Abstract
Background
Increased systolic blood pressure variability (BPV) is associated with stroke, cardiovascular disease, and dementia and mild cognitive impairment. However, prior studies assessing the relationship between BPV and dementia or mild cognitive impairment had infrequent measurement of blood pressure or suboptimal blood pressure control.
Methods and Results
We performed a post hoc analysis of the SPRINT (Systolic Blood Pressure Intervention Trial) MIND (Memory and Cognition in Decreased Hypertension) trial. The primary outcome was probable dementia during follow‐up. We defined our exposure period, during which blood pressures were collected, as the first 600 days of the trial, and outcomes were ascertained during the subsequent follow‐up. BPV was measured as tertiles of systolic blood pressure standard deviation. We fit Cox proportional hazards models to our outcome. We included 8379 patients. The mean follow‐up was 3.2±1.4 years, during which 316 (3.8%) patients developed dementia. The mean number of blood pressure measurements was 7.8, and in the tertiles of BPV, the SD was 6.3±1.6, 10.3±1.1, and 16.3±3.6 mm Hg, respectively. The rate of dementia was 2.4%, 3.6%, and 5.4% by ascending tertile, respectively (P<0.001). In the Cox models, compared with the lowest tertile of BPV, the highest tertile of BPV increased the risk of dementia in both unadjusted (hazard ratio [HR], 2.36; 95% CI, 1.77–3.15) and adjusted (HR, 1.69; 95% CI, 1.25–2.28) models.
Conclusions
In a post hoc analysis of the SPRINT MIND trial, we found that higher BPV was associated with the development of probable dementia despite excellent blood pressure control. Additional research is needed to understand how to reduce BPV and if its reduction lowers the risk of cognitive impairment and dementia.
Keywords: blood pressure variability, dementia, mild cognitive impairment
Subject Categories: Aging, Cognitive Impairment, High Blood Pressure
Increased systolic blood pressure variability (BPV) has been linked to the development of stroke, cardiovascular disease, and dementia and mild cognitive impairment (MCI), independent of mean blood pressure. 1 , 2 In addition, research has demonstrated the importance of blood pressure control for the prevention of dementia and MCI. 3 However, previous studies evaluating the relationship between BPV and dementia or MCI relied on longitudinal cohorts, with infrequent measurement of blood pressure or suboptimal blood pressure control. 2 We hypothesized that in a cohort with frequent visit‐to‐visit blood measurements and excellent blood pressure control, BPV would retain its harmful effects on cognition.
Methods
We performed a post hoc analysis of the SPRINT (Systolic Blood Pressure Intervention Trial) MIND (Memory and Cognition in Decreased Hypertension) trial, using a publicly available deidentified data set supplied by the National Heart, Lung, and Blood Institute. 4 Institutional review board approval or participant consent was not required for this post hoc analysis of deidentified data per University of Utah institutional review board policy. Our primary outcome was incident probable dementia, and secondary outcomes were MCI and the composite of dementia/MCI. The rigorous adjudication of probable dementia and MCI in the SPRINT MIND trial has previously been described and relied on both screening and adjudication at follow‐up visits and planned cognitive testing at years 2 and 4, and study closeout when it was >1 year after the year‐4 evaluation. 4 We defined our exposure period, during which blood pressures were collected at study visits, as the first 600 days of the SPRINT trial, and outcomes were recorded during the subsequent SPRINT MIND follow‐up period.
The exposure of visit‐to‐visit BPV was measured as tertiles of systolic blood pressure SD, and to improve accuracy of BPV measurement, we excluded patients with <4 blood pressure measurements. In the SPRINT trial, trained study personnel recorded seated blood pressures according to a protocol at scheduled study visits. The blood pressure used in the present study is a single value per visit that represents the average of 3 seated blood pressures at each study visit. To standardize the time between blood pressure measurements, we excluded as‐needed study visit blood pressures, and to limit the confounding of trial interventions, we also excluded the baseline visit blood pressure. We included blood pressures from up to 9 scheduled visits at 1, 2, 3, 6, 9, 12, 15, 18, and 21 months from enrollment. We calculated the SD using the formula:
We fit Cox proportional hazards models to our outcomes and a priori adjusted for patient age, sex, race/ethnicity, history of cardiovascular disease, hypertension, education, level of physical activity, current smoking status, SPRINT randomization arm, number of blood pressure measurements, and mean systolic blood pressure during the exposure. We verified the proportional hazards assumption of our final model (global test, P=0.498). We also included the interaction of randomization arm×BPV in our model and then stratified by randomization arm to explore the effect of BPV in patients with standard versus intensive blood pressure control. We subsequently tested the interactions between BPV and the other covariates in our adjusted model. As a sensitivity analysis, we confirmed our results using coefficient of variation and residual SD to generate tertiles of BPV, instead of SD. All analyses were performed in Stata 16.1 (StataCorp, College Station, TX).
