Abstract
Background
Patients with acute stroke at non‐ or primary stroke centers (PSCs) are transferred to comprehensive stroke centers for advanced treatments that reduce disability but experience significant delays in treatment and increased adjusted mortality. This study reports the results of a proactive, systematic, risk assessment of the door‐in‐door‐out process and its application to solution design.
Methods and Results
A learning collaborative (clinicians, patients, and caregivers) at 2 PSCs and 3 comprehensive stroke centers in Chicago, Illinois participated in a failure modes, effects, and criticality analysis to identify steps in the process; failures of each step, underlying causes; and to characterize each failure’s frequency, impact, and safeguards using standardized scores to calculate risk priority and criticality numbers for ranking. Targets for solution design were selected among the highest‐ranked failures. The failure modes, effects, and criticality analysis process map and risk table were completed during in‐person and virtual sessions. Failure to detect severe stroke/large‐vessel occlusion on arrival at the PSC is the highest‐ranked failure and can lead to a 45‐minute door‐in‐door‐out delay caused by failure to obtain a head computed tomography and computed tomography angiogram together. Lower risk failures include communication problems and delays within the PSC team and across the PSC comprehensive stroke center and paramedic teams. Seven solution prototypes were iteratively designed and address 4 of the 10 highest‐ranked failures.
Conclusions
The failure modes, effects, and criticality analysis identified and characterized previously unrecognized failures of the door‐in‐door‐out process. Use of a risk‐informed approach for solution design is novel for stroke and should mitigate or eliminate the failures.
Keywords: acute stroke; door‐in‐door‐out; failure modes, effects, and criticality analysis
Subject Categories: Cerebrovascular Disease/Stroke, Health Services, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- CN
criticality number
- CSC
comprehensive stroke center
- CTA
computed tomography angiography
- DIDO
door‐in‐door‐out
- FMECA
failure modes, effects, and criticality analysis
- LVO
large‐vessel occlusion
- PSC
primary stroke center
- RPN
risk prediction number
Clinical Perspective
What Is New?
Using a failure modes, effects, and criticality analysis, this analysis provides a process mapping and risk assessment of the door‐in‐door‐out processes for patients with acute stroke requiring transfer from primary to comprehensive stroke centers.
Our work reveals that the process involves >50 steps, with many failures leading to delays in the door‐in‐door‐out process.
Failure to perform an acute stroke screening scale by paramedics and/or by emergency department triage nurses upon arrival at the emergency department and failure to perform a severe stroke/large‐vessel occlusion scale at the beginning of the acute stroke diagnostic phase are 2 highly impactful underlying failures leading to door‐in‐door‐out delays.
What Are the Clinical Implications?
The increasing use of an acute stroke screening scale by paramedics and emergency department triage nurses and the routine use of a severe stroke/large‐vessel occlusion scale may significantly reduce door‐in‐door‐out delays.
Timely access to treatment can reduce disability from acute stroke. 1 Yet, treatment rates for acute ischemic stroke, which accounts for 85% of strokes, remain suboptimal. 2 , 3 , 4 , 5 An optimized stroke system that delivers the right patients to the right hospital would increase treatment rates, mitigate disability, and reduce stroke deaths by 20 000 annually nationwide. 6 Furthermore, analysis of nationwide data suggest that 13% of acute ischemic stroke admissions involve an interfacility transfer. 7 Patients with acute ischemic stroke who require transfer to a comprehensive stroke center (CSC) experience significant delays in onset‐to‐reperfusion time, 8 and transfer delay is the second most common reason (14%) next to clinical contraindications (40%) for endovascular therapy exclusion. 9 Overall, patients with acute stroke who are transferred have an increased adjusted mortality rate relative to patients directly presenting to a CSC. 10 The time from arrival to discharge for a transfer from a primary stroke center’s (PSC) emergency department (ED) is defined as the door‐in‐door‐out (DIDO) time and serves as a useful quality metric of acute stroke transfer. 11 Prior studies have noted that time from PSC arrival to CSC arrival averages 120 to 180 minutes, and PSC DIDO times are ≈100 to 120 minutes, 12 , 13 indicating that the DIDO process accounts for nearly two‐thirds of the total transfer process. 14
However, few prior studies have focused on the DIDO process of interfacility transfer. 15 , 16 , 17 , 18 Though several studies have implemented protocols to reduce DIDO times at PSCs, 12 , 17 some initiatives have, somewhat surprisingly, resulted in longer transfer times, 19 suggesting that the redesign of the interfacility transfer process needs to be fully assessed before implementation to assure that proposed solutions actually address the underlying causes of the failures. Currently, there is no standardization for critical components of the interfacility transfer process such as (1) essential information to be transmitted, (2) time goals for each step in the process, and (3) and optimal mode of transport.
Process improvement methods such as Six Sigma and Lean Management have been previously applied to myocardial infarction and stroke. 20 , 21 , 22 , 23 However, a failure modes, effects, and criticality analysis (FMECA), 24 a proactive risk assessment method that identifies the steps and then examines the causes, frequency, safeguards, and impact of the failures at each step of the process, has not been applied to many processes of acute stroke care. This article reports the results of a FMECA of the DIDO process at PSCs leading to transfers of patients with acute stroke to CSCs and their application to select targets for solution prototype design.
Methods
Any supporting data that are not within the article provided are available from the corresponding author upon request.
Study Design and Setting
The study was conducted at 2 PSCs and 3 CSCs (Table 1). Institutional review board approval was obtained at all of the centers, and all participants provided informed consent. The PSCs were Lake Forest Hospital and Rush Oak Park Hospital, community hospitals in the Chicago, Illinois suburbs, whereas the CSCs were Northwestern Memorial Hospital, Rush University Medical Center, and University of Illinois at Chicago Medical Center in Chicago, Illinois.
Table 1.
