pittsburgh results to those of the Medical Research Laboratory.
Blood for estradiol was drawn during the early follicular phase in regularly menstruating women and within 90 days of the recruitment anniversary date for women who were unable to provide a follicular phase sample or had ceased menstruating. At the University of Michigan laboratory only, estradiol assays were conducted in duplicate and measured with a modified, off‐line ACS‐180 (E2–6) immunoassay. 23
Statistical Analysis
Our first step was to compare summary statistics of the characteristics of women in the first analytic sample to (1) those who were not, and (2) to women in the second analytic sample.
In the first analytic sample, we used an empirical method to visually identify slopes over time of lipid and lipoprotein levels and the time points at which the slopes changed, with time anchored relative to the FMP. The total number of observations 11 years before the FMP and up to 16 years after the FMP for the lipid parameters across all women ranged from 13 245 to 11 776. Using LOESS plots with smoothing set at 0.70 and values anchored at the FMP, we visually identified 3 time points demarcating change in slope for TC, LDL‐C, and ApoB at 3 years before FMP, at 1 year after FMP, and 3 years after FMP; and 2 time points for triglycerides and ApoA1 at FMP and 3 years after FMP. HDL‐C had no obvious change in slope and increased linearly across time (see Figure S1 for LOESS plots). The chosen segments can be mapped onto menopausal stages of late reproductive, early transition, late transition, early postmenopause (starting after FMP), and into late postmenopause based on the Stages of Reproductive Aging Workshop +10 24 (Figure 1).
Figure 1. Illustration of menopausal stages based on the Stages of Reproductive Aging Workshop+10 24 in relationship to changes in slope of lipids in present analysis.
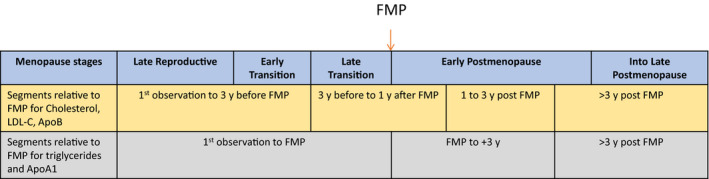
FMP indicates final menstrual period; and LDL‐C, low‐density lipoprotein cholesterol.
After the time points were visually identified, we tested whether slopes within the time points differed using piece‐wise linear mixed effect models. To be in this analysis, women had to have at least 2 lipid measures for a given lipid parameter. Note that a significant beta within segment means that the slope differs from 0. A linear mixed effect model was used to estimate the slope for HDL‐C since the association appeared to be linear relative to the FMP. We also calculated lipid slopes in the women in the carotid analysis and found the same results as in the full sample (data not shown).
The next step in the analyses examined whether age at FMP influenced the magnitude of change in lipid parameter within each time segment as noted above. Piece‐wise linear mixed effect models tested the interaction of age at FMP and time segment (including age at FMP and time segment) with no additional adjustment for covariates (model 0), adjustment for site, race/ethnicity, education, and age at baseline (model 1), and model 1 covariates plus time varying lipid lowering medications, CVD events, smoking status, body mass index, and HT (model 2). A sensitivity analysis also adjusted for time‐varying estradiol levels. They showed no differences in results and are not discussed further.
To assess whether the changes in lipids would be related to subclinical measures of IMT, adventitial diameter (AD), and carotid plaque ≥2 measured at visit 12/13, woman‐specific annual changes in the lipid in each of the identified time segment were estimated using the random effects from piece‐wise linear mixed effect models, adjusted for study site and ethnicity/race, and then regressed on each subclinical measure of carotid outcome using either linear or logistic regression as appropriate. For each subclinical carotid outcome, the interaction term of age at FMP by woman‐specific annual changes in lipids in each menopausal segment was then tested. Model 0 was conducted including age at FMP and annual changes in lipids; model 1 adjusted in addition for visit 12/13 covariates of age, systolic blood pressure, body mass index, use of blood pressure or diabetes mellitus medications, and self‐reports of stroke/myocardial infarction before visit 12/13. Model 2 adjusted for model 1 covariates plus smoking status and lipid‐lowering agents at visit 12/13.
To illustrate the significant interactions between continuous age at FMP and lipid slopes, we constructed figures based on categorizing women into 3 approximately equally sized groups according to their age at FMP: <51, 51–53, and >53 years. These figures were not subjected to formal statistical analysis.
RESULTS
Sample Characteristics
Compared to all excluded women at baseline (n=748; data not shown), women in the first analytic sample (n=1554) were more likely to be Chinese and Japanese Americans and less likely to be Hispanic or current smokers; there were no differences in educational attainment, age, body mass index, estradiol, or lipid measures, except that triglyceride levels were slightly lower, P=0.06.
In the first analytic sample, the largest racial/ethnic group was White women, followed by Black, Japanese, Chinese, and Hispanic women (Table 1 left column). As a group, the women were well educated with the majority having at least some college. At baseline, they tended to be overweight, and few were on lipid‐lowering medications or were current smokers. Lipid values on average were within the normal range. The baseline characteristics of the second analytic sample (n=890) were similar to those of the first analytic sample except that Japanese women were not included.
