Abstract
Background
Current guidelines recommend at least 6 months of antithrombotic therapy and antibiotic prophylaxis after septal‐occluding device deployment in transcatheter closure of atrial septal defect. It has been estimated that it takes ≈6 months for complete neo‐endothelialization; however, neo‐endothelialization has not previously been assessed in vivo in humans.
Methods and Results
The neointimal coverage of septal occluder devices was evaluated 6 months after implantation in 15 patients by angioscopy from the right atrium. Each occluder surface was divided into 9 areas; the levels of endothelialization in each area were semiquantitatively assessed by 4‐point grades. Device neo‐endothelialization was sufficient in two thirds of patients, but insufficient in one third. In the comparison between patients with sufficiently endothelialized devices of average grade score ≥2 (good endothelialization group, n=10) and those with poorly endothelialized devices of average grade score <2 (poor endothelialization group, n=5), those in the poor endothelialization group had larger devices deployed (27.0 mm [25.0–31.5 mm] versus 17.0 mm [15.6–22.5 mm], respectively) and progressive right heart dilatation. The endothelialization was poorer around the central areas. Moreover, the prevalence of thrombus formation on the devices was higher in the poorly endothelialized areas than in the sufficiently endothelialized areas (Grade 0, 94.1%; Grade 1, 63.2%; Grade 2, 0%; Grade 3, 1.6%).
Conclusions
Neo‐endothelialization on the closure devices varied 6 months after implantation. Notably, poor endothelialization and thrombus attachment were observed around the central areas and on the larger devices.
Keywords: angioscopy, atrial septal defect, device neo‐endothelialization
Subject Categories: Congenital Heart Disease, Imaging, Treatment
Clinical Perspective
What Is New?
This is the first study to evaluate in vivo neo‐endothelialization of atrial septal defect closure devices using angioscopy 6 months after implantation in humans.
Neo‐endothelialization of the atrial septal defect closure devices varied among the study patients; device neo‐endothelialization was sufficient in two thirds of patients, but insufficient in one third of patients.
The poor endothelialization and thrombus attachment were observed around the central areas and on larger atrial septal defect devices.
What Are the Clinical Implications?
Based on these findings, antithrombotic therapy and antibiotic prophylaxis beyond 6 months may be selectively considered in patients who were implanted with larger atrial septal defect devices.
An atrial septal defect (ASD) is one of the most common congenital cardiovascular defects. These defects often trigger heart failure caused by left to right shunt or paradoxical embolic stroke and require procedural intervention. Transcatheter closure for ASD was first performed in 1974. 1 Because of remarkable advances in device development, this treatment method using a catheter is now widely used because it is less invasive than surgical treatment. However, thrombus formation, thromboembolism, 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 and endocarditis 13 , 14 , 15 , 16 , 17 are some of the severe complications related to ASD closure devices. These complications were thought to be mainly caused by insufficient device neo‐endothelialization. Therefore, antithrombotic therapy is required to prevent thrombus formation, whereas antibiotic prophylaxis is also required to prevent endocarditis during procedures possibly causing bacteremia such as tooth extraction. The European Society of Cardiology guidelines recommend at least 6 months of antiplatelet therapy and up to 6 months of antibiotic prophylaxis after device implantation, using the phrase “until endothelialization.” 18 , 19 The American Heart Association/American College of Cardiology guidelines also recommend antibiotic prophylaxis for 6 months following implantation of prosthetic material. However, it is notable that the American Heart Association/American College of Cardiology guidelines make no mention of the recommended duration of antiplatelet therapy. 20 The minimum 6‐month duration of antithrombotic therapy has been validated in clinical practice and major trials. 3 , 21 , 22 The regimens for antithrombotic therapy in these trials were as follows: warfarin or warfarin in addition to aspirin or thienopyridine for 6 months, or 1 to 6 months of aspirin plus clopidogrel followed by aspirin monotherapy. Notably, antithrombotic therapy beyond 6 months is entirely at each clinician's discretion.
The 6‐month duration was decided on the basis of the results of a small population of animal experiments that showed that the devices were sufficiently neo‐endothelialized after 3 to 6 months. 23 , 24 , 25 To date, neo‐endothelialization has not been assessed in vivo in humans, and the evidence to support the appropriate treatment duration is inadequate. Only limited numbers of autopsy cases and device extraction cases demonstrated the extent of neo‐endothelialization of closure devices in humans. 7 , 13 , 14 , 15 , 16 , 17 , 26 , 27 , 28 , 29 , 30 Of these, only one report described sufficient endothelialization of the device, whereas the remaining reports demonstrated insufficient endothelialization in the chronic phase, which might cause endocarditis, thrombotic events, and device dislodgement.
Angioscopy is the only method available for observing the vascular surface and fully evaluating neo‐endothelialization and thrombus attachment on coronary stents. Large structures, such as the aorta, can be evaluated using a nonoccluding angioscopic system. 31 , 32 We have successfully evaluated neo‐endothelialization on an ASD closure device in a previous case. 33 The present study aimed to assess neo‐endothelialization on the occluder surfaces 6 months after implantation using angioscopy, to consider whether the current 6‐month antithrombotic therapy and antibiotic prophylaxis are appropriate, and to identify which patients need extended‐term therapy because of insufficient neo‐endothelialization.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files. The present study was performed in accordance with the ethical principles set forth in the Declaration of Helsinki. The study protocol was reviewed and approved by the institutional review board of the St. Marianna University School of Medicine (Kawasaki‐city, Kanagawa, Japan, Ethics Committee Approval No. 4036). All patients gave their written informed consent before participating in this study.
Patients
Twenty‐three consecutive patients were treated with transcatheter ASD closure at the St. Marianna University School of Medicine Hospital between July 2017 and September 2019. Of these, 4 patients aged <18 years were excluded. Nineteen adult patients were asked to participate in this study, but 4 refused. Thus, the 15 patients who gave informed consent were included in this study prospectively. Angioscopy, echocardiography, and right heart catheterization were performed 180±30 days after implantation.