Characteristics of Participating Sites
| CSC 1 | PSC 1 | CSC 2 | PSC 2 | CSC 3 | |
|---|---|---|---|---|---|
| ED beds | 54 | 17 | 57 | 21 | 33 |
| Annual ED visits | 88 299 | 30 936 | 74 124 | 38 297 | 41 355 |
| Annual strokes | 647 | 114 | 972 | 103 | 450 |
| No. of EM MDs | 49 | 31 | 30 | 17 | 42 |
| No. of stroke neurologists | 6 | 2 | 6 | 2 | 4 |
| No. of neurointerventional radiology | 3 | 0 | 2 | 0 | 2 |
| No. of neurointensivists | 8 | 0 | 8 | 0 | 3 |
| No. of vascular neurosurgeons | 2 | 1 | 1 | 0 | 3 |
CSC indicates comprehensive stroke center; ED, emergency department; EM, emergency medicine; MDs, medical doctors; and PSC, primary stroke center.
A FMECA is an engineering method, used initially in high‐risk industries such as aerospace and nuclear power, 25 , 26 but increasingly applied to health care. 27 , 28 , 29 , 30 , 31 , 32 A FMECA involves engaging stakeholders to describe and depict the steps and workflows of a process and then to identify, characterize, and rank the safety vulnerabilities or failures of each step in the process in a risk table that permits rank ordering of the failures. FMECAs help to counter most organizations’ common temptation to focus on process solutions for the most evident and visible failures, which may not be the same. 33
Study Participants
A learning collaborative was used to conduct the FMECA. Learning collaboratives have been used increasingly and effectively in health care 34 by bringing together multidisciplinary stakeholders to collectively understand, design, and implement quality and safety interventions. A learning collaborative model typically includes (1) setting a defined and targeted goal, (2) including multidisciplinary teams, (3) including content or domain experts, and (4) holding frequent sessions with sharing of data and experiential learning. 35
A broad range of learning collaborative participants, including ED physicians and nurses and the stroke coordinator from the PSCs and stroke neurologists, neuro–intensive care unit nurses, the stroke coordinator, the transfer coordinator from the CSCs, a representative from the Chicago Fire Department Emergency Medical Services (EMS), the sole public provider of emergent transportation of patients with acute stroke, a representative from a private ambulance company that provides interfacility transfers in Chicago, and patients with acute stroke who had experienced a transfer to a CSC and their caregivers were recruited. Learning collaborative members received a modest remuneration by gift certificate for participation in each FMECA and solution design session.
Process Mapping Sessions
Two in‐person sessions, scheduled well in advance to maximize participation, were held. All sessions were audio recorded. Participants were asked to focus on the DIDO process from arrival of a patient in a PSC ED to the patient leaving the PSC ED by ambulance for transfer to a CSC. Learning collaborative participants were asked to describe in their own words their roles, tasks, communications, and activities performed when providing care for an patient with acute stroke during this process. PSC participants were specifically asked to focus on the PSC ED processes for a patient with acute stroke for whom transfer to a CSC was needed, CSC participants on the processes involved in the decision about and arrangements for the transfer of a patient with acute stroke, the EMS participant on the processes at arrival in the PSC ED, and the private ambulance participant on the processes involved in arranging for and picking up the patient. Patients and caregivers were asked to describe their interactions with clinicians in the ED, including details about when discussion about a transfer was initiated, information provided about the transfer, and perceived efficiency of the transfer. Two investigators (J.L.H. and R.K.) facilitated the session by documenting each described role, task, communication, or activity by a participant on a Post‐It Note and placing the note on a white board. The notes were iteratively rearranged to represent the sequence of steps of the entire process. PSC 1 had, as part of a prior quality improvement initiative, created a process map about their ED processes for interfacility transfer of patients with acute stroke. The research team began with this process map and added information gathered from participants during the in‐person sessions to create a preliminary DIDO process map, using Microsoft Visio 2013 (Microsoft, Redmond, WA). Video‐conference sessions were held with participants for clarifications.
The preliminary process map was sent, by email, to all participants. Participants were asked to send comments via email or to print out the map and annotate it with any additions, deletions, or corrections and then scan the annotated map as a portable document format and email it back to the research team. The research team used the comments and annotated maps to further refine the process map. The refined process map was sent, by email, to all participants, and a video conference (N=1) was held with participants to reach consensus and approve the final process map (Figure) in January 2019.
Figure 1. Current state process map.
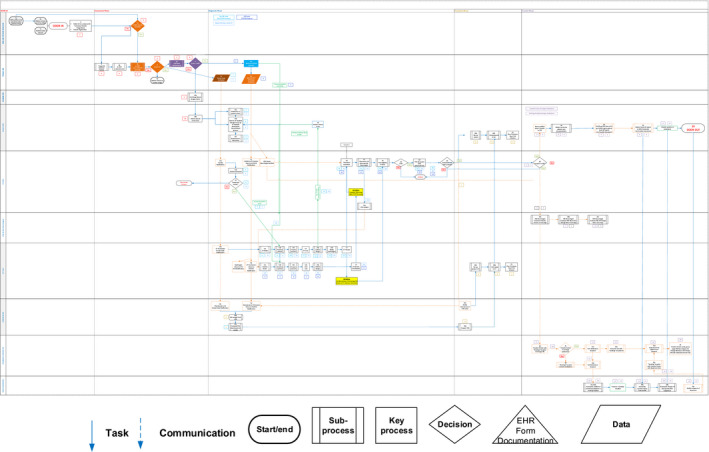
BEFAST indicates balance, eyes, face, arm, and speech test; CSC, comprehensive stroke center; CT indicates computed tomography; CTA, computed tomography angiography; ED, emergency department; EHR, electronic health record; EMS, emergency medical services; ICU, intensive care unit; IV, intravenous; LVO, large‐vessel occlusion; MD, medical doctor; RN, registered nurse; and TPA, tissue plasminogen activator.
Risk Table Completion
The final process map was used to complete, with learning collaborative members, a risk table during a series of video conferences (N=4) between January 2019 and June 2019. To complete the risk table, participants were asked for each step to systematically identify potential vulnerabilities or failures and their causes, and then estimate and score for each failure the (1) frequency, (2) impact on the DIDO process for a patient, and (3) strength of existing safeguards. A standardized FMECA risk assessment scoring matrix (Table 2) was used to score the frequency, impact, and safeguard characteristics of each failure.
Table 2.