Table 1.
Baseline Characteristics of Women With an Observed FMP With At Least 1 Lipid Value and Its Subset Also With At Least 1 Subclinical Cardiovascular Measure
| Variables | Women With Observed FMP Plus Lipids (n=1554) | Women With Observed FMP Plus Lipids and Subclinical CVD Measure (n=890) |
|---|---|---|
| Age at baseline, mean (SD), y | 46.41 (2.70) | 46.28 (2.66) |
| Age at final menstrual period, mean (SD), y | 52.07 (2.85) | 52.15 (2.87) |
| Race, n (%) | ||
| White | 668 (43.0%) | 407 (45.7%) |
| Black | 459 (29.5%) | 285 (32.0%) |
| Chinese | 147 (9.5%) | 136 (15.3%) |
| Hispanic | 108 (7.0%) | 62 (7.0%) |
| Japanese | 172 (11.1%) | 0 |
| Education, n (%) | ||
| ≤High school | 378 (24.55) | 208 (23.64) |
| Some college | 492 (31.95) | 273 (31.02) |
| College/Postgraduate | 670 (43.51) | 399 (45.34) |
| Menopausal status, n (%) | ||
| Premenopause | 884 (57.1%) | 521 (58.8%) |
| Early perimenopause | 663 (42.9%) | 365 (41.2%) |
| Lipid‐lowering medications, n (%) yes | 14 (0.9%) | 7 (0.8%) |
| Body mass index, mean (SD), kg/m2 | 28.22 (7.39) | 28.33 (7.15) |
| Smoking | ||
| Never | 913 (58.8%) | 564 (63.4%) |
| Former | 387 (24.9%) | 200 (22.5%) |
| Current | 253 (16.3%) | 126 (14.2%) |
| Estradiol, median (q1, q3), pg/mL | 55.12 (32.58, 87.08) | 55.12 (34.05, 86.25) |
| Self‐reported prior angina or myocardial infarction | 20 (1.3%) | 0 |
| Total cholesterol, mean (SD), mg/dL | 194.79 (34.49) | 192.92 (33.67) |
| High‐density lipoprotein cholesterol, mean (SD), mg/dL | 56.39 (14.21) | 56.55 (13.81) |
| Low‐density lipoprotein cholesterol, mean (SD) mg/dL | 116.34 (31.21) | 114.74 (30.22) |
| Apo A1, mean (SD), mg/dL | 149.91 (24.05) | 150.00 (24.02) |
| ApoB, mean (SD), mg/dL | 111.54 (30.07) | 109.96 (29.25) |
| Triglycerides, median (q1, q3), mg/dL | 89 (65.00, 128.00) | 89 (64.00, 127.00) |
CVD indicates cardiovascular disease; and FMP: final menstrual period.
Patterns of Change in Lipids in Relationship to FMP
Piece‐wise mixed effect models with adjustments for study site and ethnicity/race showed that for TC, LDL‐C, and ApoB, there was little or no statistically significant change up to 3 years before the FMP, but a substantial increase from 3 years before to 1 year after the FMP (see Figure 2 and Table 2). Thereafter, ApoB continued to increase from 1 year after FMP through the end of the follow‐up period, whereas TC and LDL‐C declined. Triglyceride levels increased through 3 years post‐FMP, and then declined, whereas Apo A1 levels increased in the period approaching FMP, flattened, and then declined 3 years after FMP. The pairwise comparisons of each segment within woman demonstrated that changes for TC, LDL‐C, and ApoB were larger in the time period 3 years before the FMP to 1 year compared with the time segments immediately before or after that segment (Table 2 right columns). HDL‐C increased in a linear fashion over the follow‐up period.
Figure 2. Patterns of lipids across the SWAN (Study of Women's Health Across the Nation) follow‐up period anchored at the final menstrual period showing the estimated means by the piece‐wise linear model for all but high‐density lipoprotein cholesterol, which is showing the linear model; solid lines are means and dashed lines are 95% CI; vertical lines represent FMP at Y0.
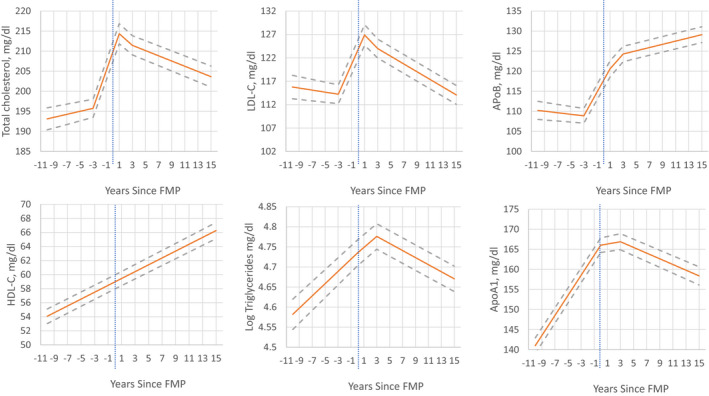
FMP indicates final menstrual period; HDL‐C, high‐density lipoprotein cholesterol; and LDL‐C, low‐density lipoprotein cholesterol.