Angioscopy
Neo‐endothelialization of the closure devices was examined by angioscopy (Visible; Fiber Tech, Tokyo, Japan) from the right atrium. Observations were made using a previously published method. 33 Each device was divided into 9 areas, and the extent of endothelialization of each area was semiquantitatively assessed with a 4‐point scoring method, as used for coronary stent evaluation: Grade 0, complete exposure of device struts (Figure 1A and 1B, Videos S1 and S2); Grade 1, sparse endothelialization (Figure 1C, Video S3); Grade 2, moderate endothelialization with visible device struts (Figure 1D, Video S4); and Grade 3, complete endothelialization with nonvisible device struts (Figure 1E, Video S5). The lower grade was adopted when a different grade score was assigned in the same area. Thrombus formation was also assessed in each area. Thrombus was defined as a red structure not removed by low‐molecular‐weight dextran flushing (Figure 1A, Video S1).
Figure 1. Definition of the endothelialization score.
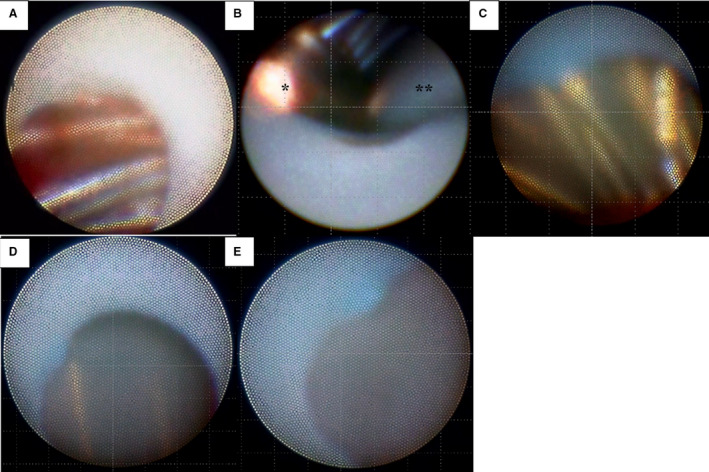
A, Grade 0, complete exposure of device struts. Thrombus attached to the exposed struts (Video S1). B, Around the central hub, the surface of the device is exposed, defined as Grade 0 (Video S2). *Central hub. **Guidewire. C, Grade 1, sparse endothelialization (Video S3). D, Grade 2, moderate endothelialization with visible device struts (Video S4). E, Grade 3, complete endothelialization with nonvisible device struts (Video S5).
Angioscopic findings were assessed by 3 experienced cardiologists who were familiar with angioscopy and blind to the implanted devices. If their opinions differed, the final findings were determined by majority decision.
Echocardiography
All patients underwent comprehensive 2‐dimensional and Doppler transthoracic echocardiography using a commercially available ultrasound system within 1 month of the procedure for angioscopy according to the American Society of Echocardiography guidelines. 34 Left ventricular end‐diastolic volume, left ventricular end‐systolic volume, ejection fraction, and left atrial maximal volume were measured using the Simpson disk method. Right ventricular end‐diastolic area and right ventricular end‐systolic area were measured from the apical 4‐chamber view, focusing on the right ventricle, according to the American Society of Echocardiography guideline for right heart assessment. 35 Right ventricular fractional area change was calculated using the following formula: ([right ventricular end‐diastolic area−right ventricular end‐systolic area]/right ventricular end‐diastolic area)×100. Tricuspid annular plane systolic excursion was determined by the distance of tricuspid lateral annulus systolic movement using M‐mode methods from the apical 4‐chamber view. The right ventricular systolic pressure was estimated using the tricuspid regurgitant pressure gradient and calculated on the basis of the modified Bernoulli equation. The right atrial pressure was estimated on the basis of the most recent American Society of Echocardiography recommendation. 35 The diameter of the inferior vena cave during expiration was assessed by epigastric longitudinal scanning.
Right Heart Catheterization
Right atrial pressure, right ventricular pressure, pulmonary arterial pressure, pulmonary capillary wedge pressure, cardiac output, and the cardiac index obtained by Fick's method were evaluated during the same procedure for angioscopy.
Statistical Analysis
Data are expressed as median and interquartile range for continuous variables and number and percentage for categorical variables. The statistical comparisons were not performed because of the small size of the analysis sample. The weighted κ was calculated to quantify reproducibility of the angioscopic assessments. All analyses were conducted using a standard statistical software program (SPSS version 24; IBM, Armonk, NY).
Results
Patients' Characteristics
The patients' baseline characteristics are shown in Table 1. The patients were 6 men and 9 women, with a median age of 67 years. The implanted devices were 9 Amplatzer Septal Occluders and 6 Figulla Flex II. The median size of the devices was 22 mm. The ECGs showed sinus rhythm in 10 patients, paroxysmal atrial fibrillation in 3 patients, and chronic atrial fibrillation in 2 patients. Three patients with paroxysmal atrial fibrillation maintained sinus rhythm at the assessments. For antithrombotic treatment, 9 patients received dual antiplatelet therapy, and 6 patients received oral anticoagulants in addition to single antiplatelet therapy. All patients had angioscopy assessment under antithrombotic medication. No patients had symptomatic thromboembolic diseases and endocarditis 6 months after implantation.
Table 1.
