Abstract
Background
Prenatal and postnatal insults can induce a physiological state that leaves offspring later in life vulnerable to subsequent challenges (stressors) eliciting cardiometabolic diseases including hypertension. In this study, we investigated whether maternal angiotensin II–induced hypertension in rats sensitizes postweaning high‐fat diet (HFD)‐elicited hypertensive response and whether this is associated with autonomic dysfunction and altered central mechanisms controlling sympathetic tone in offspring.
Methods and Results
When eating a low‐lard‐fat diet, basal mean arterial pressure of male offspring of normotensive or hypertensive dams were comparable. However, HFD feeding significantly increased mean arterial pressure in offspring of normotensive and hypertensive dams, but the elevated mean arterial pressure induced by HFD was greater in offspring of hypertensive dams, which was accompanied by greater sympathetic tone and enhanced pressor responses to centrally administrated angiotensin II or leptin. HFD feeding also produced comparable elevations in cardiac sympathetic activity and plasma levels of angiotensin II, interleukin‐6, and leptin in offspring of normotensive and hypertensive dams. Reverse transcriptase polymerase chain reaction analyses in key forebrain regions implicated in the control of sympathetic tone and blood pressure indicated that HFD feeding led to greater increases in mRNA expression of leptin, several components of the renin‐angiotensin system and proinflammatory cytokines in offspring of hypertensive dams when compared with offspring of normotensive dams.
Conclusions
The results indicate that maternal hypertension sensitized male adult offspring to HFD‐induced hypertension. Increased expression of renin‐angiotensin system components and proinflammatory cytokines, elevated brain reactivity to pressor stimuli, and augmented sympathetic drive to the cardiovascular system likely contributed.
Keywords: autonomic function, blood pressure, central nervous system, high‐fat diet, maternal gestational hypertension
Subject Categories: Hypertension, Autonomic Nervous System
Nonstandard Abbreviations and Acronyms
- AT1‐R
angiotensin II type 1 receptor
- HFD
high‐fat diet
- HR
heart rate
- LT
lamina terminalis
- LFD
low‐lard‐fat diet
- PIC
proinflammatory cytokine
- PVN
paraventricular hypothalamic nucleus
- RAS
renin‐angiotensin system
Clinical Perspective
What Is New?
These studies demonstrate that maternal gestational hypertension sensitizes the postweaning high‐fat‐diet–elicited hypertensive response in adult male offspring.
This sensitization process is integrated by upregulated mRNA expression for components of brain renin‐angiotensin system, proinflammatory cytokines and leptin, enhanced pressor responses to central angiotensin II and leptin, and elevated central sympathetic tone.
The data indicate that prenatal insults and postnatal insults can contribute synergistically to exacerbation of programming processes that lead to a vicious circle with cardiovascular diseases in their later life.
What Are the Clinical Implications?
These findings help us understand the central mechanisms of sensitized obesity‐related hypertension, and reducing brain renin‐angiotensin system activation and elevation of inflammation and leptin may be effective for treating neurogenic hypertension.
Hypertensive disorders of pregnancy, including preeclampsia and maternal gestational hypertension, affects 5% to 10% of all pregnancies in the United States. 1 , 2 Human studies have demonstrated an association between maternal hypertension and elevated blood pressure (BP) in their young children and adolescent offspring. 3 , 4 , 5 , 6 , 7 , 8 , 9 Studies using animal models have found that adult offspring from mothers with maternal hypertension also exhibit elevated BP. 10 , 11 , 12 Although multiple factors have been demonstrated to be associated with gestational hypertension‐induced increased risk for cardiovascular diseases in the offspring, the mechanisms are yet to be elucidated.
Obesity/high‐fat diet (HFD) is a key risk factor that leads to cardiovascular diseases such as hypertension. 13 The activation of the sympathetic nervous system is a major mechanism underlying both human and experimental models of obesity‐related hypertension. 14 , 15 Evidence indicates that the cause of obesity‐related hypertension is mediated primarily through neurogenic mechanisms, in which activation of renin‐angiotensin system (RAS), inflammation, and elevated levels of leptin result in reprograming the central nervous system to produce a state of enhanced sympathetic reactivity. 16 , 17 , 18 , 19 , 20 , 21 , 22 We and others have demonstrated that maternal HFD feeding induces markedly elevated renal sympathetic nerve activity and pressor responses to central angiotensin II, tumor necrosis factor (TNF)‐α, or leptin in male adult offspring. 23 , 24 Conversely, postnatal HFD feeding in offspring previously subjected to several types of experimental prenatal insults exacerbates prenatal insult‐induced metabolic‐like syndrome, impaired renal function, and the developmental programming of increased BP. 10 , 12 , 25 , 26 , 27 However, few studies have focused on the central mechanisms underlying the prenatal insult‐induced adverse effects exacerbated by a second challenge (stressor).
We previously demonstrated that the adult male offspring of hypertensive dams exhibit upregulated expression of RAS components and markers of inflammation (proinflammatory cytokines [PICs] and microglial activation) in the brain regions involved in BP regulation including structures of the lamina terminalis (LT) and paraventricular hypothalamic nucleus (PVN) along with hypertensive response sensitization to a slow‐pressor dose of angiotensin II. Either renal denervation or systemic antagonism of the RAS blocked hypertensive response sensitization and reversed the changes in brain RAS and PIC mRNA expression of the offspring of hypertensive dams. 19 , 28 , 29 Pladys and colleagues 30 reported that in fetal protein‐restricted offspring, there was increased expression of the angiotensin II receptor type 1 (AT1‐R) in the subfornical organ and the vascular organ of the LT, and that intracerebroventricular injection of an angiotensin‐converting enzyme inhibitor or an AT1‐R antagonist significantly reduced BP in these offspring. These data implicate the brain RAS and inflammation in hypertension associated with prenatal insults.
In the present study, we build on previously published literature by focusing on the central mechanisms by which a prenatal insult caused by maternal hypertension cross‐sensitizes the hypertensive response elicited by a postnatal stressor, HFD. Metabolic markers and changes in BP and autonomic function were determined. Circulating levels of angiotensin II, PIC, and leptin and putative central nervous system molecular and cellular mediators in key brain structures (ie, the LT and PVN) implicated in the control of sympathetic activity and BP were also measured. Finally, we assessed the pressor responses to intracerebroventricular administration of angiotensin II, TNF‐α, or leptin. The experimental results provide further evidence that maternal hypertension sensitizes the hypertensive response by enhancing sensitivity to brain mechanisms driving sympathetic activity in adult offspring subjected to a second physiological challenge, HFD. The findings are relevant to present‐day human lifestyle challenges.
Methods
In compliance with the ,Transparency and Openness Promotion Guidelines we will make all data related to the findings described in our article fully available without restriction.
Animals
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the University of Iowa Animal Care and Use Committee.
All animals were maintained in a temperature‐ (23±2°C) and light (12‐hours light/dark cycle)‐controlled facility. Twenty‐four female and 24 male rats (Sprague‐Dawley, 10‐week‐old, Envigo) were used for breeding. Half of the females were chronically treated with vehicle (saline) and considered as normotensive dams, while the other half were treated with angiotensin II (250 ng/kg/min SC, model 2004, 4 weeks, Alzet) throughout mating and pregnancy and considered as hypertensive dams. This dose of angiotensin II infusion induced a significant increase in BP during pregnancy (38.5±6.2 mm Hg) in dams, as described in our previous study. 29 The offspring were weighed and counted at birth, and the litter sizes reduced at 3 days of age to 8 pups, 4 male and 4 female offspring. All offspring were weaned at 3 weeks of age, and feeding began with one‐half of the animals receiving a low‐lard‐fat diet (LFD, 10% calories from lard, 3.85 kcal/g, D12450J, Research Diets Inc.) and the other one‐half receiving HFD (60% calories form lard, 5.24 kcal/g, D12492, Research Diets Inc.) for 12 weeks. This yielded 4 control/experimental groups: (1) normotensive dam+ LFD offspring, (2) normotensive dam+ HFD offspring, (3) hypertensive dam+ LFD offspring, (4) hypertensive dam+ HFD offspring. Each experimental group was composed of individual subjects that were randomly selected from different litters, and only male offspring were used in this study. A total of 44 male offspring of normotensive dams and the same number of male offspring of hypertensive dams were used in the present experiments. Food and body weight were weighed 1 time per week until the experiments began.
