Abstract
This study aimed to estimate the latest magnitudes and temporal trends of melanoma burden at the national, regional, and global levels. The data on melanoma incidence, deaths, and disability-adjusted life-years (DALYs) in 204 countries and territories between 1990 and 2019 came from the Global Burden of Disease 2019 Study. Estimated annual percentage change (EAPC) was calculated to depict the temporal trends and Spearman rank correlation was used to analyze the influential factors of EAPC. From 1990 to 2019, the incident cases of melanoma increased by 170% to 289,950, death increased by 90% to 62,840, and DALYs increased by 67% to 1,707,800 globally. The age-standardized incidence rate (ASIR) of melanoma increased globally by an average of 1.13 [95% confidence interval (CI): 0.93–1.32], while the age-standardized rates of death and DALYs both declined with the EAPC of −0.27 (95% CI: −0.36 to −0.19) and −0.49 (95% CI: −0.57 to −0.41). In 2019, the highest burden of melanoma was observed in Australasia, followed by high-income North America and Europe regions, which all presented an incremental growth in ASIR. The positive association between the EAPC in ASIR and socio-demographic index (SDI) in 2019 (ρ = 0.600, P < 0.001) suggested that countries with higher SDI have experienced a more rapid increase in ASIR of melanoma. In conclusion, the burden of melanoma is increasing globally but differed greatly across the world. Notably, the high burden areas are facing a continuing increase in incidence, which implies more targeted strategies should be taken for reducing the increasing melanoma burden.
Keywords: Disability-adjusted life-years, Epidemiology, Estimated annual percentage change, Global burden of diseases study, Incidence, Melanoma
Abbreviations: ASRs, age-standardized rates; DALYs, disability-adjusted life-years; ASIR, age-standardized incidence rate; ASDR, age-standardized death rate; SDI, Socio-demographic Index; EAPC, estimated annual percentage change; GBD, global burden of disease; UI, uncertainty interval; CI, confidence interval
Introduction
Melanoma is a malignant tumor with the most severe clinical symptoms and highest mortality rate among skin cancers [1,2]. The incidence of melanoma has increased since the early 1970s in predominantly white-skinned populations [3]. Over the past three decades, the factors affecting the burden of melanoma disease have remarkably changed worldwide. With the change in leisure-time habits such as prolonged exposure to the sun and indoor tanning experience, the dramatic increase in Ultraviolet (UV) exposure lead to increased development of melanoma [4]. Although many countries have implemented public prevention campaigns such as the SunSmart program and skin cancer screening campaign, due to the significant burden of melanoma, the long-term impact of which remains elusive during the upcoming years [5,6]. Due to differences in risk factors and prevention programs among countries and regions, the burden of melanoma has changed considerably across the world.
There are substantial disparities in melanoma burden between populations. The Global Burden of Disease (GBD) 2015 estimated that the greatest burden in melanoma fell on Australasian, North American, European, elderly and male populations [7]. After 2015, the incidence rate of melanoma continues to rise in some countries such as the United States [8], the Netherlands [9], and China [10]. Melanoma remains a significant threat to global public health with a heavy burden of incidence, prevalence, and mortality, resulting in substantial financial cost, particularly among Caucasians [11]. Fortunately, the burden of melanoma in certain countries has decreased. For example, the incidence rate of melanoma has decelerated in the United States among the younger population, while it has decreased among the total population in Hungary [12,13]. The latest spatial patterns and temporal trends of melanoma burden at global, regional and national levels are still lacking, which is essential for targeted public policy-making, such as public education on melanoma and healthcare resource allocation, particularly in countries with high incidence or increasing burden. Hence, this study aimed to comprehensively estimate the global, regional, and national burden of melanoma on incidence, deaths, disability-adjusted life-years (DALYs) in 2019 and its temporal changes from 1990 to 2019 based on the data of GBD 2019. We expect these results will help develop cost-effective prevention strategies tailored to the population with different characteristics and control the burden of melanoma.
Material and methods
Study data
Using the online Global Health Data Exchange query tool from the GBD Study 2019 (http://ghdx.healthdata.org/gbd-results-tool), we collected annual melanoma case data on incidence, deaths, DALYs, and respective age-standardized rates at global, regional, and national levels from January 1, 1990, to December 31, 2019. The GBD 2019, conducted by the Institute for Health Metrics and Evaluation (IHME), uses all the available up-to-date sources of epidemiological data and improved standardized methods to provide a comprehensive assessment of health loss across 369 diseases and injuries and 87 risk factors [14,15]. The repeatable analytical process and statistical codes for estimating melanoma burden could be obtained from the accessible supporting website (http://ghdx.healthdata.org/gbd-2019/code). The detailed data entry, processing steps and modeling estimations of GBD 2019, including its main changes compared with previous years, have been delineated in previous publications [14,16]. Here we presented the methods specific to the estimation of melanoma. Each step used in the current study to analyze the GBD database complies with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) statements [17]. The melanoma-related burden information from the GBD study was estimated based on multiple national cancer registry systems and aggregate database of cancer registries, such as Cancer Incidence in Five Continents (CI5), Surveillance, Epidemiology, and End Results (SEER), and NORDCAN [16,18]. International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes were used to define melanoma. In this study, all ICD10 codes (C43-C43.9, Z85.82-Z85.828) and ICD 9 codes (172–172.9) of melanoma were included. We also use the cases and age-standardized rates on incidence, deaths, DALYs to fully describe the impact of melanoma on a population. The rates were standardized according to the GBD world population and were reported per 100,000 person-years. The 95% uncertainty intervals (UIs) were produced for every metric using the 25th and 975th ordered 1000 draw values of the posterior distribution.
To describe the disease burden of melanoma in different geographic units, 204 countries and territories were classified into five regions (low, low-middle, middle, high-middle, and high SDI regions) according to the corresponding socio-demographic index (SDI). SDI is a composite indicator of a country's lag-distributed income per capita, average years of schooling, and the total fertility rate in females under the age of 25 years [19]. Moreover, 204 countries and territories were grouped into 21 GBD regions according to a geographic hierarchy by the GBD collaborators, such as high-income Asia Pacific, Western Europe, and Australasia (21 GBD regions were list in Table 1), which were also simplified into seven super GBD regions based on geographic location and the socioeconomic development (including Sub-Saharan Africa, North Africa and Middle East, South Asia, Southeast Asia and East Asia and Oceania, Latin America and Caribbean, Central Europe and Eastern Europe and Central Asia, High-income) [14,15].
Table 1.