Results
Of the 8563 patients who completed at least 1 cognitive assessment in the SPRINT MIND trial, we included 8379 and excluded 110 for insufficient blood pressure data, 24 for having the outcome or being right‐censored during the exposure period, and 32 for having missing demographic data or covariates in our adjusted model. Baseline demographics are shown in Table 1. During the 600‐day exposure period, the mean number of systolic blood pressure measurements was 7.8±0.6, and mean systolic blood pressure was 128.5 mm Hg. In the tertiles of BPV, the SD was 6.3±1.6, 10.3±1.1, and 16.3±3.6 mm Hg, respectively. The mean follow‐up was 3.2±1.4 years, during which 316 (3.8%) patients developed dementia. In tertiles of increasing BPV, the rate of dementia was 2.4%, 3.6%, and 5.4%, respectively (P<0.001). Similar increases were seen for the secondary outcomes (Table 1).
Table 1.
Baseline Demographics in the Full Cohort and Tertiles of BPV
| Variable | Full cohort, n=8379 | Lowest tertile of BPV, n=2796 | Middle tertile of BPV, n=2791 | Highest tertile of BPV, n=2792 | P value* |
|---|---|---|---|---|---|
| Age, y | 67.9±9.3 | 66.7±8.9 | 67.8±9.1 | 69.1±9.5 | <0.001 |
| Age ≥75 y | 2049 (24.5%) | 564 (20.2%) | 644 (23.1%) | 841 (30.1%) | <0.001 |
| Male sex | 5438 (64.9%) | 1968 (71.0%) | 1849 (66.3%) | 1603 (57.4%) | <0.001 |
| Race/ethnicity | |||||
| White | 4913 (58.6%) | 1589 (56.8%) | 1730 (62.0%) | 1594 (57.1%) | <0.001 |
| Black | 2458 (29.3%) | 807 (28.9%) | 753 (27.0%) | 898 (32.2%) | |
| Hispanic | 866 (10.4%) | 349 (12.5%) | 265 (9.5%) | 252 (9.0%) | |
| Other† | 142 (1.7%) | 51 (1.8%) | 43 (1.5%) | 48 (1.7%) | |
| History of diabetes | 137 (1.6%) | 34 (1.2%) | 49 (1.8%) | 54 (1.9%) | 0.088 |
| History of hypertension | 7779 (92.8%) | 2553 (91.3%) | 2588 (92.7%) | 2638 (94.5%) | <0.001 |
| History of peripheral vascular disease, n=8371 | 448 (5.4%) | 121 (4.3%) | 137 (4.9%) | 190 (6.8%) | <0.001 |
| History of atrial fibrillation, n=8366 | 657 (7.9%) | 198 (7.1%) | 208 (7.5%) | 251 (9.0%) | 0.017 |
| History of cardiovascular disease | 1663 (19.9%) | 494 (17.7%) | 518 (18.6%) | 651 (23.3%) | <0.001 |
| History of stroke, n=8377 | 44 (0.5%) | 14 (0.5%) | 11 (0.4%) | 19 (0.7%) | 0.325 |
| Smoking | |||||
| Never | 3713 (44.3%) | 1276 (45.6%) | 1237 (44.3%) | 1200 (43.0%) | <0.001 |
| Past | 3601 (43.0%) | 1219 (43.6%) | 1201 (43.0%) | 1181 (42.3%) | |
| Current | 1065 (12.7%) | 301 (10.8%) | 353 (12.7%) | 411 (14.7%) | |
| Alcoholism | 324 (3.9%) | 95 (3.4%) | 112 (4.0%) | 117 (4.2%) | 0.270 |
| Vigorous physical activity | |||||
| ≤1/wk | 4486 (53.6%) | 1406 (50.3%) | 1486 (53.2%) | 1594 (57.1%) | <0.001 |