Figure 1 shows the timeline of the study design. Experiment 1: At 16 to 18 weeks of age, all groups of offspring were used to evaluate basal BP and heart rate (HR) using implanted telemetric probes (n=6 per group). BP was also measured in the presence of the ganglionic blocker hexamethonium (30 mg/kg IP), the muscarinic receptor blocker atropine (8 mg/kg IP) or the β‐adrenergic receptor blocker atenolol (8 mg/kg IP) (n=6 per group). Experiment 2: In separate groups of offspring, pressor responses to intracerebroventricular injections of angiotensin II, TNF‐α, or leptin were determined through implantation of femoral artery catheters, brain lateral ventricle cannulas, and pharmacologic methods (angiotensin II, 200 ng/2 µL; TNF‐α, 200 ng/2 µL or leptin, 5 µg/2 µL; n=5–6 per group). Experiment 3: The blood and brains from separate groups of offspring with the diet treatments were collected for analyses of plasma levels of angiotensin II, interleukin‐6, and leptin (n=8–10 per group) and mRNA expression of the RAS components and PICs (n=6 per group), respectively. The structures lying along the LT (ie, the subfornical organ, the median preoptic nucleus, the vascular organ of the LT), and the PVN were used for these analyses.
Figure 1. Representative timeline of the study design.

Male offspring of normotensive (NT) or hypertensive (HT) dams were weaned at 3 weeks old and fed with a low‐lard‐fat diet (LFD) or high‐fat diet (HFD) for 12 weeks. Experiment 1 (Exp. 1): male offspring were instrumented with the telemetry transmitters after 12 weeks dietary treatment and hemodynamic and autonomic function were measured. Experiment 2 (Exp. 2): intracerebroventricular (icv) cannulas and femoral arterial catheters were implanted for assessment of pressor response to icv agents. Experiment 3 (Exp. 3): at 15 weeks of age, LFD‐ or HFD‐fed male offspring from either NT or HT dams were euthanized to collect blood and brain tissues for assessing plasma levels and mRNA expression of renin‐angiotensin system components, cytokines, or leptin, respectively.
Body Composition Measurement, Tissue Collection, and Blood Plasma Analysis
After 12 weeks of diet treatment, body composition including total body, fat, lean, and fluid mass were determined by nuclear magnetic resonance spectroscopy using a Bruker mini‐spec LF 90II instrument (Bruker Corporation, Billerica, MA). To analyze body composition, rats were placed into a restraint tube and inserted into the rodent‐sized nuclear magnetic resonance apparatus, adjusting the volume of the chamber on the basis of the size of the animal.
After decapitation of the offspring, trunk blood from all groups of offspring was collected for biochemical assays. Plasma levels of angiotensin II (Cat, CEA005Ra, Cloud Clone, Wuhan, China), interleukin‐6 (Cat, R6000B, R&D Systems, Minneapolis, MN) and leptin (Cat, M0B00, R&D Systems) were measured with commercial ELISA kits according to the manufacturers’ instructions.
At same time, visceral fat mass including inguinal, retroperitoneal, and epididymal white adipose tissue and brains were collected for measurement of visceral fat mass and analysis of mRNA expression by reverse transcriptase polymerase chain reaction, respectively.
Telemetry Probe Implantations and Measurement of BP and HR
Rat telemetric probes (HD‐S10, Data Sciences International, St. Paul, MN) were used to directly measure arterial pressure and HR in individual animals. At 15 weeks of age, all groups of offspring were anesthetized with a ketamine‐xylazine mixture (90% ketamine and 10% xylazine IP), and the femoral artery was accessed with a ventral incision. The right femoral artery was isolated, and the catheter of a telemetric probe was inserted into the vessel. Through the same ventral incision, a pocket along the right flank was formed. The body of the transmitter was slipped into the pocket and secured with tissue adhesive. The ventral incision was then closed with suture. All rats were allowed 7 days to recover from transmitter implantation surgery. Thereafter, BP and HR were telemetrically recorded and stored with the Dataquest ART data acquisition system (Data Sciences International).
Evaluation of Autonomic Functions
BP and HR were measured in the presence of the ganglionic blocker hexamethonium (30 mg/kg IP), the muscarinic receptor blocker atropine (8 mg/kg IP), or the β‐adrenergic receptor blocker atenolol (8 mg/kg IP) on 3 separate days, respectively. On the day of the experiment, rats were allowed to stabilize for at least 60 minutes, after which time BP and HR were recorded for 20 to 60 minutes before and after administration of autonomic antagonists.
Intracerebroventricular Cannula Implantation and Evaluation of the Effects of Acute Microinjection of Angiotensin II, TNF‐α, or Leptin
At 15 weeks of age, offspring were anesthetized intraperitoneally with 90% ketamine and 10% xylazine and intracerebroventricular cannulas (25 gauge) were implanted into right lateral cerebral ventricle (the coordinates 1.0 mm caudal, 1.5 mm lateral to bregma, and 4.5 mm below the skull surface) for acute bolus microinjections of vehicle (saline, 2 µL; angiotensin II, 200 ng/2 µL; TNF‐α, 200 ng/2 µL; or leptin, 5 µg/2 µL) delivered through 33‐gauge injection cannulas. After 1 week of recovery, using the same anesthetic, arterial catheters were implanted into the femoral artery for the measurement of BP. Three days later, the effects of intracerebroventricular injections of angiotensin II, TNF‐α, or leptin on BP and HR were determined on 3 separate days in unanesthetized animals using LABCHART (ADInstruments, Dunedin, New Zealand) for data acquisition.
Real‐time Reverse Transcriptase Polymerase Chain Reaction Analysis
The offspring with dietary treatments were decapitated, and the brains were quickly removed and put in iced saline for 1 minute. Then, the brain was cut into 200‐μm coronal sections, and the target tissues, including the LT and both sides of the PVN, were punched with a 15‐gauge needle stub (inner diameter, 1.5 mm). Some immediately surrounding tissue was usually included in the punch biopsies. The structures lying along the LT include the subfornical organ, median preoptic nucleus, and vascular organ of the LT. Because each of the structures lying along the LT is very small and they are located at same level of a brain section in the coronal plane, we collected these structures together and analyzed their mRNA expression as a whole. The PVN is composed of 2 subregions including the magnocellular subregion and the parvocellular subregion; we also collected both subregions and both sides of the PVN and analyzed PVN mRNA expression together. Total RNA was isolated from the LT and PVN using the Trizol method (Invitrogen, Waltham, MA) and treated with DNase I (Invitrogen). RNA integrity was checked by gel electrophoresis. Total RNA was reverse transcribed using random hexamers following the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Real‐time polymerase chain reaction was conducted using 200 to 300 ng of cDNA and 500 nM of each primer in a 20 μL reaction with iQ SYBR Green Supermix (Bio‐Rad Laboratories, Hercules, CA). Amplification cycles were conducted at 95°C for 3 minutes, followed by 40 cycles of 95°C for 15 seconds and annealing/extension at 60°C for 30 seconds. Reactions were performed in duplicate and analyzed using a C1000 thermocycler system (Bio‐Rad Laboratories). mRNA levels for RAS components (angiotensin converting enzyme, AT1‐R), proinflammatory cytokines (TNF‐α, interleukin‐1β, and interleukin‐6), microglial marker (CD11b), leptin, and GAPDH were analyzed with SYBR Green real‐time reverse transcriptase polymerase chain reaction. The values were corrected by GAPDH, and the final concentration of mRNA was calculated using the formula x=2^(−ΔΔCt), where x=fold difference relative to control. Primers were purchased from Integrated DNA Technologies (Coralville, IA). The sequences of the primers are shown in Table 1.
