Abstract
CHROMagar Staph. aureus (CSA) is a new chromogenic medium for presumptive identification of Staphylococcus aureus as mauve colonies after 24 h of incubation. We conducted a preliminary study with 100 S. aureus and 45 coagulase-negative Staphylococcus (CoNS) stock isolates plated on CSA. All S. aureus isolates yielded mauve colonies after 24 h of incubation at 37°C, while CoNS isolates grew as blue, white, or beige colonies. Culture on CSA was then prospectively compared to a conventional laboratory method, i.e., culture on 5% horse blood agar (HBA), catalase test, and latex agglutination test (HBA-catalase-latex), for isolation and presumptive identification of S. aureus from 2,000 consecutive clinical samples. Among the 310 S. aureus isolates recovered by at least one of the two methods, 296 grew as typical mauve colonies on CSA, while only 254 yielded catalase-positive, latex-positive colonies on HBA. The sensitivity of CSA was significantly higher than that of the conventional method (95.5 and 81.9%, respectively; P < 0.001) and allowed the recovery of important clinical isolates that were undetected on blood agar. The specificities of the two methods were not significantly different, although that of CSA was slightly higher (99.4% versus 98.9% for HBA-catalase-latex; P = 0.08). On the basis of its excellent sensitivity and specificity, ease of identification of positive colonies, and absence of complementary testing, CSA can be recommended as a routine plating medium for presumptive identification of S. aureus in clinical specimens.
Staphylococcus aureus remains one of the most frequently encountered bacterial pathogens and is responsible for a variety of mild to life-threatening infections. Early isolation and identification of S. aureus as an etiologic agent or indicator of potential health risk is essential for appropriate patient care. In the clinical laboratory routine, S. aureus is usually isolated on nonspecific media (e.g., blood agar) and then presumptively identified before definitive overnight characterization (11). Although immunoenzymatic and genetic assays (3, 5, 9) are available, presumptive identification of suspect colonies relies mostly on the detection of specific determinants such as clumping factor, protein A, and specific capsular antigens by agglutination of sensitized latex particles (7, 17, 19). However, this method becomes costly and time-consuming when more than one suspect morphotype is present on a plate. In an attempt to achieve isolation and presumptive identification in a single step, specific selective culture media have been proposed for use to assist in the detection of S. aureus (reviewed in reference 1). Mannitol-salt agar and related media (2, 4, 8) are widely used for specific screening of S. aureus in potentially contaminated samples. They can be supplemented with antibiotics in order to detect methicillin-resistant S. aureus (MRSA) isolates (13, 18). However, the average sensitivity and specificity of mannitol-salt agar (10, 12, 15) do not qualify it for use for early isolation and presumptive identification of S. aureus in all clinical specimens, although supplementation with egg yolk was shown to be of clinical interest (15). There is still a need for more specific media that would retain the sensitivity of blood agar and allow single-step isolation and presumptive identification of S. aureus. CHROMagar Staph. aureus (CSA) is a new selective chromogenic medium proposed for detection of S. aureus as mauve colonies after 18 to 24 h of incubation. In this study, we first evaluated with 145 clinical stock isolates the ability of CSA to differentiate S. aureus from coagulase-negative staphylococci (CoNS), which are frequently encountered in human clinical samples. Isolation and presumptive identification of S. aureus on CSA were then compared to our standard routine protocol, i.e., culture on 5% horse blood agar (HBA), catalase test and latex agglutination for clumping factor, protein A, and/or specific capsular antigens (HBA-catalase-latex), with 2,000 consecutive clinical specimens in a teaching hospital.
MATERIALS AND METHODS
Culture media.
CSA, a proprietary product, was provided for evaluation by CHROMagar Microbiology (Paris, France), as prepared 85-mm plates containing 20 ml of opaque creamy-white medium. As indicated by the manufacturer, CSA plates were stored at 4°C in a cold room and used within 4 weeks. Columbia agar supplemented with 5% horse blood (HBA) was purchased as commercially prepared plates (Becton-Dickinson, Le Pont-de-Claix, France). In our facility, HBA was preferred to sheep blood agar for general bacterial recovery because of its better capacity to support the growth of Streptococcus milleri-group streptococci and to grow Streptococcus agalactiae as beta-hemolytic colonies. All plates were incubated in air at 37°C. Brain heart infusion broth (bioMérieux, Marcy-l'Étoile, France) was used for cultures in liquid medium.