Table 2.
Annual Changes in Lipids (mg/dL) Within Each Time Segment in Relationship to the FMP Adjusted for Study Site and Ethnicity and Their Comparison Within Participant From Piece‐Wise Mixed Effect Models With Random Intercept (n=1554 Women)
| Lipid |
A. >3 y Before FMP Beta (SE) |
P Value |
B. 3 y before FMP to 1 y after FMP Beta (SE) |
P Value |
C. 1–3 y Post FMP Beta (SE) |
P Value |
D. >3 y Post FMP Beta (SE) |
P Value |
A vs B P Value |
B vs C P Value |
C vs D P Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total cholesterol | 0.375 (0.167) | 0.025 | 4.644 (0.240) | <.0001 | −1.438 (0.469) | 0.002 | −0.650 (0.106) | <.0001 | <.0001 | <.0001 | 0.127 |
| LDL‐C | −0.221 (0.156) | 0.157 | 3.166 (0.209) | <.0001 | −1.455 (0.403) | <.001 | −0.825 (0.084) | <.0001 | <.0001 | <.0001 | 0.153 |
| ApoB | −0.192 (0.134) | 0.154 | 2.923 (0.188) | <.0001 | 1.854 (0.381) | <.0001 | 0.401 (0.082) | <.0001 | <.0001 | 0.034 | <.001 |
|
A. up to FMP Beta (SE) |
P Value |
B. FMP to 3 y Post‐FMP Beta (SE) |
P Value |
C. >3 y Post‐FMP Beta (SE) |
P Value |
A vs B P Value |
B vs C P Value |
|
|---|---|---|---|---|---|---|---|---|
| Log triglycerides | 0.015 (0.002) | <.0001 | 0.014 (0.003) | <.0001 | −0.009 (0.001) | <.0001 | 0.7148 | <.0001 |
| Apo A1 | 2.504 (0.092) | <.0001 | 0.287 (0.232) | 0.215 | −0.710 (0.077) | <.0001 | <.0001 | <.001 |
| HDL‐C | 0.488 (0.018) | <.0001 |
Bold text indicates p values that are less than .05.
FMP indicates final menstrual period; HDL‐C, high‐density lipoprotein cholesterol; and LDL‐C, low‐density lipoprotein cholesterol.
Patterns of Change in Lipids According to Age at FMP
Age at FMP was associated with the change in lipid levels at some time segments relative to FMP in fully adjusted models (Models 2, Table 3). Age at FMP was unassociated with the increase in TC, LDL‐C, and triglycerides during the years immediately before FMP and in the case of TC and LDL‐C 1 year after FMP as well. However, older age at FMP was associated with a greater decline in TC and LDL‐C from 1 to 3 years post‐FMP and in triglycerides from FMP to 3 years post‐FMP. For example, during this early postmenopausal period, the annual rate of change of TC is estimated to be 0.603 mg/dL lower for each additional year of age at FMP. The older the age at FMP the greater the increase in ApoB in the segments up to 3 years before the FMP and from 3 years before to 1 year after the FMP, whereas the older the age at FMP the smaller the increase and/or greater the decline in Apo A1 before FMP and for 3 years thereafter. The pattern of significant interaction results with FMP age (continuous) is illustrated in Figure 3 for women categorized into 3 FMP age groups. Taken together, women who had an older age at FMP had less adverse changes in TC, LDL‐C, and triglycerides after FMP, but worse changes in ApoB and Apo A1 during the menopausal transition.
Table 3.
Regression Coefficients Testing the Interaction between Age at FMP and Time Segments From the Mixed Effect Model Regression Analyses
| Age at FMP by menopause status | Total cholesterol | LDL‐cholesterol | ApoB | Age at FMP by menopause status | Log triglycerides | Apo A1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β(SE) | P Value | β(SE) | P Value | β(SE) | P Value | β(SE) | P Value | β(SE) | P Value | ||
| Model 0 | |||||||||||
| First observation to 3 y before FMP | −0.062 (0.063) | 0.33 | −0.027 (0.058) | 0.65 | 0.103 (0.053) | 0.051 | First observation to FMP | −0.001 (0.001) | 0.032 | −0.184 (0.032) | <.0001 |
| 3 y before to 1 y after FMP | 0.121 (0.086) | 0.156 | 0.080 (0.072) | 0.27 | 0.229 (0.069) | <.001 | |||||
| 1–3 y post‐FMP | −0.603 (0.162) | <.001 | −0.497 (0.139) | <.001 | −0.223 (0.136) | 0.010 | FMP to 3 y post‐FMP | −0.005 (0.001) | <.0001 | −0.446 (0.084) | <.0001 |
| >3 y post‐FMP | −0.046 (0.041) | 0.26 | −0.044 (0.033) | 0.184 | −0.102 (0.032) | 0.001 | >3 y post‐FMP | 0.0005 (0.0004) | 0.26 | 0.060 (0.028) | 0.035 |
| Model 1 | |||||||||||