Patients' Characteristics
| Case No. | Age, y | Sex | BMI, kg/m2 | Closure Device | Size, mm | Antithrombotic Therapy | Rhythm |
|---|---|---|---|---|---|---|---|
| 1 | 79 | Male | 21.8 | Amplatzer | 26 | Aspirin+rivaroxaban | CAF |
| 2 | 78 | Female | 28.2 | Amplatzer | 22 | Aspirin+clopidogrel | SR |
| 3 | 76 | Male | 26 | Amplatzer | 17 | Clopidogrel+edoxaban | PAF |
| 4 | 26 | Female | 25.2 | Figulla Flex II | 16.5 | Clopidogrel+edoxaban | SR |
| 5 | 67 | Male | 26.1 | Figulla Flex II | 24 | Aspirin+clopidogrel | SR |
| 6 | 74 | Female | 21.9 | Figulla Flex II | 24 | Clopidogrel+edoxaban | SR |
| 7 | 49 | Female | 23.2 | Figulla Flex II | 12 | Aspirin+clopidogrel | PAF |
| 8 | 60 | Female | 28.2 | Amplatzer | 22 | Aspirin+clopidogrel | SR |
| 9 | 27 | Female | 23.2 | Figulla Flex II | 27 | Aspirin+clopidogrel | SR |
| 10 | 75 | Female | 19.9 | Amplatzer | 17 | Aspirin+clopidogrel | SR |
| 11 | 72 | Male | 22.8 | Amplatzer | 17 | Ticlopidine+apixaban | PAF |
| 12 | 54 | Male | 27.2 | Figulla Flex II | 27 | Aspirin+clopidogrel | SR |
| 13 | 70 | Male | 27 | Amplatzer | 36 | Clopidogrel+edoxaban | CAF |
| 14 | 67 | Female | 19.4 | Amplatzer | 13 | Aspirin+clopidogrel | SR |
| 15 | 42 | Female | 26.7 | Amplatzer | 26 | Aspirin+clopidogrel | SR |
BMI indicates body mass index; CAF, chronic atrial fibrillation; PAF, paroxysmal atrial fibrillation; and SR, sinus rhythm.
Quality of Angioscopic Evaluation
The quality of angioscopic evaluation was assessed. For interobserver reproducibility for endothelialization grade assessment, the weighted κ coefficient was 0.87 (95% CI, 0.81–0.93) between observer A and observer B, 0.85 (95% CI, 0.78–0.91) between observer B and observer C, and 0.91 (95% CI, 0.87–0.97) between observer A and observer C. For interobserver reproducibility for thrombus formation assessment, the κ coefficient was 0.78 (95% CI, 0.65–0.92) between observer A and observer B, 0.79 (95% CI, 0.66–0.92) between observer B and observer C, and 0.90 (95% CI, 0.81–0.98) between observer A and observer C. With respect to intraobserver reproducibility for endothelialization grade assessment, the weighted κ coefficients of observer A, observer B, and observer C were 0.92 (95% CI, 0.87–0.97), 0.88 (95% CI, 0.82–0.94), and 0.89 (95% CI, 0.83–0.95), respectively. For intraobserver reproducibility for thrombus formation, the κ coefficients of observer A, observer B, and observer C were 0.76 (95% CI, 0.63–0.90), 0.70 (95% CI, 0.53–0.85), and 0.89 (95% CI, 0.79–0.98), respectively.
Device Neo‐Endothelialization and Thrombus Formation 6 Months After Implantation
Neo‐endothelialization and thrombus formation in each area of all patients are shown in Figure 2. Neo‐endothelialization of the devices differed among the patients. Thrombus formation (Figure 1A, Video S1) can be found in the area marked “Th.” Thrombi were attached on the device struts, though they were not large or mobile.
Figure 2. Neo‐endothelialization and thrombus attachment 6 months after implantation.
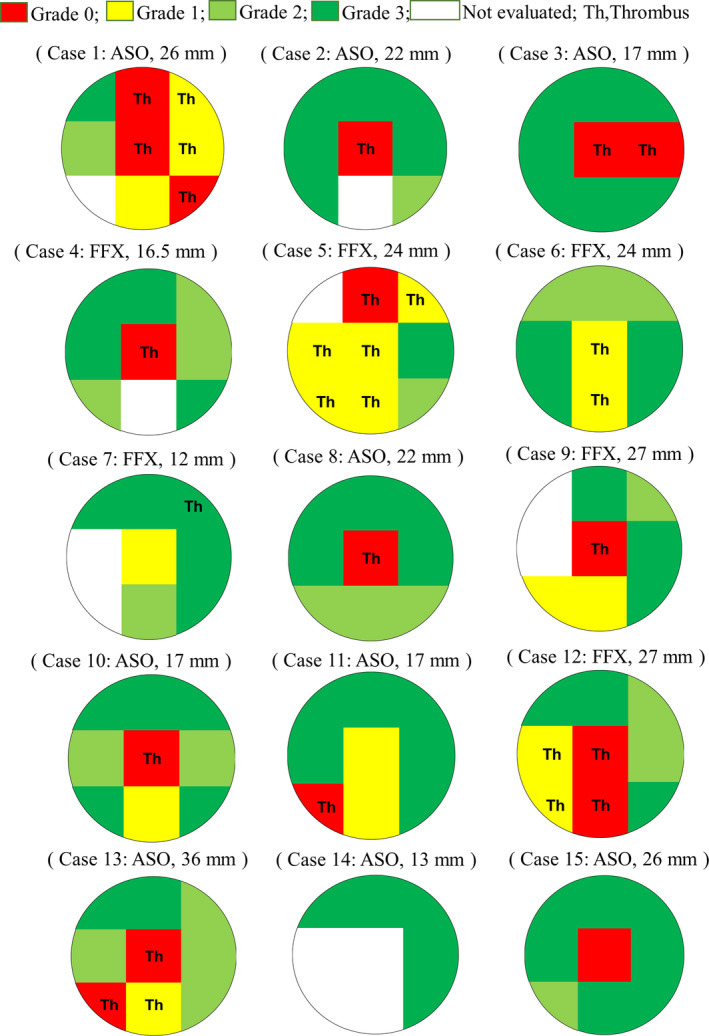
Neo‐endothelialization and thrombus attachment in each area of all patients are shown. Thrombus attachment as in Figure 1A is seen in the area marked “Th.” Neo‐endothelialization of the devices differs among the patients. ASO indicates Amplatzer Septal Occluder; FFX, Figulla Flex II; and Th, thrombus.
