Abstract
Background
Heart failure (HF) is a common complication to atrial fibrillation (AF), leading to rehospitalization and death. Early identification of patients with AF at risk for HF might improve outcomes. We aimed to derive a score to predict 1‐year risk of new‐onset HF after an emergency department (ED) visit with AF.
Methods and Results
The RE‐LY AF (Randomized Evaluation of Long‐Term Anticoagulant Therapy) registry enrolled patients with AF presenting to an ED in 47 countries, and followed them for a year. The end point was HF hospitalization and/or HF death. Among 15 400 ED patients, 9765 had no prior HF (mean age, 64.9±14.9 years). Within 1 year, new‐onset HF developed in 6.8% of patients, of whom 21% died of HF. Independent predictors of HF included left ventricular hypertrophy (odds ratio [OR], 1.47; 95% CI, 1.19–1.82), valvular heart disease (OR, 1.55; 95% CI, 1.18–2.04), smoking (OR, 1.42; 95% CI, 1.12–1.78), height (OR, 0.93; 95% CI, 0.90–0.95 per 3 cm), age (OR, 1.11; 95% CI, 1.07–1.15 per 5 years), rheumatic heart disease (OR, 1.77, 95% CI, 1.24–2.51), prior myocardial infarction (OR, 1.85; 95% CI, 1.45–2.36), remaining in AF at ED discharge (OR, 1.86; 95% CI, 1.46–2.36), and diabetes (OR, 1.33; 95% CI, 1.09–1.64). A continuous risk prediction score (LVS‐HARMED [left ventricular, valvular heart disease, smoking or other tobacco use, height, age, rheumatic heart disease, myocardial infarction, emergency department discharge rhythm, and diabetes]) had good discrimination (C statistic, 0.735; 95% CI, 0.716–0.755). Validation was conducted internally using bootstrapping (optimism‐corrected C statistic, 0.705) and externally (C statistic, 0.699). The 1‐year incidence of HF hospitalization and/or HF death across quartile groups of the score was 1.1%, 4.5%, 6.9%, and 14.4%, respectively. LVS‐HARMED also predicted incident stroke (C statistic, 0.753; 95% CI, 0.728–0.778).
Conclusions
The LVS‐HARMED score predicts new‐onset HF after an ED visit for AF. Preventative strategies should be considered in patients with high LVS‐HARMED HF risk.
Keywords: atrial fibrillation, epidemiology, heart failure, prevention, risk score, risk stratification
Subject Categories: Atrial Fibrillation, Heart Failure, Epidemiology, Secondary Prevention
Nonstandard Abbreviations and Acronyms
- AFFORD
Atrial Fibrillation and Flutter Outcomes and Risk Determination
- AIC
Akaike information criterion
- FHS
Framingham Heart Study
- LVS‐HARMED
left ventricular, valvular heart disease, smoking or other tobacco use, height, age, rheumatic heart disease, myocardial infarction, emergency department discharge rhythm, and diabetes mellitus
- RE‐LY
Randomized Evaluation of Long‐Term Anticoagulant Therapy
Clinical Perspective
What Is New?
New‐onset heart failure is a common early complication in patients with atrial fibrillation after an emergency department visit that can be predicted using the LVS‐HARMED (left ventricular, valvular heart disease, smoking or other tobacco use, height, age, rheumatic heart disease, myocardial infarction, emergency department discharge rhythm, and diabetes) risk score.
What Are the Clinical Implications?
Patients with atrial fibrillation who have a high risk of heart failure should be referred for specialist consultation after an emergency department visit for atrial fibrillation.
The global prevalence of atrial fibrillation (AF) continues to increase. 1 Heart failure (HF) is a common complication of AF 2 and independently increases the risk of death in affected patients. 3 HF is the most common cause of death among people with AF, 4 accounting for more than one third of all deaths in patients with AF worldwide. 4 However; prediction and prevention of HF have received much less attention in AF research and guidelines than stroke prevention. 5 Furthermore, despite the high risks of HF in patients with AF, no validated HF prevention programs exist for these patients. Prevention trials, targeting modifiable risk factors for HF in patients with AF are therefore needed 5 and would be feasible if patients at high risk for HF could be identified. The emergency department (ED) is a common point of first contact for patients with AF, and AF causes or contributes to a large number of AF hospitalizations every year. 6 Given the success of ED‐based interventions to improve oral anticoagulant use, the initiation of HF prevention programs in the ED might also be effective. 7 There is hope that prevention of HF in patients with AF may therefore be successfully conducted via referrals of patients with AF who have a high risk for HF from the ED to specialist care.
Previous studies have investigated risk factors for HF in patients with AF, 8 , 9 including the development of a 10‐year risk score in the FHS (Framingham Heart Study), which included age, left ventricular hypertrophy (LVH), body mass index, diabetes, significant heart murmur, and a history of myocardial infarction. 8 To the best of our knowledge, however, no previous risk prediction scores for HF in patients with AF have been developed for use in the ED, and in populations that include patients from middle‐ and low‐income countries, even though risk factors for AF and related diseases and treatments vary greatly between geographic regions. 10 Accurate prediction of incident HF in patients with AF based on variables obtained in the ED may be useful to select patients for specialist referral, in order to provide medical interventions that reduce HF hospitalizations. The aim of this analysis was to derive a model to predict 1‐year risk of new‐onset hospitalization or death from HF in the global RE‐LY AF (Randomized Evaluation of Long‐Term Anticoagulant Therapy) registry.
Methods
The analytic methods used in this article will be made available to other researchers upon reasonable request to the corresponding author. Institutional review board approval has been obtained.
Study Population and Data Collection
The RE‐LY AF registry prospectively enrolled a total of 15 400 patients with AF from 164 sites in 47 countries, representing all inhabited continents. The methods have been previously described in more detail. 4 , 10 Briefly, patients who presented to an ED with AF or atrial flutter, as identified by the treating physician, either as a primary or a secondary diagnosis, were enrolled. Patients were then assessed after 1 year, either in person or by telephone, and supplemental information was obtained from medical records. The diagnosis of HF was determined according to site discretion. All patients gave written informed consent.