. Incidence and age-standardized incidence rate per 100,000 people for melanoma in 1990 and 2019, and its estimated annual percentage change from 1990 to 2019.
| 1990 | 2019 | 1990-2019 | |||
|---|---|---|---|---|---|
| Characteristics | Incident cases No. × 103 (95% UI) | ASIR per 100,000 No. (95% UI) | Incident cases No. × 103 (95% UI) | ASIR per 100,000 No. (95% UI) | EAPC in ASIR No. (95% CI) |
| Overall | 107.38 (85.13,134.06) | 2.56 (2.05,3.25) | 289.95 (214.48,341.97) | 3.56 (2.63,4.19) | 1.13 (0.93,1.32) |
| Sex | |||||
| Males | 52.17 (34.83,70.91) | 2.71 (1.84,3.77) | 153.08 (89.76,193.33) | 4.05 (2.34,5.06) | 1.38 (1.16,1.59) |
| Females | 55.21 (41.15,72.93) | 2.48 (1.85,3.29) | 136.87 (92.69,166.59) | 3.2 (2.18,3.9) | 0.87 (0.69,1.05) |
| SDI region | |||||
| High SDI | 76.42 (57.94,95.32) | 7.86 (5.93,9.66) | 194.49 (139.7,237.65) | 12.4 (9.18,15.5) | 1.5 (1.28,1.71) |
| High-middle SDI | 22.4 (18.29,29.62) | 2.04 (1.68,2.72) | 67.63 (47.55,78.64) | 3.54 (2.48,4.1) | 2.02 (1.85,2.19) |
| Middle SDI | 4.99 (3.87,6.58) | 0.44 (0.35,0.58) | 17.3 (13.66,20.51) | 0.7 (0.55,0.83) | 1.84 (1.73,1.96) |
| Low-middle SDI | 2.1 (1.5,3.03) | 0.32 (0.23,0.44) | 6.12 (4.79,7.24) | 0.42 (0.33,0.5) | 1.03 (0.99,1.07) |
| Low SDI | 1.43 (0.92,2.25) | 0.5 (0.34,0.73) | 3.22 (2.44,4.16) | 0.51 (0.38,0.65) | 0.22 (0.14,0.3) |
| GBD region | |||||
| High-income Asia Pacific | 1.7 (1.47,2.24) | 0.88 (0.75, 1.16) | 5.74 (3.92,6.91) | 1.76 (1.2, 2.11) | 2.65 (2.38,2.93) |
| High-income North America | 39.62 (28.89,47.58) | 12.18 (8.81, 14.43) | 90 (67.86,118.88) | 16.62 (13.09, 22.68) | 0.86 (0.63,1.09) |
| Western Europe | 34.46 (27.19,44.24) | 7 (5.47, 8.92) | 99.64 (61.38,119.77) | 14.55 (9.16, 17.6) | 2.58 (2.32,2.84) |
| Australasia | 7.19 (5.61,9.42) | 31.72 (24.55, 41.32) | 18.37 (12.44,24.06) | 43.36 (30.23, 57.02) | 0.98 (0.79,1.18) |
| Tropical Latin America | 1.36 (1.07,1.82) | 1.3 (1.01, 1.73) | 4.92 (3.94,6.94) | 2.02 (1.62, 2.84) | 1.47 (1.27,1.66) |
| Andean Latin America | 0.22 (0.17,0.32) | 0.97 (0.75, 1.37) | 0.79 (0.57,1.1) | 1.37 (0.99, 1.92) | 1.41 (1.31,1.51) |
| Central Latin America | 0.68 (0.6,0.99) | 0.72 (0.62, 1.03) | 3.46 (2.71,4.72) | 1.43 (1.12, 1.95) | 2.3 (2.18,2.42) |
| Southern Latin America | 0.73 (0.63,1.08) | 1.58 (1.36, 2.32) | 2.61 (1.82,3.49) | 3.31 (2.32, 4.44) | 2.33 (2.09,2.57) |
| Caribbean | 0.21 (0.18,0.28) | 0.75 (0.65, 1.01) | 0.57 (0.45,0.75) | 1.11 (0.87, 1.46) | 1.22 (1.15,1.28) |
| Eastern Europe | 6.75 (5.61,9.82) | 2.54 (2.1, 3.69) | 17.92 (13.06,22.51) | 5.93 (4.36, 7.44) | 3.21 (2.99,3.42) |
| Central Europe | 5.1 (4.3,6.77) | 3.62 (3.06, 4.78) | 13.82 (9.94,16.81) | 7.73 (5.66, 9.4) | 2.76 (2.59,2.94) |
| Central Asia | 0.55 (0.4,0.65) | 1.12 (0.79, 1.31) | 1.01 (0.85,1.44) | 1.34 (1.13, 1.9) | -0.05 (-0.4,0.29) |
| North Africa and Middle East | 1.09 (0.64,1.56) | 0.57 (0.34, 0.8) | 3.88 (2.64,4.67) | 0.83 (0.55, 1) | 1.28 (1.2,1.36) |
| South Asia | 1.28 (0.92,1.8) | 0.21 (0.15, 0.28) | 3.77 (2.62,4.57) | 0.25 (0.18, 0.3) | 0.64 (0.59,0.7) |
| East Asia | 3.86 (2.72,5.43) | 0.4 (0.29, 0.56) | 17.35 (10.98,21.92) | 0.91 (0.58, 1.14) | 3.28 (2.97,3.58) |
| Southeast Asia | 0.75 (0.58,1.15) | 0.26 (0.21, 0.38) | 1.72 (1.36,2.39) | 0.28 (0.22, 0.39) | 0.23 (0.17,0.28) |
| Oceania | 0.02 (0.01,0.03) | 0.48 (0.34, 0.83) | 0.04 (0.02,0.06) | 0.48 (0.33, 0.74) | -0.01 (-0.04,0.02) |
| Western Sub-Saharan Africa | 0.43 (0.27,0.61) | 0.42 (0.28, 0.56) | 1.05 (0.71,1.33) | 0.45 (0.31, 0.57) | 0.34 (0.27,0.41) |
| Eastern Sub-Saharan Africa | 0.8 (0.52,1.31) | 0.83 (0.57, 1.25) | 1.94 (1.44,2.69) | 0.9 (0.66, 1.2) | 0.28 (0.22,0.33) |
| Central Sub-Saharan Africa | 0.17 (0.11,0.28) | 0.64 (0.44, 0.93) | 0.41 (0.3,0.6) | 0.67 (0.48, 0.92) | 0.2 (0.13,0.26) |
| Southern Sub-Saharan Africa | 0.4 (0.31,0.53) | 1.31 (1, 1.65) | 0.95 (0.64,1.18) | 1.59 (1.04, 1.94) | 0.72 (0.64,0.8) |
Abbreviations: No., number; ASIR, age-standardized incidence rate; UI, uncertainty interval; EAPC, estimated annual percentage change; CI, confidential interval.