| First observation to 3 y before FMP | −0.051 (0.064) | 0.43 | −0.019 (0.058) | 0.75 | 0.110 (0.053) | 0.037 | First observation to FMP | −0.001 (0.001) | 0.042 | −0.187 (0.032) | <.0001 |
| 3 y before to 1 y after FMP | 0.118 (0.086) | 0.168 | 0.075 (0.073) | 0.30 | 0.228 (0.069) | 0.001 | |||||
| 1–3 y post‐FMP | −0.611 (0.163) | <.001 | −0.506 (0.139) | <.001 | −0.241 (0.136) | 0.077 | FMP to 3 y post‐FMP | −0.006 (0.001) | <.0001 | −0.447 (0.085) | <.0001 |
| >3 y post‐FMP | −0.043 (0.041) | 0.29 | −0.040 (0.033) | 0.231 | −0.098 (0.032) | 0.002 | >3 y post‐FMP | 0.0005 (0.0004) | 0.25 | 0.058 (0.029) | 0.042 |
| Model 2 | |||||||||||
| First observation to 3 y before FMP | −0.032 (0.056) | 0.57 | −0.010 (0.051) | 0.85 | 0.129 (0.046) | 0.005 | First observation to FMP | −0.001 (0.001) | 0.061 | −0.186 (0.032) | <.0001 |
| 3 y before to 1 y after FMP | 0.158 (0.081) | 0.052 | 0.105 (0.069) | 0.126 | 0.250 (0.067) | <.001 | |||||
| 1–3 y post‐FMP | −0.525 (0.155) | <.001 | −0.384 (0.131) | 0.003 | −0.009 (0.137) | 0.95 | FMP to 3 y post‐FMP | −0.005 (0.001) | <.0001 | −0.438 (0.097) | <.0001 |
| >3 y post‐FMP | −0.071 (0.050) | 0.154 | −0.061 (0.040) | 0.131 | −0.059 (0.053) | 0.27 | >3 y post‐FMP | 0.001 (0.001) | 0.153 | −0.022 (0.054) | 0.68 |
Model 0: Adjusted for age at FMP and woman‐specific slope within time segments.
Model 1: Model 0 plus site, race, education, age at baseline.
Model 2: Model 1 plus ever smoking at baseline and time‐varying lipid‐lowering medication, cardiovascular events (stroke, heart attack, and angina), current smoker, body mass index, and hormone use.
Bold text indicates p values that are less than .05.
CVD indicates cardiovascular disease; FMP, final menstrual period; and LDL, low‐density lipoprotein.
Figure 3. Annual change in lipids according to age at final menstrual period and time segment relative to final menstrual period.
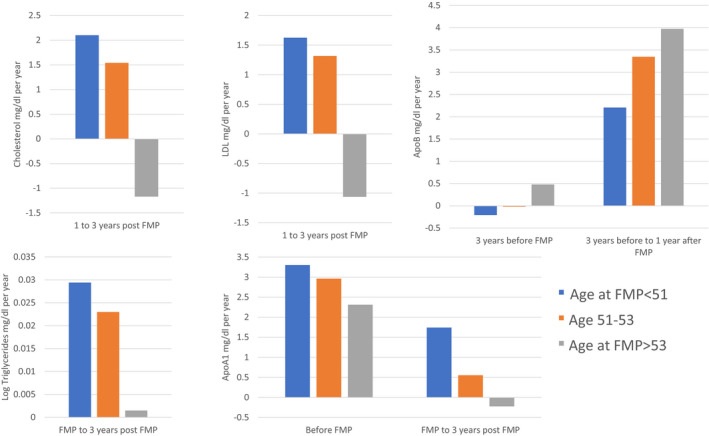
FMP indicates final menstrual period; HDL‐C, high‐density lipoprotein cholesterol; and LDL‐C, low‐density lipoprotein cholesterol.
Association of Woman‐Specific Lipid Changes With Subclinical Carotid Measures According to Age at FMP
Mean IMT and AD were 0.79 mm (SD=0.12) and 7.20 mm (SD=0.66), respectively. The number of women with carotid plaque index scores <2 and ≥2 was 658 (74%) and 227 (26%), respectively.
Tests for interactions of age at FMP and woman‐specific slopes in TC, LDL‐C, and ApoB were significant only for the period 1–3 years post‐FMP in relationship to subclinical carotid measures (Table 4). Interactions with age at FMP for these lipid measures during other time segments or for other lipid measures were not related to subclinical CVD measures. The positive beta coefficients indicate that greater lipid increases (or smaller declines) from 1 to 3 years postmenopause were associated with increasingly greater IMT, AD, and risk for carotid plaque with every year older age at FMP. Figure 4 illustrates the pattern of results for 3 women categorized into 3 FMP age groups and to whether they were in the highest, medium, and lowest tertile of lipid slopes in the early postmenopausal period. Results were consistent with the significant interactions with FMP age as a continuous variable. In the oldest FMP age group, IMT and AD increased with increasing slopes of TC, LDL‐C, and ApoB, whereas in the youngest FMP group the opposite pattern was demonstrated. For plaque index score >2 a less striking pattern was observed for oldest age at FMP group with increasing slopes of TC and LDL‐C. Thus, despite older age at FMP being associated with greater declines in TC, LDL‐C, and triglycerides during the early postmenopausal period, lipid increases during this period were still associated with higher risk for subclinical carotid disease to a greater extent among women who had an older age at FMP. Stated differently, lipid increases were associated with lower risk for subclinical carotid disease among women who had a younger age at FMP.
