Abstract
Background
No study has evaluated the impact of the additional manipulation demanded by multiple resheathing (MR) in patients undergoing transcatheter aortic valve replacement with repositionable self‐expanding valves.
Methods and Results
This study included a real‐world, multicenter registry involving 16 centers from Canada, Germany, Latin America, and Spain. All consecutive patients who underwent transcatheter aortic valve replacement with the Evolut R, Evolut PRO, and Portico valves were included. Patients were divided according to the number of resheathing: no resheathing, single resheathing (SR), and MR. The primary end point was device success. Secondary outcomes included procedural complications, early safety events, and 1‐year mortality. In 1026 patients, the proportion who required SR and MR was 23.9% and 9.3%, respectively. MR was predicted by the use of Portico and moderate/severe aortic regurgitation at baseline (both with P<0.01). Patients undergoing MR had less device success (no resheathing=89.9%, SR=89.8%, and MR=80%; P=0.01), driven by more need for a second prosthesis and device embolization. At 30 days, there were no differences in safety events. At 1 year, more deaths occurred with MR (no resheathing=10.5%, SR=8.0%, and MR=18.8%; P=0.014). After adjusting for baseline differences and center experience by annual volume, MR associated with less device success (odds ratio, 0.42; P=0.003) and increased 1‐year mortality (hazard ratio, 2.06; P=0.01). When including only the Evolut R/PRO cases (N=837), MR continued to have less device success (P<0.001) and a trend toward increased mortality (P=0.05).
Conclusions
Repositioning a self‐expanding valve is used in a third of patients, being multiple in ≈10%. MR, but not SR, was associated with more device failure and higher 1‐year mortality, regardless of the type of valve implanted.
Keywords: aortic valve stenosis, resheathing, self‐expanding valve, transcatheter aortic valve replacement
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation
Nonstandard Abbreviations and Acronyms
- MR
multiple resheathing
- NR
no resheathing
- SR
single resheathing
- TAVR
transcatheter aortic valve replacement
- THV
transcatheter heart valve
Clinical Perspective
What Is New?
Multiple resheathing is required in up to 10% of cases, with independent predictors being associated with the type of valve implanted (more with Portico) and with the presence of moderate/severe aortic regurgitation at baseline.
Multiple resheathing, but not single resheathing, was associated with worse device success, determined by a higher need for a second valve, more device embolization, and increased 1‐year mortality, regardless of the type of valve implanted.
What Are the Clinical Implications?
Multiple resheathing may not necessarily be the direct cause of the worse outcomes, but a marker of more complicated anatomical features for an optimal device implantation.
It may be reasonable for the operators to consider changing the strategy/approach or type/size of the valve before final release in cases where multiple resheathing is needed.
Since the beginning of the transcatheter aortic valve replacement (TAVR) era, there has been a continuous evolution of the transcatheter heart valves (THVs) that led to significant improvement in clinical outcomes. 1 , 2 , 3 , 4 , 5 , 6 Early generation of TAVR devices had been associated with increased risk of complications and device failure, such as moderate or severe paravalvular leak, high incidence of conduction disturbances requiring new permanent pacemaker implantation, and need for a second THV. 7 , 8 , 9 The newer‐generation devices have been designed to overcome these limitations.
Among the different self‐expanding THVs, both the Evolut R/Pro (Medtronic, Minneapolis, MN) and the Portico (Abbott, Chicago, IL) valves use a delivery system with a mechanism that allows for resheathing and recapturing of the THV before complete deployment, in case repositioning is required. This novel feature allows the operators to have 2 or even multiple attempts to position the THV, augmenting the accuracy of the valve implantation in a proper anatomical position, which has been associated with improved outcomes, including fewer conduction disturbances, less paravalvular leak, and lower need for a second device. 10
Although higher success rates have been achieved with the newer generation of self‐expanding THVs, concerns have been raised about a potentially detrimental impact of the additional maneuver with resheathing and repositioning, including more debris embolization. 11 Even though previous studies have shown no association of resheathing with impaired clinical outcomes, none of them has specifically evaluated the number of attempts per patient, and the potential of multiple resheathing (MR) on worse clinical outcomes. Therefore, the primary objective of the present study was to evaluate the incidence, predictors, and clinical impact of MR in patients treated with repositionable self‐expanding devices.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Design and Population
This was a retrospective study involving all consecutive patients undergoing TAVR with severe symptomatic aortic stenosis or degenerated aortic bioprosthesis treated with the repositionable Evolut R/PRO or Portico devices at 16 centers. A total of 1030 patients were included from Canada, Germany, Latin America, and Spain, from June 2014 to May 2020. The indication of the procedure, the techniques used, and the decision of the THV type were defined by the local heart team. Data were collected using dedicated case report forms, and remote data monitoring was performed in all cases to search and correct missing or inconsistent information. All patients gave written informed consent to the TAVR procedures, and all the local ethics committees approved the retrospective inclusion of the patients at each center. The first and last authors had full access to all the data in the study and take responsibility for their integrity and data analysis.