Stock isolates.
The 145 stock Staphylococcus strains representing 10 species (Table 1) were isolated from clinical human samples in our facility, except for two reference strains (S. aureus ATCC 25923 and Staphylococcus epidermidis ATCC 14990). Among the 100 S. aureus isolates, 26 were resistant to methicillin. One of the MRSA isolates was a small-colony variant (auxotroph for thymidine) and was unable to grow in less than 48 h on HBA. The isolates were stored frozen at −80°C; for the present study, they were thawed, seeded on blood agar, and incubated overnight at 37°C. Suspensions in saline solution were prepared from freshly grown colonies and then streaked onto CSA plates (one isolate per plate). At this stage, the color and size of colonies were evaluated after 24 h of incubation. In order to compare the output of cultures and the growth limits of S. aureus on each medium, comparative colony counts on HBA and CSA were performed for each S. aureus isolate. Suspensions were prepared from freshly grown colonies in saline solution and were adjusted to a turbidity equivalent to that of a 0.5 McFarland unit standard suspension. Dilutions were made from these suspensions, and 100 and 5 CFU of each isolate were inoculated onto HBA and CSA plates by spreading 100 μl of each dilution.
TABLE 1.
Color appearance of 145 Staphylococcus stock isolates streaked on CSA
| Organism (no. tested) | Color of isolated coloniesa |
|---|---|
| S. aureus | |
| Methicillin susceptible (74) | Mauve |
| Methicillin resistant (25) | Mauve |
| Small-colony variant (1)b | Mauvec |
| CoNS | |
| S. epidermidis (25) | Whitecd |
| S. saprophyticus (5) | Light blue |
| S. haemolyticus (5) | Light blue |
| S. hominis (3) | Whitec |
| S. lugdunensis (2) | Beige |
| S. warneri (2) | White |
| S. capitis (1) | Whitec |
| S. schleiferi (1) | White |
| S. xylosus (1) | Navy blue |
After 24 h of incubation at 37°C.
Thymidine auxotroph.
Colony diameter of <2 mm after 24 h.
Mauve background at the inoculum site.
Clinical samples.
The second arm of the study was carried out between March and August 1999 in the 800-bed Necker Enfants-Malades Hospital (Paris, France) on 2,000 consecutive clinical specimens of various origins (Table 2). Midstream urine and routine stool samples were excluded, for S. aureus was unlikely to be isolated from such specimens. Plating on CSA was added to our routine testing regimen, and a comparison of recovery for each specimen was made. Samples were inoculated in a set order: routine medium (HBA) first and then CSA. Primary inoculation was made with a loop or a swab. A straight wire was then used to streak the material to achieve isolated colonies. Quantitative cultures were performed on bronchoalveolar lavage, protected bronchial brush, and central veinous catheter (rinsed with 500 μl of saline solution) by spreading 100 μl of sample per plate. All plates were incubated at 37°C in air and examined after 24 h of incubation. Incubation was occasionally prolonged to 48 h for HBA plates only, in order to enhance differences between colony morphotypes.
TABLE 2.
Distribution of the S. aureus isolates (n = 310) among the 2,000 clinical specimens
| Specimen (no.) | No. of S. aureus strains isolated |
|---|---|
| Blood culturea (141) | 22 |
| Vascular catheter tip (135) | 15 |
| Pus | |
| Wound exudate, ulcer (205) | 49 |
| Abcess (104) | 33 |
| Ear, tympanocentesis fluid (297) | 19 |
| Eye (93) | 8 |
| Respiratory | |
| Sputum (53)b | 24 |
| Tracheal aspirate (26) | 12 |
| Bronchoalveolar lavage (40) | 10 |
| Protected bronchial brush (32) | 10 |
| Body fluids | |
| Amniotic fluid (8) | 0 |
| Ascites, cerebrospinal, peritoneal, and pleural fluids (38) | 1 |
| Drained fluid (194) | 16 |
| High vaginal swab (152) | 37 |
| Neonatalc (192) | 3 |
| Tissue sample (17) | 2 |
| Catheterized urine (154) | 21 |
| Nasal, rectal, and axillary swabsd (119) | 28 |
Positive with gram-positive cocci.