Table 4.
Beta Coefficients of the Interaction between Age at Final Menstrual Period and Woman‐Specific Slopes for Lipids in the Segment from 1 to 3 y Post‐FMP Predicting Subclinical Carotid Measures at Visits 12/13
| Age At FMP By Early Postmenopause Woman‐Specific Slope | Total Cholesterol | LDL‐Cholesterol | Apolipoprotein B | |||
|---|---|---|---|---|---|---|
| β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | |
| Intima media thickness, mm | ||||||
| Model 0 | 0.00074 (0.00022) | <.001 | 0.00074 (0.00024) | 0.002 | 0.00092 (0.00028) | 0.001 |
| Model 1 | 0.00063 (0.00021) | 0.002 | 0.00054 (0.00023) | 0.018 | 0.00056 (0.00027) | 0.042 |
| Model 2 | 0.00065 (0.00021) | 0.002 | 0.00056 (0.00023) | 0.015 | 0.00058 (0.00028) | 0.036 |
| Adventitial diameter, mm | ||||||
| Model 0 | 0.00408 (0.00121) | <.001 | 0.00514 (0.00158) | 0.001 | 0.00514 (0.00158) | 0.001 |
| Model 1 | 0.00339 (0.00112) | 0.003 | 0.00350 (0.00148) | 0.018 | 0.00350 (0.00148) | 0.018 |
| Model 2 | 0.00328 (0.001) | 0.004 | 0.00337 (0.00124) | 0.007 | 0.00349 (0.00148) | 0.018 |
| Carotid plaque (≥2) | ||||||
| Model 0 | 0.00863 (0.00429) | 0.044 | 0.00857 (0.00470) | 0.068 | 0.00890 (0.00564) | 0.115 |
| Model 1 | 0.01110 (0.00458) | 0.016 | 0.01160 (0.00499) | 0.020 | 0.00860 (0.00588) | 0.143 |
| Model 2 | 0.01170 (0.00466) | 0.021 | 0.01250 (0.00509) | 0.023 | 0.00965 (0.0059) | 0.105 |
Model 0: Adjusted for age at FMP and subject‐specific slope for lipid changes during the segment 1–3 y post‐FMP.
Model 1: Model 0 adjusted with V12 age, systolic blood pressure, body mass index, use of blood pressure medication, use of diabetes mellitus medication, and ever stroke/heart attack before V12.
Model 2: Model 1 adjusted with ever smoker and anti‐lipid medications at V12/13.
FMP indicates final menstrual period; and LDL, low‐density lipoprotein.
Bold text indicates p values that are less than .05.
Figure 4. Relationship between within‐woman slope in lipids categorized into tertiles in early postmenopause with subclinical measures according to woman's age at final menstrual period.
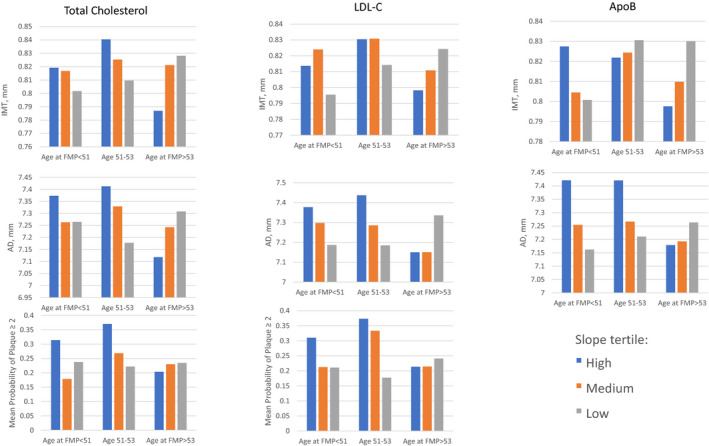
AD indicates adventitial diameter; FMP, final menstrual period; HDL‐C, high‐density lipoprotein cholesterol; IMT, intima media thickness; and LDL‐C, low‐density lipoprotein cholesterol.
DISCUSSION
The paper addresses whether age at FMP is associated with the pattern of change in lipids during the menopausal transition and the post‐menopausal years and whether the pattern of changes associated with age at FMP is associated with subsequent risk of carotid atherosclerosis.
Patterns of Change Across the Menopause Transition
Results showed that the lipid changes did not yield just 1 pattern. HDL‐C exhibited a linear increase across the follow‐up period with no obvious change relative to FMP. TC and LDL‐C levels increased dramatically throughout the menopausal transition up to the first‐year postmenopause, followed by a gradual small decline in the postmenopausal years. ApoB increased during the menopausal transition but, in contrast to cholesterol and LDL‐C, the increases continued into the postmenopausal years. Triglycerides and Apo A1 both exhibited significant increases until 3 years post‐FMP and then declined. Of interest is that ApoB, Apo A1, and triglycerides showed differences in the magnitude of annual change in the years 1–3 post‐FMP versus >3 years post‐FMP, pointing to the importance of gaining better scientific understanding of the postmenopausal stages of reproductive aging and their distinct relevance for CVD risk. Taken together, with the exception of HDL‐C, the lipid changes that occur in the perimenopause and beyond are consistent with the adverse effect of reproductive aging on changes in lipid parameters.