To evaluate the clinical impact of resheathing, patients were divided according to the use and the number of resheathing for repositioning the bioprosthesis into the following groups: no resheathing (NR), single resheathing (SR), and MR. Patients were allocated in the MR group if ≥2 resheathings were performed. Any partial or total recapture attempt was accounted. This information was confirmed by reviewing all angiographies and the reports of all procedures. The primary outcome was device success, defined by a combination of the absence of procedural death, implantation of a single prosthesis with a final mean transaortic gradient <20 mm Hg, and less than moderate paravalvular leak. Secondary outcomes were the 30‐day and cumulative mortality, the incidence of 30‐day safety events (all‐cause mortality, all stroke, life‐threatening bleeding, major vascular complication, stage 2 or 3 acute kidney injury, coronary artery obstruction requiring intervention, and valve‐related dysfunction requiring repeated procedure), and procedural complications, which included a new permanent pacemaker implantation at 30 days, new‐onset persistent left bundle‐branch block, and moderate or severe aortic regurgitation on echocardiogram at discharge. All events were assessed and reported according to the recommendation of the Valve Academic Research Consortium‐2 criteria. 12
Statistical Analysis
Baseline characteristics and an unadjusted comparison of the outcomes were performed between the 3 groups. Categorical variables were reported as total numbers of events and percentages and were compared with the χ2 test. Continuous variables were reported as mean±SD or as median with interquartile range, as appropriate, and analyzed with 1‐way ANOVA or the Kruskal‐Wallis test. Anatomical and procedural variables that differed significantly between groups were tested for their capacity of predicting the need for MR in a logistic regression model. Logistic regression was also performed to ascertain the independent effect of MR on the primary end point. Baseline variables that were significantly different between the groups and could theoretically impact device success were screened in a univariable model. Those with a P<0.1 were selected to the multivariable. In addition, patients were classified according to the absolute annual procedural volume of the institution using a self‐expanding repositionable device (≤25, 26–75, or >75 cases per year) to account for the centers' experience in the regression assessment. For the 30‐day and 1‐year mortality, survival analysis with the Kaplan‐Meier method was performed, comparing the 3 groups with the log‐rank test and pairwise method, followed by a multivariable proportional hazard regression for 1‐year mortality to assess the association of MR with the outcome after accounting for other factors. Chosen independent variables were those that differed between groups and were known by the literature to be associated with mortality. For the multivariable model, variables were included if they had a P<0.1 in the univariable. The statistical analysis results are presented as odds ratios (ORs) or hazard ratios (HRs), accordingly, with a 95% CI and P values. All analyses were performed with SPSS version 24 (IBM, Armonk, NY).
Results
Among the 1030 patients eligible for the study, 4 did not have follow‐up data and were excluded. The median follow‐up time was 394 days (interquartile range, 209–646 days). Of the studied population, 336 (32.7%) required at least 1 resheathing, being multiple in 95 (9.3%) cases, with a median of 2 attempts/patient (interquartile range, 2–3; range, 2–6) (Figure 1). Baseline clinical, echocardiographic, and multidetector computed tomography characteristics are shown in Table 1. Missing values were low (<5%) and were regarded as completely at random. Therefore, no specific analytical strategy was taken to handle them. The mean age was 81.1±7.2 years, and 44% were men, with a median Society of Thoracic Surgeons predicted risk of mortality score of 4.7 (interquartile range, 3–7). Overall, clinical baseline characteristics were well balanced between the groups, except for chronic obstructive pulmonary disease, which was more prevalent in the NR group, whereas atrial fibrillation and previous cerebrovascular disease were more prevalent in the patients in the MR group. Baseline echocardiography showed that moderate/severe aortic regurgitation was more frequent in patients in the MR group, with no difference for the severity of the aortic stenosis, as well as with respect to the multidetector computed tomography parameters. The main procedural characteristics are shown in Table 2. Resheathing and repositioning of the THV were more frequent with the Portico valve (Figure 1), and in patients with MR, more balloon predilation and postdilation were required, compared with NR and SR groups, in addition to significantly less conscious sedation. By multivariable analysis, the presence of moderate/severe aortic regurgitation at baseline and the use of the Portico valve were identified as independent predictors for the need for MR (OR, 2.33; 95% CI, 1.4–3.87; P=0.001 and OR, 2.81; 95% CI, 1.68–4.7; P<0.001, respectively) (Table 3).
Figure 1. Percentage of the need for single resheathing and multiple resheathing in the study, according to the type of transcatheter heart value (THV) implanted (A) and percentage of resheathing required, reported by previous studies for the different types of THV (B).
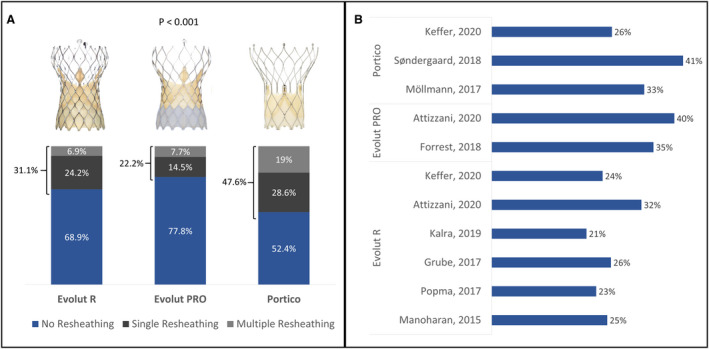
Table 1.