For this analysis, all patients with a history of HF were excluded (n=5350). Participating countries were placed into 4 income groups, based on the 2011 World Bank definitions 11 : low income: Tanzania, Kenya, Mozambique, and Uganda (n=194); low/middle income: India, Sudan, Senegal, Zambia, Cameroon, Nigeria, Egypt, and the Ukraine (n=2337); upper/middle income: Argentina, Brazil, Colombia, Ecuador, Venezuela, Chile, Russia, Latvia, Turkey, Iran, South Africa, Thailand, and China (n=2455); and high income: Japan, South Korea, Singapore, Saudi Arabia, the United Arab Emirates, Poland, Slovakia, Hungary, the Czech Republic, Bulgaria, Australia, Spain, Italy, the Netherlands, Germany, Austria, the United Kingdom, Sweden, Ireland, Canada, Denmark, and the United States (n=5064). We excluded patients with missing follow‐up data for HF hospitalizations and/or HF death (n=285), resulting in a study population of 9765 individuals.
The prediction model was based on 9321 individuals with complete data for all variables evaluated in the final prediction model. The primary end point was a composite of hospitalization for HF and/or HF death, and was retrieved from the case report forms that were completed by the study centers at the end of the follow‐up. Qualifying HF hospitalization events had a duration of ≥24 hours. Some patients died as a result of new‐onset HF without a reported HF hospitalization.
An external validation of the risk score was conducted in the previously described AFFORD (Atrial Fibrillation and Flutter Outcomes and Risk Determination) study. 12 Briefly, the AFFORD study included 623 patients presenting to the ED with AF; the aim was to derive an ED‐based clinical decision aid to identify patients with AF at low risk for adverse events. A convenience sample of patients with signs of symptoms consistent with symptomatic AF who presented between June 9, 2010, and February 28, 2013, to the Vanderbilt University Medical Center ED were recruited; AF diagnoses were confirmed with ECG.
We excluded patients with known prevalent HF or missing information, resulting in a study population of 367 patients, in whom we ascertained 17 cases of incident HF hospitalization and/or death during 365 days of follow‐up. The end point was retrieved by medical chart review conducted by 3 trained researchers and clinicians. Each chart was reviewed by multiple abstractors to ensure reliable data capture.
Statistical Analysis
Baseline characteristics were summarized and stratified by country income group, and also separately for individuals with and without subsequent HF hospitalization and/or HF death. These strata were compared using Student t tests for continuous variables and chi‐square tests for categorical variables. A logistic mixed effects regression model, with random effects for country, was used to assess the effect of baseline variables on the composite end point of HF hospitalization or death. Variables assessed for inclusion in the model were prespecified, based on the FHS risk score for HF in AF, as well as other variables plausibly related to HF in ED patients, and included age, sex, height, body mass index, systolic blood pressure, a history of smoking or other tobacco use, whether the patient remained in AF at ED discharge (to home or admission to hospital ward), LVH on ECG or echocardiogram, presence of a pacemaker, history of myocardial infarction, hypertension, rheumatic heart disease, valvular heart disease, diabetes, stroke/transient ischemic attack, or emphysema/chronic obstructive pulmonary disease. Tobacco use was defined as a reported history of smoking or use of any other tobacco product.
The Lasso method with l 1 penalization was used for variable selection. The optimal set of variables was selected by tuning the parameter lambda. 13 The model with the smallest goodness‐of‐fit statistic was chosen as the best one. Based on these selected variables, a parsimonious risk prediction model was derived in the study population. We then calculated C statistics with 95% CIs for groups of countries based on their income group, as well as by geographic region. Calibration of the score was assessed using the Hosmer‐Lemeshow goodness‐of‐fit test and depicted in a calibration plot. 14 We assessed regional variation in 2 ways: first, by running 2 logistic mixed effects regression models including the final score, one with only a random intercept effect for country and another adding a random slope effect for the final score; we then compared these models with each other using the Akaike information criterion (AIC) and the likelihood ratio test. We also calculated regression coefficients for geographic regions in a logistic mixed effects regression model with a random intercept effect for country, including the final score as well as a variable for geographic region, where North America was set as the reference region. The model was validated internally using bootstrap resampling to measure the optimism in the model. 15 The effect of medication after ED visit was assessed in a mixed effects logistic regression model including all final score components as well as the use of diuretics, calcium channel blockers, β‐blockers, angiotensin‐converting enzyme inhibitors, and digoxin, with clustering on country.
For purposes of comparison, we applied the algorithm for the FHS risk score for HF in AF to the study population. This score predicts 10‐year risk of incident HF in patients with AF. 8 We then calculated the C statistics for this score in a logistic mixed effects regression model with random intercept effects for country for the composite end point of HF hospitalization and/or HF death over the 1‐year follow‐up, in the full RE‐LY AF registry, as well as by country income group and by geographic region.
Finally, to explore whether a single score could be used to predict both HF and stroke in patients with AF in an ED setting, the C statistic for the RE‐LY AF registry–derived risk prediction score was calculated using incident stroke as the end point. Statistical analyses were performed using SAS version 9.4 for Windows (SAS Institute Inc), except for model selection and calibration, which was performed in the R package glmmixedlasso, and the external validation, which was conducted in Stata version 14.2 (StataCorp). A P value <0.05 was considered to indicate statistical significance.
Results
Baseline characteristics are reported in Table 1. The mean age of the study population was 64.9 years (SD, 14.9 years), 53.8% were men, and 17.6% had LVH on ECG or echocardiogram. At 1 year of follow‐up, the end point of HF hospitalization and/or HF death occurred in 664 of 9765 patients (6.8%). The proportion of patients with HF hospitalization and/or HF death during follow‐up was 28.0% in low‐income countries, 5.3% in low–middle‐income countries, 6.9% in upper–middle‐income countries, and 6.6% in high‐income countries. Univariable analyses of baseline characteristics in patients with and without new‐onset HF during follow‐up are reported in Table S1. Among patients with new‐onset HF, the mean age and proportion of women were higher.
Table 1.