Is Age at FMP Associated With Lipid Changes at Different Menopausal Stages?
We expected that later age at FMP would result in smaller increases in TC, LDL‐C, and ApoB during the menopausal transition and perhaps smaller increases or greater declines in the postmenopausal period. This hypothesis was only partially confirmed. Consistent with expectations, TC, LDL‐C, and triglycerides declined to a greater extent in the early postmenopausal years among women with an older age at menopause. Unexpectedly, women with a later age at FMP had greater increases in ApoB from the first observation to 1 year after the FMP and greater declines in ApoA1 from the first observation to FMP and 3 years post‐FMP. These results do not yield a consistent picture that older age at FMP positively affects lipid changes during the menopause. Our findings are consistent with an analysis in the UK Medical Research Council Survey of Health and Development. In that analysis, age at FMP was not related to trajectories of log triglycerides, LDL‐C, and HDL‐C across 3 times points from ages of 53–69 years. 25
Is Age at FMP Associated With the Relationship Between Lipid Changes Across the Menopause Transition and Subclinical Carotid Disease?
Yes, but opposite to expectations. Among women who had an older age at FMP, the magnitude of changes in TC, LDL‐C, and ApoB 1–3 years after FMP predicted greater carotid IMT, AD, and plaque index scores. Clearly, older age at FMP was not protective in terms of lipid changes associated with subclinical carotid disease during the postmenopausal period, although our earlier work showed that without consideration of age at FMP, the magnitude of changes in LDL‐C, HDL‐C, and Apo A1 within 1 year before and after FMP predicted adventitial AD and carotid plaque. 11
These findings raise a number of perplexing issues. One is the divergence of patterns of HDL‐C, Apo A1, and triglycerides changes across the transition. An ancillary SWAN study provides clues as to why this might be the case. In that study, as women traversed the menopause, they experienced an increase in their HDL‐C levels that was accompanied by decreases in large HDL particle number and size while small HDL particle and HDL triglycerides increased. 26 In addition, higher large particle concentrations and greater size were associated with lower HDL‐efflux capacity after menopause, suggesting a dysfunctionality of HDL particles regulating the reverse cholesterol transport mechanism. Interestingly, in postmenopausal women from the MESA (Multiethnic Study of Atherosclerosis) study, higher large HDL particle concentration was associated with higher IMT close to menopause but with lower IMT later in life. 27 Thus, HDL‐C and CVD risk may switch from protective to harmful during the transition. 28 , 29
Another puzzling finding is that despite the apparent benefit of later age at menopause for cholesterol and triglycerides levels in the first few postmenopausal years, this benefit did not translate into a reduced risk for subclinical carotid disease. In fact, it appears harmful. Several possibilities come to mind. One is that the benefit on lipid declines is too brief and small in magnitude and perhaps in later postmenopausal years one would observe some benefit. However, this would not explain why women who had a later age at FMP had elevated IMT, AD, and plaque index scores. Another possibility is that ApoB or the ratio of ApoB/Apo A1 is a more important determinant of later CVD risk than cholesterol and triglyceride concentrations in midlife women. In our analysis, women with a later age at FMP had greater annual increases in ApoB and greater declines in Apo A1 during the menopausal transition and did not differ thereafter from those with an earlier age at FMP. These relative changes of ApoB/Apo A1 may have offset any benefit of declines in total and LDL‐C in the early postmenopausal period. Finally, the dissociation of LDL‐C and ApoB changes after FMP may be because of smaller denser ApoB rich LDL particles being more frequent in postmenopausal women, while larger and buoyant LDL are decreased. 30
The study has a number of strengths. First, it had frequent sampling of lipids and lipoproteins through the natural menopause transition, resulting in a large number of observations on which to base its conclusions. Second, it controlled for significant covariates that would have an impact on both lipid trajectories and development of subclinical carotid disease. However, cardiovascular events were based on self‐report because SWAN did not begin to adjudicate events until later in the follow‐up. Third, it had prospective measures of menopausal status based on frequent assessments of menstrual bleeding and timing, and a multi‐ethnic cohort of women participated. Fourth, it considered the influence of aging through its analytic approach.
Limitations include that a large minority of women were not classified as having a natural menopause because of surgery, use of HT, or withdrawing from SWAN before having an FMP. Thus, conclusions are restricted to women who had a natural menopause. Women who had an early menopause, ie, before aged 42 years, were not recruited for SWAN, and the epidemiological literature suggests that early menopause, ie, perhaps before 40 or 45 years of age, might be most highly related prospectively to CVD events. This fact may also have affected the results of the UK Health and Development Study, which measured lipids only after the age of 53 years. 25 It did not contrast the value of lipids measured at a younger age versus after the FMP, which may be useful in a future analysis. In the Healthy Women Study, premenopausal risk factors even in the normal range were strong predictors of postmenopausal coronary and aortic calcification. 13 , 31 In fact, postmenopausal risk factors were unrelated to calcification in models that included premenopausal risk factors (except for blood pressure), suggesting that risk factors at presumably low risk levels at younger ages should be key targets for intervention.