Standardized Scores of FMECA to Optimize AS Door‐In‐Door‐Out Time
| Score |
Effect/impact/consequence of failure (impact) |
Frequency of failure (occurrence) |
Existing safeguard to mitigate failure (detection) |
|||
|---|---|---|---|---|---|---|
|
1 |
None |
No reason to expect failure to have any effect on safety, health, environment or mission. |
None |
1/10 000 |
Almost certain | Current control(s) almost certain to detect failure mode. Reliable controls are known with similar processes. |
|
2 |
Very low |
Minor disruption to process. Repair of failure can be quickly accomplished through verbal communication/phone call. No process delay. Example: Past medical history unknown. |
Very low |
1/5000 |
Very high |
Very high likelihood current control(s) will detect failure mode.
Example: EPIC: Automated AS screening scale that if greater than threshold requires performance of severe stroke/LVO scale. |
|
3 |
Low |
Minor disruption to process. Minor process delay (≈1–4 min). Example: Radiology technician pager not working; no AS screening scale performed in field. |
Low |
1/2000 |
High |
High likelihood current control(s) will detect failure mode.
Example: A pop‐up window with a reminder of how long the patient has been in the ED. |
|
4 |
Low to moderate |
Moderate disruption to process. Minor‐to‐moderate process delay (≈5–9 min). Example: No ED prenotification of possible AS by EMS; AS screening scale not performed in triage. |
Low to moderate |
1/1000 |
Moderately high |
Moderately high likelihood current control(s) will detect failure mode.
Example: A pop‐up window of differential diagnosis of stroke that does not require any action. |
|
5 |
Moderate |
Moderate disruption to process. Moderate process delay (≈10‐19 min). Example: Stroke symptoms not recognized by greeter/nurse; neurology resident/telestroke MD delay in responding. |
Moderate |
1/500 |
Moderate |
Moderate likelihood current control(s) will detect failure mode.
Example: Neurology MD (after EMS, triage nurse and/or ED MD) reviews history and physical exam without checklist or standard aid. |
|
6 |
Moderate to high |
Moderate disruption to process. Moderate‐to‐high process delay (≈20‐29 min). Example: Patient unable to report last known well and no family present in ED; no contact information in EMS record to gather event history from family. |
Moderate to high |
1/200 |
Low |
Low likelihood current control(s) will detect failure mode.
Example: ED MD (after EMS and/or triage nurse) gathers history of event and performs physical exam without a checklist or standard aid. |
|
7 |
High |
High disruption to process. Significant process delay (≥30 min). Example: Stroke code not activated at triage; no severe screening stroke/LVO screening scale used and patient needs to return to CT scanner for a CTA. |
High |
1/100 |
Very low |
Very low likelihood current control(s) will detect failure mode.
Example: Neurology MD gathers history of event. |
|
8 |
Very high |
Very high disruption to process. Significant process delay. Example: Walk‐in patient stroke symptoms not recognized by greeter or by triage nurse: patient waits hours before evaluation. |
Very high |
1/50 |
Remote |
Remote likelihood current control(s) will detect failure mode.
Example: CT technician asks if CTA is needed after CT. |
|
9 |
Hazard |
Potential safety, health, or environmental issue.* Example: tPA treatment delivered to nonstroke patient with hemorrhagic complication. |
Very high |
1/20 |
Very remote |
Very remote likelihood current control(s) will detect failure mode.
|
|
10 |
Hazard |
Potential safety, health, or environmental issue. † Example: Protocol violation: treatment outside 4.5‐h window or with absolute contraindication to alteplase; missed alteplase/EVT resulting in death; missed hemorrhagic stroke with herniation. |
Very high |
1/10 ‡ |
Almost impossible |
No known control(s) available to detect failure mode. Example: Stroke symptoms not recognized. |
AS indicates acute stroke; CT, computed tomography; CTA, computed tomography angiography; ED, emergency department; EMS, emergency medical services; EPIC, electronic health record; EVT, endovascular treatment; FMECA, failure modes, effects, and criticality analysis; LVO, large‐vessel occlusion; MD, medical doctor; and tPA, tissue plasminogen activator.
Patient suffers permanent damage. Patient treated past the point of full recovery and has partial brain damage.
Patient death.
Review=summation of history, physical (neurological) examination, diagnostic tests (eg, imaging, laboratory) findings.
When relevant, the research team used data from a Research Electronic Data Capture data entry form, described below, to score the characteristics of each failure. The scores were used to calculate a risk priority number (RPN) (product of frequency, impact, and strength of safeguard) and a criticality number (CN) (product of frequency by impact) for each failure, with the key difference between an RPN and a CN being the inclusion of strength of a safeguard. The RPNs and CNs permit rank ordering of the identified failures to help prioritize the most critical failures for solution prototype design.
Sources of Data for Risk Table Completion
A Research Electronic Data Capture data entry form was used by stroke coordinators at the 2 PSCs to securely enter deidentified data about all stroke cases. Abstracted data included specific time intervals in the DIDO process from patient arrival in the ED to (1) triage, (2) telestroke activation (if done), (3) start of computed tomography (CT), CT angiography (CTA), CT perfusion, and/or magnetic resonance imaging (if done), (4) initial CSC transfer center contact, (5) transfer ambulance request, and (6) transfer ambulance arrival and departure at the PSC. We also collected information on perceived delays at the PSC as determined by the stroke coordinators by chart review and included delay in triage/registration, missed or delayed stroke diagnosis, delay in CT reading or review, delay in acquisition of additional neuroimaging, delay in CSC contact, delay in ambulance arrival, delays because of paperwork/record copying before ambulance departure, medical instability, and patient/family‐related factors (eg, inability to obtain consent for treatments or authorization for transfer). For some scores (eg, frequency of telestroke physician not responding), the learning collaborative participants used these data and together discussed their perceived level of risk to reach consensus on the scores during the video conferences to complete the risk table.
Selection of Targets for Solution Development
During a video‐conference session, learning collaborative members reviewed the 10 highest‐risk failures ranked by RPN and CN and selected targets for prototype solution development. Following a period of iterative solution prototyping, an in‐person session was held, and the learning collaborative members used a standardized scoring approach to narrow the selection of prototypes for further solution development.
