Abstract
Background
It remains controversial whether long‐term clinical impact of newly diagnosed atrial fibrillation (AF) in the acute phase of acute myocardial infarction (AMI) is different from that of prior AF diagnosed before the onset of AMI.
Methods and Results
The current study population from the CREDO‐Kyoto AMI (Coronary Revascularization Demonstrating Outcome Study in Kyoto Acute Myocardial Infarction) Registry Wave‐2 consisted of 6228 patients with AMI who underwent percutaneous coronary intervention. The baseline characteristics and long‐term clinical outcomes were compared according to AF status (newly diagnosed AF: N=489 [7.9%], prior AF: N=589 [9.5%], and no AF: N=5150 [82.7%]). Median follow‐up duration was 5.5 years. Patients with newly diagnosed AF and prior AF had similar baseline characteristics with higher risk profile than those with no AF including older age and more comorbidities. The cumulative 5‐year incidence of all‐cause death was higher in newly diagnosed AF and prior AF than no AF (38.8%, 40.7%, and 18.7%, P<0.001). The adjusted hazard ratios (HRs) for mortality of newly diagnosed AF and prior AF relative to no AF remained significant with similar magnitude (HR, 1.31; 95% CI, 1.12–1.54; P<0.001, and HR, 1.32; 95% CI, 1.14–1.52; P<0.001, respectively). The cumulative 5‐year incidence of stroke decreased in the order of newly diagnosed AF, prior AF and no AF (15.5%, 12.9%, and 6.3%, respectively, P<0.001). The higher adjusted HRs of both newly diagnosed AF and prior AF relative to no AF were significant for stroke, with a greater risk of newly diagnosed AF than that of prior AF (HR, 2.05; 95% CI, 1.56–2.69; P<0.001, and HR, 1.33; 95% CI, 1.00–1.78; P=0.048, respectively). The higher stroke risk of newly diagnosed AF compared with prior AF was largely driven by the greater risk within 30 days. The higher adjusted HRs of newly diagnosed AF and prior AF relative to no AF were significant for heart failure hospitalization (HR, 1.73; 95% CI, 1.35–2.22; P<0.001, and HR, 2.23; 95% CI, 1.82–2.74; P<0.001, respectively) and major bleeding (HR, 1.46; 95% CI, 1.23–1.73; P<0.001, and HR, 1.36; 95% CI, 1.15–1.60; P<0.001, respectively).
Conclusions
Newly diagnosed AF in AMI had risks for mortality, heart failure hospitalization, and major bleeding higher than no AF, and comparable to prior AF. The risk of newly diagnosed AF for stroke might be higher than that of prior AF.
Keywords: acute myocardial infarction, anticoagulation, atrial fibrillation, percutaneous coronary intervention, stroke
Subject Categories: Anticoagulants, Atrial Fibrillation, Cerebrovascular Disease/Stroke, Myocardial Infarction, Percutaneous Coronary Intervention
Clinical Perspective
What Is New?
Atrial fibrillation (AF) newly provoked by acute myocardial infarction is associated with poor clinical outcomes, however, the different impact on clinical outcome between newly diagnosed AF and prior AF in acute myocardial infarction has not been adequately evaluated yet.
This study showed that newly diagnosed AF had comparable risk for mortality, heart failure, and major bleeding with prior AF, and higher risk for stroke than prior AF.
What Are the Clinical Implications?
Once AF is newly detected in the acute phase of acute myocardial infarction, consideration of anticoagulation therapy is mandatory in patients with high risk for stroke (CHA2DS2‐VASc score ≥2), although it should be noted that the risk of major bleeding is also high.
Atrial fibrillation (AF) often coexists in patients with acute myocardial infarction (AMI), and its incidence in the setting of AMI was reported in 6% to 21% of patients. 1 AMI can induce AF through inflammation and atrial diastolic overload, whereas rapid heart rate of AF leads to increase in oxygen demand and worsen ischemia. 2 Several studies reported that AF in the setting of AMI was associated with poor in‐hospital or midterm clinical outcomes including mortality and stroke. 1 , 3 , 4 , 5 , 6 There are 2 types of AF in the setting of AMI; prior AF diagnosed before the onset of AMI, and newly diagnosed AF emerging after the onset of AMI. The newly diagnosed AF is often self‐limited and transient. It remains controversial whether long‐term clinical impact of newly diagnosed AF during the acute phase of AMI is different from that of prior AF. 7 , 8 , 9 , 10 , 11 , 12 Patients with coronary artery disease and AF are known to be at high risk for both ischemic and bleeding events, and careful consideration would be needed for the decision to implement anticoagulation therapy concomitant with antiplatelet therapy. 13 In the current American Heart Association/American College of Cardiology/Heart Rhythm Society and European Society of Cardiology clinical guidelines, anticoagulation is recommended for patients with AMI and coexisting AF if CHA2DS2‐Vasc score ≥2. 14 , 15 Regarding antithrombotic management for newly diagnosed AF, however, the American Heart Association/American College of Cardiology/Heart Rhythm Society clinical guidelines did not make a specific recommendation, while the European Society of Cardiology guidelines recommend the same management with prior AF, but without firm scientific evidences. Comprehensive data on thrombotic and bleeding risk is still sparse in patients with newly diagnosed AF relative to those without AF or relative to those with prior AF in the current primary percutaneous coronary intervention (PCI) era.
Therefore, the purpose of this study is to clarify the baseline characteristics and prognostic impact of newly diagnosed AF compared with those with prior AF and without AF in patients with AMI undergoing PCI in a large Japanese registry in real clinical practice.
Methods
Study Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. The CREDO‐Kyoto AMI (Coronary Revascularization Demonstrating Outcome Study in Kyoto Acute Myocardial Infarction) registry Wave‐2 is a physician‐initiated, non‐company‐sponsored, multicenter registry that enrolled consecutive 6470 AMI patients who underwent coronary revascularization within 7 days of the onset of symptoms between January 2011 and December 2013 among 22 participating centers in Japan (Data S1). The relevant institutional review boards at all participating centers approved the study protocol, and written informed consent for this study was waived because of the retrospective nature of the study; however, we excluded those patients who refused participation in this study when contacted at follow‐up. This strategy is concordant with the guidelines of the Japanese Ministry of Health, Labor and Welfare.