Baseline Characteristics and Medical History
| Overall | Low‐income countries* | Lower–middle‐income countries † | Upper–middle‐income countries ‡ | High‐income countries § | |
|---|---|---|---|---|---|
| Patients, N. | N=9765 | n=193 | n=2246 | n=2358 | n=4968 |
| Age, mean±SD, y | 64.9±14.9 | 56.3±21.8 | 58.0±15.8 | 67.3±13.7 | 67.3±13.5 |
| Men, % | 53.8 | 51.8 | 49.7 | 50.2 | 57.5 |
| Weight, mean±SD, kg | 73.1±19.3 | 70.7±20.2 | 63.8±14.9 | 69.4±14.7 | 79.1±20.8 |
| Height, mean±SD, cm | 167±10.5 | 165±11.9 | 162±9.5 | 166±9.0 | 169±10.9 |
| BMI, mean±SD, kg/m2 | 26.2±5.8 | 26±6.9 | 24.3±5.2 | 25.2±4.8 | 27.5±6.1 |
| Systolic BP, mean±SD, mm Hg | 133±25 | 133±28 | 128±22 | 134±25 | 135±25 |
| Diastolic BP, mean±SD, mm Hg | 81±15 | 81±17 | 80±13 | 81±15 | 81±16 |
| Heart rate, mean±SD, beats per min | 103±31 | 99±32 | 100±28 | 101±30 | 106±33 |
| Prior diagnosis of AF, % | 59.1 | 45.6 | 39.4 | 69.5 | 63.6 |
| Paroxysmal | 35.6 | 27.3 | 21.8 | 32.4 | 41.3 |
| Persistent | 23.7 | 10.2 | 30.8 | 24.2 | 21.8 |
| Permanent | 40.7 | 62.5 | 47.4 | 43.4 | 36.8 |
| Current arrhythmia, % | |||||
| AF | 92.3 | 91.2 | 94.5 | 94.5 | 90.2 |
| Atrial flutter | 7.7 | 8.8 | 5.4 | 5.5 | 9.8 |
| Patient in AF/atrial flutter when left ED, % | 73.0 | 78.2 | 80.1 | 73.4 | 69.3 |
| Repeat visits for AF/atrial flutter complications during study period, % | 16.8 | 21.2 | 5.3 | 17.2 | 21.6 |
| Medical history, % | |||||
| Myocardial infarction, % | 11.2 | 6.7 | 14.3 | 8.1 | 11.5 |
| Coronary artery disease, % | 25.5 | 9.3 | 25.0 | 29.4 | 24.4 |
| Hypertension, % | 59.2 | 52.8 | 43.2 | 64.8 | 64.1 |
| Stroke/TIA, % | 13.2 | 12.4 | 8.5 | 18.1 | 13.1 |
| Rheumatic heart disease, % | 10.7 | 18.1 | 30.1 | 7.8 | 2.9 |
| Significant valvular heart disease, % | 18.3 | 23.3 | 42.8 | 13.4 | 9.3 |
| Mitral stenosis, % of subjects with valvular heart disease | 50.1 | 46.7 | 64.3 | 48.4 | 22.0 |
| Aortic stenosis, % of subjects with valvular heart disease | 10.7 | 2.2 | 5.2 | 10.4 | 23.1 |
| Mitral regurgitation, % of subjects with valvular heart disease | 50.2 | 73.3 | 49.5 | 52.5 | 47.9 |
| Aortic regurgitation, % of subjects with valvular heart disease | 12.7 | 20.0 | 10.7 | 14.2 | 15.1 |
| Permanent pacemaker, % | 3.8 | 0.5 | 1.7 | 3.4 | 5.0 |
| LVH, % | 17.6 | 40.9 | 11.9 | 18.4 | 18.9 |
| Pericarditis,% | 0.5 | 5.2 | 0.6 | 0.4 | 0.4 |
| Emphysema/COPD,% | 8.0 | 4.7 | 4.8 | 9.4 | 8.9 |
| Diabetes, % | 19.5 | 17.1 | 19.4 | 16.4 | 21.0 |
| Tobacco use, % | 16.0 | 11.4 | 7.0 | 19.1 | 18.8 |
| Alcohol use, standard drinks/wk, (median) mean±SD | (0) 3.3±7.7 | (3) 8.5±12.7 | (0) 1.2±5.8 | (0) 2±8.6 | (1) 3.9±7.0 |
| Prior interventions, % | |||||
| Prior cardioversions | 11.3 | 14.5 | 2.3 | 7.9 | 16.8 |
| Prior AF catheter, surgical or Maze procedure | 2.5 | 0.5 | 0.2 | 1.6 | 4.0 |
| Prior atrioventricular node ablation | 0.5 | 0.0 | 0.1 | 0.6 | 0.7 |
| Prior left atrial appendage occlusion or amputation | 0.1 | 0.0 | 0.0 | 0.1 | 0.2 |
| Medication use after ED visit, % | |||||
| β‐Blocker | 53.2 | 47.2 | 39.7 | 49.2 | 61.4 |
| CCB | 27.7 | 22.3 | 29.9 | 23.8 | 28.7 |
| ACEI | 27.6 | 34.7 | 19.9 | 24.7 | 32.2 |
| ARB | 15.3 | 12.4 | 9.8 | 18.2 | 16.6 |
| Digoxin | 25.6 | 42.5 | 48.6 | 16.7 | 18.9 |
| Diuretic | 41.0 | 41.5 | 66.0 | 26.1 | 36.7 |
| Amiodarone | 14.8 | 26.9 | 21.9 | 19.2 | 9.0 |
| Sotalol | 3.2 | 3.1 | 0.3 | 1.0 | 5.5 |
| Flecainide | 1.6 | 1.0 | 0.2 | 0.1 | 3.0 |
ACEI indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; ED, emergency department; LVH, left ventricular hypertrophy; and TIA, transient ischemic attack.
Includes Tanzania, Kenya, Mozambique, and Uganda.
Includes India, Sudan, Senegal, Zambia, Cameroon, Nigeria, Egypt, and the Ukraine.
Includes Argentina, Brazil, Colombia, Ecuador, Venezuela, Chile, Russia, Latvia, Turkey, Iran, South Africa, Thailand, and China.
Includes Japan, South Korea, Singapore, Saudi Arabia, the United Arab Emirates, Poland, Slovakia, Hungary, the Czech Republic, Bulgaria, Australia, Spain, Italy, the Netherlands, Germany, Austria, the United Kingdom, Sweden, Ireland, Canada, Denmark, and the United States.