Statistical analyzes
We chose the age-standardized rates (ASRs) of incidence, death and DALYs based on the GBD population standard age structure to quantify the melanoma burden by sex, SDI, and location from 1990 to 2019 for eliminating the effect of age composition [20]. The estimated annual percentage change (EAPC) is widely used to quantify trends of ASR from 1990 to 2019 [21], [22], [23]. The natural logarithm of the ASR could be fitted in a regression line, ln (ASR) = α + βx + ɛ, where x = calendar year. The EAPC was calculated as 100 × (exp(β)-1), and its 95% confidence interval (CI) could also be generated from the linear regression model. In this study, when the EAPC estimation and the lower boundary of its 95% CI were both >0, we considered the ASR as an increasing trend during the specified period. In contrast, when the EAPC estimation and the upper boundary of its 95% CI were both <0, the ASR was in a decreasing trend. Otherwise, the ASR was deemed to be stable over the study period.
Additionally, to explore the influential factors for EAPCs of melanoma, we evaluated the overall correlation between EAPCs and ASRs in 1990 as well as SDI in 2019 using the Spearman rank correlation (considering the non-normal distribution) at the national level. The ASRs of melanoma in 1990 reflect the disease burden at baseline and the SDI in 2019 is a composite indicator of development status. The relationship between the EAPC and ASRs of melanoma in 1990 can be used to test whether the high-burden countries pour more attention into the prevention and control of melanoma burden, and identify the key countries with high baseline burden and overall upward trend. Considering the possible non-linear relationship, the Locally Weighted Scatterplot Smoothing (LOWESS) regression was used to display more detailed information between the EAPC of ASRs and possible factors [22], which was implemented using the geom_smooth function with default parameters of the ggplot2 package. All study analyzes were performed using the R program (version 4.0.3). A two-sided P value of less than 0.05 was considered statistically significant.
Results
Estimates and variation in melanoma incidence burden
From 1990 to 2019, the number of newly diagnosed melanoma cases worldwide increased by 170%, from 107,380 (95% UI: 85,130–134,060) to 289,950 (95% UI: 214,480–341,970) (Table 1). The age-standardized incidence rate (ASIR) has increased to 3.56 cases per 100,000 persons (95% UI: 2.63–4.19) in 2019 and was highest in high SDI regions (12.4/100,000), such as New Zealand (46.56/100,000), Australia (42.74/100,000) and Netherlands (24.77/100,000) (Table 1, Fig. 1A). Alarmingly, the ASIR of melanoma significantly increased in all SDI regions between 1990 and 2019, especially in high-middle SDI regions with an EAPC of 2.02 (95% CI: 1.85–2.19) (Table 1).
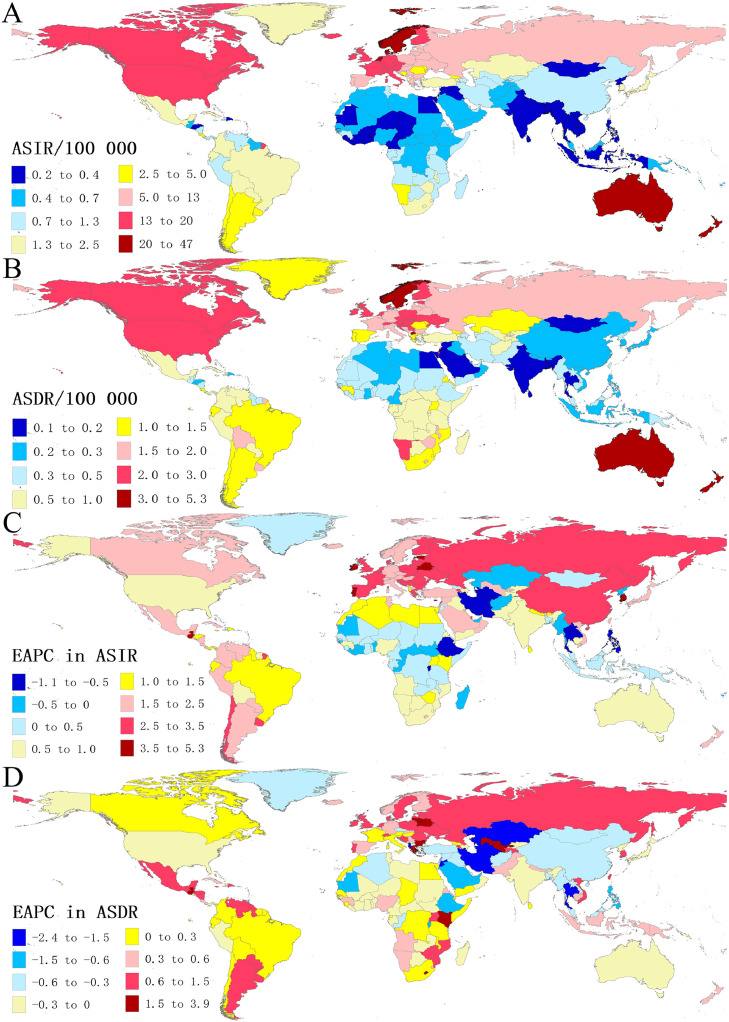
Fig. 1.
The global disease burden of melanoma in 204 countries and territories. (A) The ASIR of melanoma in 2019; (B) the ASDR of melanoma in 2019; (C) the EAPC in ASIR from 1990 to 2019; (D) the EAPC in ASDR from 1990 to 2019. ASIR, age-standardized incidence rate; ASDR, age-standardized death rate; EAPC, estimated annual percentage change.
Among the 21 GBD regions, the ASIR was greater than 14.0/100,000 in Australasia, high-income North America and Western Europe. On the contrary, the lowest ASIR was found in South Asia (0.25/100,000), followed by Southeast Asia (0.28/100,000). From 1990 to 2019, the ASIR increased in all geographical regions except for Central Asia and Oceania, with the most pronounced increase observed in East Asia (EAPC = 3.28), followed by Eastern Europe (EAPC = 3.21) and Central Europe (EAPC = 2.76) (Table 1).
As for the 204 countries and territories, the ASIR in 2019 varied considerably, with the highest observed in New Zealand (46.56/100,000), and the lowest observed in Mongolia (0.22/100,000) (Fig. 1A, Tables S3 and S4). The increasing trend in ASIR between 1990 and 2019 was observed in most countries (159/204), with the highest increase in South Korea (EAPC = 5.30; 95% CI: 4.93–5.67), followed by Belarus (EAPC = 4.77; 95% CI: 4.59–4.96) and Guatemala (EAPC = 4.60; 95% CI: 3.86–5.35) (Fig. 1C, Table S5).