Table 1.
Primer Sequences for Real‐Time PCR
| Gene | Forward primer | Reverse primer | Product size (bp) |
|---|---|---|---|
| GAPDH | TGACTCTACCCACGGCAAGTTCAA | ACGACATACTCAGCACCAGCATCA | 141 |
| ACE | GTGTTGTGGAACGAATACGC | CCTTCTTTATGATCCGCTTGA | 187 |
| AT1‐R | CTCAAGCCTGTCTACGAAAATGAG | GTGAATGGTCCTTTGGTCGT | 188 |
| TNF‐α | GCCGATTTGCCACTTCATAC | AAGTAGACCTGCCCGGACTC | 209 |
| Interleukin‐6 | GCCTATTGAAAATCTGCTCTGG | GGAAGTTGGGGTAGGAAGGA | 160 |
| Interleukin‐1β | AGCAACGACAAAATCCCT GT | GAAGACAAACCGCTTTTCCA | 209 |
| CD11b | TTACCGGACTGTGTGGACAA | AGTCTCCCACCACCAAAGTG | 239 |
| Leptin | CCAAAACCCTCATCAAGACC | GTCCAACTGTTGAAGAATGTCCC | 154 |
ACE indicates angiotensin‐converting enzyme 1; AT1‐R, angiotensin II type 1 receptor; PCR, polymerase chain reaction; and TNF‐α, tumor necrosis factor‐α.
Statistical Analysis
Mean arterial pressure (MAP) and HR, obtained from the 10 days of telemetry recordings, are presented as mean daily values and averaged daily values of the 10‐day recordings. Differences for BP were calculated for each animal on the basis of the baseline subtracted from the BP after intraperitoneal injection of hexamethonium, atenolol, and atropine or intracerebroventricular microinjection of angiotensin II (5 minutes), TNF‐α (30 minutes), and leptin (30 minutes). Likewise, differences for HR were calculated for each animal on the basis of the baseline subtracted from the HR after intraperitoneal injection of hexamethonium, atenolol, or atropine. Two‐way repeated measure ANOVA or ordinary 2‐way ANOVA was then conducted on the means of calculated differences for each of the experimental groups (factors: maternal normotensive/hypertensive and offspring LFD/HFD). After finding interactions, post hoc analyses were performed with Tukey multiple comparison tests between pairs of mean changes (Prism 9.0, GraphPad Software, La Jolla, CA). The same statistical methods were used to analyze the differences in metabolic parameters and in plasma levels and mRNA expression of the RAS components, PICs, and leptin in the trunk blood and brain regions, respectively. All data are expressed as means±SE. Statistical significance was set at P<0.05.
Results
Effect of Maternal Hypertension and HFD Feeding on Metabolic Parameters in Male Offspring
Both maternal hypertension (P=0.0197) and postweaning HFD feeding (P<0.0001) resulted in significant increases in body weight when compared with LFD offspring of normotensive dams. However, increased body weight was greater in HFD‐fed offspring of both normotensive and hypertensive dams than that in LFD offspring of hypertensive dams (F [3, 21]=62.88; P<0.0001; Figure 2 and Table 2).
Figure 2. Increases in body weight in male offspring from normotensive (NT) dams and hypertensive (HT) dams after 12 weeks (W) of low‐lard‐fat diet (LFD) or high‐fat diet (HFD) feeding.

(n=8/group; Both repeated [A] and ordinary [B] 2‐way ANOVA were used for analysis followed by Tukey’s post hoc tests. P<0.05; * vs NT‐LFD offspring; Ɨ vs HT‐LFD offspring).
Table 2.
The Metabolic Parameters in Male Offspring From Normotensive Dams or Hypertensive Dams During LFD and HFD Feeding (P<0.05; * vs Normotensive LFD Offspring; Ɨ vs Hypertensive LFD Offspring)
| Male offspring | Normotensive LFD | Normotensive HFD | Hypertensive LFD | Hypertensive HFD |
|---|---|---|---|---|
| Weaning body weight (g) | 53.2±3.5 | 48.3±3.9 | ||
| Body weight at week 12 (g) | 390.9±4.9 | 473.0±6.8*Ɨ | 406.9±7.3* | 454.9±5.4*Ɨ |
| Body weight changes (g) | 337.7±2.8 | 419.8±5.4*Ɨ | 360.2±6.4* | 407.9±4.2*Ɨ |
| Food intake (g/d) | 17.2±0.2 | 13.1±0.1*Ɨ | 17.2±0.4 | 13.0±0.1*Ɨ |
| Energy intake (calories/day) | 66.1±0.8 | 68.3±0.6 | 66.3±1.4 | 68.1±0.4 |
| Feed efficiency (mg body weight/calorie) | 60.9±1.1 | 73.4±0.5*Ɨ | 64.7±1.1* | 71.6±0.8*Ɨ |
| Total fat mass (g) | 21.6±1.8 | 41.5±3.7*Ɨ | 28.0±1.1 | 42.9±2.4*Ɨ |
| Visceral fat mass (g) | 7.9±0.5 | 18.7±1.5*Ɨ | 9.8±0.6 | 17.7±0.7*Ɨ |
| % fat | 6.3±0.4 | 9.9±0.7*Ɨ | 7.3±0.3 | 9.7±0.4*Ɨ |
| % lean | 64.7±0.2 | 61.9±0.4*Ɨ | 64.6±0.2 | 62.6±0.3*Ɨ |
| % fluid | 9.8±0.1 | 9.5±0.1 | 9.7±0.1 | 9.4±0.1 |
Food intake (g/d) was greater in the offspring from both normotensive and hypertensive dams eating the LFD than those eating the HFD (F [3, 21]=141.7; P<0.0001). However, caloric intakes (calories/d) were similar among all groups of offspring (F [3, 21]=2.015; P=0.1427). As a result, feed efficiency was higher in HFD offspring (P<0.0001) and LFD offspring of hypertensive dams (P=0.0444) when compared with LFD offspring of normotensive dams (F [3, 21]=36.63; P<0.0001; Figure 3A and Table 2).
Figure 3. Feeding efficiency (A) and fat mass (B) in male offspring from normotensive (NT) dams and hypertensive (HT) dams after low‐lard‐fat diet (LFD) or high‐fat diet (HFD) feeding.
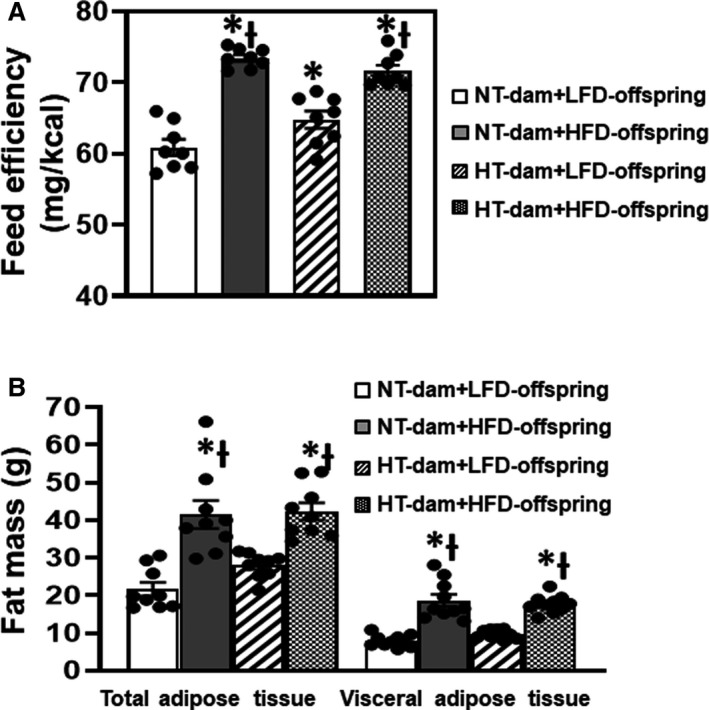
(n=8–10/group; ordinary 2‐way ANOVA was used for analysis followed by Tukey’s post hoc tests; P<0.05; * vs NT‐LFD offspring; Ɨ vs HT‐LFD offspring.