Risk Score Components and Performance
A continuous risk prediction score for new‐onset HF within 1 year of presenting to an ED with AF is reported in Table 2. The score includes the following independent predictors: LVH, valvular heart disease, history of smoking or other tobacco use, height, age, history of rheumatic heart disease, history of myocardial infarction, the patient being in AF at ED discharge, and history of diabetes. The acronym LVS‐HARMED stands for LVH, valvular heart disease, smoking or other tobacco use, height, age, rheumatic heart disease, myocardial infarction, ED discharge rhythm, and diabetes. The algorithm to calculate the LVS‐HARMED score is reported in the footnote to Table 2. Systolic blood pressure, body mass index, hypertension, presence of a pacemaker, sex, history of stroke/transient ischemic attack, and emphysema/chronic obstructive pulmonary disease did not meaningfully contribute to the optimal model and were not included in the final model. The overall discrimination, as measured by the C statistic, was 0.735 (95% CI, 0.716–0.755). Bootstrap resampling of the population with 100 samples was used to estimate the mean optimism to 0.0297, resulting in a mean optimism–corrected C statistic of 0.705. A calibration plot is presented in Figure S1.
Table 2.
Parsimonious Model for 1‐Year Risk of HF
| β | OR (95% CI) | P value | |
|---|---|---|---|
| Intercept | −0.671 | 0.4471 | |
| LVH | 0.387 | 1.473 (1.190–1.823) | 0.0007 |
| Valvular heart disease | 0.439 | 1.552 (1.183–2.035) | 0.0021 |
| Smoking/other tobacco use | 0.347 | 1.416 (1.123–1.784) | 0.0042 |
| Height, per 3 cm | −0.075 | 0.928 (0.902–0.954) | <0.0001 |
| Age, per 5 y | 0.104 | 1.110 (1.070–1.151) | <0.0001 |
| Rheumatic heart disease | 0.569 | 1.766 (1.244–2.507) | 0.0022 |
| Myocardial infarction | 0.613 | 1.847 (1.446–2.359) | <0.0001 |
| ED discharge rhythm is AF | 0.619 | 1.857 (1.464–2.355) | <0.0001 |
| Diabetes | 0.288 | 1.334 (1.085–1.640) | 0.0074 |
Includes 9321 patients and 628 events. AF indicates atrial fibrillation; ED, emergency department; HF, heart failure; LVH, left ventricular hypertrophy; and OR, odds ratio.
The LVS‐HARMED (left ventricular, valvular heart disease, smoking or other tobacco use, height, age, rheumatic heart disease, myocardial infarction, emergency department discharge rhythm, and diabetes) score is calculated as: exp (−0.671+xL+xV+xS+xH+xA+xR+xM+xE+xD)/(1+exp (−0.671+xL+xV+xS+xH+xA+xR+xM+xE+xD)), where x denotes the individual patient values, and LVS‐HARMED the specific β‐coefficient.
β‐Coefficients (P values) for the different geographic regions are as follows: North America: reference; Western Europe: −0.304 (P=0.55); Eastern Europe: 0.051 (P=0.92); Latin America: −0.749 (P=0.19); the Middle East: −0.3615 (P=0.55); Asia: −0.194 (P=0.71); and Africa: 1.395 (P=0.009).
The AFFORD study validation population had a mean age of 63.9 years (SD, 14.8), 236 (64.3%) were men, and 143 (39.0%) had LVH on ECG or echocardiogram. The external validation of the LVS‐HARMED score in this population resulted in a C statistic of 0.699 (95% CI, 0.583–0.814). A calibration plot for the AFFORD study can be found in Figure S2 and a calibration table in Table S2; the Hosmer‐Lemeshow P value was 0.22.
Risk Score Performance Among Country Income Groups and Regions
The performance of both the LVS‐HARMED and FHS risk scores for HF in AF among income groups and geographic regions are reported in Table 3, and receiver operating curves for the LVS‐HARMED score in the full registry as well as by income group can be found in Figures S3 through S7. For prediction of 1‐year risk of HF, the LVS‐HARMED score performed better than the FHS score overall and in each income group. Agreement plots and Bangdiwala statistics for the LVS‐HARMED and FHS scores are given in Figure S8. Income group–specific odds ratios (95% CI) for the predictors included in the LVS‐HARMED score are reported in Table S3. There was no significant difference in the LVS‐HARMED models without or with random slope (AIC for the model without random slope: 4298.9, AIC for the model with random slope: 4297.5; P=0.07). Regression coefficients for geographic regions are given below (Table 2). The association between LVS‐HARMED components and new‐onset HF was also assessed in a model that also included medication after ED visit (Table S4). The LVS‐HARMED coefficients were not substantially altered.
Table 3.
C Statistics for the LVS‐HARMED and FHS Score for HF in AF Among Geographic Region and Income Group Levels
| LVS‐HARMED | FHS | Patients, No.* | Events, No. (%) | |||
|---|---|---|---|---|---|---|
| C statistic | 95% CI | C statistic | 95% CI | |||
| Overall | 0.735 | 0.716 to 0.755 | 0.603 | 0.579 to 0.627 | 9765 | 664 (6.80%) |
| Region | ||||||
| North America | 0.710 | 0.658 to 0.763 | 0.650 | 0.594 to 0.706 | 1291 | 90 (7.0%) |
| Latin America | 0.632 | 0.537 to 0.727 | 0.582 | 0.477 to 0.686 | 689 | 33 (4.8%) |
| Western Europe | 0.797 | 0.749 to 0.844 | 0.718 | 0.657 to 0.780 | 1528 | 78 (5.1%) |
| Eastern Europe | 0.704 | 0.644 to 0.765 | 0.654 | 0.591 to 0.717 | 1030 | 73 (7.1%) |
| Middle East | 0.745 | 0.662 to 0.828 | 0.677 | 0.577 to 0.778 | 604 | 35 (5.8%) |
| Africa | 0.795 | 0.732 to 0.857 | 0.400 | 0.321 to 0.479 | 367 | 79 (21.5%) |
| Asia | 0.703 | 0.672 to 0.733 | 0.611 | 0.575 to 0.647 | 4256 | 276 (6.5%) |
| Income group | ||||||
| Low‐income countries* | 0.847 | 0.782 to 0.913 | 0.381 | 0.284 to 0.478 | 193 | 54 (28.0) |
| Lower–middle‐income countries † | 0.708 | 0.660 to 0.756 | 0.537 | 0.480 to 0.594 | 2246 | 119 (5.3) |
| Upper–middle‐income countries ‡ | 0.689 | 0.648 to 0.730 | 0.602 | 0.557 to 0.646 | 2358 | 163 (6.9) |
| High‐income countries § | 0.733 | 0.707 to 0.759 | 0.667 | 0.637 to 0.698 | 4968 | 328 (6.6) |
AF indicates atrial fibrillation; FHS, Framingham Heart Study; HF, heart failure; and LVS‐HARMED, left ventricular, valvular heart disease, smoking or other tobacco use, height, age, rheumatic heart disease, myocardial infarction, emergency department discharge rhythm, and diabetes.