Results
FMECA Participants
A total of 21 clinicians, 3 patients with acute stroke who had experienced a transfer to a CSC, and 2 caregivers (Table 3) participated in the FMECA, beginning in November 2018 and ending in June 2019. The solution prototype design was then undertaken by the research team with iterative reviews and feedback by the learning collaborative clinician members until June 2020.
Table 3.
Learning Collaborative Participants by Site and Role
| Sites | Roles | |||||
|---|---|---|---|---|---|---|
| Stroke Coordinator | ED Physician | ED Nurse | Transfer Coordinator | Stroke Neurologist | Neuro ICU Nurse | |
| PSC 1 | 1 | 3 | 1 | |||
| PSC 2 | 1 | 1 | 1 | |||
| CSC 1 | 1 | … | 2 | … | ||
| CSC 2 | 1 | 1 | 1 | 1 | ||
| CSC 3 | 1 | 1 | 1 | 1 | ||
| Total | 5 | 4 | 2 | 2 | 4 | 2 |
| Patients | Caregivers | Chicago EMS | Private Ambulance | |||
| 3 | 2 | 1 | 1 | |||
CSC indicates comprehensive stroke center; ED, emergency department; and PSC, primary stroke center.
FMECA Process Map and Risk Table
The total number of steps by phase, as shown in Table 4, in the DIDO process ranges from 49 to 65 and varies by type of acute stroke (ischemic versus hemorrhagic). The full process map is shown in the Figure. The risk table was completed using data from the Research Electronic Data Capture database about 259 acute stroke cases at both PSCs.
Table 4.
Number of Steps by Phase of Door‐In‐Door‐Out
| Phases | |||||
|---|---|---|---|---|---|
| Tasks and activities | Assessment | Diagnostic | Treatment | Transfer | |
| Stroke screening scale | EMS or Triage | 8 | |||
| After triage | 10 | ||||
| Severe stroke or LVO screening before CT | Performed | 8 | |||
| Not Performed | 20 | ||||
| Severe stroke or LVO screening before CTA | Performed | 14 | |||
| Not Performed | 28 | ||||
| Alteplase in ED | 7 | ||||
| Alteplase in CT scanner | 7 | ||||
| Ambulance organized by transfer center | 20 | ||||
| Ambulance organized by sending hospital | 20 | ||||
| Total no. of steps | 49–65 | ||||
CT indicates computed tomography; CTA, computed tomography angiography; ED, emergency department; EMS, emergency medical services; and LVO, large‐vessel occlusion.
The 10 highest‐risk failures ranked by RPN and CN are shown in Table 5. Failure to perform a severe stroke/large‐vessel occlusion (LVO) scale (heretofore LVO scale) at the beginning of the diagnostic phase and instead relying on clinical judgement, contributes to the highest ranked failure of not recognizing the need to obtain a head CT and CTA during a single transfer of the patient to the CT scanner suite, early in the diagnostic phase. This failure (Figure, step 21a) has a major impact on the DIDO process, increasing the DIDO time by up to 45 minutes, based on stroke registry data (DIDO time, whether the patient had an LVO) and electronic health record data (whether the patient was taken directly to the CT scanner for a CT and CTA together). Relying on clinician assessment and judgement, waiting to review the CT scan results, or waiting until a telestroke consultation has occurred before ordering a CTA are underlying causes of this impactful failure of the DIDO process. Lack of recognition of signs/symptoms suggestive of acute stroke by EMS personnel (for patients arriving by ambulance) and/or by the ED greeter or triage nurse during the initial assessment phase were also highly ranked failures by RPN, primarily because of the lack of any potential safeguards. These failures have many downstream effects because they result in failure to perform an acute stroke screening scale and failure to perform an LVO screen, further delaying the recognition of acute stroke, particularly LVO. Delay in initiating a telestroke consultation was also a highly ranked failure because of its significant impact on the transfer process; a telestroke must occur to initiate the process. Underlying causes are the telestroke neurologist not answering the call or the PSC ED team failing to initiate a telestroke consultation. Failures of more modest risk or criticality occur in the processes of arranging for the transfer, with underlying causes including the transfer center telephone line being busy and the call being sent to voicemail (Figure, step 28a), the stroke neurologist providing inaccurate information to the transfer center, such as an incorrect name of the sending hospital, leading to the ambulance being dispatched to the wrong hospital (Figure, step 28a), and wrong level (basic life support versus advanced life support) of ambulance being dispatched for the transfer because of failure to update the ambulance request after a deterioration in the patient’s clinical status (Figure, step 32). Both the frequency (range, 4–8) and impact ranges from 5 (moderate [10–19‐minute delay]) to 8 (very high [disruption in process can lead to nonpermanent harm requiring significant intervention]) for these failures. Many communication failures also occur between and within the PSC’s ED team and the CSC (eg, ED physicians and CSC stroke neurologists, ED and CSC nurses, CSC neurologist, and CSC intensive care unit nurses) and with the ambulance team, and between the sending PSC’s ED team and the CSC’s transfer center. Although the failures are generally less impactful (impact scores of 3–5), they are of high frequency (frequency scores 7–9). The remaining failures, which involve all phases of the DIDO process (assessment phase: stroke code activation, diagnostic phase: CT results not uploaded in the picture archiving and communications system, treatment phase: ED nurse inserts an intravenous line, and transfer phase: EMS paramedic prepares patient for transfer) have impact scores of 3 to 4 and frequency scores that are generally <5, with modest safeguards (scores 5–6), resulting in lower RPNs and CNs.
Table 5.