Total adipose tissue mass (F [3, 24]=16.06; P<0.0001) and visceral adipose mass (F [3, 27]=32.49; P<0.0001) were significantly increased after 12 weeks of HFD feeding in offspring from both normotensive and hypertensive dams when compared with those with LFD (Figure 3B). Likewise, increased body fat composition (F [3, 19]=10.90; P=0.0002) and decreased lean composition (F [3, 19]=17.62; P<0.0001) were evident in HFD‐fed offspring. Although the body weight and feed efficiency were also significantly increased in LFD offspring of hypertensive dams, total adipose tissue mass, visceral adipose mass, and the body composition were not altered when compared with LFD offspring of normotensive dams (P>0.05) (Table 2).
Effect of Maternal Hypertension on Increases in BP and HR Induced by Postweaning HFD
Maternal hypertension had no effects on basal MAP (112.7±1.0 versus 112.6±0.9 mm Hg) and HR (334.1±5.4 versus 329.3±4.6 beats/min). HFD feeding significantly elevated basal MAP in offspring of both normotensive (120.3±1.3 mm Hg) and hypertensive dams (128.7±1.6 mm Hg) when compared with those receiving LFD feeding (F [3, 19]=17.62; P<0.0001). However, the increases in MAP were greater in offspring of hypertensive dams than those in offspring of normotensive dams (P=0.0012; Figures 4A and 4B). Dietary treatment had no effect on the HR of any group of offspring (F [3, 15]=0.5287; P=0.6693) (Figure 4C and 4D).
Figure 4. Daily and averaged mean arterial pressure (MAP) and heart rate (HR) in male offspring from normotensive (NT) dams and hypertensive (HT) dams.
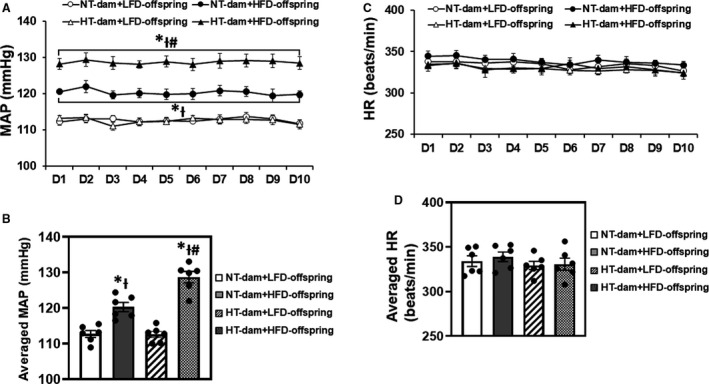
Twelve weeks of high‐fat diet (HFD) feeding beginning from weaning significantly increased MAP in male offspring from either NT dams or HT dams, but the offspring of HT dams showed a greater increase in MAP (A and B). HRs were comparable in all groups of offspring (C and D). (n=6/group; both repeated [A and C] and ordinary [B and D] 2‐way ANOVA was used for analysis followed by Tukey’s post hoc tests. P<0.05; * vs NT‐LFD offspring; Ɨ vs HT‐LFD offspring; # vs NT‐HFD offspring).
Changes in Autonomic Function After Diet Treatment
Ganglionic blockade resulted in a significant reduction in BP and HR in HFD‐fed offspring when compared with LFD‐fed offspring of either normotensive dams (BP: −36.2±1.7 versus −23.4±2.9 mm Hg; P=0.0241; HR: −30.1±8.1 versus −2.0±6.3 beats/min; P=0.0180) or hypertensive dams (−51.9±3.7 versus −26.8±2.1 mm Hg; P<0.0001; HR: −35.7±4.7 versus −3.4±5.6 beats/min; P=0.0066). However, the reduction in BP, but not in HR (P>0.05), was greater in offspring of hypertensive dams than that in offspring of normotensive dams (P=0.0059), suggesting that exposure in utero to maternal hypertension sensitized the hypertensive response elicited by HFD feeding by increasing sympathetic outflow from the central nervous system but produced a similar increase in cardiac sympathetic tone in HFD‐fed offspring from normotensive and hypertensive dams (F [3, 15]=21.04; P<0.0001; Figure 5A and 5B). Furthermore, β‐adrenergic receptor antagonism significantly decreased HR in HFD offspring of both normotensive dams (−55.5±9.6 versus −28.7±5.0 beats/min; P=0.034) and hypertensive dams (−57.9±4.5 versus −21.4±1.9 beats/min; P=0.0038; Figure 5C) and resulted in a slight and comparable reduction in BP in all groups of offspring (Figure 5D). This also suggests that HFD similarly increased cardiac sympathetic tone in HFD‐fed offspring (F [3, 15]=9.135; P=0.0011; Figure 5C). In contrast, HR and BP responses to muscarinic receptor antagonism were similar in all groups (normotensive‐dams, 112.6±10.8 versus 94.8±11.5 beats/min; hypertensive dams, 103.4±13.5 versus 114.0±16.8 beats/min), indicating that neither maternal hypertension nor postweaning HFD had an effect on cardiac vagal tone (F [3, 15]=0.4454; P=0.7241; Figure 5E and 5F).
Figure 5. The changes in autonomic function in male offspring from normotensive (NT) dams or hypertensive (HT) dams after 12 weeks of low‐lard‐fat diet (LFD) or high‐fat diet (HFD) feeding.
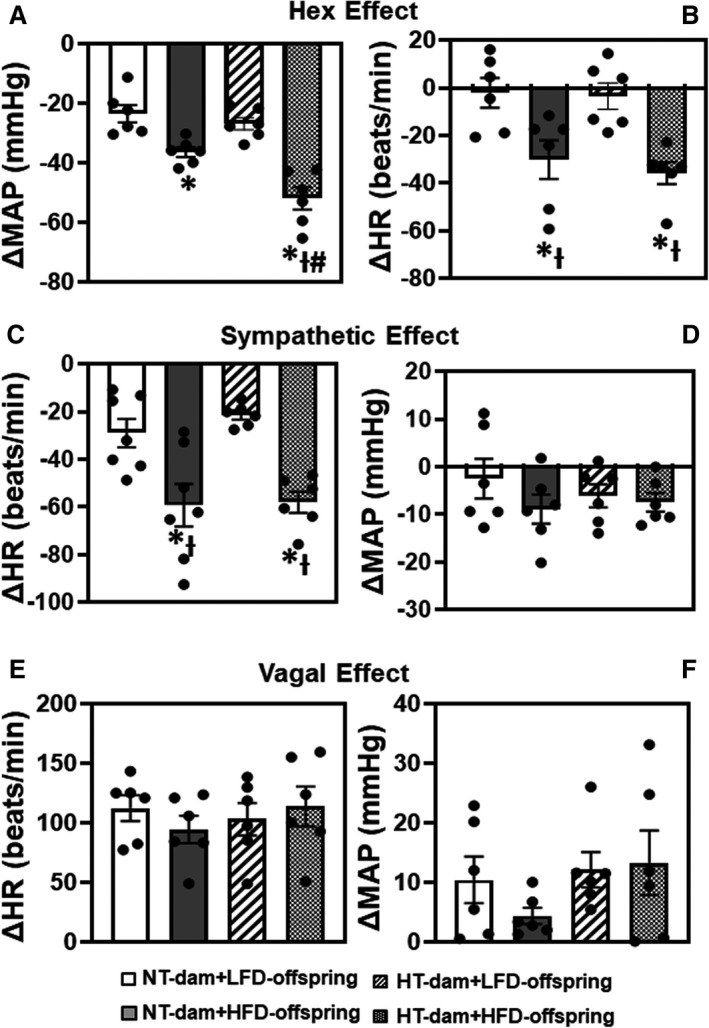
The autonomic parameters including centrally driven sympathetic tone (A, B), cardiac sympathetic activity (C, D) and cardiac vagal activity (E, F) were obtained by intraperitoneal injection of hexamethonium (Hex), atenolol, and atropine, respectively. (n=6/group; ordinary 2‐way ANOVA was used for analysis followed by Tukey’s post hoc tests. P<0.05; * vs NT‐LFD offspring; Ɨ vs HT‐LFD offspring; # vs NT‐HFD offspring).