Estimates and variation in melanoma deaths and DALYs burden
Globally, approximately 62,840 (95% UI: 46,320–71,000) individuals died due to melanoma in 2019, which increased by 90% from 33,080 deaths (95% UI: 27,830–43,090) in 1990 (Table S1). Likely, melanoma was responsible for 1,707,800 global DALYs (95%UI: 1,295,600–1,997,500) and increased by 67% from 1,025,700 DALYs in 1990 (Table S2). In 2019, the estimated age-standardized death rate (ASDR) was 0.79 per 100,000 population, with the ASR of DALYs of 20.81/100,000. The ASDR and ASR of DALYs showed a downward trend from 1990 to 2019 globally (EAPC in ASDR = −0.27; EAPC in ASR of DALYs = −0.49), apart from the low-middle SDI regions (Tables S1 and S2).
Melanoma-related deaths and DALYs were both highest in high SDI regions in 2019, such as Western Europe, High-income North America. The corresponding highest ASRs were also observed in high SDI regions, followed by high-middle SDI and low SDI regions. The ASDR in 2019 exceeding 2/100,000 was observed in Australasia, High-income North America and Central Europe. The ASR of DALYs was more than 50/100,000 in Australasia, High-income North America, Central Europe, Western Europe, and Eastern Europe (Tables S1 and S2).
The ASDR in 2019 was highest in New Zealand (5.21/100,000) and exceeded 3/100,000 in Australia, Norway, North Macedonia and Sweden (Fig. 1B, Table S3). The ASDR was less than 0.17/100,000 in Mongolia, Egypt, Sri Lanka, and India (Fig. 1B, Table S4). We also found that the top-five highest ASR of DALYs were in New Zealand(152.05/100,000), Australia(124.66/100,000), Norway(100.14/100,000), Sweden(92.55/100,000), and Netherlands(88.35/100,000) (Table S3, Fig. S1A). Although the ASDR and ASR of DALYs both presented a downward trend globally, 102 countries presented a climbing trend in ASDR, and 87 countries in ASR of DALYs. Guatemala had the largest increases in both ASDR and ASR of DALYs (EAPC = 3.88, 95% CI: 3.13–4.63; EAPC = 3.27, 95% CI: 2.63–3.92), followed by Belarus and Greece (Table S5, Figs. 1D and S1B).
The variation of global melanoma burden in sex and age
Globally, in 2019, the ASRs of incidence, death and DALYs in men were 1.27-times, 1.56-times, and 1.44-times as high as those in women. From 1990 to 2019, the increase of ASIR in males (EAPC = 1.38, 95% CI: 1.16–1.59) was more obvious than those in females (EAPC = 0.87; 95% CI, 0.69–1.05), while the decreases in ASDR and ASR of DALYs were significantly greater in women than in men (Tables 1, S1 and S2). The gender disparity in the disease burden of melanoma presented a slight widening trend.
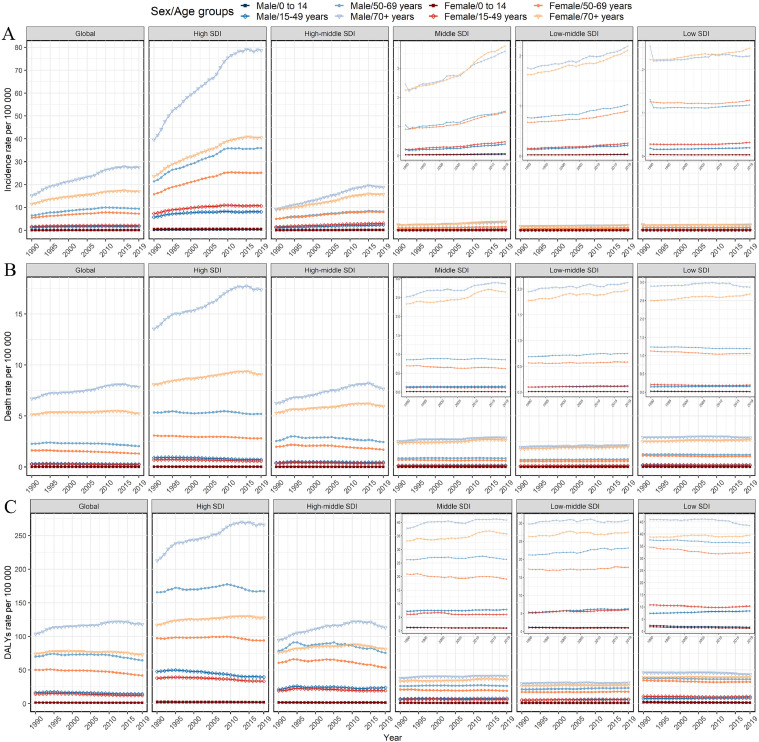
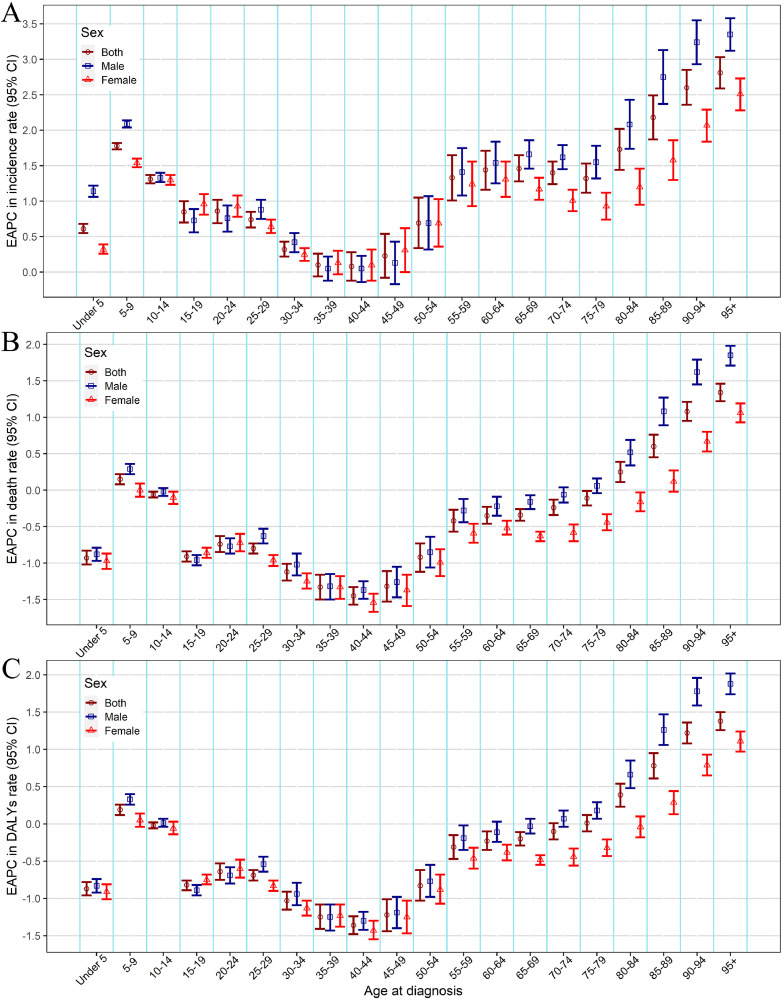
We also analyzed the incidence rate, death rate and DALYs rate for males and females in the different age groups and SDI regions. Figs. 2 and S2 showed that prominent incidence rate, death rate and DALYs rate occurred in the older 70 age group, especially among males and high SDI regions. From 1990 to 2019, the incidence rate increased in almost all age groups, especially among the population aged over 80 years (Fig. 3A). Further, we found that the death rate and DALYs rate showed a downward trend in age groups younger than 80 years old, especially among the people aged 30–49 years, whereas showed an upward trend among the population aged over 80 years (Fig. 3B,C).