Highest‐Risk Failures of the Door‐In‐Door‐Out Process and Solution Prototypes
| Phase of care | Step | Potential failures | Examples of failure causes |
Effect/ consequence |
Risk priority number (F×I×S) | Criticality no. | Solution prototypes | Estimated change in RPN | |
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic | 1 | Decision about need for CTA |
|
|
Very high disruption to process (Delay: 45–60 min) |
640 (1) (108×8) |
80 (2) (10×8) |
|
240 (6×8×5) |
| Assessment | 2 | Patient arrives in ED by EMS |
|
|
Hazard, potential permanent harm | 576 (2) |
72 (3) |
No solution recommended | N/A |
| Assessment | 3 | Triage RN patient assessment |
|
|
Hazard, potential permanent harm | 567 (3) | 81 (1) | No solution recommended | N/A |
| Diagnostic | 4 | Telestroke evaluation initiation |
|
|
Very high disruption to process (Delay: 45–60 min) | 448 (4) (88×7) | 64 (4) (8×8) |
|
128 (4×8×4) |
| Diagnostic | 5 | ED charge nurse assigns room or sends to CT |
|
|
Very high disruption to process (Delay: 45–60 min) | 400 (5) (10×5×8) | 50 (7) (10×5) |
|
200 (8×5×5) |
| Transfer | 6 | Transfer center arranges ambulance |
|
|
Very high disruption to process (Delay: 45–60 min) | 392 (6) | 56 (5) |
Not addressed |
N/A |
| Diagnostic | 7 | Telestroke consultation during patient evaluation |
|
|
Moderate disruption to process (Delay: 10–19 min) | 350 (7) (10×5×7) | 50 (7) (10×5) |
|
180 (6×5×6) |
| Diagnostic | 8 | CT scan delays |
|
|
High disruption to process (Delay: >30 min) | 336 (8) | 42 (9) | Not addressed | N/A |
| Transfer | 9 | Sending ED hospital arranges ambulance |
|
|
High disruption to process (Delay: >30 min) | 336 (9) | 42 (9) | Not addressed | N/A |
| Treatment | 10 | Alteplase administration delay |
|
|
Hazard, potential permanent harm | 324 (10) | 56 (4) | Not addressed | N/A |
BEFAST indicates balance, eyes, far; BUN, blood urea nitrogen; CSC, comprehensive stroke center; CT, computed tomography; CTA, computed tomography angiography; ED, emergency department; EMS, emergency medical services; BEFAST, balance, eyes, face, arm and speech test; MD, medical doctor; LVO, large‐vessel occlusion; N/A, Not Applicable; PSC, primary stroke center; RN, registered nurse; RPN, risk prediction number; and tPA, tissue plasminogen activator.
Solution Prototypes Informed by the FMECA
Although an obvious solution for not recognizing the need to obtain a CT/CTA might be to perform a CTA on all patients with suspected acute stroke, most PSCs and non–stroke center hospitals have CT scan capacity limitations and lack technicians and radiologists with the requisite experience and availability 24 hours a day/7 days a week. Therefore, the learning collaborative recommended development of a prototype that includes (1) performance and documentation in the electronic health record of an acute stroke scale score on all patients with suspected stroke and, if the acute stroke scale meets a predetermined threshold, a decision support tool prompting (2) performance and documentation in the electronic health record of an LVO scale and, if the LVO is positive, a decision support tool prompting (3) a combined CT/CTA order. When articulating this solution prototype, the learning collaborative further identified a potential delay in obtaining a CT/CTA if renal function studies are required before injecting CTA contrast material. The learning collaborative proposed to include an additional decision support tool to the CT/CTA order to clarify the limited group of patients who need renal function studies. Furthermore, the learning collaborative recognized that most PSC hospitals, although having an ED/radiology policy that prioritizes use of the CT scanner for head CTs of patients with acute stroke, do not have a policy for prioritization of CTAs. Therefore, the combined CT/CTA order solution will also trigger prioritization of the CT scanner.
With regard to initial recognition of signs and symptoms suggestive of acute stroke (Table 5, failures 2 and 3), the learning collaborative recognized that some acute stroke signs and symptoms are uncommon and may not be recognized by clinicians. Furthermore, EMS paramedics 36 and ED triage staff, with diverse levels of experience and expertise, assess and triage patients rapidly, often relying on patient/witness report of signs and symptoms. However, the accuracy and quality of such reports can vary considerably or not be available. The learning collaborative chose not to recommend any solution prototypes to address these failures.
On the other hand, the learning collaborative easily proposed a solution to mitigate delay in reaching a telestroke neurologist. The underlying identified cause was that the neurologist was involved in a telestroke consultation with another PSC. The recommended solution is to automate forwarding of the unanswered call to a backup on‐call telestroke neurologist.
Estimated Changes in RPN from Preliminary Solutions
The final column in Table 5 shows estimated potential change in RPN of a preliminary solution, proposed by the learning collaborative. We re‐estimated the score for the frequency, impact, and strength of safeguard characteristics of the failure using the standardized FMECA risk‐scoring matrix (Table 2). For example, for the step 1, highest‐ranked failure, the proposed solution is to create a direct‐to‐CT/CTA pathway by performing an acute stroke screening scale and, if positive, an LVO scale on all patients with suspected stroke. The solution would likely reduce the frequency by 4 levels (from 10–6) of failing to obtain a CT/CTA. However, the impact of the failure should it occur (eg, because stroke was not suspected, and therefore no acute stroke scale was performed) is unchanged, but the strength of the safeguard for obtaining a CT/CTA because of the decision support is likely enhanced (from 8–5). For step 4, telestroke evaluation initiation, the solution reduces the frequency of the failure (from 8–4), the impact of the failure, should it still occur, is unchanged, yet the strength of the safeguard is improved (from 7‐4). Similarly, for steps 5 and 7, the frequencies will be reduced, and strength of the safeguard will improve modestly.
Discussion
This study reveals that failure to perform an LVO scale in patients with suspected stroke at the beginning of the DIDO diagnostic phase can increase DIDO time by up to 45 minutes. This highly impactful failure has not been previously reported. Failure to perform an LVO scale, which, if positive, suggests that performance of the CT and CTA should be combined during a single transfer to the CT scanner suite. By failing to conduct a CT/CTA during a single transfer to the CT scanner suite adds many additional steps and time to the DIDO process. Currently, LVO scales are not routinely used by clinicians in many hospitals 37 despite recommendations for their use in guidelines. 38 The study also identified that failure to perform an acute stroke screening scale by the EMS paramedic and/or by the ED triage nurse upon arrival at the ED contributes significantly to delays in the DIDO process, confirming findings of prior studies, 39 , 40 , 41 , 42 including our own prior study that conducted FMECAs to compare the door‐to‐needle processes at an academic hospital and a community hospital. Diagnostic factors, such as knowledge and experience (eg, querying patients about last known well time), diagnostic skills (eg, neurological examinations skills), and cognitive biases (eg, framing, anchor) were similarly identified as underlying causes of identified failures in the DIDO process. 43
The DIDO process is important because more patients with acute stroke are likely to require transfer from a PSC or non–stroke center hospital to a CSC as a result of advances in stroke treatment. 44 , 45 Although delays in treatment, 8 transfer, 7 , 9 and less favorable outcomes 7 , 10 have been documented for patients with acute stroke who are transferred, the underlying causes of the delays in the DIDO process have not been previously characterized.