Effect of Intracerebroventricular Injection of Angiotensin II, TNF‐α, or Leptin on BP in Diet‐Treated Offspring
Either maternal hypertension or HFD feeding alone significantly elevated pressor responses to intracerebroventricular angiotensin II (F [3, 12]=20.58; P<0.0001; Figure 6A and 6B) and to TNF‐α (F [3, 12]=8.826; P=0.0023; Figure 6C and 6D). As compared with LFD‐fed offspring, HFD feeding significantly elevated pressor responses to intracerebroventricular leptin (F [3, 12]=43.93; P<0.0001; Figure 6E and 6F). Furthermore, the increases in pressor responses to angiotensin II (P=0.0024) or leptin (P=0.0041) were significantly greater in HFD‐fed offspring of hypertensive dams than that in HFD‐fed offspring of normotensive dams (Figure 6A and 6E).
Figure 6. The pressor effects of intracerebroventricular (icv) injection of angiotensin (ANG) II (A, B), tumor necrosis factor (TNF)‐α (C, D) or leptin (E, F) in male offspring from normotensive (NT) dams or hypertensive (HT) dams after 12 weeks of low‐lard‐fat diet (LFD) or high‐fat diet (HFD) feeding.
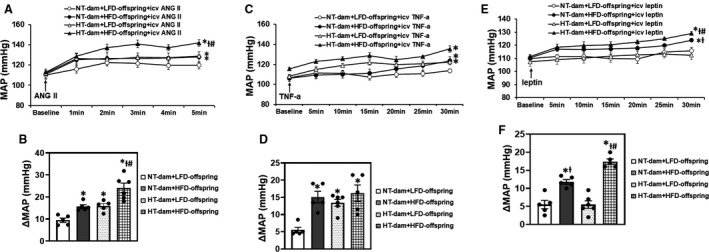
Group data (A, C, E) showing blood pressure responses to icv injection of the pressor agents. Bar graphs (B, D, F) showing changes in mean arterial pressure (MAP). (n=5–6/group; ordinary 2‐way ANOVA was used for analysis followed by Tukey’s post hoc tests. P<0.05; * vs NT‐LFD offspring; Ɨ vs HT‐LFD offspring; # vs NT‐HFD offspring).
Effect of Maternal Hypertension and HFD Feeding on Plasma Levels of Angiotensin II, Interleukin‐6, and Leptin in Offspring
Both maternal hypertension and HFD feeding increased plasma levels of angiotensin II (F [3, 19]=6.327; P=0.0037; Figure 7A). Maternal BP during pregnancy had no effect on plasma interleukin‐6 or leptin levels (Figure 7B and 7C). However, HFD feeding significantly elevated plasma levels of interleukin‐6 (F [3, 20]=6.601; P=0.0028; Figure 7B) and leptin (F [3, 24]=9.309; P=0.0003; Figure 7C) in offspring of both hypertensive and normotensive dams.
Figure 7. Comparisons of plasma angiotensin (ANG) II (A), interleukin‐6 (IL‐6) (B) and leptin (C) in male offspring from normotensive (NT) dams or hypertensive (HT) dams after 12 weeks of low‐lard‐fat diet (LFD) or high‐fat diet (HFD) feeding.

(n=8–10/per group; ordinary 2‐way ANOVA was used for analysis followed by Tukey’s post hoc tests. P<0.05; * vs NT‐LFD offspring; Ɨ vs HT‐LFD offspring.
Effect of Maternal Hypertension and Postweaning HFD on mRNA Expression of RAS Components, PICs, and Leptin in the Brain
In LT tissues, reverse transcriptase polymerase chain reaction analysis revealed that maternal hypertension resulted in a significant increase in mRNA expression of angiotensin converting enzyme (P=0.0437), AT1‐R (P<0.0001), and TNF‐α (P=0.0498) in the offspring fed LFD when compared with LFD offspring of normotensive dams (Figure 8A). HFD feeding in offspring of hypertensive dams produced a significant increase in mRNA expression of angiotensin converting enzyme (P=0.0108) and enhanced AT1‐R (P=0.0007), TNF‐α (P<0.0001), and leptin (P=0.0199) expression (Figure 8A) when compared with HFD offspring of normotensive dams who only exhibited a significant increase in mRNA expression of TNF‐α (P=0.0255), interleukin‐6 (P=0.0489), and leptin (P=0.0358).
Figure 8. Comparison of the mRNA expression of renin‐angiotensin system components, proinflammatory cytokines and leptin in the lamina terminalis (LT; A) and paraventricular nucleus (PVN; B) of male offspring from both normotensive (NT) and hypertensive (HT) dams after 12 weeks of low‐lard‐fat diet (LFD) or high‐fat diet (HFD) feeding.
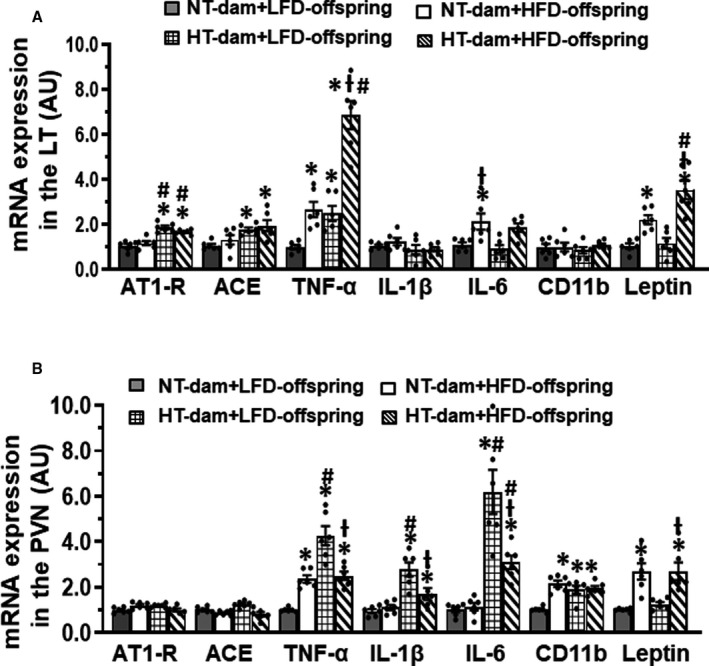
(n=6/group; ordinary 2‐way ANOVA was used for analysis followed by Tukey’s post hoc tests; P<0.05; * vs NT‐LFD offspring; Ɨ vs HT‐LFD offspring; # vs NT‐HFD offspring.