Includes 35 samples from patients with cystic fibrosis.
Gastric aspirate, placenta, meconium, ear, and umbilical swab.
Detection of S. aureus carriage.
Presumptive identification of S. aureus.
The criteria for presumptive identification of S. aureus were defined as follows: (i) on CSA, well-separated mauve colonies after 24 h of incubation, without further testing, and (ii) on HBA, colonial appearance after 24 to 48 h of incubation (11, 12) and Gram stain morphology, positive catalase test (ID color Catalase; bioMérieux), and positive agglutination for Pastorex Staph Plus (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France), as previously described (14). Pastorex Staph Plus allows simultaneous detection of clumping factor, protein A, and two S. aureus-specific capsular and was shown to identify most S. aureus isolates, including MRSA lacking clumping factor and/or protein A (17). On HBA plates with mixed staphylococcal flora, one colony of each suspected different morphotype was tested for catalase and by latex agglutination. All plates were inspected by the same technologist.
Confirmatory identification.
S. aureus identification was confirmed by a positive tube-coagulase test, as previously described (11). Colonies from primary recovery plates presumptively identified as S. aureus were inoculated into brain heart infusion broth and incubated overnight. The cultures were then mixed with citrated rabbit plasma (bioMérieux) and incubated at 37°C. Clotting was evaluated after 4 h, and after 24 h if negative after 4 h. When required, staphylococci and micrococci were identified by the API ID32 STAPH system (bioMérieux) and in-house complementary tests based on conventional phenotypic characteristics (12).
Statistical analysis.
Differences in sensitivities, specificities, and predictive values of the two methods were evaluated by the Yates-corrected χ2 test.
RESULTS
Colonial appearance of S. aureus and other microorganisms on CSA.
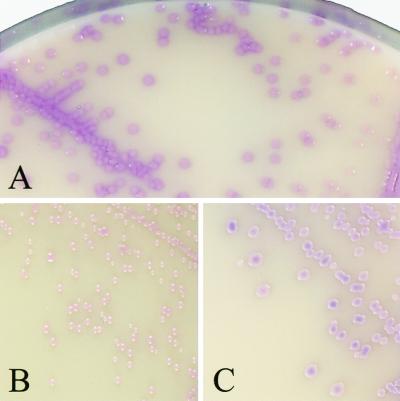
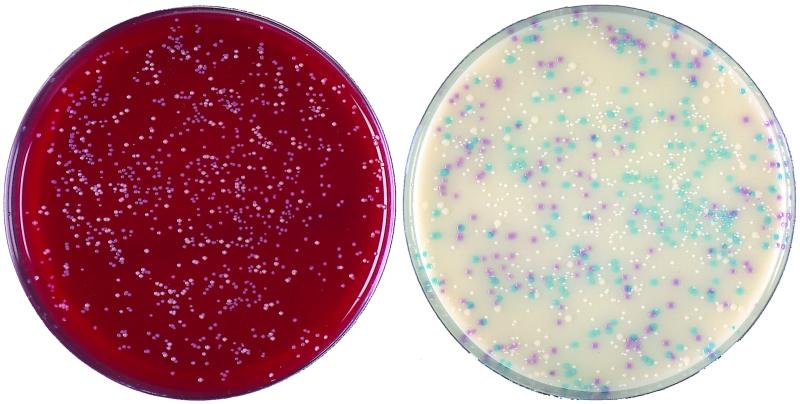
We first evaluated the colonial appearance of 145 Staphylococcus stock isolates on CSA. All 100 S. aureus isolates yielded mauve (positive) colonies, which were usually 2 to 3 mm in diameter after 24 h of incubation (Fig. 1A). A mat halo surrounded the S. aureus colonies, analogous to that observed on Tween 80-supplemented media when lipase-producing bacteria are present. Colonies of the small-colony variant MRSA were smaller (0.5 to 1 mm), although they were clearly visible and mauve after 24 h. Colonies of other MRSA and methicillin-susceptible S. aureus isolates (n = 25 and 74, respectively) displayed no particular difference in terms of size, shape, and color. Except for that of the small-colony variant which did not grow on HBA after 24 h, the colony counts performed on both media were identical (data not shown). When CSA was challenged with low inocula (ca. 5 CFU/plate), its capacity to detect S. aureus was similar to that of HBA. The CoNS stock isolates tested grew as different shades of blue, white, or beige colonies (Table 1), as did most CoNS isolated from clinical specimens during the second part of the study. Figure 2 shows the comparative growth of a mixed gram-positive flora on HBA and CSA after 24 h of incubation. Several staphylococcal morphotypes are seen on HBA, each having required catalase and latex tests, while mauve colonies readily identified S. aureus on