Baseline Clinical, Echocardiographic, and Computed Tomography Characteristics of the Study Population
| Characteristics |
Overall (n=1026) |
NR (n=686) |
SR (n=245) |
MR (n=95) |
P Value |
|---|---|---|---|---|---|
| Clinical variables | |||||
| Age, y | 81±7 | 80.8±7.5 | 81.6±6.5 | 81.6±7.1 | 0.23 |
| Male sex | 452 (44.1) | 304 (44.3) | 99 (40.4) | 49 (51.6) | 0.17 |
| NYHA class ≥III | 616 (60) | 412 (60.1) | 150 (61.2) | 54 (56.8) | 0.76 |
| Hypertension | 889 (86.6) | 595 (86.7) | 215 (87.8) | 79 (83.2) | 0.53 |
| Diabetes mellitus | 346 (33.7) | 221 (32.2) | 91 (37.1) | 34 (35.8) | 0.34 |
| COPD | 216 (21.1) | 161 (23.5) | 43 (17.6) | 12 (12.6) | 0.02 |
| Coronary artery disease | 495 (48.2) | 342 (49.9) | 114 (46.5) | 39 (41.1) | 0.23 |
| Previous CABG | 125 (12.2) | 76 (11.1) | 37 (15.1) | 12 (12.6) | 0.25 |
| Previous valve surgery | 128 (12.5) | 77 (11.2) | 37 (15.1) | 14 (14.7) | 0.23 |
| Previous Afib | 339 (33) | 227 (33.1) | 70 (28.6) | 42 (44.2) | 0.02 |
| Prior pacemaker | 141 (14.1) | 98 (14.6) | 29 (12.3) | 14 (15.4) | 0.63 |
| Prior RBBB | 87 (8.8) | 61 (9.1) | 21 (9) | 5 (5.5) | 0.51 |
| Prior LBBB | 119 (12) | 82 (12.2) | 24 (10.3) | 13 (14.3) | 0.57 |
| Cerebrovascular disease | 91 (8.9) | 54 (7.9) | 22 (9) | 15 (15.8) | 0.04 |
| Peripheral artery disease | 181 (17.6) | 118 (17.2) | 51 (20.8) | 12 (12.6) | 0.18 |
| STS‐PROM score, % | 4.7 (3–7) | 4.7 (3.1–7.1) | 4.8 (2.9–6.9) | 4.8 (3.2–7) | 0.9 |
| Hemoglobin, g/dL | 11.7±1.8 | 11.7±1.8 | 11.7±1.7 | 11.9±1.8 | 0.33 |
| Creatinine, mg/dL | 1.2±0.74 | 1.2±0.8 | 1.1±0.5 | 1.2±0.7 | 0.08 |
| Echocardiographic variables* | |||||
| LVEF, % | 56.1±12.4 | 56±12.6 | 56.2±12.1 | 55.8±12.1 | 0.95 |
| Aortic gradient, mm Hg | 43.1±17.2 | 43.3±18.2 | 42.2±14.9 | 43.5±15.9 | 0.68 |
| Aortic valve area, cm2 | 0.72±0.34 | 0.72±0.37 | 0.71±0.28 | 0.71±0.22 | 0.88 |
| Moderate/severe aortic regurgitation | 151 (15.1) | 91 (13.6) | 35 (14.5) | 25 (26.6) | 0.004 |
| Moderate/severe mitral regurgitation | 180 (18) | 117 (17.6) | 46 (19.2) | 17 (17.9) | 0.86 |
| Pulmonary hypertension | 484 (57.3) | 334 (59.9) | 110 (53.9) | 40 (48.2) | 0.07 |
| MDCT variables † | |||||
| Annulus perimeter, mm | 73.8±8.9 | 73.9±9 | 73±8.4 | 75.5±9 | 0.09 |
| Eccentricity index | 0.18±0.09 | 0.19±0.09 | 0.18±0.09 | 0.19±0.08 | 0.6 |
| Agatston calcium score ‡ , § | 2464±1572 | 2462±1593 | 2416±1450 | 2395±1555 | 0.9 |
Values are number (percentage), mean±SD, or median (interquartile range). Afib indicates atrial fibrillation; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; LBBB, left bundle‐branch block; LVEF, left ventricular ejection fraction; MDCT, multidetector computed tomography; MR, multiple resheathing; NR, no resheathing; NYHA, New York Heart Association; RBBB, right bundle‐branch block; SR, single resheathing; and STS‐PROM, Society of Thoracic Surgeons predicted risk of mortality.
Preprocedural echocardiogram data were available for 98% of the patients.
Preprocedural MDCT was available for 90% of the patients.
Compared with the natural log transformation of the variable for normalization.
Data on Agatston calcium score were available for 592 patients overall.
Table 2.
Procedural Characteristics of the Study Population
| Characteristics |
Overall (n=1026) |
NR (n=686) |
SR (n=245) |
MR (n=95) |
P Value | SR vs NR | MR vs NR | MR vs SR |
|---|---|---|---|---|---|---|---|---|
| Procedural characteristic | ||||||||
| Transfemoral approach | 918 (89.5) | 608 (88.6) | 223 (91) | 87 (91.6) | 0.45 | … | … | … |
| Conscious sedation | 584 (57) | 417 (60.9) | 130 (53.1) | 37 (38.9) | <0.001 | 0.03 | <0.001 | 0.02 |
| Valve‐in‐valve procedure | 99 (9.6) | 59 (8.6) | 29 (11.8) | 11 (11.6) | 0.27 | … | … | … |
| Predilatation | 466 (45.4) | 296 (43.1) | 116 (47.3) | 54 (56.8) | 0.03 | 0.26 | 0.01 | 0.012 |
| Postdilatation | 277 (27) | 166 (24.2) | 71 (29) | 40 (42.1) | 0.001 | 0.14 | <0.001 | 0.02 |
| Prosthesis type | ||||||||
| Evolut R | 720 (70.2) | 496 (72.3) | 174 (71) | 50 (52.6) | <0.001 | |||
| Evolut PRO | 117 (11.4) | 91 (13.3) | 17 (6.9) | 9 (9.5) | 0.002 | <0.001 | 0.005 | |
| Portico | 189 (18.4) | 99 (14.4) | 54 (22) | 36 (37.9) | ||||
| Prosthesis size* | ||||||||
| Small | 460 (44.8) | 300 (43.7) | 121 (49.4) | 39 (41.1) | 0.45 | |||
| Medium | 463 (45.1) | 313 (45.6) | 102 (41.6) | 48 (50.5) | … | … | … | |
| Large | 103 (10) | 73 (10.6) | 22 (9.0) | 8 (8.4) | ||||
| No. of resheathings | 0 (0–1) | 0 | 1 | 2 (2–3) | <0.001 | … | … | … |
Values are number (percentage) or median (interquartile range). MR indicates multiple resheathing; NR, no resheathing; and SR, single resheathing.