Includes Tanzania, Kenya, Mozambique, and Uganda; 193 patients and 54 events.
Includes India, Sudan, Senegal, Zambia, Cameroon, Nigeria, Egypt, and the Ukraine; 2246 patients and 119 events.
Includes Argentina, Brazil, Colombia, Ecuador, Venezuela, Chile, Russia, Latvia, Turkey, Iran, South Africa, Thailand and China; 2358 patients and 163 events.
Includes Japan, South Korea, Singapore, Saudi Arabia, the United Arab Emirates, Poland, Slovakia, Hungary, the Czech Republic, Bulgaria, Australia, Spain, Italy, the Netherlands, Germany, Austria, the United Kingdom, Sweden, Ireland, Canada, Denmark and the United States; 4968 patients and 328 events.
The observed frequencies of HF hospitalizations, HF deaths, and the composite end point of HF hospitalization and/or HF death across quartiles of the score and income groups are given in Table 4 and Figure. In the top quartile of the score, the incidence of HF hospitalization and/or HF death was high in all income groups: 75.0% in low‐income countries, 10.5% in lower–middle‐income countries, 12.5% in upper–middle‐income countries, and 13.9% in high‐income countries.
Table 4.
Observed Outcomes by Quartiles of Predicted LVS‐HARMED Risk Among Income Groups*
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Total | |
|---|---|---|---|---|---|
| Full study population | |||||
| Total, N | 2330 | 2330 | 2331 | 2330 | 9321 |
| HF death, n (%) | 3 (0.13) | 32 (1.37) | 36 (1.54) | 62 (2.66) | 133 (1.43) |
| HF hospitalization, n (%) | 24 (1.03) | 77 (3.30) | 137 (5.88) | 299 (12.83) | 537 (5.76) |
| Total HF (hospitalization and/or HF death), n (%) | 26 (1.12) | 104 (4.46) | 162 (6.95) | 336 (14.42) | 628 (6.74) |
| Predicted score range | (0.0032, 0.0310) | (0.0310, 0.0515) | (0.0515, 0.0847) | (0.0847, 0.8477) | (0.0032, 0.8477) |
| Low‐income countries † | |||||
| Total, N | 44 | 44 | 44 | 44 | 176 |
| HF death, n (%) | 0 (0.00) | 1 (2.27) | 3 (6.82) | 1 (2.27) | 5 (2.84) |
| HF hospitalization, n (%) | 3 (6.82) | 3 (6.82) | 14 (31.82) | 33 (75.00) | 53 (30.11) |
| Total HF (hospitalization and/or HF death), n (%) | 3 (6.82) | 3 (6.82) | 14 (31.82) | 33 (75.00) | 53 (30.11) |
| Predicted score range | (0.0097, 0.0639) | (0.0639, 0.1330) | (0.1330, 0.4623) | (0.4623, 0.8477) | (0.0097, 0.8477) |
| Lower–middle‐income countries ‡ | |||||
| Total, N | 532 | 532 | 532 | 532 | 2128 |
| HF death, n (%) | 1 (0.19) | 10 (1.88) | 16 (3.01) | 21 (3.95) | 48 (2.26) |
| HF hospitalization, n (%) | 6 (1.13) | 8 (1.50) | 16 (3.01) | 39 (7.33) | 69 (3.24) |
| Total HF (hospitalization and/or HF death), n (%) | 7 (1.32) | 17 (3.20) | 30 (5.64) | 56 (10.53) | 110 (5.17) |
| Predicted score range | (0.0055, 0.0266) | (0.0266, 0.0412) | (0.0412, 0.0622) | (0.0622, 0.3905) | (0.0055, 0.3905) |
| Upper–middle‐income countries § | |||||
| Total, N | 566 | 567 | 567 | 566 | 2266 |
| HF death, n (%) | 4 (0.71) | 6 (1.06) | 11 (1.94) | 21 (3.71) | 42 (1.85) |
| HF hospitalization, n (%) | 8 (1.41) | 22 (3.88) | 33 (5.82) | 59 (10.42) | 122 (5.38) |
| Total HF (hospitalization and/or HF death), n (%) | 12 (2.12) | 28 (4.94) | 40 (7.05) | 71 (12.54) | 151 (6.66) |
| Predicted score range | (0.0032, 0.0361) | (0.0361, 0.0590) | (0.0590, 0.0921) | (0.0921, 0.3764) | (0.0032, 0.3764) |
| High‐income countries ǁ | |||||
| Total, N | 1187 | 1188 | 1188 | 1188 | 4751 |
| HF death, n (%) | 0 (0.00) | 8 (0.67) | 9 (0.76) | 21 (1.77) | 38 (0.80) |
| HF hospitalization, n (%) | 8 (0.67) | 51 (4.29) | 81 (6.82) | 153 (12.88) | 293 (6.17) |
| Total HF (hospitalization and/or HF, n (%) death) | 8 (0.67) | 55 (4.63) | 86 (7.24) | 165 (13.89) | 314 (6.61) |
| Predicted score range | (0.0039, 0.0313) | (0.0313, 0.0529) | (0.0529, 0.0876) | (0.0876, 0.4300) | (0.0039, 0.4300) |
HF indicates heart failure.
Includes patients without missing values for the LVS‐HARMED (left ventricular, valvular heart disease, smoking or other tobacco use, height, age, rheumatic heart disease, myocardial infarction, emergency department discharge rhythm, and diabetes) score.
Includes Tanzania, Kenya, Mozambique, and Uganda.
Includes India, Sudan, Senegal, Zambia, Cameroon, Nigeria, Egypt, and the Ukraine.
Includes Argentina, Brazil, Colombia, Ecuador, Venezuela, Chile, Russia, Latvia, Turkey, Iran, South Africa, Thailand, and China.