In PVN tissues, maternal hypertension elicited a significant increase in mRNA expression of TNF‐α, interleukin‐1β, interleukin‐6, and CD11b in the offspring fed LFD when compared with the LFD offspring of normotensive dams (all components P<0.0001; Figure 8B). HFD feeding increased interleukin‐1β (P=0.049) and interleukin‐6 (P=0.0422) expression only in offspring of hypertensive dams but not in offspring of normotensive dams, while HFD feeding increased expression of TNF‐α, CD11b, and leptin in offspring of both normotensive (P=0.0037, 0.0004, and 0.0035, respectively) and hypertensive (P=0.0016, 0.0002, and 0.0032, respectively) dams (Figure 8B). Interestingly, the levels of expression of PICs in HFD‐fed offspring of both normotensive (P<0.0001) and hypertensive (TNF‐α, P=0.0003; interleukin‐1β, P=0.0041; interleukin‐6, P=0.0075) dams were lower than that in LFD offspring of hypertensive dams (Figure 8B).
Discussion
The goals of the current studies were to determine if experimental maternal hypertension sensitized the hypertensive response elicited by HFD feeding and to investigate the mechanisms mediating this type of synergism. The major findings of the present study were that in postweaning HFD‐fed offspring, maternal gestational hypertension induced (1) a sensitized HFD‐elicited hypertensive response, (2) greater centrally driven sympathetic tone, (3) enhanced pressor responses to centrally administered angiotensin II and leptin, and (4) a greater increase in mRNA expression of leptin and inflammatory markers in key brain cardiovascular nuclei. The sensitized BP responses were accompanied by increased sympathetic drive to the heart and vasculature and elevated plasma levels of angiotensin II, interleukin‐6, and leptin, but these effects did not differ between HFD‐fed offspring from either normotensive or hypertensive dams. The results indicate that the exaggerated centrally driven sympathetic activity, the enhanced pressor responses to central leptin and RAS or PIC components, and the sustained upregulated expression of related genes in the brain are likely responsible for the sensitized hypertensive response to HFD in offspring of hypertensive dams.
Maternal gestational hypertension affects up to 10% of pregnancies and has a significant impact on maternal and fetal health immediately and in the long term. 6 It has been established that in human and animal models, the offspring of mothers with maternal hypertension exhibit higher BP from an early age and on into adulthood. 1 , 3 , 4 , 8 , 9 , 12 Numerous factors have been implicated in the pathogenesis of the developmental programming of hypertension, including renal or endothelial dysfunction, activation of the RAS, and inflammation. 31 Recent studies show that prenatal insults exaggerate the cardiometabolic responses of offspring to postnatal adverse factors. The prenatal and postnatal challenges (stressors) can be viewed as “first hits” and “second hits,” respectively. Adverse gestational events as first hits reprogram homeostatic controls of BP so that a second hit results in exacerbated cardiometabolic diseases. 10 , 12 , 25 , 26 , 27 We have previously demonstrated the ability of maternal gestational hypertension to sensitize the response to angiotensin II–induced hypertension in male offspring. 29 The present study was to test the hypothesis that this effect of maternal gestational hypertension extends to another second hit commonly encountered in today’s world, that is, eating a HFD.
Much of the global increase in incidence of hypertension is related to the rise in consumption of Western diets. In obese humans and in animal models of diet‐induced obesity, increased sympathetic nervous system activity and BP have been demonstrated to be associated with RAS activation, elevation of inflammation, and increased leptin peripherally and centrally. 13 , 16 , 19 , 32 , 33 Consistent with this, we found that HFD‐fed offspring exhibited increased BP, elevated levels of angiotensin II, interleukin‐6, and leptin in the plasma and increased centrally driven sympathetic activity. The increased BP and central sympathetic tone were greater in HFD‐fed offspring of hypertensive dams when compared with HFD‐fed offspring of normotensive dams. Our data suggest that the sensitizing effect of maternal hypertension on the expression and increased reactivity of central sympathetic nervous system components that drive increased sympathetic tone accounts for these findings.
Sensitization of hypertensive responses is not without precedent. Several studies have shown that prenatal hypertension, maternal HFD feeding, or exposure of pregnant dams to nicotine significantly enhances the pressor response of offspring to angiotensin II infusions. 24 , 29 , 34 , 35 Furthermore, postnatal HFD exacerbates perinatal programming of hypertension vulnerability induced by prenatal dexamethasone exposure, maternal high fructose consumption, NG‐nitro‐L‐arginine methyl ester‐induced maternal hypertension or endothelial nitric oxide synthase gene knockout. 10 , 12 , 25 , 26 , 27 The present study extended observations by showing that maternal gestational hypertension impairs the offspring’s ability to cope with a HFD as a challenge because HFD increases systemic activation of the RAS, inflammation, and leptin, all of which act through systemic‐central and humoral‐neural coupling to increase central sympathetic activity and, in turn, increase sympathetic drive to blood vessels and increase BP. 32 , 36 , 37
Obesity‐related hypertension is mediated primarily through neurogenic mechanisms. 15 The LT and PVN receive and integrate information initially derived from systemic humoral and neural afferent signals. 38 , 39 In the central neural network, RAS components, inflammatory factors and leptin act on these structures to alter neural processing and storage information to establish a new level of sympathetic nervous system basal activity and reactivity to stressors, such as seen with obesity‐related hypertension. 16 , 19 , 32 , 40 To explore the central mechanisms underlying maternal hypertension sensitization of postweaning HFD‐induced increase in BP, we determined the pressor effects of intracerebroventricular application of angiotensin II, TNF‐α, or leptin in offspring. We found that although LFD‐fed offspring of hypertensive dam had a normal baseline BP, they exhibited increased pressor responses to intracerebroventricular angiotensin II and to TNF‐α. This is consistent with previous studies showing that maternal HFD elevated the pressor responses to central administration of either angiotensin II, TNF‐α, or leptin. 23 , 24 Furthermore, we found that in HFD‐fed offspring, the pressor responses to these agents were significantly increased, and the increased pressor effects to angiotensin II or leptin were greater in offspring of hypertensive dams when compared with offspring of normotensive dams. The results indicate that maternal hypertension sensitizes pressor responses and enhances brain sensitivity to these prohypertensive factors, which are responsible for increased sympathetic drive and the sensitized hypertensive response in HFD‐fed offspring.
The finding of central sensitization of central pressor responses in the HFD‐fed offspring of hypertensive dams received further support from the results of studies characterizing the gene expression of leptin, PICs, and RAS components in critical forebrain regions. Messages for both RAS components and PICs were upregulated in the LT and PVN of the LFD‐fed offspring of hypertensive dams, and HFD feeding led to an even greater increase in messages for leptin, PICs, and several components of the RAS in these offspring of hypertensive dams. The results suggest that maternal gestational hypertension–induced sustained increases in brain PICs and RAS components to enhance central nervous system activity may provide a physiological foundation for the maintained sensitized state produced by postnatal HFD. Consistent with the present finding, our previous studies demonstrated the roles for central RAS activation and inflammation in mediating sensitization of angiotensin II–induced hypertension in offspring from maternal hypertensive or HFD dams. The adult male offspring showed upregulated expression of both RAS components and PICs in the LT and PVN. Systemic blockade of the RAS, inflammation, or renal denervation blocked hypertensive response sensitization and reversed the changes in RAS and PIC mRNA expression in brain cardiovascular nuclei in the offspring of hypertensive or HFD dam. 19 , 24 , 28 , 29 , 35 Taken together, these studies suggest that a common central mechanism is responsible for an exaggerated hypertensive response when prenatally challenged offspring are exposed to a second stressor later in life.