Fig. 2.
Age-specific rates of melanoma burden by sex and SDI regions from 1990 to 2019. (A) Incidence rate; (B) death rate; (C) DALYs rate. SDI, Socio-demographic index; DALYs, disability-adjusted life-years.
Fig. 3.
The change of the burden of melanoma by different sexes and age groups, from 1990 to 2019. (A) EAPC in incidence rate, (B) EAPC in death rate, (C) EAPC in DALYs rate. EAPC, estimated annual percentage change; DALYs, disability-adjusted life-years.
Influential factors for EAPCs
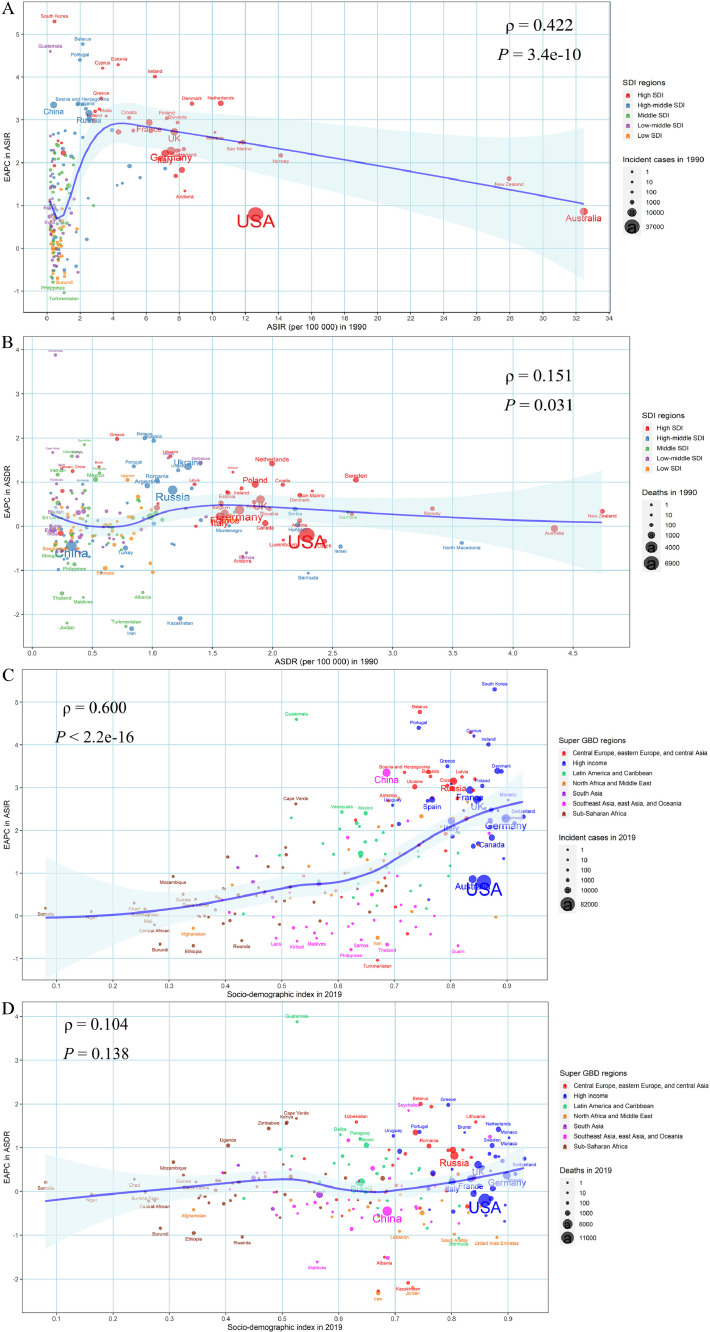
We found a positive relationship between the EAPC in ASIR and initial ASIR when the ASIR was below 4/100,000 at the national level in 1990 (ρ = 0.422, P < 0.001), which showed the countries with higher baseline ASIR usually had a higher increase in ASIR (EAPC of ASIR > 0) (Fig. 4A). However, when the baseline ASIR was greater than 4/100,000, the association was negative, but the EAPC was still greater than zero (Fig. 4A). In addition, the EAPC in ASIR was positively associated with the SDI in 2019 (ρ = 0.600, P < 0.001), which indicated that the higher SDI regions even presented a faster growth in ASIR (EAPC of ASIR > 0) (Fig. 4C). Surprisingly, the EAPC of melanoma ASDR showed a slight correlation with the baseline ASDR (ρ = 0.151, P = 0.031) while there was no significant trend at different SDI in 2019 (ρ = 0.104, P = 0.138) (Fig. 4B,D). Meanwhile, a significant association between EAPC in age-standardized DALYs rate and age-standardized DALYs rate in 1990 (ρ = 0.177, P = 0.011), as well as that between EAPC in age-standardized DALYs rate and SDI in 2019 (ρ = 0.137, P = 0.049) were found in our study (Fig. S3).
Fig. 4.
Influential factors for the EAPCs in age-standardized burden rate of melanoma from 1990 to 2019 at the national level. (A) EAPC in ASIR and ASIR in 1990; (B) EAPC in ASDR and ASDR in 1990; (C) EAPC in ASIR and SDI in 2019; (D) EAPC in ASDR and SDI in 2019. The blue line was an adaptive association fitted with adaptive Loess regression based on all data points. The ρ indices and p values presented were derived from the Spearman rank correlation. EAPC, estimated annual percentage change; ASIR, age-standardized incidence rate; ASDR, age-standardized death rate; SDI, Socio-demographic index (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Discussion
In this study, we comprehensively reported the most up-to-date estimates of spatial patterns and temporal trends of the incidence, death, and DALYs associated with melanoma worldwide. The variations and trends of melanoma burden differed greatly across the world. Our study showed that the highest ASRs of melanoma burden were in high SDI regions and males in 2019. Moreover, although there was a slight decrease in ASDR and ASR of DALYs, the ASIR of melanoma burden dramatically increased globally in the past three decades. This is important for increasing the world's attention to reducing the burden of melanoma especially for countries with a high or increasing burden.