| 1–4/wk | 2767 (33.0%) | 993 (35.5%) | 902 (32.3%) | 872 (31.2%) | |
| ≥5/wk | 1126 (13.4%) | 397 (14.2%) | 403 (14.5%) | 326 (11.7%) | |
| Aspirin use | 4313 (51.5%) | 1422 (50.9%) | 1428 (51.2%) | 1463 (52.4%) | 0.481 |
| Retired | 5036 (60.1%) | 1558 (55.7%) | 1689 (60.5%) | 1789 (64.1%) | <0.001 |
| Education | |||||
| Less than college/other | 5053 (60.3%) | 1611 (57.6%) | 1655 (59.3%) | 1787 (64.0%) | <0.001 |
| College | 1227 (14.6%) | 437 (15.6%) | 412 (14.8%) | 378 (13.5%) | |
| Grad school | 2099 (25.1%) | 748 (26.8%) | 724 (25.9%) | 627 (22.5%) | |
| Randomized to intensive blood pressure arm | 4214 (50%) | 1498 (53.3%) | 1412 (50.3%) | 1304 (46.4%) | <0.001 |
| Mean no. of antihypertensive medications during follow‐up | 2.7±1.2 | 2.5±1.1 | 2.7±1.2 | 3.0±1.2 | <0.001 |
| No. of blood pressure measurements | 7.8±0.7 | 7.8±0.7 | 7.8±0.7 | 7.7±0.7 | 0.002 |
| Mean systolic blood pressure during the exposure | 128.5±9.8 | 126.2±9.7 | 128.1±9.3 | 131.2±9.8 | <0.001 |
| Percentage of study visits with hypotension, SBP <90 or DBP <50 mm Hg | 2.1±8.5 | 1.2±6.9 | 1.7±7.7 | 3.4±10.3 | <0.001 |
| Systolic blood pressure at the beginning of the exposure | 128.4±14.6 | 126.1±11.4 | 127.9±13.1 | 131.1±18.1 | <0.001 |
| Systolic blood pressure at the end of the exposure | 127.6±14.9 | 125.9±11.6 | 127.2±13.4 | 129.6±18.5 | <0.001 |
| Standard deviation of systolic blood pressure | 10.9±4.7 | 6.3±1.6 | 10.3±1.1 | 16.3±3.6 | <0.001 |
P value is for the comparison between the tertiles of BPV, tested with the χ2 test for binary variables and ANOVA for interval variables. Binary variables are presented as n (%) and interval variables as mean±SD. BPV indicates blood pressure variability; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
SPRINT.
In the Cox models, the highest tertile of BPV, compared with the lowest, increased the risk of dementia in both unadjusted (hazard ratio [HR], 2.36; 95% CI, 1.77–3.15) and adjusted (adjusted HR, 1.69; 95% CI, 1.25–2.28) models (Table 2). For the secondary outcomes, we found that the highest tertile of BPV was associated with both MCI (adjusted HR, 1.40; 95% CI, 1.14–1.71), and the composite of dementia/MCI (adjusted HR, 1.43; 95% CI, 1.20–1.71) (Table 2). The interaction term between randomization arm and BPV was not significant (P=0.557), indicating that the effect of BPV was present in both the standard and intensive blood pressure control arms. The Kaplan‐Meier curves for the tertiles of BPV after stratification by randomization arm are shown in the Figure and demonstrate a consistent relationship between higher BPV and the risk of dementia in both randomization arms.
Table 2.