Small=Evolut R/PRO 23/26 and Portico 23/25; medium=Evolut R/PRO 29 and Portico 27/29; and large=Evolut R/PRO 34.
Table 3.
Univariable and Multivariable Logistic Regression for MR
| Univariable | Multivariable* | ||||
|---|---|---|---|---|---|
| Variables | OR (95% CI) | P Value | Variable | OR (95% CI) | P Value |
| Aortic regurgitation † | 2.25 (1.37–3.69) | 0.001 | Aortic regurgitation † | 2.33 (1.4–3.87) | 0.001 |
| Balloon predilation | 1.66 (1.08–2.54) | 0.02 | Balloon predilation | 1.21 (0.74–2) | 0.45 |
| Evolut PRO ‡ | 1.12 (0.53–2.34) | 0.77 | … | … | … |
| Portico ‡ | 3.15 (1.98–5.01) | <0.001 | Portico ‡ | 2.81 (1.68–4.7) | <0.001 |
MR indicates multiple resheathing; and OR, odds ratio.
A total of 1003 (97.8%) cases were included in a complete case analysis (more details in Table S3).
Moderate or severe aortic regurgitation at baseline.
Evolut R/PRO as reference.
Procedural and Clinical Outcomes
Overall, device success was achieved in 89% of cases, with a lower rate in the patients in the MR group in comparison to the other 2 groups (80% versus 89.9% versus 89.8%; P=0.01), and this was mostly driven by a higher need of a second valve and more prosthesis embolization (Table 4). No differences in procedural death or other intraprocedural complication rates were observed. The incidence of new‐onset persistent left bundle‐branch block was higher in the patients in the MR group, although the need for new permanent pacemaker implantation was similar. On multivariable regression analysis, variables that independently impacted the device success were moderate/severe aortic regurgitation at baseline (OR, 0.47; 95% CI, 0.3–0.76; P=0.002) and MR (OR, 0.42; 95% CI, 0.23–0.74; P=0.003) (Table 5).
Table 4.
Comparison of Procedural and 30‐Day Outcomes Between the Groups
| Variables |
Overall (n=1026) |
NR (n=686) |
SR (n=245) |
MR (n=95) |
P Value* | SR vs NR | MR vs NR | MR vs SR |
|---|---|---|---|---|---|---|---|---|
| Procedural outcomes | ||||||||
| Device success | 913 (89) | 617 (89.9) | 220 (89.8) | 76 (80) | 0.01 | 0.95 | 0.004 | 0.02 |
| Procedural death | 29 (2.8) | 21 (3.1) | 4 (1.6) | 4 (4.2) | 0.36 | … | … | … |
| Need of a second valve | 23 (2.2) | 4 (0.6) | 9 (3.7) | 10 (10.5) | <0.001 | 0.001 | <0.001 | 0.01 |
| Prosthesis embolization | 15 (1.5) | 3 (0.4) | 5 (2) | 7 (7.4) | <0.001 | 0.02 | <0.001 | 0.02 |
| Tamponade | 20 (1.9) | 9 (1.3) | 9 (3.7) | 2 (2.1) | 0.07 | … | … | … |
| Coronary obstruction | 8 (0.8) | 6 (0.9) | 2 (0.8) | 0 | 0.66 | … | … | … |
| Aortic rupture | 4 (0.4) | 3 (0.4) | 1 (0.4) | 0 | 0.81 | … | … | … |
| 30‐d outcomes | ||||||||
| All‐cause death † | 36 (3.6) | 25 (3.7) | 5 (2.1) | 6 (6.4) | 0.15 | … | … | … |
| Combined early safety | 157 (15.3) | 108 (15.7) | 35 (14.3) | 14 (14.7) | 0.85 | … | … | … |
| Stroke | ||||||||
| All stroke | 24 (2.4) | 18 (2.6) | 5 (2) | 1 (1.1) | 0.6 | … | … | … |
| Disabling stroke | 15 (1.5) | 10 (1.5) | 4 (1.6) | 1 (1.1) | 0.93 | … | … | … |
| Major vascular complication | 56 (5.5) | 37 (5.4) | 14 (5.7) | 5 (5.4) | 0.98 | … | … | … |
| Life‐threatening bleeding | 42 (4.1) | 24 (3.5) | 14 (5.7) | 4 (4.3) | 0.33 | … | … | … |
| Acute kidney injury (stages 2 and 3) | 61 (6) | 42 (6.2) | 12 (4.9) | 7 (7.4) | 0.65 | … | … | … |
| New permanent pacemaker | 154 (15.2) | 96 (14.2) | 42 (17.2) | 16 (17) | 0.47 | … | … | … |
| New‐onset persistent LBBB | 192 (19.2) | 111 (16.6) | 52 (21.7) | 29 (30.9) | 0.002 | 0.08 | 0.001 | 0.08 |
| Moderate/severe aortic regurgitation | 30 (3.1) | 18 (2.8) | 8 (3.5) | 4 (4.5) | 0.65 | … | … | … |
| Aortic gradient, mm Hg | 8.5±5.3 | 8.5±5.3 | 8.3±4.8 | 9.6±6.6 | 0.15 | … | … | … |
Data are given as number (percentage) or mean±SD. LBBB indicates left bundle‐branch block; MR, multiple resheathing; NR, no resheathing; and SR, single resheathing.
Overall P value.
Kaplan‐Meier event probability estimates (log‐rank).