In summary, the present study suggests that older age of menopause has little benefit on lipid patterns during the transition and may have some benefit post‐FMP. However, those post‐FMP benefits in lipids are paradoxically associated with risk for subclinical carotid disease. These findings are restricted to women who have a natural menopause within the normal age range of FMP. They suggest that it is unlikely that early age at menopause is associated with elevated risk for CVD events through more adverse changes in lipids.
Sources of Funding
The SWAN study has grant support from the National Institutes of Health, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research and the National Institutes of Health Office of Research on Women's Health (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Institute of Nursing Research, Office of Research on Women’s Health, or the National Institutes of Health.
Disclosures
Dr Jackson reports research funding from Amgen; editorial board membership: circulation: Cardiovascular Quality and Outcomes; consulting: American College of Cardiology and McKesson, Inc.; expert witness for DeBlase Brown Everly LLP.; and royalties for UpToDate. Dr. Thurston receives consultant fees from Astellas, Pfizer, and Virtue Health. The remaining authors have no disclosures to report.
Supporting information
Figure S1
Acknowledgments
We thank the study staff at each site and all the women who participated in SWAN: Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, Principal Investigator (PI) 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Sherri‐Ann Burnett‐Bowie, PI 2020 – present; Joel Finkelstein, PI 1999 – 2020; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Imke Janssen, PI 2020 – present; Howard Kravitz, PI 2009 – 2020; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Elaine Waetjen and Monique Hedderson, PIs 2020 – present; Ellen Gold, PI 1994 ‐ 2020; University of California, Los Angeles – Arun Karlamangla, PI 2020 – present; Gail Greendale, PI 1994 ‐ 2020; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Rebecca Thurston, PI 2020 – present; Karen Matthews, PI 1994 ‒ 2020. NIH Program Office: National Institute on Aging, Bethesda, MD – Rosaly Correa‐de‐Araujo 2020 ‒ present; Chhanda Dutta 2016‐ present; Winifred Rossi 2012–2016; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers. Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 ‒ present; Kim Sutton‐Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA ‐ Sonja McKinlay, PI 1995 – 2001. Steering Committee: Susan Johnson, Current Chair. Chris Gallagher, Former Chair
For Sources of Funding and Disclosures, see page 12.
References
- 1. Muka T, Oliver‐Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all‐cause mortality: a systematic review and meta‐analysis. JAMA Cardiol. 2016;1:767–776. doi: 10.1001/jamacardio.2016.2415 [DOI] [PubMed] [Google Scholar]
- 2. Gold EB, Crawford SL, Avis NE, Crandall CJ, Matthews KA, Waetjen LE, Lee JS, Thurston R, Vuga M, Harlow SD. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178:70–83. doi: 10.1093/aje/kws421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu D, Chung H‐F, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019;4:e553–e564. doi: 10.1016/S2468-2667(19)30155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506–e532. doi: 10.1161/CIR.0000000000000912 [DOI] [PubMed] [Google Scholar]
- 5. Kok HS, van Asselt KM, van der Schouw YT, van der Tweel I, Peeters PHM, Wilson PWF, Pearson PL, Grobbee DE. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976–1983. doi: 10.1016/j.jacc.2005.12.066 [DOI] [PubMed] [Google Scholar]
- 6. Parente RC, Faerstein E, Celeste RK, Werneck GL. The relationship between smoking and age at the menopause: a systematic review. Maturitas. 2008;61:287–298. doi: 10.1016/j.maturitas.2008.09.021 [DOI] [PubMed] [Google Scholar]
- 7. Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the study of women's health across the nation. Am J Epidemiol. 2009;169:1352–1361. doi: 10.1093/aje/kwp043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–646. doi: 10.1056/NEJM198909073211004 [DOI] [PubMed] [Google Scholar]
- 9. Bonithon‐Kopp C, Scarabin PY, Darne B, Malmmejac A, Guize L. Menopause‐related changes in lipoproteins and some other cardiovascular risk factors. Int J Epidemiol. 1990;19:42–48. doi: 10.1093/ije/19.1.42 [DOI] [PubMed] [Google Scholar]
- 10. Matthews KA, Crawford SL, Chae CU, Everson‐Rose SA, Sowers MF, Sternfeld B, Sutton‐Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matthews KA, El Khoudary SR, Brooks MM, Derby CA, Harlow SD, Barinas‐Mitchell EJ, Thurston RC. Lipid changes around the final menstrual period predict carotid subclinical disease in postmenopausal women. Stroke. 2017;48:70–76. doi: 10.1161/STROKEAHA.116.014743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joakimsen O, Bonaa KH, Stensland‐Bugge E, Jacobsen BK. Population‐based study of age at menopause and ultrasound assessed carotid atherosclerosis: the Tromso study. J Clin Epidemiol. 2000;53:525–530. doi: 10.1016/S0895-4356(99)00197-3 [DOI] [PubMed] [Google Scholar]