Event Rate of the Outcomes by Tertiles of Systolic Standard Deviation and Cox Proportional Hazards Models Fit to the Outcomes
| Outcome | Tertile of systolic standard deviation (range in mm Hg) | Event rate, n, % | Unadjusted hazard ratio (95% CI) | P value | Adjusted hazard ratio* (95% CI) | P value |
|---|---|---|---|---|---|---|
| Probable dementia | Lowest (0.5–8.5) | 67/2796, 2.4% | Ref | … | Ref | … |
| Middle (8.5–12.3) | 99/2791, 3.6% | 1.51 (1.11–2.06) | 0.009 | 1.39 (1.02–1.91) | 0.037 | |
| Highest (12.3–41.1) | 150/2792, 5.4% | 2.36 (1.77–3.15) | <0.001 | 1.69 (1.25–2.28) | 0.001 | |
| Mild cognitive Impairment | Lowest (0.5–8.5) | 169/2796, 6.0% | Ref | … | Ref | … |
| Middle (8.5–12.3) | 195/2791, 7.0% | 1.18 (0.96–1.45) | 0.119 | 1.13 (0.92–1.40) | 0.237 | |
| Highest (12.3–41.1) | 264/2792, 9.5% | 1.64 (1.36–2.00) | <0.001 | 1.40 (1.14–1.71) | 0.002 | |
| Composite of probable dementia and mild cognitive impairment | Lowest (0.5–8.5) | 218/2796, 7.8% | Ref | … | Ref | … |
| Middle (8.5–12.3) | 264/2791, 9.5% | 1.24 (1.04–1.48) | 0.020 | 1.17 (0.97–1.40) | 0.094 | |
| Highest (12.3–41.1) | 370/2792, 13.3% | 1.94 (1.51–2.11) | <0.001 | 1.43 (1.20–1.71) | <0.001 |
Adjusted for patient age, sex, race/ethnicity, history of cerebrovascular disease, hypertension, education, level of physical activity, smoking status, percentage of study visits during the exposure with hypotension (SBP <90 or DBP <50 mm Hg), mean SBP during the exposure, and randomization arm. DBP indicates diastolic blood pressure; Ref, reference; and SBP, systolic blood pressure.
Figure 1. Kaplan‐Meier curves showing failure rates for probable dementia in the first 1500 days of follow‐up after stratification by the standard vs intensive blood pressure reduction arm in SPRINT.
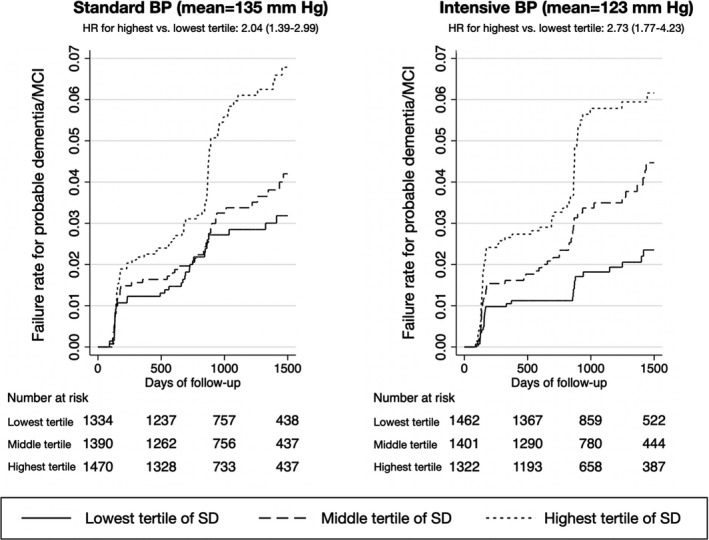
BP indicates blood pressure; HR, hazard ratio; MCI, mild cognitive impairment; and SPRINT, Systolic Blood Pressure Intervention Trial.
We tested the interactions between BPV and the other covariates in our adjusted model and found that none had a significant interaction (all P>0.1). In the sensitivity analysis, where we used coefficient of variation and residual standard deviation to make tertiles of BPV, we found consistent results. For example, in the adjusted model fit to our primary outcome, the HR for dementia in the top tertile of coefficient of variation was 1.53 (95% CI, 1.15–2.05), whereas in the top tertile of residual standard deviation it was 1.81 (95% CI, 1.33–2.46).