After excluding 21 patients who refused the study participation and 221 patients who received coronary artery bypass grafting, the current study population consisted of 6228 AMI patients who underwent PCI, and was divided into 3 groups according to the presence or absence of AF, and types of AF; newly diagnosed AF, prior AF, and no AF (Figure 1).
Figure 1. Study flow chart.

AF indicates atrial fibrillation; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; CREDO‐Kyoto AMI Registry Wave‐2, Coronary Revascularization Demonstrating Outcome study in Kyoto AMI Registry Wave‐2; and PCI, percutaneous coronary intervention.
Definitions for Baseline Characteristics and Outcome Measures
We defined newly diagnosed AF as presumably newly developed AF documented during index hospitalization for AMI. Prior AF included all types of AF (paroxysmal, persistent, or permanent) diagnosed before admission for AMI. Prior AF was regarded as present when the diagnosis was indicated in the hospital charts in the participating centers. Other baseline clinical characteristics, such as hypertension, current smoking, heart failure, prior myocardial infarction, and chronic obstructive pulmonary disease were regarded as present when these diagnoses were documented in the hospital charts. Diabetes was defined as treatment with oral hypoglycemic agents or insulin, prior clinical diagnosis of diabetes, glycated hemoglobin level ≥6.5%, or non‐fasting blood glucose level ≥200 mg/dL. Peripheral vascular disease was regarded as present when carotid, aortic, or other peripheral vascular diseases were being treated or scheduled for surgical or endovascular interventions. Renal function was evaluated by the estimated glomerular filtration rate calculated by the Modification of Diet in Renal Disease formula modified for Japanese patients. 16
The outcome measures in this study were all‐cause death, cardiovascular death, myocardial infarction, stroke, hospitalization for heart failure, major bleeding, and any coronary revascularization. Death was regarded as cardiac in origin unless obvious non‐cardiac causes could be identified. Any death during the index hospitalization for AMI was regarded as cardiac death. Cardiovascular death included cardiac death and other vascular death related to stroke, renal disease, and vascular disease. Myocardial infarction was defined according to the Academic Research Consortium definition. 17 Stroke was defined as ischemic or hemorrhagic stroke with neurological symptoms lasting >24 hours. Hospitalization for heart failure was defined as de novo hospitalization or prolongation of hospitalization due to heart failure requiring intravenous treatment. Major bleeding was defined according to the Bleeding Academic Research Consortium classification of type 3 or 5. 18 Any coronary revascularization included either PCI or coronary artery bypass grafting for any reasons. The clinical event committee adjudicated all the events for the outcome measures (Data S2).
Data Collection for Baseline Characteristics and Follow‐Up Events
Baseline clinical, angiographic and procedural data were collected from medical charts or hospital databases according to the pre‐specified definitions by the experienced clinical research coordinators from an independent clinical research organization (Research Institute for Production Development, Kyoto, Japan) (Data S3). Follow‐up data were collected from the hospital charts and/or by contacting with patients, their relatives or family physicians between January 2018 and December 2019. Median follow‐up duration was 5.5 years (interquartile range: 3.6–6.6 years). Complete 1‐, 3‐, and 5‐year follow‐up information was obtained in 96.5%, 93.6%, and 83.1% of patients, respectively.
Statistical Analysis
Categorical variables were presented as values and percentages, and were compared using the chi‐square test. Continuous variables were presented as mean±SD or median and interquartile range and were compared using the analysis of variance or Kruskal‐Wallis test according to their distributions. Cumulative incidences of the outcome measures were estimated with the Kaplan‐Meier method, and the differences were assessed with the log‐rank test. We also performed a landmark analysis at 30 days to estimate the cumulative incidence of the outcome measures within or beyond 30 days after index PCI for AMI. The cumulative incidence of a given event beyond 30 days was estimated by the Kaplan‐Meier method among patients who were free from the event at 30 days. The effects of the newly diagnosed AF group and the prior AF group relative to the no AF group for the outcome measures were estimated by the Cox proportional hazard models and were expressed as hazard ratios (HRs) and their 95% CIs. In the multivariable Cox proportional hazard models in the entire follow‐up period, we incorporated dummy‐coded AF status together with the 28 clinically relevant risk‐adjusting variables listed in Table 1 without model selection procedures in consistent with our previous report. 19 Continuous risk‐adjusting variables were dichotomized by clinically meaningful reference values to make proportional hazard assumptions robust and to be consistent with our previous reports. 20 , 21 The missing values for the risk‐adjusting variables were imputed as “normal” in the binary classification, because data should have been available if abnormalities were suspected. Proportional hazard assumptions for the primary variable (newly diagnosed AF, prior AF, and no AF) and the risk‐adjusting variables were assessed on the plots of log (time) versus log [−log (survival)] stratified by the variable. The assumptions were verified to be acceptable for all the variables except for ST‐segment–elevation myocardial infarction, which was included as the stratification variable in the Cox proportional hazard models. We did not construct the multivariable models for the landmark analyses, because the number of patients with events was too small to construct the models within 30 days, and the multivariable models beyond 30 days were similar to those in the entire follow‐up period.
Table 1.