Includes Japan, South Korea, Singapore, Saudi Arabia, the United Arab Emirates, Poland, Slovakia, Hungary, the Czech Republic, Bulgaria, Australia, Spain, Italy, the Netherlands, Germany, Austria, the United Kingdom, Sweden, Ireland, Canada, Denmark, and the United States.
Figure 1. Observed heart failure (HF) hospitalizations and HF deaths by quartile of LVS‐HARMED (left ventricular, valvular heart disease, smoking or other tobacco use, height, age, rheumatic heart disease, myocardial infarction, emergency department discharge rhythm, and diabetes) score.
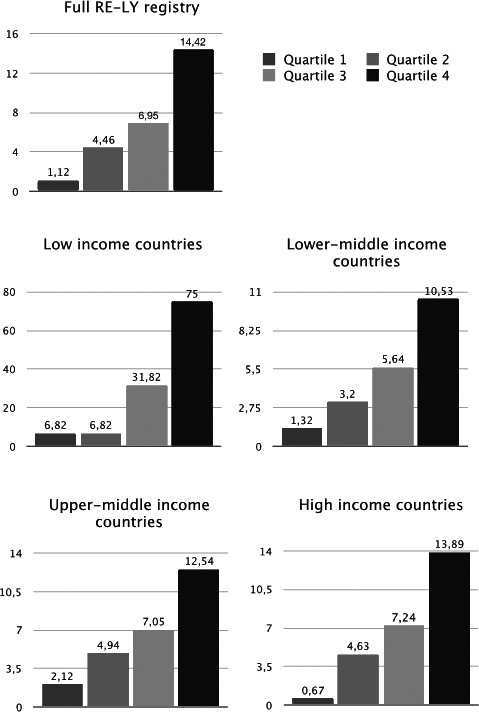
Observed HF hospitalizations and HF deaths across quartiles of the LVS‐HARMED score for 1‐year risk of new‐onset HF after an emergency consultation for atrial fibrillation among country income groups. RE‐LY indicates Randomized Evaluation of Long‐Term Anticoagulant Therapy.
In the full cohort, the composite end point of HF hospitalization and/or death occurred in 14.4% of the upper quartile; there were HF hospitalizations in 12.8%, and 2.7% died from HF. There was evidence of good fit in the full population (calibration slope, 1.07; Hosmer‐Lemeshow, P=0.22 [Figure S1]), as well as among income groups (Figures S9 through S12). The bootstrap validated estimate of the calibration slope was 0.934. By comparison, among RE‐LY AF registry patients who had follow‐up data for HF hospitalizations and/or HF death, but were excluded from the current analysis because of a history of HF (n=5169), 29.6% were either hospitalized for (26.1%) or died (7.5%) of HF within 1 year.
We also assessed the discrimination of the LVS‐HARMED score for prediction of 1‐year stroke risk. In the logistic mixed effects regression model with random intercept effect for country, the C statistic for the stroke end point was 0.755 (95% CI, 0.730–0.780).
Discussion
New‐onset HF is common in patients following an ED visit for AF, in all countries, regardless of income level. Within 1 year, 1 in 17 patients with AF without prior HF will be hospitalized for HF, and 21% of these patients will die. We derived a 1‐year prediction score for new‐onset HF in a population of ED patients with AF (LVS‐HARMED; www.phri.ca/LVS‐HARMED) using 9 variables that can easily be obtained for most patients in an ED. LVS‐HARMED identifies one quarter of patients presenting to the ED with AF who have a 1 in 7 risk of death or hospitalization attributable to new‐onset HF in the following year. These patients would likely benefit from referral to specialist care upon ED discharge and might benefit from interventions that target known modifiable HF risk factors. 16
HF is associated with substantial mortality, reduced quality of life, and economic costs. 17 With accurate risk prediction, it may be possible to prevent HF onset in AF, using a variety of interventions. In the ACTIVE‐I (Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events) trial, treatment with the angiotensin receptor blocker irbesartan reduced hospitalizations for HF in patients with AF. 18 In the population as a whole and among HF cases, less than half of the patients were using a renin‐aldosterone‐angiotensin system blocker at baseline and improved HF prediction and could therefore lead to improved medical therapy in a substantial number of patients. Risk factor management in individuals with a high risk of HF has been shown to be both effective, reducing the risk of new‐onset left ventricular dysfunction or HF by almost half, and also cost‐efficient in the STOP‐HF (St Vincent’s Screening to Prevent Heart Failure) trial. 19 , 20 Several of the predictors identified in the LVS‐HARMED score may be suitable targets for intervention. The risk associated with LVH was high, as was the prevalence of LVH, particularly in low‐income countries. Effective treatment of known causes of LVH, such as hypertension and valvular heart disease, 21 as well as treatments that improve prognosis after myocardial infarction, may therefore be effective in reducing the risk of HF in patients with AF, particularly in low‐income countries. A history of myocardial infarction was more prevalent in lower–middle‐income countries, where use of β‐blockers, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers, which improve prognosis after high‐risk myocardial infarctions, 22 was the least prevalent.