- 13. Matthews KA, Kuller LH, Sutton‐Tyrrell K, Chang Y. Change in cardiovascular risk factors during the perimenopause and postmenopause and carotid artery atherosclerosis in healthy women. Stroke. 2001;32:1104–1111 [DOI] [PubMed] [Google Scholar]
- 14. Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold E, Greendale G, Evans D, Neer R, Matthews K, Sherman S, et al. SWAN: a multi‐center, multi‐ethnic community‐based cohort study of women and the menopausal transition. In: Lobo R, Marcus R, Kelsey J, eds. Menopause: Biology and Pathology. New York: Academic Press; 2000. [Google Scholar]
- 15. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima‐media thickness and plaque consensus (2004‐2006‐2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290‐296. doi: 10.1159/000343145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson T, Sutton‐Tyrrell K, Wildman RP, Kao A, Fitzgerald SG, Shook B, Tracy RP, Kuller LH, Brockwell S, Manzi S. Progression of carotid intima‐media thickness and plaque in women with systemic lupus erythematosus. Arthritis Rheum. 2008;58:835–842. doi: 10.1002/art.23196 [DOI] [PubMed] [Google Scholar]
- 17. Wendelhag I, Gustavsson T, Suurkula M, Berglund G, Wikstrand J. Ultrasound measurement of wall thickness in the carotid artery: fundamental principles and description of a computerized analysing system. Clin Physiol. 1991;11:565–577. doi: 10.1111/j.1475-097X.1991.tb00676.x [DOI] [PubMed] [Google Scholar]
- 18. Sutton‐Tyrrell K, Wolfson SK Jr, Thompson T, Kelsey SF. Measurement variability in duplex scan assessment of carotid atherosclerosis. Stroke. 1992;23:215–220. doi: 10.1161/01.STR.23.2.215 [DOI] [PubMed] [Google Scholar]
- 19. Steiner P, Freidel J, Bremner W, Stein E. Standardization of micromethods for plasma cholesterol, triglyceride, and HDL‐cholesterol with the lipid clinics' methodology. J Clin Chem Clin Biochem. 1981;19:850 [Google Scholar]
- 20. Warnick GR, Albers JJ. A comprehensive evaluation of the heparin‐manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. doi: 10.1016/S0022-2275(20)41577-9 [DOI] [PubMed] [Google Scholar]
- 21. Izawa S, Okada M, Matsui H, Horital Y. A new direct method for measuring HDL‐choleterol, which does not produce any biased results. J Med Pharm Sci. 1997;37:1385–1388 [Google Scholar]
- 22. Freidwald WT, Levy RI, Frederickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma. Clin Chem. 1972;18:499–501 [PubMed] [Google Scholar]
- 23. van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–3282 [DOI] [PubMed] [Google Scholar]
- 24. Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. Executive summary of the stages of reproductive aging workshop+10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Keeffe LM, Kuh D, Fraser A, Howe LD, Lawlor D, Hardy R. Age at period cessation and trajectories of cardiovascular risk factors across mid and later life. Heart. 2020;106:499–505. doi: 10.1136/heartjnl-2019-315754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. El Khoudary SR, Chen X, Nasr A, Billheimer J, Brooks MM, McConnell D, Orchard T, Crawford S, Matthews KA, Rader DJ. HDL (High‐Density Lipoprotein) subclasses, lipid content, and function trajectories across the menopause transition: SWAN‐HDL Study. Arterioscler Thromb Vasc Biol. 2021;41(2):951–961. doi: 10.1161/ATVBAHA.120.315355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El Khoudary SR, Ceponiene I, Samargandy S, Stein JH, Li D, Tattersall MC, Budoff MJ. HDL (High‐Density Lipoprotein) metrics and atherosclerotic risk in women. Arterioscler Thromb Vasc Biol. 2018;38:2236–2244. doi: 10.1161/ATVBAHA.118.311017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woodard GA, Brooks MM, Barinas‐Mitchell E, Mackey RH, Matthews KA, Sutton‐Tyrrell K. Lipids, menopause, and early atherosclerosis in Study of Women's Health Across the Nation Heart women. Menopause. 2011;18:376–384. doi: 10.1097/gme.0b013e3181f6480e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. El Khoudary SR, Wang L, Brooks MM, Thurston RC, Derby CA, Matthews KA. Increase HDL‐C level over the menopausal transition is associated with greater atherosclerotic progression. J Clin Lipidol. 2016;10:962–969. doi: 10.1016/j.jacl.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fonseca MIH, da Silva IT, Ferreira SRG. Impact of menopause and diabetes on atherogenic lipid profile: is it worth to analyse lipoprotein subfractions to assess cardiovascular risk in women? Diabetol Metab Syndr. 2017;9:22. doi: 10.1186/s13098-017-0221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matthews KA, Kuller LH, Chang Y, Edmundowicz D. Premenopausal risk factors for coronary and aortic calcification: a 20‐year follow‐up in the healthy women study. Prev Med. 2007;45:302–308. doi: 10.1016/j.ypmed.2007.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