Factors Affecting Device Neo‐Endothelialization
The poor endothelialization group included the patients with an average grade score <2, and the good endothelialization group included those with an average grade score ≥2 (Table 2). The poor endothelialization group consisted of 5 patients (patients 1, 5, 9, 12, and 13), and the good endothelialization group consisted of 10 patients (patients 2, 3, 4, 6, 7, 8, 10, 11, 14, and 15). Compared with the good endothelialization group, device size was larger in the poor endothelialization group (27.0 mm [25.0–31.5] versus 17.0 [15.6–22.5 mm], respectively). The right ventricular end‐diastolic area, right ventricular end‐systolic area, right atrium area, and the diameter of the inferior vena cava were larger, and right ventricular systolic pressure was higher in the poor endothelialization group than in the good endothelialization group. The proportion of patients with chronic atrial fibrillation was higher in the poor endothelialization group.
Table 2.
Factors Affecting Device Neo‐Endothelialization
| All, N=15 | Poor Endothelialization Group, n=5 | Good Endothelialization Group, n=10 | |
|---|---|---|---|
| Patients' characteristics | |||
| Age, y | 67 (49–75) | 67 (40.5–74.5) | 69.5 (47.3–75.3) |
| Sex, men/women | 6/9 | 4/1 | 2/8 |
| BMI, kg/m2 | 25.2 (21.9–27.0) | 26.1 (22.5–27.1) | 24.2 (21.4–27.0) |
| Rhythm, SR/PAF/CAF | 10/3/2 | 3/0/2 | 7/3/0 |
| Closure device, ASO/FFX | 9/6 | 2/3 | 7/3 |
| Device size, mm | 22.0 (17.0–26.0) | 27.0 (25.0–31.5) | 17.0 (15.6–22.5) |
| Antithrombotic therapy, DAPT/SAPT+OAC | 9/6 | 3/2 | 6/4 |
| CHA2DS2‐VASc score | 3.0 (2.0–4.0) | 2.0 (0.5–3.5) | 3.0 (3.0–5.25) |
| Blood examination | |||
| WBC, ×103/μL | 5.40 (4.30–5.90) | 5.90 (5.35–6.45) | 5.20 (4.08–5.75) |
| Hb, g/dL | 12.7 (11.7–14.0) | 14.0 (12.2–15.8) | 12.1 (11.0–13.1) |
| Plt, ×103/μL | 214 (187–276) | 195 (163–325) | 223 (194–286) |
| D‐dimer, μg/mL | 0.60 (0.30–1.15) | 0.50 (0.30–1.00) | 0.60 (0.35–1.40) |
| Cr, mg/dL | 0.76 (0.61–1.02) | 0.92 (0.68–1.04) | 0.68 (0.60–0.92) |
| eGFR, mL/min | 69.3 (55.0–75.6) | 67.3 (55.2–85.5) | 71.5 (54.5–75.8) |
| HbA1c, % | 5.5 (5.3–5.6) | 5.5 (5.1–6.9) | 5.5 (5.3–5.6) |
| LDL‐C, mg/dL | 116 (96–121) | 103 (92–128) | 116 (82–120) |
| CRP, mg/dL | 0.04 (0.03–0.05) | 0.04 (0.03–0.07) | 0.04 (0.03–0.07) |
| NT‐proBNP, pg/mL | 109 (78–251) | 82 (74–1554) | 114.5 (80.4–130.8) |
| Hemodynamics | |||
| SBP, mm Hg | 128 (121–142) | 132 (113–139) | 128 (120–148) |
| DBP, mm Hg | 70 (61–80) | 77 (63–86) | 66 (60–76) |
| HR, per min | 65 (61–68) | 65 (63–68) | 66 (60–71) |
| SPAP, mm Hg | 28 (25–35) | 33 (23–41) | 28 (26–32) |
| DPAP, mm Hg | 11 (10–15) | 13 (10–20) | 11 (9–14) |
| MPAP, mm Hg | 17 (15–23) | 21 (15–28) | 17 (15–22) |
| RA, mm Hg | 6 (5–8) | 7 (5–7) | 6 (5–8) |
| PCWP, mm Hg | 11 (9–16) | 16 (11–22) | 11 (8–14) |
| CO, L/min | 5.6 (3.3–6.1) | 5.8 (3.9–6.3) | 5.0 (3.2–6.2) |
| CI, L/min per m2 | 2.9 (2.0–3.7) | 2.9 (2.2–3.6) | 3.1 (2.0–3.8) |
| Echocardiography findings | |||
| LVEDV, mL | 91 (75–100) | 100 (71–107) | 85 (76–93) |
| LVESV, mL | 30 (23–36) | 34 (25–38) | 30 (22–37) |
| EF (%) | 65 (61–71) | 66 (60–71) | 65 (61–71) |
| LAVI, mL/m2 | 44 (34–55) | 49 (35–65) | 37 (31–50) |
| RVEDA, cm2 | 23 (16–28) | 27 (25–31) | 18 (16–26) |
| RVESA, cm2 | 14 (9–17) | 17 (15–20) | 11 (8–15) |
| RVFAC, % | 39 (36–46) | 38 (29–44) | 40 (36–46) |
| Right atrial area, cm2 | 17 (14–23) | 20 (18–33) | 15 (11–19) |
| TAPSE, mm | 21 (17–22) | 21 (17–22) | 19 (17–24) |
| TR grade, none, trivial/mild/moderate/severe | 5/7/2/1 | 1/3/0/1 | 4/4/2/0 |
| RVSP, mm Hg | 26 (22–30) | 30 (28–34) | 23 (22–29) |
| IVC, mm | 13 (10–18) | 18 (14–20) | 11 (9–14) |
ASO indicates Amplatzer Septal Occluder; BMI, body mass index; CAF, chronic atrial fibrillation; CI, cardiac index; CO, cardiac output; Cr, creatinine; CRP, C‐reactive protein; DAPT, dual antiplatelet therapy; DBP, diastolic blood pressure; DPAP, diastolic pulmonary arterial pressure; EF, ejection fraction (calculated by Simpson's method); eGFR, estimated glomerular filtration rate; FFX, Figulla Flex 2; Hb, hemoglobin; HbA1c, hemoglobin A1c; HR, heart rate; IVC, inferior vena cava; LAVI, left atrial volume index; LDL‐C, low‐density lipoprotein cholesterol; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; MPAP, mean pulmonary arterial pressure; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OAC, oral anticoagulant; PAF, paroxysmal atrial fibrillation; PCWP, pulmonary capillary wedge pressure; Plt, platelets; RA, right atrium; RVFAC, right ventricular fraction area change; RVEDA, right ventricular end‐diastolic area; RVESA, right ventricular end‐systolic area; RVSP, right ventricular systolic pressure; SAPT, single antiplatelet therapy; SBP, systolic blood pressure; SPAP, systolic pulmonary arterial pressure; SR, sinus rhythm; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitant; and WBC, white blood cell.