In 2019, the highest ASIR, ASDR and ASR of DALYs were all observed in high SDI regions, especially Australasia and High-income North America regions. The reasons for the high burden of melanoma in Australasia and High-income North America had not yet been fully elucidated, and may include a predominantly fair-skinned population, high ambient UV radiation, an outdoor lifestyle and culture of tanning [24], [25], [26]. In addition, in terms of national differences, we found that among 204 countries, the top five countries with the highest ASDR and ASR of DALYs in 2019 were New Zealand, Australia, Norway, Sweden and the Netherlands, which was consistent with the GBD 2015 estimates [7]. Considering the global burden of melanoma (incidence, death, and DALYs), Europe was one of the most affected places in the world [27], as our study found that Norway, Sweden and Netherlands were in the top five ASDR and ASR of DALYs for melanoma. Moreover, in addition to New Zealand and Australia, there are three European countries in the top five ASIR countries: Netherlands, Norway and Denmark. Previous studies have presented that the incidence of melanoma ranked fourth among 25 major cancers in Europe in 2018 [12]. These indicate that melanoma has already become a significant health care problem across Europe.
From 1990 to 2019, the increasing trend in ASIR was observed in all SDI regions, while decreasing trends in ASDR and ASR of DALYs were observed in all SDI regions except for the low-middle SDI regions. Although the low-middle SDI regions have a growth trend of burden, the burden of these regions in 2019 is still the lowest among the five SDI regions because there has a relatively low baseline burden. However, for low SDI regions, the declining trend in ASDR and ASR of DALYs may be partly due to the limited data source of melanoma there. The increasing trend in ASIR was observed in most countries (159/204), with the highest increase in South Korea, followed by Belarus and Guatemala. One reason for the increase in incidence rates may be the earlier detection and diagnosis of melanoma, which was not detected earlier in the past [28]. A study demonstrated that in South Korea, cutaneous melanoma occurred mainly at acral sites (such as the palm or sole or nails or toenails), making it easy to find pathological changes earlier [29]. Another study noted that the obviously increased ASIR of melanoma in South Korea may be associated with a national screening program for cancer and other common diseases that began in 1999, although the absolute increase of melanoma was slight [30]. Indeed, Germany has initiated a national screening program (SCREEN project) for malignant melanoma since 2008, and showed it could lead to substantial increases in incidence [31]. While there is a suspicion that there is currently “overdiagnosis”, this has not been proven [32]. Besides, compared with the increase or stability of mortality in previous studies [33,34], the latest data showed that the ASDR of melanoma decreased globally. This may be related to the development of health care and medical technology, such as early detection through skin cancer screenings, better diagnostic tools, and the approval of immunotherapy and molecularly targeted therapies for advanced melanoma [35], [36], [37].
Sex differences were observed in the distribution of melanoma [38]. We found that melanoma-related ASIR, ASDR, and ASR of DALYs were higher in males than in females worldwide in 2019, which was consistent with the GBD 2015 estimates [7]. Of note, over the past 30 years, males have a higher growth in ASIR, whereas females have a higher decline in ASDR and ASR of DALYs. This suggests the gender disparity in the disease burden of melanoma is further widening. Thus, more attention should be paid to the prevention and management of melanoma in males. Studies have shown that more than 80% of melanomas could be attributed to UV exposure [39]. There are different UV exposure habits between males and females. Males spent more time in the sun and had less UV protection, while females were more likely to avoid the sun and use sunscreen [40]. Other studies indicated that females attached great importance to skin health status, actively conducted skin examination, which could detect tumors at a younger age, and responded more effectively to treatment [41,42]. However, males were significantly older at the time of diagnosis, and their survival rate was worse than that in females, with a worsening trend in time [43]. Other behavioral and lifestyle factors that may affect gender differences in the risk of melanoma included alcohol and diet [44].
This study also analyzed the incidence rate, death rate and DALYs rate in the different age groups and SDI regions. Our results showed that the age-specific rates for incidence, death, and DALYs all peaked at elderly people over 70 years old, especially in the high SDI regions. The higher burden observed in elderly people may be partly due to peak incidence rates by a structural and physiologic decline that occurred as a natural consequence of aging [45]. Besides, the aging of the global population may explain some of the increase in ASIR of melanoma worldwide [46]. Furthermore, we found that the DALYs rate largely decreased among the population aged 30–49 years from 1990 to 2019, which may be related to decreasing sun exposure in children following intensive preventive campaigns in some countries [47].
We further discussed the relationship between the EAPCs in melanoma burden and baseline burden in 1990 and SDI in 2019, which has not been previously reported. As shown in the results, among countries with relatively low baseline burden, the higher the baseline burden, the more obvious the upward trend of ASIR. However, among countries with relatively high baseline burden, such as Australia, New Zealand, Norway, and the United States, EAPC of ASIR showed a downward trend as the baseline ASIR increased. This phenomenon could be explained as: the countries with low melanoma burden are unlikely to make the prevention and control of melanoma as a high priority due to limited economic conditions, especially in poorer regions [48]. The experience of melanoma intervention in countries with relatively slow ASIR growth, such as the SunSmart program in Australia and the sunscreen campaign in the United States, is worth learning from countries such as South Korea, Belarus, and Guatemala that have seen rapid growth in the burden rate of melanoma [5,13]. In addition, some studies report that socioeconomic status remains closely associated with the incidence and prognosis of cancer [49,50]. In our study, our results showed that the annualized increasing trend of ASIR due to melanoma was associated with the increasing SDI, which indicated that countries with higher SDI have experienced a more rapid increase in ASIR of melanoma from 1990 to 2019. Possible reasons include: because of adequate economic and health resources, the higher SDI countries with a predominantly fair-skinned population have witnessed early detection through screening, improved accurate diagnoses, effective cancer registration system, and increased UV radiation caused by changes in behavior and lifestyle [26,36].
GBD studies provide a high-quality estimate of the global cancer burden, but some shortcomings need to be stressed. First, as for other disease burden estimation, the burden of melanoma is reconstructed by mathematical models based on GBD 2019, and bias could not be avoided in fitting the unavailable data [14]. Second, the heterogeneity in quality and quantity of data sources, and differences in diagnostic methods among countries or territories in the GBD study may affect our analysis results. Moreover, in our study, the spatial and temporal trends of histopathological subtypes of melanoma (like superficial spreading melanoma and acral lentiginous melanoma) were not further quantified and analyzed due to the inaccessibility of relevant data. Finally, this study lacks an estimate of underlying risk factors of melanoma, such as sunburns, the number of melanocytic nevi, indoor tanning, because those risk factors for melanoma were not included in the GBD database. Future research should focus on this aspect and try our best to expand potential risk factors for diseases in the GBD study, which will help guide different countries and regions to formulate specific prevention and control policies for reducing the burden of melanoma.