Baseline Characteristics
| Newly diagnosed AF (N=489) | Prior AF (N=589) | No AF (N=5150) | P value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, y | 74.4±11.2 | 74.8±10.5 | 68.1±12.3 | <0.001 |
| Age ≥75 y* | 264 (54%) | 339 (58%) | 1706 (33%) | <0.001 |
| Men* | 343 (70%) | 403 (68%) | 3927 (76%) | <0.001 |
| Body mass index, kg/m2 | 23.2±3.4 | 23.1±3.8 | 23.8±3.6 | <0.001 |
| Body mass index <25.0 kg/m2 * | 360 (74%) | 441 (75%) | 3483 (68%) | <0.001 |
| Hypertension* | 379 (78%) | 478 (81%) | 4184 (81%) | 0.13 |
| Diabetes* | 201 (41%) | 209 (36%) | 1840 (36%) | 0.06 |
| Treated with insulin | 35 (7.2%) | 49 (8.3%) | 303 (5.9%) | 0.045 |
| Current smoking* | 126 (26%) | 135 (23%) | 1865 (36%) | <0.001 |
| Heart failure (prior and/or current)* | 255 (52%) | 303 (51%) | 1459 (28%) | <0.001 |
| Left ventricular ejection fraction (%) | 47.9±13.7 | 51.3±13.8 | 55.4±12.2 | <0.001 |
| Left ventricular ejection fraction ≤40% | 120 (27%) | 113 (22%) | 517 (11%) | <0.001 |
| Mitral regurgitation grade 3/4 | 75 (17%) | 110 (21%) | 360 (7.6%) | <0.001 |
| Prior myocardial infarction* | 45 (11%) | 92 (16%) | 522 (10%) | <0.001 |
| Prior stroke* | 77 (16%) | 151 (26%) | 512 (9.9%) | <0.001 |
| Prior ischemic stroke | 67 (14%) | 131 (22%) | 406 (7.9%) | <0.001 |
| Prior hemorrhagic stroke | 12 (2.5%) | 22 (3.7%) | 112 (2.2%) | 0.059 |
| Peripheral vascular disease* | 25 (5.1%) | 45 (7.6%) | 237 (4.6%) | 0.005 |
| eGFR <30 mL/min per 1.73 m2 not on dialysis* | 58 (12%) | 64 (11%) | 279 (5.4%) | <0.001 |
| Dialysis* | 14 (2.9%) | 34 (5.8%) | 175 (3.4%) | 0.009 |
| Prior gastrointestinal bleeding | 13 (2.7%) | 39 (6.6%) | 144 (2.8%) | <0.001 |
| Chronic obstructive pulmonary disease* | 22 (4.5%) | 39 (6.6%) | 176 (3.4%) | <0.001 |
| Malignancy* | 54 (11%) | 80 (14%) | 556 (11%) | 0.12 |
| Liver cirrhosis* | 9 (1.8%) | 18 (3.1%) | 107 (1.8%) | 0.27 |
| Anemia* (hemoglobin <11 g/dL) | 85 (17%) | 101 (17%) | 580 (11%) | <0.001 |
| Thrombocytopenia* (platelet <106/μL) | 15 (3.1%) | 20 (3.4%) | 101 (2.0%) | 0.03 |
| White blood cell counts, /μL | 10 859±4212 | 9405±3594 | 9827±3592 | <0.001 |
| CHADS2 score | 2.6±1.3 | 2.8±1.4 | 2.0±1.2 | <0.001 |
| CHADS2 score ≥1 | 476 (97%) | 569 (97%) | 4786 (93%) | <0.001 |
| CHA2DS2‐Vasc score | 3.9±1.7 | 4.2±1.8 | 3.0±1.7 | <0.001 |
| CHA2DS2‐Vasc score ≥2 | 455 (93%) | 542 (92%) | 4103 (80%) | <0.001 |
| ARC‐HBR | 349 (71%) | 487 (83%) | 2161 (42%) | <0.001 |
| Presentation, angiographic, and procedural characteristics | ||||
| STEMI † | 400 (82%) | 410 (70%) | 3815 (74%) | <0.001 |
| Cardiogenic shock (Killip IV)* | 145 (30%) | 144 (25%) | 632 (12%) | <0.001 |
| Cardiopulmonary arrest on arrival | 34 (7.0%) | 33 (5.6%) | 183 (3.6%) | <0.001 |
| Intra‐aortic balloon pump use | 175 (36%) | 127 (22%) | 821 (16%) | <0.001 |
| Percutaneous cardiopulmonary support use | 34 (7.0%) | 28 (4.8%) | 130 (2.5%) | <0.001 |
| Peak creatine kinase, U/L | 2580 (1106–4785) | 1240 (437–2871) | 1336 (439–3037) | <0.001 |
| Infarct related artery location | <0.001 | |||
| Left anterior descending artery | 205 (42%) | 232 (39%) | 2324 (45%) | |
| Left circumflex artery | 76 (16%) | 94 (16%) | 731 (14%) | |
| Right coronary artery | 167 (34%) | 232 (39%) | 1919 (37%) | |
| Left main coronary artery | 38 (7.8%) | 23 (3.9%) | 152 (3.0%) | |
| Coronary artery bypass graft | 3 (0.6%) | 8 (1.4%) | 24 (0.5%) | |
| Anterior wall infarction* | 243 (50%) | 257 (44%) | 2484 (48%) | 0.35 |
| Multivessel disease* | 320 (65%) | 322 (55%) | 2909 (57%) | <0.001 |
| Target of proximal left anterior descending artery* | 277 (57%) | 272 (46%) | 2847 (55%) | <0.001 |
| Target of unprotected left main coronary artery* | 51 (10%) | 29 (4.9%) | 244 (4.7%) | <0.001 |
| Medication at discharge | ||||
| Aspirin | 482 (99%) | 565 (96%) | 5074 (99%) | <0.001 |
| Thienopyridine | 469 (96%) | 546 (93%) | 5032 (98%) | <0.001 |
| Oral anticoagulation | 139 (28%) | 322 (55%) | 326 (6.3%) | <0.001 |
| Warfarin | 115 (24%) | 275 (47%) | 317 (6.2%) | <0.001 |
| DOAC | 25 (5.1%) | 47 (8.0%) | 9 (0.2%) | <0.001 |
| Statins* | 361 (74%) | 420 (71%) | 4346 (84%) | <0.001 |
| β‐blocker* | 260 (53%) | 329 (56%) | 2650 (52%) | 0.11 |
| ACEI or ARB* | 313 (64%) | 370 (63%) | 3959 (77%) | <0.001 |
| ACEI | 177 (36%) | 167 (28%) | 2065 (40%) | <0.001 |
| ARB | 138 (28%) | 209 (35%) | 1939 (38%) | <0.001 |
| Nitrate | 81 (17%) | 108 (18%) | 986 (19%) | 0.36 |
| Calcium channel blocker* | 101 (21%) | 168 (29%) | 1259 (25%) | 0.01 |
| Proton pump inhibitor or Histamine 2 blocker* | 419 (86%) | 496 (84%) | 4350 (85%) | 0.76 |
Continuous variables were expressed as mean±SD, or median (interquartile range). Categorical variables were expressed as number (percentage). Values are missing for body mass index in 146 patients, for left ventricular ejection fraction in 649 patients, for mitral regurgitation in 505 patients, eGFR in 15 patients, for hemoglobin level in 13 patients, for platelet count in 19 patients, for white blood cell counts in 20 patients, and for peak creatine kinase in 83 patients. ACEI indicates angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; ARC‐HBR, The Academic Research Consortium for High Bleeding Risk; CABG, coronary artery bypass grafting; DOAC, direct oral anticoagulants; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention; and STEMI, ST‐segment elevation myocardial infarction.