Table 5.
Univariable and Multivariable Logistic Regression for Device Success
| Univariable | Multivariable* | ||||
|---|---|---|---|---|---|
| Variables | OR (95% CI) | P Value | Variable | OR (95% CI) | P Value |
| COPD | 0.71 (0.45–1.11) | 0.13 | … | … | … |
| Aortic regurgitation † | 0.44 (0.27–0.7) | <0.001 | Aortic regurgitation † | 0.47 (0.3–0.76) | 0.002 |
| Balloon predilation | 1.01 (0.68–1.5) | 0.95 | … | … | … |
| Balloon postdilation | 0.88 (0.57–1.36) | 0.58 | … | … | … |
| Evolut PRO ‡ | 0.99 (0.54–1.8) | 0.96 | … | … | … |
| Portico ‡ | 1.67 (0.93–3.02) | 0.08 | Portico ‡ | 1.89 (0.97–3.67) | 0.06 |
| Multiple resheathing § | 0.45 (0.26–0.78) | 0.004 | Multiple resheathing | 0.42 (0.23–0.74) | 0.003 |
| SE‐THV center annual volume <25 cases § , || | 1.47 (0.87–2.5) | 0.15 | SE‐THV center annual volume <25 cases || | 1.58 (0.91–2.74) | 0.11 |
| SE‐THV center annual volume 26–75 cases § , || | 1.5 (0.95–2.38) | 0.08 | SE‐THV center annual volume 26–75 cases || | 1.28 (0.77–2.12) | 0.35 |
COPD indicates chronic obstructive pulmonary disease; OR, odds ratio; and SE‐THV, self‐expanding transcatheter heart valve.
A total of 1003 (97.8%) cases were included in a complete case analysis (more details in Table S4).
Moderate or severe aortic regurgitation at baseline.
Evolut R as the reference.
Interaction between center annual volume and multiple resheathing (P=0.45).
SE‐THV center annual volume >75 cases as reference.
At 30 days, there was a similar rate of all‐cause death, stroke, and other safety events among the groups (Table 3). At 1 year, MR was associated with increased mortality in comparison to NR and SR cases (18.8% versus 10.5% versus 8.0%, respectively; P=0.014) (Figure 2). As shown in Table 6, after adjusting for differences in baseline characteristics and center volume on a multivariable proportional hazard model, chronic obstructive pulmonary disease (HR, 1.74; 95% CI, 1.11–2.73; P=0.03), the need for MR (HR, 2.06; 95% CI, 1.18–3.6; P=0.01), and lower center volume were independently associated with cumulative mortality (HR, 1.89; 95% CI, 1.06–3.36; P=0.03). Table S1 shows the rate of MR by the centers' annual volume. No interaction in the regression models was found between center volume and MR for neither device success (P=0.45 for interaction) nor 1‐year mortality (P=0.13 for interaction). In a sensitivity analysis, excluding the 187 Portico cases, MR with the Evolut R/PRO continued to be associated with less device success and with a trend toward increased mortality at 1 year (Table S2 and Figure S1). Tables S3 through S5 show the number of cases included in each regression analysis.
Figure 2. Comparison of the Kaplan‐Meier cumulative mortality curves at 1 year among the groups.
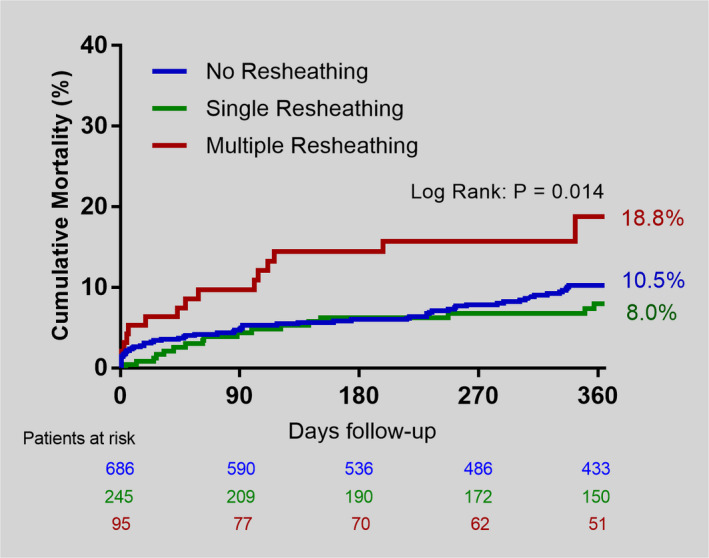
In pairwise log‐rank comparison, there was a significant difference between no resheathing (NR) vs multiple resheathing (MR) (P=0.02), and between single resheathing (SR) vs MR (P=0.005). No difference was observed between NR vs SR (P=0.3).
Table 6.
Univariable and Multivariable Proportional Hazard Regression for the Cumulative Mortality at 1 Year
| Univariable | Multivariable* | ||||
|---|---|---|---|---|---|
| Variables | HR (95% CI) | P Value | Variables | HR (95% CI) | P Value |
| COPD | 1.65 (1.07–2.55) | 0.03 | COPD | 1.74 (1.11–2.73) | 0.03 |
| Afib † | 1.44 (0.96–2.16) | 0.08 | Afib † | 1.49 (0.98–2.73) | 0.06 |
| Cerebrovascular disease | 1.19 (0.62–2.28) | 0.61 | … | … | … |
| Aortic regurgitation ‡ | 1.07 (0.62–1.86) | 0.81 | … | … | … |
| Evolut PRO § | 0.78 (0.4–1.52) | 0.47 | … | … | … |
| Portico § | 0.75 (0.43–1.31) | 0.32 | … | … | … |
| Multiple resheathing || | 1.92 (1.11–3.32) | 0.02 | Multiple resheathing | 2.06 (1.18–3.6) | 0.01 |
| SE‐THV center annual volume <25 cases || , ¶ | 1.72 (0.97–3.05) | 0.06 | SE‐THV center annual volume <25 cases ¶ | 1.89 (1.06–3.36) | 0.03 |
| SE‐THV center annual volume 26–75 cases || , ¶ | 1.36 (0.8–2.33) | 0.26 | SE‐THV center annual volume 26–75 cases ¶ | 1.33 (0.77–2.3) | 0.3 |
Afib indicates atrial fibrillation; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; and SE‐THV, self‐expanding transcatheter heart valve.