Conclusion
In conclusion, the melanoma burden and its trends were unevenly distributed among different sexes, age groups and geographic locations. This study revealed that the burden of melanoma was still pronounced in high SDI regions, males, and aged populations in 2019 worldwide. Furthermore, the ASIR of melanoma global burden showed a substantial increase trend, although in some SDI regions, the ASDR and ASR of DALYs presented a slight decrease trend from 1990 to 2019. The overall burden of melanoma is very staggering. Based on the estimates and variation in melanoma burden, more detailed prevention and control strategies such as conducting related health education, decreasing UV exposure, and screening susceptible populations, should be implemented especially in regions and countries with a high or increasing incidence rate.
CRediT authorship contribution statement
Zhen Li: Data curation, Formal analysis, Methodology. Yuan Fang: Data curation, Formal analysis, Methodology. Hui Chen: Data curation, Formal analysis, Methodology. Tongchao Zhang: Data curation, Formal analysis, Methodology. Xiaolin Yin: Data curation, Formal analysis, Methodology. Jinyu Man: Data curation, Formal analysis, Methodology. Xiaorong Yang: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Validation. Ming Lu: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to thank the countless individuals who have contributed to the global burden of disease study 2019 in various capacities.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 82103912, 82173591, and 81973116); the China Postdoctoral Science Foundation (2021M700080); the Shandong Provincial Natural Science Foundation (Grant No. ZR2020QH302); and the National Key Research and Development Program of China (Grant No. 2017YFC0907003). The funders were not involved in the collection, analysis, or interpretation of data, or the writing or submitting of this report.
Data availability statement
All data could be extracted from in the online GBD repository, http://ghdx.healthdata.org/gbd-results-tool.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2021.11.013.
Contributor Information
Xiaorong Yang, Email: yangxiaorong@sdu.edu.cn.
Ming Lu, Email: lvming@sdu.edu.cn.
Appendix. Supplementary materials
References
- 1.Linares MA, Zakaria A, Nizran P. Skin cancer. Prim. Care. 2015;42(4):645–659. doi: 10.1016/j.pop.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 2.da Cruz AT, Hunger A, de Melo FHM, Monteiro AC, Pare GC, Lai D, et al. miR-138-5p induces aggressive traits by targeting Trp53 expression in murine melanoma cells, and correlates with poor prognosis of melanoma patients. Neoplasia. 2021;23(8):823–834. doi: 10.1016/j.neo.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schadendorf D, van Akkooi ACJ, Berking C, Griewank KG, Gutzmer R, Hauschild A, et al. Melanoma. Lancet. 2018;392(10151):971–984. doi: 10.1016/S0140-6736(18)31559-9. [DOI] [PubMed] [Google Scholar]
- 4.Carr S, Smith C, Wernberg J. Epidemiology and risk factors of melanoma. Surg. Clin. N. Am. 2020;100(1):1–12. doi: 10.1016/j.suc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Tabbakh T, Volkov A, Wakefield M, Dobbinson S. Implementation of the SunSmart program and population sun protection behavior in Melbourne, Australia: results from cross-sectional summer surveys from 1987 to 2017. PLoS Med. 2019;16(10) doi: 10.1371/journal.pmed.1002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boniol M, Autier P, Gandini S. Melanoma mortality following skin cancer screening in Germany. Bmj Open. 2015;5(9) doi: 10.1136/bmjopen-2015-008158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimkhani C, Green AC, Nijsten T, Weinstock MA, Dellavalle RP, Naghavi M, et al. The global burden of melanoma: results from the global burden of disease study 2015. Br. J. Dermatol. 2017;177(1):134–140. doi: 10.1111/bjd.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal P, Knabel P, Fleischer AB. United States burden of melanoma and non-melanoma skin cancer from 1990 to 2019. J. Am. Acad. Dermatol. 2021;85(2):388–395. doi: 10.1016/j.jaad.2021.03.109. [DOI] [PubMed] [Google Scholar]
- 9.Leeneman B, Schreuder K, Uyl-de Groot CA, van Akkooi ACJ, Haanen J, Wakkee M, et al. Stage-specific trends in incidence and survival of cutaneous melanoma in the Netherlands (2003-2018): A nationwide population-based study. Eur. J. Cancer. 2021;154:111–119. doi: 10.1016/j.ejca.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Bai RH, Huang H, Li MM, Chu M. Temporal trends in the incidence and mortality of skin malignant melanoma in China from 1990 to 2019. J. Oncol. 2021:2021. doi: 10.1155/2021/9989824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krensel M, Schafer I, Augustin M. Cost-of-illness of melanoma in Europe - a systematic review of the published literature. J. Eur. Acad. Dermatol. Venereol. 2019;33(3):504–510. doi: 10.1111/jdv.15315. [DOI] [PubMed] [Google Scholar]
- 12.Liszkay G, Kiss Z, Gyulai R, Olah J, Hollo P, Emri G, et al. Changing trends in melanoma incidence and decreasing melanoma mortality in Hungary between 2011 and 2019: a nationwide epidemiological study. Front. Oncol. 2020;10 doi: 10.3389/fonc.2020.612459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulson KG, Gupta D, Kim TS, Veatch JR, Byrd DR, Bhatia S, et al. Age-specific incidence of melanoma in the United States. Jama Dermatol. 2020;156(1):57–64. doi: 10.1001/jamadermatol.2019.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. 2016;388(10062):E19–E23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Zhang T, Zhang H, Sang S, Chen H, Zuo X. Temporal trend of gastric cancer burden along with its risk factors in China from 1990 to 2019, and projections until 2030: comparison with Japan, South Korea, and Mongolia. Biomark. Res. 2021;9(1):84. doi: 10.1186/s40364-021-00340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HD, Abbas KM, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, et al. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1160–1203. doi: 10.1016/S0140-6736(20)30977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Yang X, He W, Ye W. Burden of pancreatic cancer along with attributable risk factors in Europe between 1990 and 2019, and projections until 2039. Int. J. Cancer. 2021;149(5):993–1001. doi: 10.1002/ijc.33617. [DOI] [PubMed] [Google Scholar]