Relationship Between Device Size and Neo‐Endothelialization
The average endothelialization score was lower for the large devices with diameter ≥24 mm (n=7; 1.7 [1.3–2.1]) than for the small devices with diameter ≤22 mm (n=8; 2.3 [2.2–2.6]) (Figure 3).
Figure 3. Relationship between device size and neo‐endothelialization.
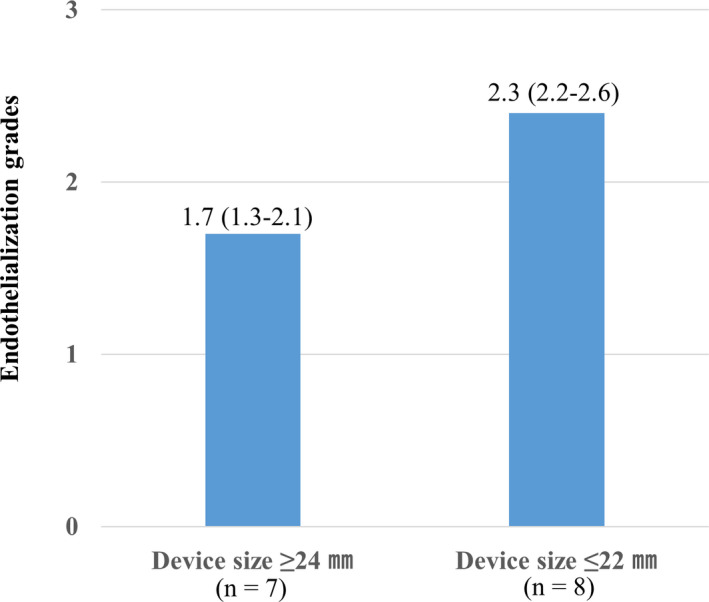
The average endothelialization grade score is lower for the large devices with diameter ≥24 mm (n=7; 1.7 [1.3–2.1]) than for the small devices with diameter ≤22 mm (n=8; 2.3 [2.2–2.6]).
Relationship Between the Location of the Device and Neo‐Endothelialization
The rate of insufficient endothelialization with Grade 0 or 1 in each area is shown in Figure 4. Neo‐endothelialization around central areas, especially near the hub (Figure 1B, Video S2), was insufficient in all cases.
Figure 4. Relationship between the location of the device and neo‐endothelialization.
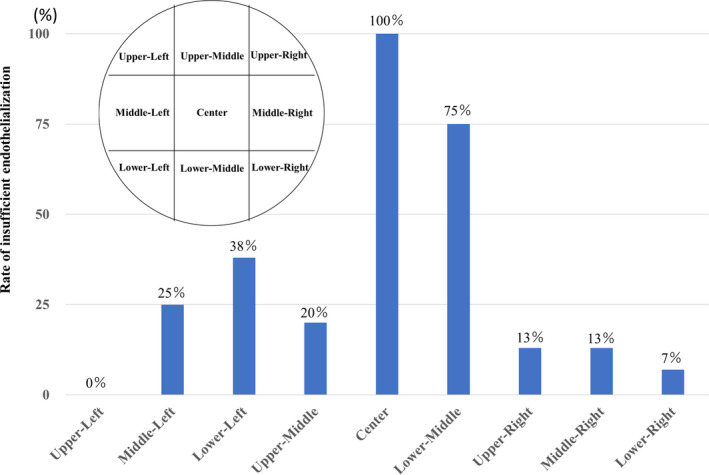
The rate of insufficient endothelialization with Grade 0 or 1 of each area is shown. Neo‐endothelialization around central areas, especially near the hub, is insufficient in all cases.
Relationship Between Thrombus Formation and Neo‐Endothelialization
The relationship between neo‐endothelialization and thrombus formation is shown in Figure 5. Thrombus attachment was found more frequently in the poorly endothelialized areas with Grades 0 (94.4%) and 1 (57.1%) than in the well‐endothelialized areas with Grades 2 (0%) and 3 (1.6%).
Figure 5. Relationship between thrombus formation and neo‐endothelialization.
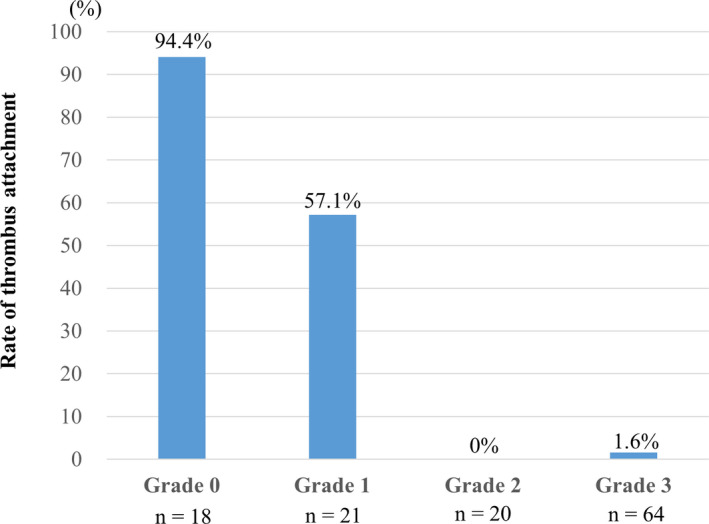
The relationship between neo‐endothelialization and thrombus formation is shown. Thrombus attachment is found more frequently in the poorly endothelialized areas with Grades 0 (94.4%) and 1 (57.1%) than in the well‐endothelialized areas with Grades 2 (0%) and 3 (1.6%).