Health care delivery, despite being considered a high‐risk industry, 46 does not routinely apply engineering risk assessment methods. The purpose of conducting this FMECA was to proactively, systematically, and comprehensively document the DIDO process steps and rank order the steps with the most impactful failures rather than merely attending to the most obvious failures. This approach further describes the underlying causes of each failure because solutions that do not directly address the underlying causes typically do not result in sustained improvement.
It is not surprising that many of the transfer phase steps (eg, arranging for an ambulance, hand‐offs to clinicians at the CSC) of the DIDO process are replete with delays, considering that many of the steps, executed at PSCs (eg, contacting an ambulance) or that involve interactions (eg, patient information required by CSC for transfer) between PSCs and CSCs, lack standardization. Although some CSCs have a formal transfer center, staffed usually by a nurse who arranges transfers for all types of patients, including patients with acute stroke to the CSC, other CSCs do not, and the PSC or non–stroke center hospital make the arrangements for the transfer. Although the number of steps is similar, the failures and their underlying causes and characteristics differ.
This study underscores previously identified risks of interprofessional and interfacility communications, in general, 47 for the delivery of health care, and more specifically during the DIDO process. 37 Similar themes of the need for improved quality of communications, including improved sharing of information within a team as well as across the organizations involved in a transfer (EMS, PSC, ambulance company, CSC) with a focus on direct verbal and written communication between clinicians and staff, updates about a patient’s status, and acknowledgment of receipt of information.
Finally, specific examples of potential targets for solution development, identified by the learning collaborative and directly resulting from the findings of the FMECA and risk table, are described, and we have estimated the potential change in RPN if the solution were to be implemented. The learning collaborative is currently designing, developing, and iteratively testing the solutions, using a human (user)‐centered design approach 48 and will be implementing the solutions and gathering outcomes data during the next year. The pilot implementation study will provide actual data to calculate the change in RPN.
This study has several limitations. Although the learning collaborative included representatives of all professions and professional levels of clinicians involved in the DIDO process from diverse PSCs and CSCs in the Chicago area, the identified failures, their causes, and their ranking by risk and criticality may not be fully generalizable, particularly to nonurban settings. Although a FMECA is considered to be a robust engineering methodology, some of the data used to complete the risk table to estimate the RPN and CN (eg, frequency of delay in gathering required information for patient hand‐off to ambulance paramedic) are not routinely measured and therefore had to be appraised by the learning collaborative participants. As in any observational study, there may be omissions and biases of the participants; however, we believe this limitation is mitigated by the multiple and diverse clinicians, patients, and caregivers from multiple health systems.
Conclusions
This study, by using a learning collaborative approach that includes a wide range of clinicians from multiple health systems involved in the DIDO process, as well as patients with acute stroke who experienced a transfer and their caregivers, to apply a proactive systematic risk assessment method, FMECA, created a process map of the steps undertaken to accomplish the DIDO process, used the map for learning collaborative members to systematically and comprehensively identify and characterize the failures in the DIDO process, and described the underlying causes of the identified failures, leading to estimates of risk and criticality of each failure and a rank ordering of the failures. The FMECA revealed a multitude of underlying causes of delay, including some previously unrecognized, such as the failure to perform a CTA immediately after performing a CT in patients with an LVO. Rank ordering of the failures permits the selection of targets for solution design efforts on the highest risk failures, which can lead to significant and potentially permanent patient harm. Furthermore, the details of each failure, specifically its characterization and identification of underlying causes, are invaluable for the design of robust and highly reliability solutions to mitigate and/or eliminate significant delays in DIDO.
Sources of Funding
None.
Disclosures
None.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau‐Sepulveda MV, Pan W, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. DOI: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 2. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJH, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. DOI: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 3. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et al. Stent‐retriever thrombectomy after intravenous t‐PA vs. t‐PA alone in stroke. N Engl J Med. 2015;372:2285–2295. DOI: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 4. Sacchetti DC, Cutting SM, McTaggart RA, Chang AD, Hemendinger M, Mac Grory B, Siket MS, Burton T, Thompson B, Rostanski SK, et al. Perfusion imaging and recurrent cerebrovascular events in intracranial atherosclerotic disease or carotid occlusion. Int J Stroke. 2018;13:592–599. DOI: 10.1177/1747493018764075. [DOI] [PubMed] [Google Scholar]
- 5. Prabhakaran S, Ruff I, Bernstein RA. Intervention for acute stroke‐reply. JAMA. 2015;314:626–627. DOI: 10.1001/jama.2015.7851. [DOI] [PubMed] [Google Scholar]