CSA. Several S. epidermidis isolates produced small white colonies with a mauve background in regions of denser growth, not to be confused with the actual color of individualized colonies. Micrococcus spp. grew as yellow to brown colonies, except for five Micrococcus luteus isolates which yielded mauve colonies (Fig. 1B). None of the numerous gram-negative organisms isolated on HBA grew on CSA. Enterococcus isolates yielded blue colonies, as did Listeria monocytogenes. Diphtheroids grew on CSA as minute white colonies, except for ANF group Corynebacterium isolates, which produced pinpoint-like red colonies that were barely visible after 24 h. Candida albicans was the only yeast species recovered on CSA, as white colonies.
FIG. 1.
Colonial appearance of S. aureus and of two false-positive isolates on CSA. Colonies were plated out from suspensions of S. aureus (A), M. luteus (B), and S. cohnii (C). CSA plates were incubated for 24 h at 37°C. Magnification, ×2.
FIG. 2.
Colonies plated out from a central venous catheter tip. (Left) Columbia agar supplemented with 5% horse blood; (right) CSA. Mauve colonies of S. aureus are readily differentiated from three other morphotypes on the chromogenic medium; light blue, smaller white, and larger white colonies were identified as Staphylococcus haemolyticus, S. epidermidis, and Staphylococcus warneri, respectively. Plates were incubated for 24 h at 37°C and are shown at their actual size.
Recovery of S. aureus from clinical isolates.
During the second part of this work, the performances of CSA and HBA-catalase-latex were compared in laboratory routine. A total of 310 S. aureus strains were isolated on at least one medium from the 2,000 consecutive clinical specimens. All 310 isolates were coagulase positive after 4 h, regardless of the medium from which they had been isolated. The distribution of the isolates in the different specimens is indicated in Table 2. On HBA, 254 isolates yielded catalase-positive, latex-positive colonies and were confirmed as S. aureus (sensitivity, 81.9%) (Table 3). A total of 1,157 latex agglutination tests were performed, representing a mean of 4.55 tests for each S. aureus isolate actually identified by the conventional method. On CSA, 296 S. aureus isolates grew as typical mauve colonies (sensitivity, 95.5%; P < 0.001). The majority (n = 53) of the 56 falsely negative isolates missed on HBA but not on CSA were either masked or inhibited by a gram-negative associated flora (n = 45) or were unable to grow (n = 8) on primary plating, probably because of a low inoculum. When subcultured from CSA to HBA, those 53 isolates grew as typical staphylococcal colonies. The remaining three isolates falsely negative on HBA were subcultured from CSA to HBA and yielded latex-negative colonies. Among the 14 falsely negative isolates missed on CSA but identified on HBA, 1 grew as blue-violet colonies (suggesting the superposition of mauve and blue colors) and proved to be coagulase positive. Six grew as creamy-white colonies with a mauve background, and these isolates originated from mucoid sputa of patients with cystic fibrosis. Subculture of such colonies on CSA restored typical mauve morphotypes, which were then identified as S. aureus by a coagulase test. Seven isolates originated as scant colonies on HBA, and no S. aureus growth was observed on the corresponding CSA plates, but subculture from HBA to CSA yielded typical mauve colonies.