Discussion
Main Findings
This is the first study to evaluate neo‐endothelialization of ASD closure devices using angioscopy 6 months after implantation in the human heart in vivo. The results can be summarized as follows: (1) neo‐endothelialization of the devices varied among the study patients, with device neo‐endothelialization sufficient in two thirds of patients, but insufficient in one third of patients; (2) patients with right heart remodeling who were treated with a large device might show poor endothelialization; (3) endothelialization of the central areas was insufficient; and (4) thrombus attachment was marked in the insufficiently endothelialized areas.
Angioscopy is a useful device for evaluating vessels and implanted devices and is superior to contrast‐enhanced computed tomography and intravascular ultrasound in the assessment of neo‐endothelialization after coronary stent deployment. 36 Transthoracic echocardiography was performed in all patients, and transesophageal echocardiography was performed in Patient 1 in the good endothelialization group and Patient 2 in the poor endothelialization group; however, both types of echocardiography could not identify obvious differences on the surfaces. Therefore, angioscopy was the only imaging modality that could depict the extent of neo‐endothelialization on the closure devices in vivo. No complications occurred during angioscopy in this study.
Antithrombotic therapy and antibiotic prophylaxis are required until sufficient endothelialization following implantation of atrial septal closure devices. It is assumed that the endothelialization is completed around 6 months after implantation. This is based on the results of a small number of animal experiments. 23 , 24 , 25 However, one study demonstrated that endothelialization varied among the kinds of animals; thus, applying the results from the animal experiments to the human clinical situation has nonnegligible limitations. 25 In addition, some reports of autopsy and device extraction cases reported insufficient neo‐endothelialization in humans beyond 6 months after implantation. 7 , 13 , 14 , 15 , 16 , 17 , 26 , 27 , 28 , 29 , 30 However, in these studies, most of the cases had problems such as endocarditis and dislodgement. The present study provides a better assessment of the actual situation, because all study patients had no device‐related issues. Moreover, the present study demonstrated insufficient neo‐endothelialization in one third of patients, which shows that evidence for the duration of 6 months for antiplatelet and antibiotic prophylaxis recommended by the guidelines is lacking.
The assessment of device neo‐endothelialization is important not only for the ASD closure device, but also for the newer closure devices such as for patent foramen ovale and a left atrial appendage. Angioscopy can play a major role in demonstrating the extent of neo‐endothelialization of these devices in the real world.
Individual Variability of Neo‐Endothelialization of Closure Devices
It has been reported that insufficient endothelialization might cause endocarditis and device dislodgement in cases undergoing surgical extraction. 7 , 13 , 14 , 15 , 16 , 17 Several autopsy cases showed extremely poor endothelialization 6 months or more after implantation. 26 , 27 , 28 , 29 , 30 In the present study, insufficient neo‐endothelialization 6 months after implantation was seen in one third of the study patients. Accordingly, insufficient neo‐endothelialization is not rare, but most cases are benign, and some clinical problems might occur in only a small proportion.
Thrombosis After Transcatheter ASD Closure
Kutty et al reported that the incidence of symptomatic stroke after transcatheter ASD closure was 3%, with a median observation period of 10 years 2 ; however, patients aged <40 years accounted for 60% of their study population. The incidence of thrombosis might be higher in the real world because the population is aging and includes a greater number of older patients. Moreover, when asymptomatic device thrombosis was included, the incidence was 2% to 27%, 9 which is not that low. The prevalence of device thrombosis is presumed to be relatively high until completion of neo‐endothelialization, and thrombosis is prone to occur soon after implantation. Meanwhile, some studies demonstrated a lower prevalence of device thrombosis at 6 months or later. 3 , 5 , 7 Several case reports also showed the prevalence of severe stroke and pulmonary embolism at 6 months or later and a large thrombus attached around the central hub in 2 cases. 11 , 12
In the present study, angioscopy demonstrated poor neo‐endothelialization around the central hub and a high frequency of thrombus attachment in the poorly endothelialized areas.
Endocarditis After Transcatheter ASD Closure
Endocarditis after transcatheter ASD closure is uncommon 2 , 6 , 13 ; the vegetation is typically observed on poorly endothelialized device surfaces. Of 22 reported cases, 13 , 14 , 15 , 16 , 17 10 described the size of deployed devices. Seven cases used devices with a diameter ≥24 mm. The remaining 3 cases were infants, and the implanted devices had diameters <24 mm, which was large for their body proportions. 13 In the present study, neo‐endothelialization was insufficient in patients with device diameter ≥24 mm, which was consistent with the results of the earlier case reports.
Duration of Antithrombotic Therapy and Antibiotic Prophylaxis
As previously described, the European Society of Cardiology guidelines recommend at least 6 months of antiplatelet therapy and up to 6 months of antibiotic prophylaxis after device implantation. 18 , 19 The American Heart Association/American College of Cardiology guidelines also recommend antibiotic prophylaxis for 6 months, but there is no mention of the recommended duration of antiplatelet therapy. 20
The results of the present study and the earlier case reports suggested that implanted devices with diameter ≥24 mm might have a risk for thrombosis and endocarditis caused by insufficient neo‐endothelialization. The results also suggested that neo‐endothelialization was poor in the central areas of the device 6 months after implantation. Therefore, stopping antithrombotic therapy and antibiotic prophylaxis at 6 months after implantation in all cases may be inappropriate.