A total of 1026 (100%) cases were included in a complete case analysis (more details in Table S5).
Afib indicates atrial fibrillation at baseline.
Moderate or severe aortic regurgitation at baseline.
Evolut R as the reference.
Interaction between center annual volume and multiple resheathing (P=0.13).
SE‐THV center annual volume >75 cases as reference.
Discussion
The main findings of this real‐world registry of patients undergoing TAVR with repositionable self‐expanding THV were as follows: (1) resheathing was required in a third of patients, being multiple in ≈10% of them; (2) independent predictors of MR were moderate/severe aortic regurgitation at baseline and implantation of a Portico valve; (3) MR was associated with lower device success, a higher rate of prosthesis embolization, and the need for a second valve, irrespective of the THV implanted; and (4) no differences were seen with respect to the combined early safety events, although MR was an independent predictor of increased midterm mortality.
To the best of our knowledge, this is the first study to specifically address the need for resheathing during TAVR using a self‐expanding device, according to the number of attempts/patients. Although prior studies in the TAVR field have shown resheathing rates of ≈30%, similar to our research, there has been a considerable variation among them according to valve type, ranging from 21% to 41%. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Of note, MR, defined as the need for ≥2 partial or total recapture of the device, was required in 28% of all resheathing cases (≈10% overall), somewhat lower than the 38% reported by a recent smaller study. 13 More important, in our research, MR was more frequent with a Portico versus Evolut R/Pro device, and by multivariable analysis, the use of a Portico THV increased ≈3‐fold the chances for MR. This has also been consistent with the literature, as shown in the recent Portico‐I trial, where 41% of the cases needed at least 1 resheathing. 16 One might argue that the Portico's lower radial force might play a role in the higher resheathing rates. 22 Yet, all but one of the participating centers had more experience with the Evolut P/Pro device than with the Portico. The overall lower experience with the Portico could have also played a role in the higher need for repositioning. The first‐generation delivery system could have also contributed to these findings because the new Flexnav system that showed improved outcomes in the recent PORTICO IDE trial is recalled for a more stable deployment. 23 Nonetheless, this should be further evaluated in future studies.
Other anatomical factors that have been argued as possibly related to the need for repositioning have also been evaluated, such as the severity of aortic stenosis determined by transaortic gradient, valve orifice area, and aortic valve calcification, in addition to annulus size and eccentricity index. Yet, no significant correlation of such factors with the need for resheathing was found. Of note, a recent large study also failed to demonstrate an association of these variables with the use of the repositioning feature with both the Evolut R and PRO devices, 14 highlighting that predicting the need for recapturing and repositioning self‐expanding devices might not be so evident. In this regard, however, apart from the valve type, we identified moderate or severe aortic regurgitation at baseline to be independently associated with the need for MR. Most likely, the greater pulse pressure, generally accompanied by larger stroke volumes, could translate into a less stable delivery of the valve, explaining the finding.
Although the clinical outcomes during TAVR procedures have unquestionably been improved with the current generation of devices, including lower profile delivery systems and the possibility to reposition the THV, there is controversy on whether resheathing and redeploying the valve could jeopardize clinical outcomes. Therefore, although repositioning the THV might ensure proper implants, generally, at a higher position to reduce conduction disturbances and to improve hemodynamics, this could augment the instrumentation of the aorta, potentially increasing cerebrovascular events and device embolization. Nonetheless, recent larger studies using various repositionable devices did not show an association of this maneuver with poorer clinical outcomes, including stroke, in accordance with our findings. 16 , 18 , 24 , 25 Notably, a recent study evaluating histological and histomorphometric data of elements captured from filters of patients undergoing TAVR using embolic protection filters showed a much higher amount of debris among those in whom THV was repositioned. 11 The real impact of such findings is still unclear. Whether the use of embolic protection devices in patients with an anticipated higher risk for MR (eg, patients with moderate/severe aortic regurgitation at baseline) could reduce neurologic events needs to be further evaluated in proper design studies.
Another important aspect is that prior research has not explicitly evaluated the number of resheathing and recapture during TAVR and the potential impact on clinical outcomes. In the present study, we were able to determine that in up to 10% of the patients, MR was necessary, and it was significantly associated with less device success and increased 1‐year mortality. Although the overall device success in our study was ≈90%, similar to what is found in most of the literature for self‐expanding THV, 16 , 18 , 24 , 25 in those patients requiring MR it went down to 80%. These results were driven by higher device embolization rates and need for a second THV in the MR group. Notably, previous studies have consistently demonstrated with the various THVs that valve embolization and, eventually, the need for a second device significantly increased mortality. 26 , 27 , 28 It is important to mention that even though the Portico device was associated with higher MR need, the negative impact of MR was seen regardless of the type of THV implanted.