Discussion
In a post hoc analysis of the SPRINT MIND trial, we found that visit‐to‐visit BPV was associated with the development of probable dementia, MCI, and the composite of both. This association was present in both the standard and intensive target blood pressure randomization arms, indicating residual risk in the intensive arm, some of which may be attributable to BPV. The implication of these findings is that BPV has an impact on cognition independent of mean blood pressure. Prior longitudinal studies have found similar findings, but either relied on blood pressure readings at study visits separated by years, 5 , 6 , 7 , 8 daily readings for less than a month, 9 or dementia adjudication that was not as robust. 10 In addition, the existing data are largely from community‐dwelling cohorts with higher mean blood pressure than the SPRINT MIND cohort. 2 We show that despite the excellent blood pressure control in the SPRINT MIND trial, BPV remains a risk factor for developing cognitive impairment, which is a key outcome for older adults and warrants additional preventative research and routine screening in primary care. 11
There are several potential explanations for the association between increased BPV and dementia or MCI. Although increased BPV has been associated with a higher risk of stroke, 12 which is associated with dementia, we did not find a difference in the rate of stroke in the tertiles of BPV. Secondary to the stiffer arteries that can cause increased BPV, BPV has been linked to intermittent cerebral hypoperfusion, which can predispose to vascular dementia. 2 A study also showed an association between increased BPV and neurofibrillary tangle pathology on autopsy, which is the hallmark of Alzheimer disease, as opposed to vascular dementia. 8 Hypotension has been identified as a potential risk factor for the development of dementia. 13 Although we found that hypotension at study visits was more common in patients in higher tertiles of BPV, we adjusted for hypotension in our multivariate models, and the association between increased BPV and dementia remained significant.
Ultimately, the exact mechanism by which increased BPV may cause dementia remains uncertain, and increased BPV may be an epiphenomenon of another unidentified causal mechanism. Although post hoc analyses can identify a potential risk factor, there is always unmeasured confounding. A randomized clinical trial that attempts to lower the variability and mean of blood pressure, and with the end point of dementia or MCI, will be necessary to determine if BPV is a viable treatment target. Because both short‐term and longer‐term visit‐to‐visit increased BPV have both been associated with dementia and cardiovascular events, 9 , 14 to identify patients with increased BPV for such a trial, the inclusion criteria could have a qualifying period with 12 or 24 hours of ambulatory blood pressure monitoring. Prior research has shown that calcium channel blockers can reduce BPV, but the goal of reducing both systolic blood pressure mean and variability will require sophisticated medication titration. 15 Before a clinical trial can address the question of the therapeutic effects of lowering BPV, additional research is needed to determine how BPV will be reduced and if that reduction is safe.
Our study has several limitations, including that it is a post hoc analysis of a trial that was not designed to answer the proposed hypothesis. There are not identical exposures between patients, because the frequency of blood pressure measurement was dependent on the randomization arm, which may have introduced bias, although the mean number of measurements in the standard versus intensive arm did not differ significantly (7.8 versus 7.8 measurements, respectively; P=0.53), and the difference in the tertiles of BPV was not meaningfully different. We also were not able to examine neuroimaging mediators of the observed association, such as chronic microvascular disease or brain atrophy, because repeat brain magnetic resonance imaging was only available in a subset of patients, and the final study magnetic resonance imaging was at 48 months. The strengths of our study are that the outcome of dementia was rigorously adjudicated in the SPRINT MIND trial, patients had excellent blood pressure control, and we had an average of 7.8 blood pressure readings available for determining BPV.
Conclusions
In the SPRINT MIND study, blood pressure variability during the first 600 days was associated with subsequent development of probable dementia or MCI, despite excellent blood pressure control. The practical implication of this finding is that additional research is needed to understand how to reduce blood pressure variability and if its reduction lowers the risk of cognitive impairment and dementia.
Sources of Funding
Dr de Havenon reports National Institutes of Health/National Institute of Neurological Disorders and Stroke funding (K23NS105924).
Disclosures
Dr de Havenon has received investigator‐initiated clinical research funding from Regeneron and AMAG pharmaceuticals. The remaining authors have no disclosures to report.
Acknowledgments
The authors acknowledge the National Heart, Lung, and Blood Institute (NHLBI) and the SPRINT investigators for making the trial's data set publicly available. This article was prepared using SPRINT MIND Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of SPRINT MIND or the NHLBI.
For Sources of Funding and Disclosures, see page 5.