TABLE 3.
Recovery of S. aureus from 2,000 clinical specimensa
| Method | No. of:
|
Sensitivity (%) | |
|---|---|---|---|
| True-positive isolates | False-negative isolates | ||
| Standardb | 254 | 56 | 81.9 |
| CSAc | 296 | 14 | 95.5 |
A total of 310 isolates were recovered by at least one method (sensitivity, 100%).
Suggestive morphology on HBA, catalase, and latex agglutination.
Mauve colony on CSA after 24 h of incubation.
Specificity of S. aureus presumptive identification.
As shown in Table 4, the specificities of both methods were high (98.9 and 99.4% for HBA-catalase-latex and CSA, respectively) and not significantly different (P = 0.08). A total of 18 and 8 false-positive strains were isolated on HBA and CSA, respectively. They consisted of Staphylococcus lugdunensis (n = 5), Staphylococcus schleiferi (n = 4), Staphylococcus cohnii (n = 4), Staphylococcus saprophyticus (n = 3), S. epidermidis (n = 1), and Kocuria (formerly Micrococcus) kristinae (n = 1) on HBA (catalase- and latex-positive colonies). On CSA, false-positive isolates consisted of M. luteus (n = 5) and S. cohnii (n = 3). Falsely positive M. luteus colonies on CSA (Fig. 1B) were pink-mauve, convex, and smaller than typical S. aureus colonies (Fig. 1A) after 24 h of incubation, and they lacked the mat halo observed around S. aureus colonies. The three false-positive S. cohnii isolates yielded violet colonies after 24 h (Fig. 1C), which in our opinion were not different enough from typical mauve colonies of S. aureus and therefore were considered suspect. All colonies falsely positive with the conventional method were subcultured on CSA, and none of them grew as mauve colonies. Conversely, the three S. cohnii isolates falsely positive on CSA were also falsely positive (latex-positive) on HBA. The five M. luteus isolates that were mauve on CSA were latex negative when grown on HBA. The negative predictive values (Table 4) of the two methods were significantly different (96.8 and 99.2% for HBA-catalase-latex and CSA, respectively; P < 0.001), demonstrating a better capacity of CSA to exclude the presence of S. aureus in clinical specimens.
TABLE 4.
Specificities and negative predictive values of the two methods
| Method | No. of:
|
Specificity (%)a | Negative predictive value (%)b | |
|---|---|---|---|---|
| True-negative results | False-positive results | |||
| Standard | 1,672 | 18c | 98.9 | 96.8 |
| CSA | 1,680 | 8d | 99.4 | 99.2 |
(Number of true negative results/number of negative samples) × 100.
[Number of true negative results/(number of true negative results + number of false negative isolates)] × 100.
S. lugdunensis, n = 5; S. schleiferi, n = 4; S. cohnii, n = 4; S. saprophyticus; n = 3; S. epidermidis, n = 1, K. kristinae, n = 1.
M. luteus, n = 5, S. cohnii, n = 3.
DISCUSSION
The first stage of this study, performed with stock bacteria, indicated the ability of CSA to grow a variety of clinical S. aureus isolates as typical positive colonies, including MRSA. Conversely, CoNS isolates, including species frequently encountered in clinical specimens, did not yield positive colonies. The similar yields of S. aureus from cultures on HBA and CSA demonstrated that there was no inhibitory effect of CSA on the growth of S. aureus compared to HBA, even when the medium was challenged with very low inocula. The performance of CSA was then compared in routine analysis of clinical specimens to our routine protocol (HBA-catalase-latex) for isolation and presumptive identification of S. aureus. Good separation added to easy distinction of mauve from blue or white colonies (Fig. 2) was of considerable help for identification of suspect colonies. The difference in sensitivity observed between plating on CSA and the conventional method (Table 3) appeared to result from the complete inhibition of gram-negative bacterial growth on CSA. This was demonstrated by the recovery on CSA of 53 S. aureus isolates that were unable to grow on primary plating on HBA and were associated with abundant gram-negative flora, especially Pseudomonas aeruginosa and Escherichia coli. In such cases, the selectivity of CSA was an asset for the recovery of several clinically important S. aureus isolates. On HBA, only three S. aureus isolates were negative for the Pastorex Staph Plus agglutination, confirming the excellent sensitivity of this test, as previously reported (17, 19). Except for one strain which yielded atypical blue-violet colonies, all S. aureus isolates grew as typical mauve colonies on CSA, either on primary plating of clinical specimens (n = 296) or by subculture of falsely negative isolates (n = 13). Obstacles to the recovery of all isolates on primary plating on CSA appeared to be related mainly to very low inocula, possibly due to the set order of inoculation (first HBA and then CSA) and to the nature of some sputum specimens from patients with cystic fibrosis. In this setting, S. aureus colonies were atypical on primary plating, although they were typically mauve when subcultured. We have no explanation for this phenomenon and recommend that usage of CSA should be further evaluated in the particular setting of cystic fibrosis.