Risk‐adjusting variables selected for the Cox proportional hazard models.
Risk‐adjusting variable as the stratification variable for the Cox proportional hazard models.
Statistical analyses were conducted with JMP 14.0 software (SAS Institute, Inc., Cary, North California) and R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria). All statistical analyses were 2‐tailed, and the threshold of P values for significance was P<0.05.
Results
Baseline Characteristics
Among 6228 AMI patients who received PCI, there were 489 patients (7.9%) with newly diagnosed AF, 589 patients (9.5%) with prior AF, and 5150 patients (82.7%) with no AF (Figure 1).
Patients with newly diagnosed AF and prior AF had similar baseline characteristics, who had significantly higher risk profile than those with no AF including older age and higher prevalence of comorbidities (Table 1). The mean CHA2DS2‐Vasc score was significantly higher in newly diagnosed AF and prior AF than in no AF (3.9±1.7, 4.2±1.8, and 3.0±1.7), although majority of patients in all the 3 groups had high thrombotic risk score (CHA2DS2‐Vasc score ≥2: 93% in newly diagnosed AF, 92% in prior AF, and 80% in no AF). Patients with newly diagnosed and prior AF also had higher prevalence of high bleeding risk than patients with no AF (Academic Research Consortium for High Bleeding Risk: 71% in newly diagnosed AF, 83% in prior AF, and 42% in no AF) (Table 1).
Regarding the clinical presentation, angiographic characteristics, and procedural characteristics, patients with newly diagnosed AF had larger infarct size as indicated by the lower left ventricular ejection fraction, and higher peak creatine kinase level, and had higher risk features with greater prevalence of ST‐segment–elevation myocardial infarction, cardiogenic shock, and use of hemodynamic support devise than those with prior AF and no AF.
Despite their high thrombotic risk, only 28% of patients in newly diagnosed AF and 55% of those in prior AF had received anticoagulation therapy at hospital discharge from the index hospitalization. Dual antiplatelet therapy had been implemented in the vast majority of patients. The prescription rate of β‐blocker was not different regardless of AF, while other evidence based medications such as statins and angiotensin converting enzyme inhibitors/angiotensin II receptor blockers were less often prescribed in patients with newly diagnosed AF and prior AF than in those with no AF (Table 1).
Clinical Outcomes
During median follow‐up of 5.5 (3.6–6.6) years, the cumulative 5‐year incidence of all‐cause death was 38.8% in newly diagnosed AF, 40.7% in prior AF, and 18.7% in no AF (Log‐rank P<0.001) (Figure 2). The cumulative incidence of all‐cause death was consistently higher in newly diagnosed AF and prior AF than in no AF both within and beyond 30 days after index AMI (Figure 2 and Tables S1, S2). Even after adjusting for confounders, the higher HRs of newly diagnosed AF and prior AF relative to no AF remained significant for all‐cause death with similar magnitude of HRs in newly diagnosed AF and prior AF (HR: 1.31, 95% CI: 1.12–1.54, P<0.001, and HR: 1.32, 95% CI: 1.14–1.52, P<0.001, respectively) (Table 2). Findings were consistent for cardiovascular death (Table 2, Figure 3, Figure S1, and Tables S1, S2).
Figure 2. Kaplan‐Meier event curves for all‐cause death.

A, During the entire follow‐up period, and (B) Landmark analysis at 30‐day. Crude HRs and 95% CIs were indicated with reference to no AF. AF indicates atrial fibrillation; and HR, hazard ratio.
Table 2.