- 6. Alberts MJ, Wechsler LR, Jensen ME, Latchaw RE, Crocco TJ, George MG, Baranski J, Bass RR, Ruff RL, Huang J, et al. Formation and function of acute stroke‐ready hospitals within a stroke system of care recommendations from the brain attack coalition. Stroke. 2013;44:3382–3393. DOI: 10.1161/STROKEAHA.113.002285. [DOI] [PubMed] [Google Scholar]
- 7. Ali SF, Fonarow G, Liang L, Xian Y, Smith EE, Bhatt DL, Schwamm L. Rates, characteristics, and outcomes of patients transferred to specialized stroke centers for advanced care. Circ Cardiovasc Qual Outcomes. 2018;11:e003359. DOI: 10.1161/CIRCOUTCOMES.116.003359. [DOI] [PubMed] [Google Scholar]
- 8. McTaggart RA, Moldovan K, Oliver LA, Dibiasio EL, Baird GL, Hemendinger ML, Haas RA, Goyal M, Wang TY, Jayaraman MV. Door‐in‐door‐out time at primary stroke centers may predict outcome for emergent large vessel occlusion patients. Stroke. 2018;49:2969–2974. DOI: 10.1161/STROKEAHA.118.021936. [DOI] [PubMed] [Google Scholar]
- 9. Prabhakaran S, Ward E, John S, Lopes DK, Chen M, Temes RE, Mohammad Y, Lee VH, Bleck TP. Transfer delay is a major factor limiting the use of intra‐arterial treatment in acute ischemic stroke. Stroke. 2011;42:1626–1630. DOI: 10.1161/STROKEAHA.110.609750. [DOI] [PubMed] [Google Scholar]
- 10. Rinaldo L, Brinjikji W, McCutcheon BA, Bydon M, Cloft H, Kallmes DF, Rabinstein AA. Hospital transfer associated with increased mortality after endovascular revascularization for acute ischemic stroke. J NeuroIntervent Surg. 2017;9:1166–1172. DOI: 10.1136/neurintsurg-2016-012824. [DOI] [PubMed] [Google Scholar]
- 11. Wang TY, Nallamothu BK, Krumholz HM, Li S, Roe MT, Jollis JG, Jacobs AK, Holmes DR, Peterson ED, Ting HH. Association of door‐in to door‐out time with reperfusion delays and outcomes among patients transferred for primary percutaneous coronary intervention. JAMA. 2011;305:2540–2547. DOI: 10.1001/jama.2011.862. [DOI] [PubMed] [Google Scholar]
- 12. McTaggart RA, Yaghi S, Cutting SM, Hemendinger M, Baird GL, Haas RA, Furie KL, Jayaraman MV. Association of a primary stroke center protocol for suspected stroke by large‐vessel occlusion with efficiency of care and patient outcomes. JAMA Neurol. 2017;74:793–800. DOI: 10.1001/jamaneurol.2017.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng FC, Low E, Andrew E, Smith K, Campbell BCV, Hand PJ, Crompton DE, Wijeratne T, Dewey HM, Choi PM. Deconstruction of interhospital transfer workflow in large vessel occlusion: real‐world data in the thrombectomy era. Stroke. 2017;48:1976–1979. DOI: 10.1161/STROKEAHA.117.017235. [DOI] [PubMed] [Google Scholar]
- 14. Prabhakaran S. Out through the in door: interhospital stroke transfers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11:e005005. DOI: 10.1161/CIRCOUTCOMES.118.005005. [DOI] [PubMed] [Google Scholar]
- 15. Sun CH, Nogueira RG, Glenn BA, Connelly K, Zimmermann S, Anda K, Camp D, Frankel MR, Belagaje SR, Anderson AM, et al. "Picture to puncture": a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013;127:1139–1148. DOI: 10.1161/CIRCULATIONAHA.112.000506. [DOI] [PubMed] [Google Scholar]
- 16. Goyal M, Almekhlafi MA, Fan L, Menon BK, Demchuk AM, Yeatts SD, Hill MD, Tomsick T, Khatri P, Zaidat OO, et al. Evaluation of interval times from onset to reperfusion in patients undergoing endovascular therapy in the Interventional Management of Stroke III trial. Circulation. 2014;130:265–272. DOI: 10.1161/CIRCULATIONAHA.113.007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kodankandath TV, Wright P, Power PM, De Geronimo M, Libman RB, Kwiatkowski T, Katz JM. Improving transfer times for acute ischemic stroke patients to a comprehensive stroke center. J Stroke Cerebrovasc Dis. 2017;26:192–195. DOI: 10.1016/j.jstrokecerebrovasdis.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 18. Menon BK, Almekhlafi MA, Pereira VM, Gralla J, Bonafe A, Davalos A, Chapot R, Goyal M, Investigators SS . Optimal workflow and process‐based performance measures for endovascular therapy in acute ischemic stroke: analysis of the Solitaire FR thrombectomy for acute revascularization study. Stroke. 2014;45:2024–2029. DOI: 10.1161/STROKEAHA.114.005050. [DOI] [PubMed] [Google Scholar]
- 19. Goyal M, Jadhav AP, Bonafe A, Diener H, Mendes Pereira V, Levy E, Baxter B, Jovin T, Jahan R, Menon BK, et al. Analysis of workflow and time to treatment and the effects on outcome in endovascular treatment of acute ischemic stroke: results from the SWIFT PRIME randomized controlled trial. Radiology. 2016;279:888–897. DOI: 10.1148/radiol.2016160204. [DOI] [PubMed] [Google Scholar]
- 20. Parikh SV, Jacobi JA, Chu E, Addo TA, Warner JJ, Delaney KA, McGuire DK, deLemos JA, Cigarroa JE, Murphy SA, et al. Treatment delay in patients undergoing primary percutaneous coronary intervention for ST‐elevation myocardial infarction: a key process analysis of patient and program factors. Am Heart J. 2008;155:290–297. DOI: 10.1016/j.ahj.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 21. Kelly EW, Kelly JD, Hiestand B, Wells‐Kiser K, Starling S, Hoekstra JW. Six sigma process utilization in reducing door‐to‐balloon time at a single academic tertiary care center. Prog Cardiovasc Dis. 2010;53:219–226. DOI: 10.1016/j.pcad.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 22. Ford AL, Williams JA, Spencer M, McCammon C, Khoury N, Sampson TR, Panagos P, Lee JM. Reducing door‐to‐needle times using Toyota's lean manufacturing principles and value stream analysis. Stroke. 2012;43:3395–3398. DOI: 10.1161/STROKEAHA.112.670687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khare RK, Nannicelli AP, Powell ES, Seivert NP, Adams JG, Holl JL. Use of risk assessment analysis by failure mode, effects, and criticality to reduce door‐to‐balloon time. Ann Emerg Med. 2013;62:388–398.e12. DOI: 10.1016/j.annemergmed.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 24. Rausand M. System Analysis Failure Modes, Effects, and Criticality Analysis . System Reliability Theory, 2nd, ed. Wiley; 2004. [Google Scholar]
- 25. Jordan WE. Failure modes, effects and criticality analyses. 1972.