Leaving the ED in AF was the strongest single predictor of incident HF hospitalization and/or death. Confounding‐by‐indication likely explains this association at least to some degree, since sicker patients may be less likely to receive rhythm control therapies in the ED, as are patients with a longer history of AF. However, there is still uncertainty regarding the benefit of rhythm control in this population, and it is possible that persistence of AF at the time of ED discharge could be causally related to incident HF. 23 In the AF‐CHF (Atrial Fibrillation and Congestive Heart Failure) and AFFIRM (Atrial Fibrillation Follow‐up Investigation of Rhythm Management) trials there was no lack of reduction in adverse cardiac outcomes in the rhythm control arms. 24 , 25 Patients in these studies had sinus rhythm maintained using antiarrhythmic medications, which had both toxicity and relatively modest efficacy. Catheter ablation for AF has been associated with reduced incidence of HF in a Danish nationwide registry study, 26 and the CASTLE‐AF (Catheter Ablation versus Standard Conventional Therapy in Patients With Left Ventricular Dysfunction and Atrial Fibrillation) trial reported a reduction in a composite end point of death and HF hospitalization in patients with established HF. 27 Recent results from EAST‐AFNET (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial), with reduction in a composite end point of mortality, stroke, and hospitalization for HF or acute coronary syndrome, as well as a nonsignificant association between early rhythm control therapy and reduced risk of HF hospitalization, is in line with these results. 28 As yet, it has not been determined whether the superior rhythm control strategy inherent in ablation could reduce new‐onset HF in patients with AF. 29 Further analyses of published studies 30 and future studies, such as the RAFT‐AF (Randomized Ablation‐based Atrial Fibrillation Rhythm Control Versus Rate Control Trial in Patients With Heart Failure and High Burden Atrial Fibrillation) trial, are needed to determine this. 31
Predictors for HF in patients with AF have been previously studied in smaller cohorts, notably in the FHS (n=725), which derived a prediction algorithm for the 10‐year risk of HF in AF. 8 Several predictors (age, history of myocardial infarction or diabetes, left ventricular hypertrophy, and evidence of significant valvular disease) are included in both the FHS prediction score and LVS‐HARMED, and many of the same variables also predict HF in the general population. 32 The RE‐LY AF registry provides complementary data to the Framingham analysis by including an ED population and patients from countries representing a range of national income, with variations in the prevalence risk factors for both AF and HF. In contrast to the FHS, the RE‐LY AF registry evaluates early, 1‐year outcomes in patients following an ED visit with AF, identifying a group of patients who might benefit from early postdischarge referral to specialist care. 19 In this time frame and among ED patients, the LVS‐HARMED score had somewhat better discrimination than the FHS score, which is intended to be used to predict 10‐year risk. Furthermore, the LVS‐HARMED score also had good discrimination for a stroke outcome. 33 , 34
Strengths and Limitations
The RE‐LY AF registry includes patients from 47 different countries and data from an ED setting. This has permitted us to generate a score that is based on variables that are easy to obtain in the ED and is valid worldwide. Many risk scores are developed in less diverse settings; healthcare access and practices vary widely among the included countries, as do underlying conditions. Despite this, the score had good calibration and discrimination among income groups and geographic regions. Some score components, such as rheumatic heart disease, likely contribute more to the discrimination of the score in some settings, whereas other components likely play a larger role in others. Similarly, factors leading to hospital admission for HF differ among the countries included in the RE‐LY registry. No particular testing such as N‐terminal pro–brain natriuretic peptide, chest radiograph, or echocardiogram was required to confirm the diagnosis; this would have introduced a bias where high‐income countries or in some settings individual patients with sufficient resources to pay for diagnostic testing, would be over‐represented as cases. As it is, we would argue that the LVS‐HARMED score will in each setting identify the cases that are relevant in that setting. Regrettably, time‐to‐event data were not captured––the events were recorded as a binary variable at the end of a 1‐year follow‐up. Individuals from the highest‐income countries are over‐represented in the RE‐LY AF registry, but there appears to have been sufficient numbers from low‐income and low/middle‐income countries to highlight important differences concerning these populations. However, the smaller number of individuals in these countries precluded dividing the sample into derivation and validation cohorts. Instead, the risk score was validated internally in the full population using bootstrap resampling, as recommended by Steyerberg et al, 14 and showed good discrimination after correction of optimism. External validation of the risk score in the AFFORD cohort yielded similar results. Furthermore, the discrimination of the LVS‐HARMED score was acceptable or better in all income groups and geographic regions, demonstrating generalizability of the score to a variety of settings. Rather than creating an integer‐based points score, our prediction model weighted predictor variables by their beta‐coefficients (rounded to 3 decimals), in order to avoid unnecessary dichotomization of predictors, which may introduce bias and reduce statistical power. 35 Because of the diverse setting of the RE‐LY population, the score does not include biomarkers. Future studies are needed to ascertain whether the addition of biomarkers may improve the discrimination of LVS‐HARMED in settings where biomarker use is feasible.
Conclusions
The risk of new‐onset HF is high in patients following an ED visit for AF in all countries, regardless of economic status. This risk can be quantified using the LVS‐HARMED score. Preventative strategies should be considered in patients with high LVS‐HARMED HF risk.
Sources of Funding
Dr Johnson is supported by governmental funding within the Swedish National Health Services, the Swedish Society of Medicine, the Swedish Heart and Lung Association,and the Bergqvist Foundation. Dr Johnson and Dr Engström are supported by the Swedish Heart and Lung Foundation. Dr Ezekowitz is funded from the Sharpe‐Strumia foundation or Bryn Mawr Hospital. Dr Conen holds a McMaster University Department of Medicine Mid‐Career Research Award; his work is supported by the Hamilton Health Sciences RFA Strategic Initiative Program. Dr McIntyre holds fellowship awards from the Canadian Institutes for Health Research (CIHR) and the Canadian Stroke Prevention Intervention Network (C‐SPIN). Dr Healey holds the Stuart Connolly Chair in Cardiology Research at the Population Health Research Institute, and the Salim Yusuf Chair at Hamilton Health Sciences. Dr Barrett is partially funded by National Institutes of Health (NIH)/ National Institute on Drug Abuse (NIDA) CTN0099 ED INNOVATION study and CDC/Tennessee Department of Health Services contract: 34301‐29420. The AFFORD study was funded by NIH grant K23 HL102069 from the National Heart, Lung and Blood Institute, Bethesda, Maryland. Dr McNaughton is partially funded by the NIH (R21HL140381), Veterans Affairs (IIR‐19‐134), and Pfizer. The RE‐LY AF registry was funded by Boehringer‐Ingelheim.
Disclosures
None.