Should we extend antithrombotic therapy beyond 6 months for all patients treated with larger devices? Our answer is no, because the incidence of symptomatic thrombosis is low, and antithrombotic therapy might trigger side effects such as bleeding. Extended‐term antithrombotic therapy might be carefully examined in patients with thrombotic risk factors such as old age, hypertension, hyperlipidemia, diabetes mellitus, and a larger implanted device. If the thrombotic risks are high and the bleeding risk is low, extended antithrombotic therapy might be considered. Antibiotic prophylaxis may be considered for invasive procedures for 6 months or more for all patients implanted with larger devices, because it seems safe and not harmful to the patients.
Study Limitations
As for limitations, the present study was conducted in a single center, the number of the study patients was small, and children were excluded. Larger studies are needed to determine if there are differences in endothelialization with respect to the closure devices, antithrombotic therapy, blood test results, and hemodynamics. In addition, angioscopy showed only the right atrial side of the devices, not the left atrial side. However, differences in neo‐endothelialization between the right atrial side and the left atrial side were not reported by the earlier published autopsy studies, extracted devices, and animal experimental studies. Accordingly, neo‐endothelialization of the right atrial side is considered to be similar to that of the left atrial side. Finally, it was not technically possible to evaluate all 9 areas with angioscopy in all patients.
Conclusions
This is the first study to evaluate neo‐endothelialization of ASD closure devices in the human heart in vivo. Neo‐endothelialization of ASD closure devices 6 months after implantation varied among the study patients. In particular, poor endothelialization and thrombus attachment were observed in the central areas and on the larger devices. Prescription of individually optimized extended antithrombotic therapy and antibiotic prophylaxis beyond 6 months might be carefully considered in patients implanted with the larger devices.
Sources of Funding
This work was supported by a Grant‐in‐Aid for Scientific Research from the Japan Society for the Promotion of Science.
Disclosures
None.
Supporting information
Supplementary Video Legends
Videos S1–S5
Acknowledgments
The authors thank Dr Fukamachi of the Division of Cardiology, Department of Medicine, Nihon University School of Medicine, for his excellent technical assistance.
(J Am Heart Assoc. 2021;10:e019282. DOI: 10.1161/JAHA.120.019282.)
For Sources of Funding and Disclosures, see page 10.
References
- 1. King TD, Mills NL. Nonoperative closure of atrial septal defects. Surgery. 1974;75:383–388. [PubMed] [Google Scholar]
- 2. Kutty S, Hazeem AA, Brown K, Danford CJ, Worley SE, Delaney JW, Danford DA, Latson LA. Long‐term (5‐ to 20‐year) outcomes after transcatheter or surgical treatment of hemodynamically significant isolated secundum atrial septal defect. Am J Cardiol. 2012;109:1348–1352. DOI: 10.1016/j.amjcard.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 3. Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. 2004;43:302–309. DOI: 10.1016/j.jacc.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 4. Anzai H, Child J, Natterson B, Krivokapich J, Fishbein MC, Chan VK, Tobis JM. Incidence of thrombus formation on the CardioSEAL and the Amplatzer interatrial closure devices. Am J Cardiol. 2004;93:426–431. DOI: 10.1016/j.amjcard.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 5. Chessa M, Carminati M, Butera G, Bini RM, Drago M, Rosti L, Giamberti A, Pomè G, Bossone E, Frigiola A. Early and late complications associated with transcatheter occlusion of secundum atrial septal defect. J Am Coll Cardiol. 2002;39:1061–1065. DOI: 10.1016/S0735-1097(02)01711-4. [DOI] [PubMed] [Google Scholar]
- 6. Masura J, Gavora P, Podnar T. Long‐term outcome of transcatheter secundum‐type atrial septal defect closure using Amplatzer septal occluders. J Am Coll Cardiol. 2005;45:505–507. DOI: 10.1016/j.jacc.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 7. Kreutzer J, Ryan CA, Gauvreau K, Van Praagh R, Anderson JM, Jenkins KJ. Healing response to the Clamshell device for closure of intracardiac defects in humans. Catheter Cardiovasc Interv. 2001;54:101–111. DOI: 10.1002/ccd.1248. [DOI] [PubMed] [Google Scholar]
- 8. Raghu A, Kawalsky D, Feldman M. Embolic stroke due to a left atrial thrombus two years after placement of an atrial septal defect closure device. Am J Cardiol. 2006;98:1294–1296. DOI: 10.1016/j.amjcard.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 9. Divchev D, Schaefer A, Fuchs M, Breymann T, Drexler H, Meyer GP. Thrombus formation on an atrial septal defect closure device: a case report and review of the literature. Eur J Echocardiogr. 2007;8:53–56. [DOI] [PubMed] [Google Scholar]
- 10. Knepp MD, Rocchini AP, Lloyd TR, Aiyagari RM. Long‐term follow up of secundum atrial septal defect closure with the Amplatzer septal occluder. Congenit Heart Dis. 2010;5:32–37. DOI: 10.1111/j.1747-0803.2009.00358.x. [DOI] [PubMed] [Google Scholar]
- 11. Belgrave K, Cardozo S. Thrombus formation on Amplatzer septal occluder device: pinning down the cause. Case Rep Cardiol. 2014;2014:457850. DOI: 10.1155/2014/457850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murala J, Sassalos P, Si MS. 'Near death' thromboembolic episode following device closure of atrial septal defect. Interact Cardiovasc Thorac Surg. 2016;23:340–341. DOI: 10.1093/icvts/ivw145. [DOI] [PubMed] [Google Scholar]
- 13. Amedro P, Soulatges C, Fraisse A. Infective endocarditis after device closure of atrial septal defects: case report and review of the literature. Catheter Cardiovasc Interv. 2017;89:324–334. DOI: 10.1002/ccd.26784. [DOI] [PubMed] [Google Scholar]