Finally, there was a trend toward more early deaths for the MR group, that on the midterm follow‐up was significant, with 2‐fold greater mortality compared with NR or SR cases. It is difficult to conclude whether MR was the causative factor of the increased mortality at 1 year. One would expect a procedure‐related event to rather impact early outcomes. Nevertheless, most of the Kaplan‐Meier curve separation occurred during the first 120 days, a period more sensitive to the consequences of a procedural issue. Alternatively, MR could be merely a marker of more complicated anatomical features for proper device implantation, leading to more prosthesis embolization, which, in turn, compromises the results. This was highlighted by a higher need for predilatation and postdilatation, and less use of conscious sedation in the MR group. Therefore, we suggest that in patients in whom the operator has difficulties positioning the THV after >2 attempts, it may be reasonable to consider switching to a different size or even to another type of bioprosthesis before final deployment.
The present study has several limitations. Its retrospective nature makes it prone to biases related to this type of study design. Thus, although other studies in the literature support our results, they should be perceived as exploratory and confirmed by further research. Moreover, there was no central adjudication of events or a central core laboratory to assess pre‐TAVR and post‐TAVR imaging examinations, even though all participating centers have well‐developed TAVR programs with experienced heart teams. Nevertheless, there was some heterogeneity in experience among the centers, which was taken into account as a cofactor in the multivariable analysis, according to each center's annual procedural volume with self‐expanding valves. Also, we see this variation of experience among centers to better reflect the real world of TAVR practice, increasing the external validity of our findings. Another limitation was that the exact causes of the resheathing were not available, which could have also played a role in explaining the results. However, a recent study has not seen an association of the cause of resheathing with outcomes. 14 Finally, most of the cases included in the study were performed before the more widespread use of current techniques using specific gantry angles for THV deployment, aiming higher implants, and using more precise positioning. Thus, future studies with more contemporary techniques may be warranted to further confirm our findings.
In conclusion, repositioning a self‐expanding valve during TAVR is used in a third of patients, being multiple in ≈10% of them, which was predicted by the presence of moderate/severe aortic regurgitation at baseline and implantation of a Portico valve. MR, but not SR, was associated with more device failure and increased 1‐year mortality, regardless of the type of transcatheter valve implanted.
Sources of Funding
None.
Disclosures
Dr Rodés‐Cabau has received institutional research grants from Medtronic (significant), Edwards Lifesciences (significant), and Boston Scientific (significant). Dr Ribeiro has served as proctor for Edwards Lifescience, Medtronic, and Boston Scientific (significant). Dr Nombela‐Franco has served as proctor for Abbott (significant) and has received speaker honoraria from Edwards Lifesciences (modest). Dr Amat Santos is proctor for Boston Scientific (significant) and has received institutional research grants from Medtronic, Abbott, and Boston Scientific (significant). Dr Mangione has served as proctor for Edwards Lifescience and Medtronic (modest). Dr Pessoa de Melo has served as proctor for Medtronic (significant). Dr Tumeleiro has served as proctor for Medtronic (significant). The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5
Figure S1
Supplementary material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020682
For Sources of Funding and Disclosures, see page 9.
See Editorial by Bagur
References
- 1. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. DOI: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 2. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. DOI: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. DOI: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 4. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, et al. Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. DOI: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 5. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med. 2014;370:1790–1798. DOI: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 6. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med. 2019;380:1706–1715. DOI: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 7. Généreux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, Smith C, Serruys PW, Kappetein AP, Leon MB. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions. J Am Coll Cardiol. 2012;59:2317–2326. DOI: 10.1016/j.jacc.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 8. Athappan G, Patvardhan E, Tuzcu EM, Svensson LG, Lemos PA, Fraccaro C, Tarantini G, Sinning J‐M, Nickenig G, Capodanno D, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement. J Am Coll Cardiol. 2013;61:1585–1595. DOI: 10.1016/j.jacc.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 9. Ribeiro HB, Orwat S, Hayek SS, Larose É, Babaliaros V, Dahou A, Le Ven F, Pasian S, Puri R, Abdul‐Jawad Altisent O, et al. Cardiovascular magnetic resonance to evaluate aortic regurgitation after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:577–585. DOI: 10.1016/j.jacc.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 10. Jilaihawi H, Zhao Z, Du R, Staniloae C, Saric M, Neuburger PJ, Querijero M, Vainrib A, Hisamoto K, Ibrahim H, et al. Minimizing permanent pacemaker following repositionable self‐expanding transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:1796–1807. DOI: 10.1016/j.jcin.2019.05.056. [DOI] [PubMed] [Google Scholar]
- 11. Seeger J, Romero M, Schuh C, Virmani R, Wöhrle J. Impact of repositioning during transcatheter aortic valve replacement on embolized debris. J Invasive Cardiol. 2019;31:282–288. [PubMed] [Google Scholar]