- 26. Prabhakaran S, Khorzad R, Brown A, Nannicelli AP, Khare R, Holl JL. Academic‐community hospital comparison of vulnerabilities in door‐to‐needle process for acute ischemic stroke. Circ Cardiovasc Qual Outcomes. 2015;8:S148–S154. DOI: 10.1161/CIRCOUTCOMES.115.002085. [DOI] [PubMed] [Google Scholar]
- 27. Coles G, Fuller B, Nordquist K, Kongslie A. Using failure mode effects and criticality analysis for high‐risk processes at three community hospitals. Jt Comm J Qual Patient Saf. 2005;31:132–140. DOI: 10.1016/S1553-7250(05)31018-X. [DOI] [PubMed] [Google Scholar]
- 28. McElroy LM, Collins KM, Koller FL, Khorzad R, Abecassis MM, Holl JL, Ladner DP. Operating room to intensive care unit handoffs and the risks of patient harm. Surgery. 2015;158:588–594. DOI: 10.1016/j.surg.2015.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McElroy LM, Khorzad R, Nannicelli AP, Brown AR, Ladner DP, Holl JL. Failure mode and effects analysis: a comparison of two common risk prioritisation methods. BMJ quality & safety. 2016;25:329–336. DOI: 10.1136/bmjqs-2015-004130. [DOI] [PubMed] [Google Scholar]
- 30. Thornton VL, Holl JL, Cline DM, Freiermuth CE, Sullivan DT, Tanabe P. Application of a proactive risk analysis to emergency department sickle cell care. West J Emerg Med. 2014;15:446–458. DOI: 10.5811/westjem.2014.4.20489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu H‐C. FMEA for proactive healthcare risk analysis: a systematic literature review. In: Improved FMEA Methods for Proactive Healthcare Risk Analysis. Springer; 2019:15–45. [Google Scholar]
- 32. Pollack TA, Illuri V, Khorzad R, Aleppo G, Oakes DJ, Holl JL, Wallia A. Risk assessment of the hospital discharge process of high‐risk patients with diabetes. BMJ Open Qual. 2018;7:e000224. DOI: 10.1136/bmjoq-2017-000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Process analysis tools: failure modes and effects analysis (FMEA) . http://www.asq.org/learn‐about‐quality/process‐analysis‐tools/overview/fmea.html. Published 2009. Accessed July 20, 2021.
- 34. Berenholtz SM, Pronovost PJ, Lipsett PA, Hobson D, Earsing K, Farley JE, Milanovich S, Garrett‐Mayer E, Winters BD, Rubin HR, et al. Eliminating catheter‐related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014–2020. DOI: 10.1097/01.CCM.0000142399.70913.2F. [DOI] [PubMed] [Google Scholar]
- 35. IHI's Collaborative Model for Achieving Breakthrough Improvement. Boston: Institute for Healthcare Improvement; 2003. [Google Scholar]
- 36. Tennyson JC, Michael SS, Youngren MN, Reznek MA. Delayed recognition of acute stroke by emergency department staff following failure to activate stroke by emergency medical services. West J Emerg Med. 2019;20:342–350. DOI: 10.5811/westjem.2018.12.40577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hayes M, Schlundt D, Bonnet K, Vogus TJ, Kripalani S, Froehler MT, Ward MJ. Tales from the trips: a qualitative study of timely recognition, treatment, and transfer of emergency department patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28:1219–1228. DOI: 10.1016/j.jstrokecerebrovasdis.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 39. Abdullah AR, Smith EE, Biddinger PD, Kalenderian D, Schwamm LH. Advance hospital notification by EMS in acute stroke is associated with shorter door‐to‐computed tomography time and increased likelihood of administration of tissue‐plasminogen activator. Prehosp Emerg Care. 2008;12:426–431. DOI: 10.1080/10903120802290828. [DOI] [PubMed] [Google Scholar]
- 40. Busby L, Owada K, Dhungana S, Zimmermann S, Coppola V, Ruban R, Horn C, Rochestie D, Khaldi A, Hormes JT, et al. CODE FAST: a quality improvement initiative to reduce door‐to‐needle times. J Neurointerv Surg. 2016;8:661–664. DOI: 10.1136/neurintsurg-2015-011806. [DOI] [PubMed] [Google Scholar]
- 41. McKinney JS, Mylavarapu K, Lane J, Roberts V, Ohman‐Strickland P, Merlin MA. Hospital prenotification of stroke patients by emergency medical services improves stroke time targets. J Stroke Cerebrovasc Dis. 2013;22:113–118. DOI: 10.1016/j.jstrokecerebrovasdis.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 42. Medoro I, Cone DC. An analysis of EMS and ED detection of stroke. Prehosp Emerg Care. 2017;21:476–480. DOI: 10.1080/10903127.2017.1294222. [DOI] [PubMed] [Google Scholar]
- 43. Singh H, Sittig DF. Advancing the science of measurement of diagnostic errors in healthcare: the Safer Dx framework. BMJ Qual Saf. 2015;24:103–110. DOI: 10.1136/bmjqs-2014-003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zaidat OO, Lazzaro M, McGinley E, Edgell RC, Nguyen T, Linfante I, Janjua N. Demand‐supply of neurointerventionalists for endovascular ischemic stroke therapy. Neurology. 2012;79:S35–S41. DOI: 10.1212/WNL.0b013e31826957ef. [DOI] [PubMed] [Google Scholar]
- 45. Cloft HJ, Rabinstein A, Lanzino G, Kallmes DF. Intra‐arterial stroke therapy: an assessment of demand and available work force. Am J Neuroradiol. 2009;30:453–458. DOI: 10.3174/ajnr.A1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hudson P. Applying the lessons of high risk industries to health care. Qual Saf Health Care. 2003;12:i7–12. DOI: 10.1136/qhc.12.suppl_1.i7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jeffs L, Lyons RF, Merkley J, Bell CM. Clinicians' views on improving inter‐organizational care transitions. BMC Health Serv Res. 2013;13:289. DOI: 10.1186/1472-6963-13-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kachirskaia IMK, Neuwirth E. Human‐centered design and performance improvement: better together. NEJM Catalyst. 2018. https://catalyst.nejm.org/doi/full/10.1056/CAT.18.0144 Accessed July 20, 2021. [Google Scholar]