Clinical Outcomes
| End points | Rhythm | N of patients with event (cumulative 5‐y incidence) | Unadjusted HR [95% CI] | P value | Adjusted HR [95% CI] | P value | |
|---|---|---|---|---|---|---|---|
| All‐cause death | Newly diagnosed AF | 202 | (38.8%) | 2.42 [2.08–2.81] | <0.001 | 1.31 [1.12–1.54] | <0.001 |
| Prior AF | 255 | (40.7%) | 2.47 [2.16–2.83] | <0.001 | 1.32 [1.14–1.52] | <0.001 | |
| No AF | 1080 | (18.7%) | Reference | Reference | |||
| Cardiovascular death | Newly diagnosed AF | 135 | (27.7%) | 2.61 [2.17–3.14] | <0.001 | 1.29 [1.06–1.57] | 0.01 |
| Prior AF | 168 | (30.0%) | 2.65 [2.24–3.14] | <0.001 | 1.34 [1.12–1.60] | 0.001 | |
| No AF | 640 | (11.8%) | Reference | Reference | |||
| Myocardial infarction | Newly diagnosed AF | 31 | (7.0%) | 1.06 [0.74–1.53] | 0.74 | 1.01 [0.70–1.48] | 0.94 |
| Prior AF | 49 | (9.3%) | 1.37 [1.02–1.85] | 0.04 | 1.16 [0.85–1.58] | 0.36 | |
| No AF | 369 | (7.1%) | Reference | Reference | |||
| Stroke | Newly diagnosed AF | 60 | (15.5%) | 2.64 [2.02–3.44] | <0.001 | 2.05 [1.56–2.69] | <0.001 |
| Prior AF | 56 | (12.9%) | 1.93 [1.47–2.54] | <0.001 | 1.33 [1.00–1.78] | 0.048 | |
| No AF | 284 | (6.3%) | Reference | Reference | |||
| Ischemic stroke | Newly diagnosed AF | 50 | (12.7%) | 2.61 [1.93–3.54] | <0.001 | 1.95 [1.42–2.68] | <0.001 |
| Prior AF | 51 | (10.8%) | 2.13 [1.58–2.89] | <0.001 | 1.45 [1.06–1.99] | 0.02 | |
| No AF | 251 | (4.7%) | Reference | Reference | |||
| Hemorrhagic stroke | Newly diagnosed AF | 17 | (3.2%) | 2.37 [1.42–3.97] | 0.001 | 2.08 [1.22–3.54] | 0.007 |
| Prior AF | 11 | (2.5%) | 1.23 [0.66–2.29] | 0.52 | 0.90 [0.47–1.71] | 0.74 | |
| No AF | 97 | (1.9%) | Reference | Reference | |||
| Hospitalization for heart failure | Newly diagnosed AF | 79 | (19.9%) | 2.58 [2.03–3.28] | <0.001 | 1.73 [1.35–2.22] | <0.001 |
| Prior AF | 136 | (28.0%) | 3.63 [2.99–4.40] | <0.001 | 2.23 [1.82–2.74] | <0.001 | |
| No AF | 431 | (8.0%) | Reference | Reference | |||
| Major bleeding | Newly diagnosed AF | 323 | (35.9%) | 2.14 [1.81–2.52] | <0.001 | 1.46 [1.23–1.73] | <0.001 |
| Prior AF | 189 | (34.0%) | 1.93 [1.65–2.26] | <0.001 | 1.36 [1.15–1.60] | <0.001 | |
| No AF | 1010 | (19.4%) | Reference | Reference | |||
| Any coronary revascularization | Newly diagnosed AF | 110 | (28.1%) | 0.93 [0.76–1.13] | 0.44 | 0.89 [0.73–1.09] | 0.27 |
| Prior AF | 122 | (25.0%) | 0.79 [0.66–0.95] | 0.01 | 0.77 [0.63–0.93] | 0.01 | |
| No AF | 1514 | (31.1%) | Reference | Reference | |||
Cumulative incidence was estimated by Kaplan‐Meier method, and was represented with that at 5‐year. Number of patients with event and HRs with 95% CIs were estimated throughout the entire follow‐up period by the Cox proportional hazard models. AF indicates atrial fibrillation; and HR, hazard ratio.
Figure 3. Kaplan‐Meier event curves for cardiovascular death, hospitalization for heart failure, myocardial infarction, and any coronary revascularization.

A, Cardiovascular death, (B) Hospitalization for heart failure, (C) Myocardial infarction, and (D) Any coronary revascularization. Crude HRs and 95% CIs were indicated with reference to no AF. AF indicates atrial fibrillation; and HR, hazard ratio.
Regarding hospitalization for heart failure, the cumulative 5‐year incidence was 19.9% in newly diagnosed AF, 28.0% in prior AF, and 8.0% in no AF (Log‐rank P<0.001) (Figure 3). After adjusting for confounders, the higher HRs of newly diagnosed AF and prior AF relative to no AF for hospitalization for heart failure remained significant (HR: 1.73, 95% CI: 1.35–2.22, P<0.001, and HR: 2.23, 95% CI: 1.82–2.74, P<0.001, respectively) (Table 2, Figure 3).
For myocardial infarction, there was no significantly higher adjusted HRs of newly diagnosed AF and prior AF relative to no AF (Table 2, Figure 3). For any coronary revascularization, the lower adjusted HR of prior AF relative to no AF was significant, while the lower adjusted HR of newly diagnosed AF relative to no AF was not significant (Table 2, Figure 3).
The cumulative 5‐year incidence of stroke decreased in the order of newly diagnosed AF, prior AF and no AF (15.5%, 12.9%, and 6.3%, respectively, Log‐rank P<0.001) (Figure 4 and Figures S2, S3). Relative to no AF, long‐term risk of stroke in newly diagnosed AF (HR: 2.05, 95% CI: 1.56–2.69, P<0.001) was numerically greater than that in prior AF (HR: 1.33, 95% CI: 1.00–1.78, P=0.048) (Table 2). The cumulative incidence of stroke at 30 days was much higher in newly diagnosed AF than in prior AF and no AF (4.5%, 1.8%, and 1.5% respectively, Log‐rank P<0.001) (Figure 4 and Table S1). Beyond 30 days, the cumulative incidences of stroke in newly diagnosed AF and prior AF were comparable, and much higher than that in no AF (Figure 4 and Table S2).
Figure 4. Kaplan‐Meier event curves for stroke and major bleeding.