- 14. Zahr F, Katz WE, Toyoda Y, Anderson WD. Late bacterial endocarditis of an Amplatzer atrial septal defect occluder device. Am J Cardiol. 2010;105:279–280. DOI: 10.1016/j.amjcard.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 15. Slesnick TC, Nugent AW, Fraser CD Jr, Cannon BC. Images in cardiovascular medicine. Incomplete endothelialization and late development of acute bacterial endocarditis after implantation of an Amplatzer septal occluder device. Circulation. 2008;117:e326–e327. DOI: 10.1161/CIRCULATIONAHA.107.754069. [DOI] [PubMed] [Google Scholar]
- 16. Nguyen AK, Palafox BA, Starr JP, Gates RN, Berdjis F. Endocarditis and incomplete endothelialization 12 years after Amplatzer septal occluder deployment. Tex Heart Inst J. 2016;43:227–231. DOI: 10.14503/THIJ-14-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim DJ, Shim CY, You SC, Lee SH, Hong GR. Late bacterial endocarditis and abscess formation after implantation of an Amplatzer septal occluder device. Circulation. 2015;131:e536–e538. DOI: 10.1161/CIRCULATIONAHA.115.016339. [DOI] [PubMed] [Google Scholar]
- 18. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke‐Baerwolf C, Kaemmerer H, Kilner P, et al.; Task Force on the Management of Grown‐up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG) . ESC Guidelines for the management of grown‐up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915–2957. [DOI] [PubMed] [Google Scholar]
- 19. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, et al.; ESC Scientific Document Group . 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio‐Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–3128. DOI: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 20. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e698–e800. DOI: 10.1161/CIR.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 21. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators . Long‐term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. 2017;377:1022–1032. DOI: 10.1056/NEJMoa1610057. [DOI] [PubMed] [Google Scholar]
- 22. Lee PH, Song J‐K, Kim JS, Heo R, Lee S, Kim D‐H, Song J‐M, Kang D‐H, Kwon SU, Kang D‐W, et al. Cryptogenic stroke and high‐risk patent foramen ovale: the DEFENSE‐PFO trial. J Am Coll Cardiol. 2018;71:2335–2342. DOI: 10.1016/j.jacc.2018.02.046. [DOI] [PubMed] [Google Scholar]
- 23. Lock JE, Rome JJ, Davis R, Van Praagh S, Perry SB, Van Praagh R, Keane JF. Transcatheter closure of atrial septal defects. Experimental studies. Circulation. 1989;79:1091–1099. DOI: 10.1161/01.CIR.79.5.1091. [DOI] [PubMed] [Google Scholar]
- 24. Sharafuddin MJ, Gu X, Titus JL, Urness M, Cervera‐Ceballos JJ, Amplatz K. Transvenous closure of secundum atrial septal defects: preliminary results with a new self‐expanding nitinol prosthesis in a swine model. Circulation. 1997;95:2162–2168. DOI: 10.1161/01.CIR.95.8.2162. [DOI] [PubMed] [Google Scholar]
- 25. Jalal Z, Seguela PE, Baruteau AE, Benoist D, Bernus O, Villemain O, Boudjemline Y, Iriart X, Thambo JB. Role of animal models for percutaneous atrial septal defect closure. J Thorac Dis. 2018;10:S2966–S2974. DOI: 10.21037/jtd.2018.07.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sigler M, Jux C, Ewert P. Histopathological workup of an Amplatzer atrial septal defect occluder after surgical removal. Pediatr Cardiol. 2006;27:775–776. DOI: 10.1007/s00246-006-1413-1. [DOI] [PubMed] [Google Scholar]
- 27. Astroulakis Z, El‐Gamel A, Hill JM. Failed endothelialisation of a percutaneous atrial septal defect closure device. Heart. 2008;94:580. DOI: 10.1136/hrt.2007.135251. [DOI] [PubMed] [Google Scholar]
- 28. Chen F, Zhao X, Zheng X, Chen S, Xu R, Qin Y. Incomplete endothelialization and late dislocation after implantation of an Amplatzer septal occluder device. Circulation. 2011;124:e188–e189. DOI: 10.1161/CIRCULATIONAHA.110.991836. [DOI] [PubMed] [Google Scholar]
- 29. Kawamura A, Kigasawa H, Kamma H. Autopsy findings of Amplatzer septal occluder at 5 months after closure of atrial septal defect: how long does it take to be endothelialized? J Invasive Cardiol. 2013;25:E167–E168. [PubMed] [Google Scholar]
- 30. Chessa M, Butera G, Frigiola A, Carminati M. Endothelialization of ASD devices for transcatheter closure: possibility or reality? Int J Cardiol. 2004;97:563–564. DOI: 10.1016/j.ijcard.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 31. Komatsu S, Ohara T, Takahashi S, Takewa M, Minamiguchi H, Imai A, Kobayashi Y, Iwa N, Yutani C, Hirayama A, et al. Early detection of vulnerable atherosclerotic plaque for risk reduction of acute aortic rupture and thromboemboli and atheroemboli using non‐obstructive angioscopy. Circ J. 2015;79:742–750. DOI: 10.1253/circj.CJ-15-0126. [DOI] [PubMed] [Google Scholar]
- 32. Komatsu S, Yutani C, Ohara T, Takahashi S, Takewa M, Hirayama A, Kodama K. Angioscopic evaluation of spontaneously ruptured aortic plaques. J Am Coll Cardiol. 2018;71:2893–2902. [DOI] [PubMed] [Google Scholar]
- 33. Tanabe Y, Sato Y, Izumo M, Ishibashi Y, Higuma T, Harada T, Akashi YJ. Endothelialization of an Amplatzer septal occluder device 6 months post implantation: is this enough time? An in vivo angioscopic assessment. J Invasive Cardiol. 2019;31:E44. [DOI] [PubMed] [Google Scholar]
- 34. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. DOI: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 35. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. DOI: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 36. Nojima Y, Adachi H, Ihara M, Kurimoto T, Okayama K, Sakata Y, Nanto S. Comparison of neointimal coverage between durable‐polymer everolimus‐eluting stents and bioresorbable‐polymer everolimus‐eluting stents 1 year after implantation using high‐resolution coronary angioscopy. Catheter Cardiovasc Interv. 2019;94:204–209. DOI: 10.1002/ccd.28095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video Legends
Videos S1–S5