- 21.Yang X, Zhang T, Zhang H, Sang S, Chen H, Zuo X. Temporal trend of gastric cancer burden along with its risk factors in China from 1990 to 2019, and projections until 2030: comparison with Japan, South Korea, and Mongolia. Biomark. Res. 2021;9(1):84. doi: 10.1186/s40364-021-00340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X, Chen H, Zhang D, Shen L, An G, Zhao S. Global magnitude and temporal trend of infective endocarditis, 1990–2019: results from the global burden of disease study. Eur. J. Prev. Cardiol. 2021 doi: 10.1093/eurjpc/zwab184. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Zhang TC, Yin XL, Man JY, Yang XR, Lu M. Magnitude and temporal trend of acne vulgaris burden in 204 countries and territories from 1990 to 2019: a analysis from the global burden of disease study 2019. Br. J. Dermatol. 2021 doi: 10.1111/bjd.20882. [DOI] [PubMed] [Google Scholar]
- 24.Thrift AP, Gudenkauf FJ. Melanoma incidence among non-hispanic whites in all 50 us states from 2001 through 2015. J. Natl. Cancer Inst. 2020;112(5):533–539. doi: 10.1093/jnci/djz153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aitken JF, Youlden DR, Baade PD, Soyer HP, Green AC, Smithers BM. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995–2014. Int. J. Cancer. 2018;142(8):1528–1535. doi: 10.1002/ijc.31141. [DOI] [PubMed] [Google Scholar]
- 26.Leiter U, Keim U, Garbe C. Epidemiology of skin cancer: update 2019. Adv. Exp. Med. Biol. 2020;1268:123–139. doi: 10.1007/978-3-030-46227-7_6. [DOI] [PubMed] [Google Scholar]
- 27.Sommariva A, Forsea AM, Agius D, Ascierto PA, Bastiaannet E, Borgognoni L, et al. Quality assurance in melanoma care: the EU-MELACARE study. Eur. J. Surg. Oncol. 2018;44(11):1773–1778. doi: 10.1016/j.ejso.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Garbe C, Keim U, Gandini S, Amaral T, Katalinic A, Hollezcek B, et al. Epidemiology of cutaneous melanoma and keratinocyte cancer in white populations 1943-2036. Eur. J. Cancer. 2021;152:18–25. doi: 10.1016/j.ejca.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 29.Oh CM, Cho H, Won YJ, Kong HJ, Roh YH, Jeong KH, et al. Nationwide trends in the incidence of melanoma and non-melanoma skin cancers from 1999 to 2014 in South Korea. Cancer Res. Treat. 2018;50(3):729–737. doi: 10.4143/crt.2017.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SJ, Oh CM, Kim BW, Woo SJ, Cho H, Park KH. Nationwide incidence of ocular melanoma in South Korea by using the national cancer registry database (1999–2011) Invest. Ophthalmol. Vis. Sci. 2015;56(8):4719–4724. doi: 10.1167/iovs.15-16532. [DOI] [PubMed] [Google Scholar]
- 31.Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. Documenting melanoma incidence and mortality from 2008 to 2013. Dtsch. Arztebl. Int. 2015;112(38):629–634. doi: 10.3238/arztebl.2015.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutzner H, Jutzi TB, Krahl D, Krieghoff-Henning EI, Heppt MV, Hekler A, et al. Overdiagnosis of melanoma - causes, consequences and solutions. J. Dtsch. Dermatol. Ges. 2020;18(11):1236–1243. doi: 10.1111/ddg.14233. [DOI] [PubMed] [Google Scholar]
- 33.Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br. J. Dermatol. 2014;170(1):11–19. doi: 10.1111/bjd.12492. [DOI] [PubMed] [Google Scholar]
- 34.Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28(6):1005–1011. (Review) [PubMed] [Google Scholar]
- 35.Trager MH, Queen D, Samie FH, Carvajal RD, Bickers DR, Geskin LJ. Advances in prevention and surveillance of cutaneous malignancies. Am. J. Med. 2020;133(4):417–423. doi: 10.1016/j.amjmed.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. (Article) [DOI] [PubMed] [Google Scholar]
- 37.Frankel AE, Eskiocak U, Gill JG, Yuan S, Ramesh V, Froehlich TW, et al. Digoxin plus trametinib therapy achieves disease control in BRAF wild-type metastatic melanoma patients. Neoplasia. 2017;19(4):255–260. doi: 10.1016/j.neo.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bellenghi M, Puglisi R, Pontecorvi G, De Feo A, Care A, Mattia G. Sex and gender disparities in melanoma. Cancers. 2020;12(7) doi: 10.3390/cancers12071819. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones OT, Ranmuthu CKI, Hall PN, Funston G, Walter FM. Recognising skin cancer in primary care. Adv. Ther. 2020;37(1):603–616. doi: 10.1007/s12325-019-01130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sideris E, Thomas SJ. Patients' sun practices, perceptions of skin cancer and their risk of skin cancer in rural Australia. Health Promot. J. Aust. 2020;31(1):84–92. doi: 10.1002/hpja.253. [DOI] [PubMed] [Google Scholar]
- 41.Wright CY, Kapwata T, Singh E, Green AC, Baade P, Kellett P, et al. Trends in melanoma mortality in the population groups of South Africa. Dermatology. 2019;235(5):396–399. doi: 10.1159/000500663. [DOI] [PubMed] [Google Scholar]
- 42.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 43.El Sharouni MA, Witkamp AJ, Sigurdsson V, van Diest PJ, Louwman MWJ, Kukutsch NA. Sex matters: men with melanoma have a worse prognosis than women. J. Eur. Acad. Dermatol. Venereol. 2019;33(11):2062–2067. doi: 10.1111/jdv.15760. [DOI] [PubMed] [Google Scholar]
- 44.Yang DD, Salciccioli JD, Marshall DC, Sheri A, Shalhoub J. Trends in malignant melanoma mortality in 31 countries from 1985 to 2015. Br. J. Dermatol. 2020;183(6):1056–1064. doi: 10.1111/bjd.19010. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Wang Y, Wang L, Yin P, Lin Y, Zhou M. Burden of melanoma in China, 1990–2017: findings from the 2017 global burden of disease study. Int. J. Cancer. 2020;147(3):692–701. doi: 10.1002/ijc.32764. [DOI] [PubMed] [Google Scholar]
- 46.Rudnicka E, Napierala P, Podfigurna A, Meczekalski B, Smolarczyk R, Grymowicz M. The world health organization (WHO) approach to healthy ageing. Maturitas. 2020;139:6–11. doi: 10.1016/j.maturitas.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raimondi S, Suppa M, Gandini S. Melanoma epidemiology and sun exposure. Acta Derm. Venereol. 2020;100:250–258. doi: 10.2340/00015555-3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang XR, Man JY, Chen H, Zhang TC, Yin XL, He QF, et al. Temporal trends of the lung cancer mortality attributable to smoking from 1990 to 2017: a global, regional and national analysis. Lung Cancer. 2021;152:49–57. doi: 10.1016/j.lungcan.2020.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Gibson JAG, Dobbs TD, Griffiths R, Song J, Akbari A, Whitaker S, et al. The association of smoking and socioeconomic status on cutaneous melanoma: a population-based, data-linkage, case-control study. Br. J. Dermatol. 2020;182(5):1136–1147. doi: 10.1111/bjd.18526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fidler MM, Soerjomataram I, Bray F. A global view on cancer incidence and national levels of the human development index. Int. J. Cancer. 2016;139(11):2436–2446. doi: 10.1002/ijc.30382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data could be extracted from in the online GBD repository, http://ghdx.healthdata.org/gbd-results-tool.