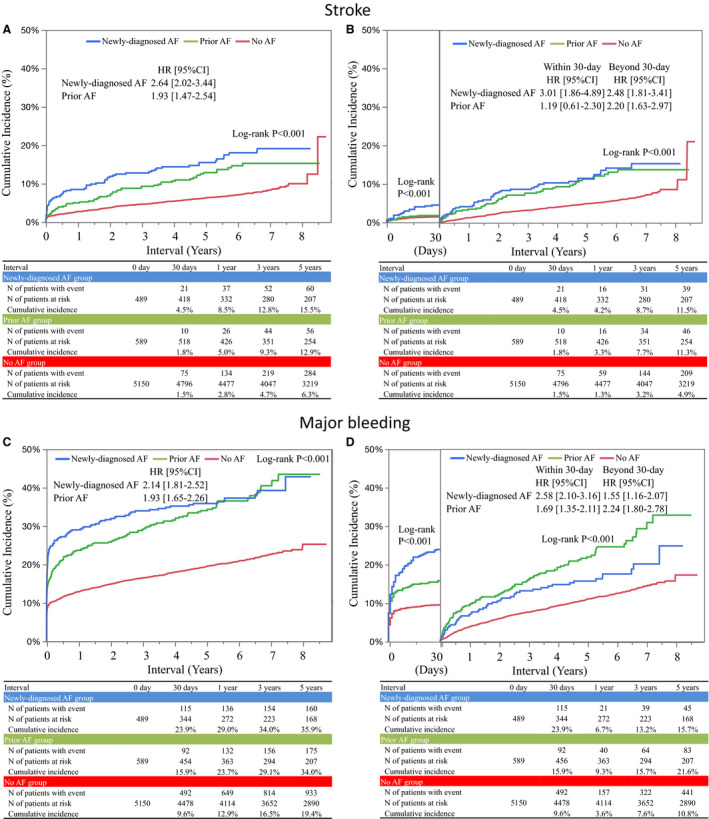
A, Stroke during the entire follow‐up period, (B) Landmark analysis at 30‐day for stroke, (C) Major bleeding during the entire follow‐up period, and (D) Landmark analysis at 30‐day for major bleeding. Crude HRs and 95% CIs were indicated with reference to no AF. AF indicates atrial fibrillation; and HR, hazard ratio.
The cumulative 5‐year incidences of major bleeding were significantly higher in newly diagnosed AF and prior AF than in no AF (35.9%, 34.0%, and 19.4%, respectively, Log‐rank P<0.001) (Figure 4). The higher adjusted HRs of newly diagnosed AF and prior AF relative to no AF remained significant for major bleeding (HR: 1.46, 95% CI: 1.23–1.73, P<0.001, and HR: 1.36, 95% CI: 1.15–1.60, P<0.001, respectively) (Table 2). Within 30 days, the cumulative incidence of major bleeding was higher in newly diagnosed AF than in prior AF, while beyond 30 days, it was higher in prior AF than in newly diagnosed AF (Figure 4 and Tables S1, S2).
Discussion
The main findings of the present study were as follows; (1) Newly diagnosed AF was found in 7.9% of patients during index hospitalization in AMI patients who underwent PCI; (2) Newly diagnosed AF had risks for mortality, heart failure hospitalization, and major bleeding higher than no AF, and comparable to prior AF; (3) The risk of newly diagnosed AF for stroke might be higher than that of prior AF; (4) Only less than one‐third of patients with newly diagnosed AF had received anticoagulation therapy at discharge from index hospitalization, although most of the patients had CHA2DS2‐Vasc score ≥2.
The prevalence of newly diagnosed AF in the acute phase of AMI in the present study (7.9%) was consistent with those reported in previous studies (3.7–10.3%), indicating that newly diagnosed AF during the acute phase of AMI is not rare in daily clinical practice. 8 , 9 , 11 , 12 AF, newly diagnosed AF in particular, had adverse effects on hemodynamics through tachycardia, atrioventricular dyssynchrony, and reduced cardiac output. 1 , 4 , 22 Indeed, the prevalence of cardiogenic shock, and use of hemodynamic support devises was higher in patients with AF, newly diagnosed AF in particular, than in patients without AF. Thus, AF would trigger hemodynamic compromise, while hemodynamic compromise might beget AF. 2 The previous reports indicated that AMI induces AF through inflammation, catecholamine drive, and necrosis, and therefore, hemodynamic compromise in AMI might be important for the development of AF. 12 , 22
Patients with AF were older and more often had comorbidities than patients without AF. Therefore, it has been well known that AF coexisting with AMI was associated with higher risk of acute and long‐term mortality than no AF. 4 , 5 However, it remains controversial whether long‐term clinical impact of newly diagnosed AF during acute phase of AMI, which is often self‐limited and transient, is different from prior AF diagnosed before the onset of AMI. 7 , 8 , 9 , 10 , 11 , 12 In this study including a large number of AMI patients who received coronary revascularization, patients with newly diagnosed AF had long‐term risks of mortality, heart failure hospitalization, and major bleeding comparable to those with prior AF, which were much higher than that in those without AF. One of the reasons for this poor prognosis of newly diagnosed AF might be partly explained by the relatively large infarct size, and hemodynamic instability in those patients. Another reason might be that patients with newly diagnosed AF and prior AF had similar baseline characteristics with high thrombotic and bleeding risk features. On the other hand, the risk for any coronary revascularization was not higher in patients with newly diagnosed AF and significantly lower in those with prior AF as compared with that in those without AF. The reason for this unexpected finding was unclear. However, one of the possible explanations might be related to the higher prevalence of elderly patients in both AF groups than in the no AF group. It would be likely that attending physicians tended to avoid coronary revascularization in older patients with many comorbidities due to high procedural risk.