Supporting information
Table S1–S4
Figure S1–S12
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017735.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Zheng ZJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. DOI: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta‐analysis. BMJ. 2016;354:i4482. DOI: 10.1136/bmj.i4482. [DOI] [PubMed] [Google Scholar]
- 3. Chamberlain AM, Redfield MM, Alonso A, Weston SA, Roger VL. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail. 2011;4:740–746. DOI: 10.1161/CIRCHEARTFAILURE.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, Commerford P, Jansky P, Avezum A, Sigamani A, et al.; Registry R‐LAF and Cohort Study I . Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet. 2016;388:1161–1169. DOI: 10.1016/S0140-6736(16)30968-0. [DOI] [PubMed] [Google Scholar]
- 5. Ko D, Schnabel RB, Trinquart L, Benjamin EJ. The changing landscape of atrial fibrillation: time to target heart failure prevention. JACC Heart Fail. 2017;5:561–564. DOI: 10.1016/j.jchf.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 6. Rozen G, Hosseini SM, Kaadan MI, Biton Y, Heist EK, Vangel M, Mansour MC, Ruskin JN. Emergency department visits for atrial fibrillation in the United States: trends in admission rates and economic burden from 2007 to 2014. J Am Heart Assoc. 2018;7:e009024. DOI: 10.1161/JAHA.118.009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parkash R, Magee K, McMullen M, Clory M, D’Astous M, Robichaud M, Andolfatto G, Read B, Wang J, Thabane L, et al. The Canadian Community Utilization of Stroke Prevention Study in Atrial Fibrillation in the Emergency Department (C‐CUSP ED). Ann Emerg Med. 2019;73:382–392. DOI: 10.1016/j.annemergmed.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 8. Schnabel RB, Rienstra M, Sullivan LM, Sun JX, Moser CB, Levy D, Pencina MJ, Fontes JD, Magnani JW, McManus DD, et al. Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail. 2013;15:843–849. DOI: 10.1093/eurjhf/hft041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, Kowey PR, Mahaffey KW, Hylek E, Peterson ED, et al. Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail. 2017;5:44–52. DOI: 10.1016/j.jchf.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 10. Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P, Zhu J, Jansky P, Sigamani A, Morillo CA, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE‐LY Atrial Fibrillation Registry. Circulation. 2014;129:1568–1576. DOI: 10.1161/CIRCULATIONAHA.113.005451. [DOI] [PubMed] [Google Scholar]
- 11. World Bank . World Bank list of economies 2011. http://wdronline.worldbank.org/worldbank/a/incomelevel. Accessed 13 July, 2017.
- 12. Barrett TW, Storrow AB, Jenkins CA, Abraham RL, Liu D, Miller KF, Moser KM, Russ S, Roden DM, Harrell FE, et al. The AFFORD clinical decision aid to identify emergency department patients with atrial fibrillation at low risk for 30‐day adverse events. Am J Cardiol. 2015;115:763–770. DOI: 10.1016/j.amjcard.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schelldorfer J, Meier L, Buhlmann P. GLMMLasso: an algorithm for high‐dimensional generalized linear mixed models using l1‐penalization. J Comput Graph Stat. 2014;23:460–477. [Google Scholar]
- 14. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–138. DOI: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. DOI: 10.1016/S0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 16. Chatterjee NA, Chae CU, Kim E, Moorthy MV, Conen D, Sandhu RK, Cook NR, Lee IM, Albert CM. Modifiable risk factors for incident heart failure in atrial fibrillation. JACC Heart Fail. 2017;5:552–560. DOI: 10.1016/j.jchf.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. DOI: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 18. Investigators AI , Yusuf S, Healey JS, Pogue J, Chrolavicius S, Flather M, Hart RG, Hohnloser SH, Joyner CD, Pfeffer MA, et al. Irbesartan in patients with atrial fibrillation. N Engl J Med. 2011;364:928–938. DOI: 10.1056/NEJMoa1008816. [DOI] [PubMed] [Google Scholar]
- 19. Ledwidge M, Gallagher J, Conlon C, Tallon E, O’Connell E, Dawkins I, Watson C, O’Hanlon R, Bermingham M, Patle A, et al. Natriuretic peptide‐based screening and collaborative care for heart failure: the STOP‐HF randomized trial. JAMA. 2013;310:66–74. DOI: 10.1001/jama.2013.7588. [DOI] [PubMed] [Google Scholar]
- 20. Ledwidge MT, O'Connell E, Gallagher J, Tilson L, James S, Voon V, Bermingham M, Tallon E, Watson C, O'Hanlon R, et al. Cost‐effectiveness of natriuretic peptide‐based screening and collaborative care: a report from the STOP‐HF (St Vincent's Screening TO Prevent Heart Failure) study. Eur J Heart Fail. 2015;17:672–679. DOI: 10.1002/ejhf.286. [DOI] [PubMed] [Google Scholar]
- 21. Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108:7–13. DOI: 10.7326/0003-4819-108-1-7. [DOI] [PubMed] [Google Scholar]
- 22. Bahit MC, Kochar A, Granger CB. Post‐myocardial infarction heart failure. JACC Heart Fail. 2018;6:179–186. DOI: 10.1016/j.jchf.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 23. Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. 2016;13:131–147. DOI: 10.1038/nrcardio.2015.191. [DOI] [PubMed] [Google Scholar]
- 24. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. DOI: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 25. Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. DOI: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 26. Modin D, Claggett B, Gislason G, Hansen ML, Worck R, Johannessen A, Hansen J, Svendsen JH, Pallisgaard JL, Schou M, et al. Catheter ablation for atrial fibrillation is associated with lower incidence of heart failure and death. Europace. 2020;22:74–83. DOI: 10.1093/europace/euz264. [DOI] [PubMed] [Google Scholar]
- 27. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379:492. DOI: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 28. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. Early rhythm‐control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. DOI: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 29. Jaïs P, Cauchemez B, Macle L, Daoud E, Khairy P, Subbiah R, Hocini M, Extramiana F, Sacher F, Bordachar P, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. DOI: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 30. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. DOI: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhythm Control ‐ Catheter Ablation With or Without Anti‐arrhythmic Drug Control of Maintaining Sinus Rhythm Versus Rate Control With Medical Therapy and/or Atrio‐ventricular Junction Ablation and Pacemaker Treatment for Atrial Fibrillation. https://clinicaltrials.gov/ct2/show/NCT01420393. Accessed 5 July, 2021.
- 32. Yang H, Negishi K, Otahal P, Marwick TH. Clinical prediction of incident heart failure risk: a systematic review and meta‐analysis. Open Heart. 2015;2:e000222. DOI: 10.1136/openhrt-2014-000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van den Ham HA, Klungel OH, Singer DE, Leufkens HG, van Staa TP. Comparative performance of ATRIA, CHADS2, and CHA2DS2‐VASc risk scores predicting stroke in patients with atrial fibrillation: results from a National Primary Care Database. J Am Coll Cardiol. 2015;66:1851–1859. DOI: 10.1016/j.jacc.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 34. Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: Nationwide cohort study. BMJ. 2011;342:d124. DOI: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. DOI: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1–S4
Figure S1–S12