References
- 1. Muntner P, Whittle J, Lynch AI, Colantonio LD, Simpson LM, Einhorn PT, Levitan EB, Whelton PK, Cushman WC, Louis GT, et al. Visit‐to‐visit variability of blood pressure and coronary heart disease, stroke, heart failure and mortality: a cohort study. Ann Intern Med. 2015;163:329–338. DOI: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma Y, Tully PJ, Hofman A, Tzourio C. Blood pressure variability and dementia: a state‐of‐the‐art review. Am J Hypertens. 2020;33:1059–1066. DOI: 10.1093/ajh/hpaa119. [DOI] [PubMed] [Google Scholar]
- 3. Ou Y‐N, Tan C‐C, Shen X‐N, Xu W, Hou X‐H, Dong Q, Tan L, Yu J‐T. Blood pressure and risks of cognitive impairment and dementia. Hypertension. 2020;76:217–225. DOI: 10.1161/HYPERTENSIONAHA.120.14993. [DOI] [PubMed] [Google Scholar]
- 4. SPRINT MIND Investigators for the SPRINT Research Group , Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–561. DOI: 10.1001/jama.2018.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Middelaar T, van Dalen JW, van Gool WA, van den Born B‐JH, van Vught LA, Moll van Charante EP, Richard E. Visit‐to‐visit blood pressure variability and the risk of dementia in older people. J Alzheimers Dis. 2018;62:727–735. DOI: 10.3233/JAD-170757. [DOI] [PubMed] [Google Scholar]
- 6. Alpérovitch A, Blachier M, Soumaré A, Ritchie K, Dartigues J‐F, Richard‐Harston S, Tzourio C. Blood pressure variability and risk of dementia in an elderly cohort, the Three‐City Study. Alzheimers Dement. 2014;10:S330–S337. DOI: 10.1016/j.jalz.2013.05.1777. [DOI] [PubMed] [Google Scholar]
- 7. Ma Y, Wolters FJ, Chibnik LB, Licher S, Ikram MA, Hofman A, Ikram MK. Variation in blood pressure and long‐term risk of dementia: a population‐based cohort study. PLoS Med. 2019;16:e1002933. DOI: 10.1371/journal.pmed.1002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma Y, Blacker D, Viswanathan A, van Veluw SJ, Bos D, Vernooij MW, Hyman BT, Tzourio C, Das S, Hofman A. Visit‐to‐visit blood pressure variability, neuropathology, and cognitive function. Neurology. 2021;96:e2812–e2823. DOI: 10.1212/WNL.0000000000012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oishi E, Ohara T, Sakata S, Fukuhara M, Hata J, Yoshida D, Shibata M, Ohtsubo T, Kitazono T, Kiyohara Y, et al. Day‐to‐day blood pressure variability and risk of dementia in a general Japanese elderly population: the Hisayama Study. Circulation. 2017;136:516–525. DOI: 10.1161/CIRCULATIONAHA.116.025667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rouch L, Cestac P, Sallerin B, Piccoli M, Benattar‐Zibi L, Bertin P, Berrut G, Corruble E, Derumeaux G, Falissard B, et al. Visit‐to‐visit blood pressure variability is associated with cognitive decline and incident dementia. Hypertension. 2020;76:1280–1288. DOI: 10.1161/HYPERTENSIONAHA.119.14553. [DOI] [PubMed] [Google Scholar]
- 11. Scuteri A, Benetos A, Sierra C, Coca A, Chicherio C, Frisoni GB, Gasecki D, Hering D, Lovic D, Manios E, et al. Routine assessment of cognitive function in older patients with hypertension seen by primary care physicians: why and how—a decision‐making support from the working group on ‘hypertension and the brain’ of the European Society of Hypertension and from the European Geriatric Medicine Society. J Hypertens. 2021;39:90–100. DOI: 10.1097/HJH.0000000000002621. [DOI] [PubMed] [Google Scholar]
- 12. de Havenon A, Fino NF, Johnson B, Wong K‐H, Majersik JJ, Tirschwell D, Rost N. Blood pressure variability and cardiovascular outcomes in patients with prior stroke: a secondary analysis of PRoFESS. Stroke. 2019;50:3170–3176. DOI: 10.1161/STROKEAHA.119.026293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moretti R, Torre P, Antonello RM, Manganaro D, Vilotti C, Pizzolato G. Risk factors for vascular dementia: hypotension as a key point. Vasc Health Risk Manag. 2008;4:395–402. DOI: 10.2147/vhrm.s2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mancia G. Short‐ and long‐term blood pressure variability. Hypertension. 2012;60:512–517. DOI: 10.1161/HYPERTENSIONAHA.112.194340. [DOI] [PubMed] [Google Scholar]
- 15. Webb AJ, Rothwell PM. Effect of dose and combination of antihypertensives on interindividual blood pressure variability: a systematic review. Stroke. 2011;42:2860–2865. DOI: 10.1161/STROKEAHA.110.611566. [DOI] [PubMed] [Google Scholar]