In this study, both newly diagnosed AF and prior AF were associated with significantly higher risk for stroke than no AF. In the recent American Heart Association/American College of Cardiology/Heart Rhythm Society clinical guidelines for AF, anticoagulation is recommended as class I indication for patient with acute coronary syndrome and AF with CHA2DS2‐Vasc score ≥2. 14 It is based on 3 randomized controlled trials that demonstrated a lower risk of bleeding events with a comparable risk of cardiovascular events with dual therapy with P2Y12 receptor blocker and anticoagulant as compared with triple antithrombotic therapy. 23 , 24 , 25 However, these trials included only patients with prior AF. For patients with newly diagnosed AF during the acute phase of AMI, European Society of Cardiology clinical guidelines for ST‐segment–elevation myocardial infarction also recommend anticoagulation as class IIa indication, if CHA2DS2‐Vasc score ≥2, 15 although the recommendation was based on relatively old studies in which primary PCI was not prevalent. 1 , 3 Despite the guideline recommendation, anticoagulation was not widely implemented in real‐world clinical practice. 7 , 12 , 26 Indeed, in the present study, only less than one‐third of patients with newly diagnosed AF had received anticoagulation therapy, although most of the patients had CHA2DS2‐Vasc score ≥2. A previous study from the SWEDEHEART (Swedish Web‐system for Enhancement and Development of Evidence‐based Care in Heart Disease Evaluated According to Recommended Therapies) registry reported that patients with newly diagnosed AF who resumed sinus rhythm at discharge still had substantially higher risk of stroke than patients without AF, although they had lower risk of stroke than those with newly diagnosed AF who had AF at discharge. 11 In the present study, patient characteristics and long‐term risk of stroke were similar in patients with newly diagnosed and prior AF. Therefore, it would be reasonable to implement anticoagulation therapy in patients with newly diagnosed AF if CHA2DS2‐Vasc score ≥2 as recommended in the European Society of Cardiology guidelines. 15 In the present study, long‐term risk of stroke in newly diagnosed AF was numerically greater than that in prior AF. The higher stroke risk of newly diagnosed AF compared with prior AF was largely driven by the greater risk within 30 days of AMI. We might have to consider implementing anticoagulation therapy as soon as AF is newly detected. However, in the acute phase of AMI, the risk of major bleeding was also very high in newly diagnosed AF patients. Dual therapy with P2Y12 receptor blocker and reduced dose of direct oral anticoagulant might be a reasonable option, but further investigations are obviously needed to define optimal antithrombotic therapy in this setting.
Limitations
Several limitations of this study should be considered. First, due to the retrospective and observational study design, there might be unmeasured confounders for estimating the long‐term risk of cardiovascular events, although we attempted an extensive multivariable adjustment. Second, the diagnosis of AF was based on the physicians' diagnosis and records in the hospital charts. Therefore, very short duration of AF could have been overlooked, or newly diagnosed AF could actually have been undiagnosed paroxysmal AF before the onset of AMI. Third, ECGs were not evaluated at discharge or during follow‐up. We did not know how many patients with newly diagnosed AF resumed sinus rhythm, which was reported to be associated with lower risk, 27 , 28 and how many patients with no AF or newly diagnosed AF with sinus rhythm at discharge developed AF after discharge. Fourth, the definition of stroke included not only cardiogenic cerebral infarction derived from AF, but also other types of stroke such as atherosclerotic ischemic stroke, lacunar stroke, and cardiogenic stroke due to thrombus in the left ventricle. Additionally, in some patients with stroke that developed during hospitalization, AF could occur after the onset of stroke because of sympathetic nerve activation or hypovolemia induced by stroke. Finally, the low prevalence of anticoagulation might be attributed to the study period of 2011 to 2013, when the use of direct oral anticoagulant and dual therapy with P2Y12 receptor blocker and direct oral anticoagulant were not common for patients with AF undergoing PCI.
Conclusions
Newly diagnosed AF in AMI had risks for mortality, heart failure hospitalization, and major bleeding higher than no AF, and comparable to prior AF. The risk of newly diagnosed AF for stroke might be higher than that of prior AF.
Sources of Funding
This work was supported by an educational grant from the Research Institute for Production Development (Kyoto, Japan).
Disclosures
Dr Shiomi reports personal fees from Boston Scientific, personal fees from Abbot Vascular, and personal fees from Daiichi Sankyo. Dr Morimoto reports modest honoraria from Bayer and Kowa, and modest expert witness from Boston Scientific and Sanofi. Dr Tamura reports personal fees from Daiichi Sankyo and personal fees from Bayer. Dr Furukawa reports personal fees from Daiichi Sankyo, personal fees from Bayer, personal fees from Bristol Myers Squibb, personal fees from Pfizer, and personal fees from Boehringer Ingelheim. Dr Nakagawa reports modest research grant from Abbott Vascular and Boston Scientific, and modest honoraria from Abbott Vascular, Bayer, and Boston Scientific. Dr Kimura reports significant honoraria from Abbott Vascular, and modest honoraria from Astellas, AstraZeneca, Bayer, Boston Scientific, Kowa, and Sanofi. The remaining authors have no disclosures to report.
Supporting information
Datas S1–S3
Tables S1–S2
Figures S1–S3
Acknowledgments
We appreciate the support and collaboration of the co‐investigators participating in the CREDO‐Kyoto AMI Registry Wave‐2. We are indebted to the clinical research coordinators for data collection.
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.121.021417
For Sources of Funding and Disclosures, see page 11.