In this study, both methods were shown to be highly specific. The 18 false positives with the conventional method included isolates of clumping factor-producing staphylococci (S. lugdunensis and S. schleiferi) and other CoNS known to occasionally show falsely positive latex agglutination (6, 17). Only eight false-positive isolates of infrequently isolated species (S. cohnii and M. luteus) grew on CSA. As the presence of a mat halo around S. aureus colonies on CSA was not indicated as discriminant by the manufacturer, we did not take it into account. However, this characteristic could be of diagnostic interest, since there was no halo around the M. luteus colonies as opposed to S. aureus colonies. The white color and smaller size of individualized colonies of S. epidermidis were clearly distinct from the color and size positive colonies despite a mauve background in the regions of dense, confluent growth. With a little experience, the difference between isolates producing a mauve background and those growing as mauve colonies was straightforward. Still, we recommend that workers who are using CSA for the first time include a mauve background-producing S. epidermidis isolate (e.g., strain ATCC 14990) for comparison with S. aureus.
The excellent negative predictive value in the absence of mauve colonies on CSA was particularly appreciated when screening for S. aureus in specimens contaminated with skin flora, which often requires several catalase and latex tests with the conventional method (Fig. 2). This suggests that CSA supplemented with antibiotics could be an interesting alternative to selective media currently used for screening of MRSA in hospitalized patients (13, 18, 16).
Our results indicate that primary plating on CSA provides a convenient and time-saving method for the presumptive identification of S. aureus in routine clinical microbiology. Interpretation of colony colors is easy and false positives are rare when the plates are incubated for 24 h. The selectivity of CSA, resulting in the absence of growth of gram-negative organisms, allows rate of recovery of S. aureus higher than that with HBA, and definitive identification requires only a tube-coagulase or thermonuclease test. Plating on CSA is an excellent alternative to conventional multistep techniques presently used for the isolation and presumptive identification of S. aureus.
ACKNOWLEDGMENTS
We thank Alain Rambach and CHROMagar Microbiology for providing us with the CHROMagar Staph. aureus medium. We are grateful to Claire Poyart for the kind gift of strains and to Michel Simonet and Colin R. Tinsley for their helpful comments and critical reading of the manuscript.