The association between maternal gestational hypertension and metabolic markers other than BP, such as body weight, weight gain, body composition, lipid profile, and glucose and insulin metabolism, is less clear and often contradictory. Several studies have shown that maternal gestational hypertension in women who deliver at term was associated with higher systolic BP in the offspring, but not with cardiometabolic risk factors. 3 , 7 In contrast, Tripathi and colleagues reported that maternal gestational hypertension was associated with generally better cardiometabolic health in offspring mid‐childhood. This was contrary to that author’s hypothesis that maternal gestational hypertension might be predictive of a worse overall metabolic profile. 9 Furthermore, in the offspring either exposure to intrauterine growth restriction 25 or maternal gestational hypertension, 12 postnatal HFD treatment had no effects on metabolic function in males while female offspring manifested a full spectrum of a metabolic‐like syndrome phenotype, suggesting a sex‐specific regulation of combined abnormal intrauterine environment and postnatal adverse factors on metabolic function. In the present study, we found that maternal hypertension produced significant increases in body weight, fat mass, and feed efficiency in both LFD and postweaning HFD‐fed male offspring, and that HFD enhanced these metabolic markers. However, the heightened metabolic markers along with increased levels of plasma angiotensin II, interleukin‐6, and leptin were not different between HFD‐fed offspring from normotensive or hypertensive dams. Although the results may represent an exacerbated effect of maternal hypertension on metabolic function in LFD offspring and in postweaning HFD offspring, the mixed effects and absence of measurements for glucose and insulin metabolism prevent us drawing a conclusion and warrant further investigation.
There are several limitations in this study. First, we used maternal infusion of angiotensin II to induce maternal gestational hypertension. It has been shown that activation of the maternal RAS in various animal models including infusion of angiotensin II in our study is an etiologic factor in maternal hypertension and preeclampsia. However, upregulation of tissue angiotensin II, but not circulating angiotensin II, in the maternal part of the placenta of rats represents an important growth factor for trophoblast invasion and migration, and fetal sheep appear less responsive to infused angiotensin II than pregnant ewes because of a greater fetal metabolic clearance rate that lowers plasma angiotensin II levels in fetal sheep. 41 , 42 Therefore, it is likely that the sensitizing effect exhibiting in offspring of maternal hypertension induced by infusion of angiotensin II in the present study is mainly attributed to maternal hypertension and preeclampsia. However, long‐term infusion of angiotensin II in pregnant ewes has been demonstrated to have a direct impact on fetuses through altering uteroplacental blood flow and fetal gas exchange. 43 Based on these data, in our maternal gestational hypertension model, it is possible that increased circulating angiotensin II in rat dams may have a direct impact on the fetus, and we cannot rule out this possibility. In the future, the maternal hypertension model induced by infusion of aldosterone (low circulating renin and angiotensin II) will be used to differentiate the impacts of maternal hypertension on fetuses or direct effect of circulating angiotensin II on fetuses. Second, we did not determine the enzymatic activity or expression of protein associated with leptin and RAS and PIC components in the brain cardiovascular control related nuclei. Leptin and RAS and PIC components bind to their receptors and enhance activity in the brain to elevate sympathetic activity and increase BP. In this study, we determined the gene expression in the brain and levels of these agents in the plasma. Interestingly, although the pressor effects to leptin or angiotensin II were greater in HFD‐fed offspring of hypertensive dams than that in LFD offspring of hypertensive dams, the mRNA expression of PICs in the PVN was even reduced in the former when compared with that in the latter, while leptin expression was not upregulated in the LT and PVN of LFD offspring of hypertensive dams. Therefore, more studies assessing the enzymatic activity, protein levels, and activation of second messenger mechanisms related to these agents in the brain nuclei are needed to confirm the functional significance of the changes in gene expression. Also, the pathway between the LT and the hypothalamic nuclei involved in processing signals associated with the RAS, PICs, and leptin that initiate the maternal hypertension and HFD‐induced sensitization should be specifically studied in the future. Third, we do not present female offspring data in this article. In fact, we carried out parallel experiments in male and female offspring at the same time and conditions and found similar but not identical results to those reported here for males. Females from mothers with gestational hypertension displayed a sensitized hypertensive response to HFD, but it was lower. Because of different mechanisms underlying the sensitizing effect of maternal hypertension on dietary‐treated male and female offspring, we made a strategic decision to publish the male and female data separately.
In conclusion, this study highlights how maternal gestational hypertension adversely affects vulnerability to expression of hypertension in the next generation exposed to overnutrition. The study demonstrated that maternal gestational hypertension sensitizes postweaning HFD‐induced hypertension, probably through upregulation of leptin, PICs, and RAS components in key brain areas involved in BP regulation that enhances brain sensitivity and sympathetic drive to the cardiovascular system. This study provides insights into a specific central mechanism by which prenatal and postnatal insults converge to interact and synergistically contribute to exacerbation of programming processes that lead to a vicious circle with cardiovascular diseases in offspring later in life.
Sources of Funding
This work was supported by the NIH grants HL‐139575 (Drs Johnson and Xue), HL‐139521 & HL‐155091 (Dr Wei), HL‐136149 (Dr Felder).
Disclosures
None.
Acknowledgments
Author contributions: Drs Xue and Johnson designed the experiments; Drs Xue and Yu, F. Guo, and T. Beltz performed experiments and analyzed data; Dr Xue wrote the manuscript; Drs Xue, Yu, Wei, Felder, and Johnson revised the manuscript. All authors read and approved the final manuscript.
This manuscript was sent to Francis Miller, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 13.
Contributor Information
Baojian Xue, Email: baojian-xue@uiowa.edu.
Alan Kim Johnson, Email: alan-johnson@uiowa.edu.
References
- 1. Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre‐eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:391–403. DOI: 10.1016/j.bpobgyn.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 2. Ananth CV, Keyes KM, Wapner RJ. Pre‐eclampsia rates in the United States, 1980–2010: age‐period‐cohort analysis. BMJ. 2013;347:f6564. DOI: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fraser A, Nelson SM, Macdonald‐Wallis C, Sattar N, Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62:614–620. DOI: 10.1161/HYPERTENSIONAHA.113.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herrera‐Garcia G, Contag S. Maternal preeclampsia and risk for cardiovascular disease in offspring. Curr Hypertens Rep. 2014;16:475. DOI: 10.1007/s11906-014-0475-3. [DOI] [PubMed] [Google Scholar]
- 5. Miettola S, Hartikainen AL, Vaarasmaki M, Bloigu A, Ruokonen A, Jarvelin MR, Pouta A. Offspring's blood pressure and metabolic phenotype after exposure to gestational hypertension in utero. Eur J Epidemiol. 2013;28:87–98. DOI: 10.1007/s10654-013-9763-5. [DOI] [PubMed] [Google Scholar]
- 6. Paauw ND, van Rijn BB, Lely AT, Joles JA. Pregnancy as a critical window for blood pressure regulation in mother and child: programming and reprogramming. Acta Physiol (Oxf). 2017;219:241–259. DOI: 10.1111/apha.12702. [DOI] [PubMed] [Google Scholar]