- 12. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM , Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es G‐A , et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium‐2 consensus document. Eur Heart J. 2012;33:2403–2418. DOI: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 13. Kefer J, Maes F, Renkin J, Kautbally S, De Meester C, Delacour M, Pouleur A‐C. Resheathing of self‐expanding bioprosthesis: impact on procedural results, clinical outcome and prosthetic valve durability after transcatheter aortic valve implantation. IJC Hear Vasc. 2020;26:100462. DOI: 10.1016/j.ijcha.2019.100462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Attizzani GF, Dallan LAP, Markowitz A, Yakubov SJ, Deeb GM, Reardon MJ, Forrest JK, Mangi AA, Huang J, Popma JJ. Impact of repositioning on outcomes following transcatheter aortic valve replacement with a self‐expandable valve. JACC Cardiovasc Interv. 2020;13:1816–1824. DOI: 10.1016/j.jcin.2020.04.028. [DOI] [PubMed] [Google Scholar]
- 15. Forrest JK, Mangi AA, Popma JJ, Khabbaz K, Reardon MJ, Kleiman NS, Yakubov SJ, Watson D, Kodali S, George I, et al. Early outcomes with the evolut PRO repositionable self‐expanding transcatheter aortic valve with pericardial wrap. JACC Cardiovasc Interv. 2018;11:160–168. DOI: 10.1016/j.jcin.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 16. Søndergaard L, Rodés‐Cabau J, Hans‐Peter Linke A, Fichtlscherer S, Schäfer U, Kuck K‐H, Kempfert J, Arzamendi D, Bedogni F, Asch FM, et al. Transcatheter aortic valve replacement with a repositionable self‐expanding prosthesis: the PORTICO‐I trial 1‐year outcomes. J Am Coll Cardiol. 2018;72:2859–2867. DOI: 10.1016/j.jacc.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 17. Möllmann H, Linke A, Holzhey DM, Walther T, Manoharan G, Schäfer U, Heinz‐Kuck K, Van Boven AJ, Redwood SR, Kovac J, et al. Implantation and 30‐day follow‐up on all 4 valve sizes within the portico transcatheter aortic bioprosthetic family. JACC Cardiovasc Interv. 2017;10:1538–1547. DOI: 10.1016/j.jcin.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 18. Popma JJ, Reardon MJ, Khabbaz K, Harrison JK, Hughes GC, Kodali S, George I, Deeb GM, Chetcuti S, Kipperman R, et al. Early clinical outcomes after transcatheter aortic valve replacement using a novel self‐expanding bioprosthesis in patients with severe aortic stenosis who are suboptimal for surgery: results of the Evolut R U.S. Study. JACC Cardiovasc Interv. 2017;10:268–275. DOI: 10.1016/j.jcin.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 19. Grube E, Van Mieghem NM, Bleiziffer S, Modine T, Bosmans J, Manoharan G, Linke A, Scholtz W, Tchétché D, Finkelstein A, et al. Clinical outcomes with a repositionable self‐expanding transcatheter aortic valve prosthesis: the international FORWARD study. J Am Coll Cardiol. 2017;70:845–853. [DOI] [PubMed] [Google Scholar]
- 20. Kalra SS, Firoozi S, Yeh J, Blackman DJ, Rashid S, Davies S, Moat N, Dalby M, Kabir T, Khogali SS, et al. Initial experience of a second‐generation self‐expanding transcatheter aortic valve. JACC Cardiovasc Interv. 2017;10:276–282. DOI: 10.1016/j.jcin.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 21. Manoharan G, Walton AS, Brecker SJ, Pasupati S, Blackman DJ, Qiao H, Meredith IT. Treatment of symptomatic severe aortic stenosis with a novel resheathable supra‐annular self‐expanding transcatheter aortic valve system. JACC Cardiovasc Interv. 2015;8:1359–1367. DOI: 10.1016/j.jcin.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 22. Kumar S, Moseman B, Vietmeier K. Stent geometry and radial force comparison of Portico vs CoreValve (abstr). Circulation. 2014;130:A16952. [Google Scholar]
- 23. O’Brien SM, Cohen DJ, Rumsfeld JS, Brennan JM, Shahian DM, Dai D, Holmes DR, Hakim RB, Thourani VH, Peterson ED, et al. Variation in hospital risk‐adjusted mortality rates following transcatheter aortic valve replacement in the United States. Circ Cardiovasc Qual Outcomes. 2016;9:560–565. DOI: 10.1161/CIRCOUTCOMES.116.002756. [DOI] [PubMed] [Google Scholar]
- 24. Rao G, Sheth S, Donnelly J, Scatola A, Tariq U, Laighold S, Grines C, Rutkin B. Early real‐world experience with corevalve evolut pro and r systems for transcatheter aortic valve replacement. J Interv Cardiol. 2019;2019:1–8. DOI: 10.1155/2019/1906814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Makkar RR, Cheng W, Waksman R, Satler LF, Chakravarty T, Groh M, Abernethy W, Russo MJ, Heimansohn D, Hermiller J, et al. Self‐expanding intra‐annular versus commercially available transcatheter heart valves in high and extreme risk patients with severe aortic stenosis (PORTICO IDE): a randomised, controlled, non‐inferiority trial. Lancet. 2020;396:669–683. DOI: 10.1016/S0140-6736(20)31358-1. [DOI] [PubMed] [Google Scholar]
- 26. Kim W‐K, Schäfer U, Tchetche D, Nef H, Arnold M, Avanzas P, Rudolph T, Scholtz S, Barbanti M, Kempfert J, et al. Incidence and outcome of peri‐procedural transcatheter heart valve embolization and migration: the TRAVEL registry (TranscatheteR HeArt Valve EmboLization and Migration). Eur Heart J. 2019;40:3156–3165. DOI: 10.1093/eurheartj/ehz429. [DOI] [PubMed] [Google Scholar]
- 27. Makkar RR, Jilaihawi H, Chakravarty T, Fontana GP, Kapadia S, Babaliaros V, Cheng W, Thourani VH, Bavaria J, Svensson L, et al. Determinants and outcomes of acute transcatheter valve‐in‐valve therapy or embolization: a study of multiple valve implants in the U.S. PARTNER trial (Placement of AoRTic TraNscathetER Valve Trial Edwards SAPIEN Transcatheter Heart Valve). J Am Coll Cardiol. 2013;62:418–430. DOI: 10.1016/j.jacc.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 28. De Brito FS, Carvalho LA, Sarmento‐Leite R, Mangione JA, Lemos P, Siciliano A, Caramori P, São Thiago L, Grube E, Abizaid A. Outcomes and predictors of mortality after transcatheter aortic valve implantation: results of the Brazilian registry. Catheter Cardiovasc Interv. 2015;85:E153–E162. DOI: 10.1002/ccd.25778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figure S1