References
- 1. Schmitt J, Duray G, Gersh BJ, Hohnloser SH. Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. 2009;30:1038–1045. doi: 10.1093/eurheartj/ehn579 [DOI] [PubMed] [Google Scholar]
- 2. Vermond RA, Van Gelder IC, Crijns HJ, Rienstra M. Does myocardial infarction beget atrial fibrillation and atrial fibrillation beget myocardial infarction? Circulation. 2015;131:1824–1826. doi: 10.1161/CIRCULATIONAHA.115.016595 [DOI] [PubMed] [Google Scholar]
- 3. Siu CW, Jim MH, Ho HH, Miu R, Lee SWL, Lau CP, Tse HF. Transient atrial fibrillation complicating acute inferior myocardial infarction: implications for future risk of ischemic stroke. Chest. 2007;132:44–49. doi: 10.1378/chest.06-2733 [DOI] [PubMed] [Google Scholar]
- 4. Jabre P, Roger VL, Murad MH, Chamberlain AM, Prokop L, Adnet F, Jouven X. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta‐analysis. Circulation. 2011;123:1587–1593. doi: 10.1161/CIRCULATIONAHA.110.986661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garg L, Agrawal S, Agarwal M, Shah M, Garg A, Patel B, Agarwal N, Nanda S, Sharma A, Cox D. Influence of atrial fibrillation on outcomes in patients who underwent primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction. Am J Cardiol. 2018;121:684–689. doi: 10.1016/j.amjcard.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 6. Luo J, Xu S, Li H, Li Z, Liu B, Qin X, Gong M, Shi B, Wei Y. Long‐term impact of new‐onset atrial fibrillation complicating acute myocardial infarction on heart failure. ESC Heart Fail. 2020;7:2762–2772. doi: 10.1002/ehf2.12872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeymer U, Annemans L, Danchin N, Pocock S, Newsome S, Van de Werf F, Medina J, Bueno H. Impact of known or new‐onset atrial fibrillation on 2‐year cardiovascular event rate in patients with acute coronary syndromes: results from the prospective EPICOR Registry. Eur Heart J Acute Cardiovasc Care. 2019;8:121–129. doi: 10.1177/2048872618769057 [DOI] [PubMed] [Google Scholar]
- 8. Jabre P, Jouven X, Adnet F, Thabut G, Bielinski SJ, Weston SA, Roger VL. Atrial fibrillation and death after myocardial infarction: a community study. Circulation. 2011;123:2094–2100. doi: 10.1161/CIRCULATIONAHA.110.990192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang C‐L, Chen P‐C, Juang H‐T, Chang C‐J. Adverse outcomes associated with pre‐existing and new‐onset atrial fibrillation in patients with acute coronary syndrome: a retrospective cohort study. Cardiol Ther. 2019;8:117–127. doi: 10.1007/s40119-019-0136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Biasco L, Radovanovic D, Moccetti M, Rickli H, Roffi M, Eberli F, Jeger R, Moccetti T, Erne P, Pedrazzini G. New‐onset or pre‐existing atrial fibrillation in acute coronary syndromes: two distinct phenomena with a similar prognosis. Rev Esp Cardiol. 2019;72:383–391. doi: 10.1016/j.rec.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 11. Batra G, Svennblad B, Held C, Jernberg T, Johanson P, Wallentin L, Oldgren J. All types of atrial fibrillation in the setting of myocardial infarction are associated with impaired outcome. Heart. 2016;102:926–933. doi: 10.1136/heartjnl-2015-308678 [DOI] [PubMed] [Google Scholar]
- 12. Braga CG, Ramos V, Martins J, Arantes C, Abreu G, Vieira C, Salgado A, Gaspar A, Azevedo P, Álvares Pereira M, et al. Impact of atrial fibrillation type during acute coronary syndromes: clinical features and prognosis. Rev Port Cardiol. 2015;34:403–410. doi: 10.1016/j.repc.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 13. Dewilde WJM, Janssen PWA, Verheugt FWA, Storey RF, Adriaenssens T, Hansen ML, Lamberts M, Ten Berg JM. Triple therapy for atrial fibrillation and percutaneous coronary intervention: a contemporary review. J Am Coll Cardiol. 2014;64:1270–1280. doi: 10.1016/j.jacc.2014.06.1193 [DOI] [PubMed] [Google Scholar]
- 14. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1016/j.jacc.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 15. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393 [DOI] [PubMed] [Google Scholar]
- 16. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034 [DOI] [PubMed] [Google Scholar]
- 17. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Van Es G‐A, Gabriel Steg P, Morel M‐angèle, Mauri L, Vranckx P, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 18. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 19. Goto K, Nakai K, Shizuta S, Morimoto T, Shiomi H, Natsuaki M, Yahata M, Ota C, Ono K, Makiyama T, et al. Anticoagulant and antiplatelet therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am J Cardiol. 2014;114:70–78. doi: 10.1016/j.amjcard.2014.03.060 [DOI] [PubMed] [Google Scholar]
- 20. Kimura T, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, Iwabuchi M, Shizuta S, Shiomi H, Tada T, Tazaki J, et al. Long‐term safety and efficacy of sirolimus‐eluting stents versus bare‐metal stents in real world clinical practice in Japan. Cardiovasc Interv Ther. 2011;26:234–245. doi: 10.1007/s12928-011-0065-0 [DOI] [PubMed] [Google Scholar]
- 21. Kimura T, Morimoto T, Furukawa Y, Nakagawa Y, Shizuta S, Ehara N, Taniguchi R, Doi T, Nishiyama K, Ozasa N, et al. Long‐term outcomes of coronary‐artery bypass graft surgery versus percutaneous coronary intervention for multivessel coronary artery disease in the bare‐metal stent era. Circulation. 2008;118:S199–S209. doi: 10.1161/CIRCULATIONAHA.107.735902 [DOI] [PubMed] [Google Scholar]
- 22. Fukunami M. Transient atrial fibrillation during acute myocardial infarction is a predictor of poor outcomes. Circ J. 2016;80:1534–1536. doi: 10.1253/circj.CJ-16-0529 [DOI] [PubMed] [Google Scholar]
- 23. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454 [DOI] [PubMed] [Google Scholar]
- 24. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, Van Eickels M, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594 [DOI] [PubMed] [Google Scholar]
- 25. Dewilde WJM, Oirbans T, Verheugt FWA, Kelder JC, De Smet BJGL, Herrman J‐P, Adriaenssens T, Vrolix M, Heestermans AACM, Vis MM, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open‐label, randomised, controlled trial. Lancet. 2013;381:1107–1115. doi: 10.1016/S0140-6736(12)62177-1 [DOI] [PubMed] [Google Scholar]
- 26. Lopes RD, Li L, Granger CB, Wang TY, Foody JM, Funk M, Peterson ED, Alexander KP. Atrial fibrillation and acute myocardial infarction: antithrombotic therapy and outcomes. Am J Med. 2012;125:897–905. doi: 10.1016/j.amjmed.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 27. Axelrod M, Gilutz H, Plakht Y, Greenberg D, Novack L. Early atrial fibrillation during acute myocardial infarction may not be an indication for long‐term anticoagulation. Angiology. 2020;71:559–566. doi: 10.1177/0003319720908760 [DOI] [PubMed] [Google Scholar]
- 28. Su KJ, Lin WY, Lin WS, Lin CS, Cheng CC, Liou JT, Ho CH, Yang SP, Cheng SM, Hung Y. Prognostic effect of restoring sinus rhythm in patients with new‐onset atrial fibrillation during acute coronary syndrome. Acta Cardiol Sin. 2021;37:155–165. doi: 10.6515/ACS.202103_37(2).20200915A [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Datas S1–S3
Tables S1–S2
Figures S1–S3