- 7. Rice MM, Landon MB, Varner MW, Casey BM, Reddy UM, Wapner RJ, Rouse DJ, Tita ATN, Thorp JM, Chien EK, et al. Pregnancy‐associated hypertension and offspring cardiometabolic health. Obstet Gynecol. 2018;131:313–321. DOI: 10.1097/AOG.0000000000002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Staley JR, Bradley J, Silverwood RJ, Howe LD, Tilling K, Lawlor DA, Macdonald‐Wallis C. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: Findings from a prospective study. J Am Heart Assoc. 2015;4: e001422. DOI: 10.1161/JAHA.114.001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tripathi RR, Rifas‐Shiman SL, Hawley N, Hivert M‐F, Oken E. Hypertensive disorders of pregnancy and offspring cardiometabolic health at midchildhood: project viva findings. J Am Heart Assoc. 2018;7(3): DOI: 10.1161/JAHA.117.007426. DOI: 10.1161/JAHA.117.007426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen HE, Lin YJ, Lin IC, Yu HR, Sheen JM, Tsai CC, Huang LT, Tain YL. Resveratrol prevents combined prenatal n(g)‐nitro‐l‐arginine‐methyl ester (l‐name) treatment plus postnatal high‐fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient‐sensing signals, oxidative stress and gut microbiota. J Nutr Biochem. 2019;70:28–37. DOI: 10.1016/j.jnutbio.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 11. Guo Q, Feng X, Xue H, Jin S, Teng X, Duan X, Xiao L, Wu Y. Parental renovascular hypertension‐induced autonomic dysfunction in male offspring is improved by prenatal or postnatal treatment with hydrogen sulfide. Front Physiol. 2019;10:1184. DOI: 10.3389/fphys.2019.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Longo M, Refuerzo JS, Mann L, Leon M, Moussa HN, Sibai BM, Blackwell SC. Adverse effect of high‐fat diet on metabolic programming in offspring born to a murine model of maternal hypertension. Am J Hypertens. 2016;29:1366–1373. DOI: 10.1093/ajh/hpw088. [DOI] [PubMed] [Google Scholar]
- 13. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity‐induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. DOI: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fu Q. Sex differences in sympathetic activity in obesity and its related hypertension. Ann N Y Acad Sci. 2019;1454:31–41. DOI: 10.1111/nyas.14095. [DOI] [PubMed] [Google Scholar]
- 15. Head GA, Lim K, Barzel B, Burke SL, Davern PJ. Central nervous system dysfunction in obesity‐induced hypertension. Curr Hypertens Rep. 2014;16:466. DOI: 10.1007/s11906-014-0466-4. [DOI] [PubMed] [Google Scholar]
- 16. de Kloet AD, Krause EG, Shi PD, Zubcevic J, Raizada MK, Sumners C. Neuroimmune communication in hypertension and obesity: a new therapeutic angle? Pharmacol Ther. 2013;138:428–440. DOI: 10.1016/j.pharmthera.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin‐renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303:H197–206. DOI: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim K, Burke SL, Head GA. Obesity‐related hypertension and the role of insulin and leptin in high‐fat‐fed rabbits. Hypertension. 2013;61:628–634. DOI: 10.1161/HYPERTENSIONAHA.111.00705. [DOI] [PubMed] [Google Scholar]
- 19. Xue B, Zhang Y, Johnson AK. Interactions of the brain renin‐angiotensin‐system (RAS) and inflammation in the sensitization of hypertension. Front Neurosci. 2020;14:650. DOI: 10.3389/fnins.2020.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin‐induced increases in renal sympathetic activity but not its metabolic effects. Hypertension. 2013;61:737–744. DOI: 10.1161/HYPERTENSIONAHA.111.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi P, Diez‐Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. DOI: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shi Z, Li B, Brooks VL. Role of the paraventricular nucleus of the hypothalamus in the sympathoexcitatory effects of leptin. Hypertension. 2015;66:1034–1041. DOI: 10.1161/HYPERTENSIONAHA.115.06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prior LJ, Davern PJ, Burke SL, Lim K, Armitage JA, Head GA. Exposure to a high‐fat diet during development alters leptin and ghrelin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2014;63:338–345. DOI: 10.1161/HYPERTENSIONAHA.113.02498. [DOI] [PubMed] [Google Scholar]
- 24. Zhang YP, Huo YL, Fang ZQ, Wang XF, Li JD, Wang HP, Peng W, Johnson AK, Xue B. Maternal high‐fat diet acts on the brain to induce baroreflex dysfunction and sensitization of angiotensin II‐induced hypertension in adult offspring. Am J Physiol Heart Circ Physiol. 2018;314:H1061–H1069. DOI: 10.1152/ajpheart.00698.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Intapad S, Dasinger JH, Johnson JM, Brown AD, Ojeda NB, Alexander BT. Male and female intrauterine growth‐restricted offspring differ in blood pressure, renal function, and glucose homeostasis responses to a postnatal diet high in fat and sugar. Hypertension. 2019;73:620–629. DOI: 10.1161/HYPERTENSIONAHA.118.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tain YL, Lee WC, Wu KLH, Leu S, Chan JYH. Maternal high fructose intake increases the vulnerability to post‐weaning high‐fat diet‐induced programmed hypertension in male offspring. Nutrients. 2018;10:56. DOI: 10.3390/nu10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tain YL, Sheen JM, Yu HR, Chen CC, Tiao MM, Hsu CN, Lin YJ, Kuo KC, Huang LT. Maternal melatonin therapy rescues prenatal dexamethasone and postnatal high‐fat diet induced programmed hypertension in male rat offspring. Front Physiol. 2015;6:377. DOI: 10.3389/fphys.2015.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson AK, Xue B. Central nervous system neuroplasticity and the sensitization of hypertension. Nat Rev Nephrol. 2018;14:750–766. DOI: 10.1038/s41581-018-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xue B, Yin H, Guo F, Beltz TG, Thunhorst RL, Johnson AK. Maternal gestational hypertension‐induced sensitization of angiotensin II hypertension is reversed by renal denervation or angiotensin‐converting enzyme inhibition in rat offspring. Hypertension. 2017;69:669–677. DOI: 10.1161/HYPERTENSIONAHA.116.08597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–1049. DOI: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- 31. Dasinger JH, Davis GK, Newsome AD, Alexander BT. Developmental programming of hypertension: Physiological mechanisms. Hypertension. 2016;68:826–831. DOI: 10.1161/HYPERTENSIONAHA.116.06603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Git KC, Adan RA. Leptin resistance in diet‐induced obesity: the role of hypothalamic inflammation. Obes Rev. 2015;16:207–224. DOI: 10.1111/obr.12243. [DOI] [PubMed] [Google Scholar]
- 33. Kalupahana NS, Moustaid‐Moussa N. The renin‐angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev. 2012;13:136–149. DOI: 10.1111/j.1467-789X.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- 34. Xiao D, Xu Z, Huang X, Longo LD, Yang S, Zhang L. Prenatal gender‐related nicotine exposure increases blood pressure response to angiotensin ii in adult offspring. Hypertension. 2008;51:1239–1247. DOI: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang XF, Li JD, Huo YL, Zhang YP, Fang ZQ, Wang HP, Peng W, Johnson AK, Xue B. Blockade of angiotensin‐converting enzyme or tumor necrosis factor‐alpha reverses maternal high‐fat diet‐induced sensitization of angiotensin ii hypertension in male rat offspring. Am J Physiol Regul Integr Comp Physiol. 2020;318:R351–R359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65:1126–1133. DOI: 10.1161/HYPERTENSIONAHA.114.05112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood‐borne proinflammatory cytokines. Hypertension. 2013;62:118–125. DOI: 10.1161/HYPERTENSIONAHA.113.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dampney RA. Central neural control of the cardiovascular system: current perspectives. Adv Physiol Educ. 2016;40:283–296. DOI: 10.1152/advan.00027.2016. [DOI] [PubMed] [Google Scholar]
- 39. Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. DOI: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 40. Shi Z, Pelletier NE, Wong J, Li B, Sdrulla AD, Madden CJ, Marks DL, Brooks VL. Leptin increases sympathetic nerve activity via induction of its own receptor in the paraventricular nucleus. Elife. 2020;9. DOI: 10.7554/eLife.55357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hering L, Herse F, Geusens N, Verlohren S, Wenzel K, Staff AC, Brosnihan KB, Huppertz B, Luft FC, Muller DN, et al. Effects of circulating and local uteroplacental angiotensin II in rat pregnancy. Hypertension. 2010;56:311–318. DOI: 10.1161/HYPERTENSIONAHA.110.150961. [DOI] [PubMed] [Google Scholar]
- 42. Rosenfeld CR, Gresores A, Roy TA, Magness RR. Comparison of ANG II in fetal and pregnant sheep: metabolic clearance and vascular sensitivity. Am J Physiol. 1995;268:E237–247. DOI: 10.1152/ajpendo.1995.268.2.E237. [DOI] [PubMed] [Google Scholar]
- 43. Stevens AD, Lumbers ER. The effects of long‐term infusions of angiotensin II into the pregnant ewe on uterine blood flow and on the fetus. J Cardiovasc Pharmacol. 1999;34:824–830. DOI: 10.1097/00005344-199912000-00009. [DOI] [PubMed] [Google Scholar]