REFERENCES
- 1.Baird R M, Lee W H. Media used in the detection and enumeration of Staphylococcus aureus. Int J Food Microbiol. 1995;26:15–24. doi: 10.1016/0168-1605(93)e0028-p. [DOI] [PubMed] [Google Scholar]
- 2.Blair E B, Emerson J S. A new medium, salt plasma agar, for the isolation of Staphylococcus aureus. Am J Clin Pathol. 1967;47:30–39. doi: 10.1093/ajcp/47.1.30. [DOI] [PubMed] [Google Scholar]
- 3.Brakstad O G, Aasbakk K, Maeland J A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol. 1992;30:1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman G H. The significance of sodium chloride in studies of staphylococci. J Bacteriol. 1945;50:201–203. doi: 10.1128/JB.50.2.201-203.1945. [DOI] [PubMed] [Google Scholar]
- 5.Davis T E, Fuller D D. Direct identification of bacterial isolates in blood cultures by using a DNA probe. J Clin Microbiol. 1991;29:2193–2196. doi: 10.1128/jcm.29.10.2193-2196.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felten A, Lepage E, Lagrange P. Critical analysis of tests for rapid detection of Staphylococcus aureus, Pastorex Staph plus, Slidex Staph-kit and Staph aureus in clinical isolates. Pathol Biol. 1995;43:471–476. [PubMed] [Google Scholar]
- 7.Fournier J M, Bouvet A, Mathieu D, Nato F, Boutonnier A, Gerbal R, Brunengo P, Saulnier C, Sagot N, Slizewicz B. New latex reagent using monoclonal antibodies to capsular polysaccharide for reliable identification of both oxacillin-susceptible and oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993;31:1342–1344. doi: 10.1128/jcm.31.5.1342-1344.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunn B A, Dunkelberg W E, Creitz J R. Clinical evaluation of 2% LSM medium for primary isolation and identification of staphylococci. Am J Clin Pathol. 1972;57:230–240. doi: 10.1093/ajcp/57.2.236. [DOI] [PubMed] [Google Scholar]
- 9.Guzmàn C A, Guardati M C, Fenoglio D, Coratza G, Pruzzo C, Satta G. A novel immunoenzymatic assay for the identification of Staphylococcus aureus strains negative for coagulase and protein A. J Clin Microbiol. 1992;30:1194–1197. doi: 10.1128/jcm.30.5.1194-1197.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones E M, Bowker K E, Cooke R, Marshall R J, Reeves D S, MacGowan A P. Salt tolerance of EMRSA-16 and its effect on the sensitivity of screening cultures. J Hosp Infect. 1997;35:59–62. doi: 10.1016/s0195-6701(97)90168-7. [DOI] [PubMed] [Google Scholar]
- 11.Kloos W E, Bannermann T L. Staphylococcus and Micrococcus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 282–298. [Google Scholar]
- 12.Koneman E W, Allen S D, Janda W M, Schreckenberger P C, Winn W C., Jr . Staphylococci and related organisms. In: Koneman E W, Allen S D, Janda W M, Schreckenberger P C, Winn W C Jr, editors. Color atlas and textbook of diagnostic microbiology. 5th ed. Philadelphia, Pa: Lippincott-Raven; 1997. pp. 539–576. [Google Scholar]
- 13.Lally R T, Ederer M N, Woolfrey B F. Evaluation of mannitol salt agar with oxacillin as a screening medium for methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1985;22:501–504. doi: 10.1128/jcm.22.4.501-504.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luijendijk A, van Belkum A, Verbrugh H, Kluytmans J. Comparison of five tests for identification of Staphylococcus aureus from clinical samples. J Clin Microbiol. 1996;34:2267–2269. doi: 10.1128/jcm.34.9.2267-2269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merlino J, Gill R, Robertson G J. Application of lipovitellin-salt-mannitol agar for screening, isolation, and presumptive identification of Staphylococcus aureus in a teaching hospital. J Clin Microbiol. 1996;34:3012–3015. doi: 10.1128/jcm.34.12.3012-3015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry P L, Coombs G W, Boehm J D, Pearman J W. A rapid (20 h) solid screening medium for detecting methicillin-resistant Staphylococcus aureus. J Hosp Infect. 1998;40:67–72. doi: 10.1016/s0195-6701(98)90027-5. [DOI] [PubMed] [Google Scholar]
- 17.Personne P, Bes M, Lina G, Vandenesch F, Brun Y, Etienne J. Comparative performances of six agglutination kits assessed by using typical and atypical strains of Staphylococcus aureus. J Clin Microbiol. 1997;35:1138–1140. doi: 10.1128/jcm.35.5.1138-1140.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Enk R A, Thompson K D. Use of a primary isolation medium for recovery of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1992;30:504–505. doi: 10.1128/jcm.30.2.504-505.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkerson M, McAllister S, Miller J M, Heiter B J, Bourbeau P P. Comparison of five agglutination tests for identification of Staphylococcus aureus. J Clin Microbiol. 1997;35:148–151. doi: 10.1128/jcm.35.1.148-151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]