Abstract
Background
This study was designed to assess the efficacy of Bifidobacterium animalis ssp. lactis (Bl-04) for prevention of rhinovirus colds and to explore the interactions between the probiotic, the viral infection, the host response and the host microbiome.
Methods
The effect of ingestion of the probiotic Bl-04 was evaluated in a randomized, double-blinded rhinovirus (RV) challenge study. Healthy volunteers recruited from a university community in USA were randomized 1:1 using a computer generated code to ingest either Bl-04 (n=165) or placebo (n=169) for 28 days and were then challenged with RV-A39, and followed for 14 days. All study interactions and sample collection occurred in dedicated clinical research space. The primary analysis was the effect of the probiotic on the incidence of RV-associated illness. (Trial registration: NCT02679807, study complete).
Findings
The first cohort of volunteers was randomized on March 14, 2016 and the last (5th) cohort was randomized on March 12, 2018. Sixty-three (56%, 95% CI [47%; 66%]) of the 112 subjects in the active group and 60 (50%,95% CI [41%; 59%]) of the 120 subjects in the placebo group had a protocol-defined rhinovirus-associated illness (χ2=0·91, p=0·34). The point estimate of the difference in illness (active-placebo) is 6.3% (95% CI -6.7;19.1). There were no adverse events that were judged as definitely or probably related to the study product.
Interpretation
In this study there was no effect of orally administered Bl-04 on the occurrence of RV-associated illness.
Funding
Danisco Sweeteners Oy (now IFF Health & Biosciences).
Key Words: probiotic, rhinovirus, upper respiratory infection, innate immunity
Research in context.
Evidence before this study
Bifidobacterium animalis ssp. Lactis, Bl-04, has been previously evaluated in both natural and experimental infection models of viral respiratory infection. These studies suggested that the probiotic might have beneficial effects on viral respiratory infection in general and rhinovirus infection in particular. The observed effects were not definitive, however, and there was no evidence for a mechanism of action for the probiotic.
Added value of this study
This large study in the rhinovirus challenge model found no evidence of a clinical effect of the probiotic on rhinovirus infection or rhinovirus-associated illness. Furthermore, the probiotic did not appear to have effects on the host or the host response.
Implications of all the available evidence
The rhinovirus challenge model has been shown to have predictive value for subsequent studies of effectiveness in the general population. The current evidence suggests that orally administered Bl-04 does not have a beneficial treatment effect on rhinovirus infections or illness. The absence of definitive effects of the probiotic on the host and host response suggest that the current understanding of potential mechanisms of action for probiotics in viral respiratory infection should be further evaluated.
Alt-text: Unlabelled box
1. Introduction
Probiotics, “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” are among the most commonly consumed dietary supplements [1,[2], [3], [4]]. A variety of probiotic preparations have been studied for different human illnesses with some evidence of a beneficial effect on various diarrheal and gastrointestinal disorders [5,6]. The results of studies using probiotics for the treatment or prevention of viral respiratory illnesses have; however, been inconsistent [5,7]. These inconsistent results may be due to the variable quality of the study designs and results reporting of the clinical trials [2]. These trials have generally not addressed potential mechanisms of action in humans, and the proposed mechanism of action of probiotics as treatment for viral respiratory disease have been based primarily on animal models [8,9].
The experimental RV challenge model has been used for many years as a tool for the exploration of pathogenesis, prevention and treatment of these infections in human volunteers in a well-controlled, clinically relevant setting. Previous studies of a probiotic, Bifidobacterium animalis, subsp. lactis Bl-04 (Bl-04), in the experimental challenge model were directed at examining the effect of the probiotic on the nasal innate immune response to the viral infection [10]. Modest but inconsistent immune effects were noted and associated exploratory analyses suggested effects on viral shedding. Another exploratory analysis from the study found apparent effects of the nasal microbiota, unrelated to probiotic consumption, on the clinical and immunologic response to the RV infection [11]. The purpose of the present study was to further explore in a larger challenge study the effects of Bl-04 on clinical outcomes associated with RV infection as well as assess the virologic, immunologic, and microbiomic basis for any observed effect.
2. Methods
Subjects: Healthy adult volunteers, 18-60 years old, were recruited by posted advertisements from the University of Virginia community. Written informed consent was obtained prior to study participation in a form approved by the Human Investigations Committee of the University of Virginia (FWA#:00006183) and the study was conducted in compliance with Good Clinical Practices and in accordance with the Declaration of Helsinki. The trial was registered in clinicaltrials.gov (NCT02679807) and the study protocol is available at that site. Subjects were compensated for participation. Volunteers susceptible to RV type 39, as evidenced by a serum neutralizing antibody titer of ≤.1:4, were invited to participate. Volunteers who had significant underlying respiratory or gastrointestinal disease or had an acute illness were excluded.
Study material and randomization: The active supplement was Bifidobacterium animalis ssp. lactis Bl-04 (Danisco US, Madison, WI). The daily dose of probiotic was provided in a sachet containing a minimum of 2 × 109 colony forming units of Bl-04 mixed with 1g of sucrose as a carrier. The placebo was provided as a sachet containing 1g of sucrose that was identical to the study product in appearance, smell, and taste. Volunteers enrolled in the study were randomized 1:1 to the probiotic or placebo group. The randomization sequence was created by the study sponsor using nQuery Advisor software (version 7.0.1490.0) using a block size of 6. All study personnel involved in the conduct and analysis of the study were blinded to the randomization scheme until the study database was locked. Volunteers were assigned sequential subject numbers as they enrolled in the study and then provided numbered study product corresponding to their subject number.
Conduct of the study: The study design was similar to that described in a previous study [10]. Study cohorts were conducted in the Spring of 2016, 2017, and 2018 and in the Fall of 2016 and 2017. All cohorts were conducted in an identical fashion; the schedule for study activities is outlined in Supplemental Material Table 1. On day -28 of the study, all eligible volunteers had specimens collected and were then randomized to active or placebo supplement to be taken daily through study day 14. Volunteers returned to the study site on days -21, -14, and -7 to replenish supplies of study product and to monitor compliance with the study protocol. During the supplementation period, days -28 to 14, the volunteers were instructed to avoid ingestion of other probiotics or probiotic containing foods and were prohibited from taking antibiotics or anti-inflammatory agents.
Assessment of compliance and blinding: Compliance with study supplements was assessed by counting used sachets of the study products and by qPCR for Bl-04 on stool specimens collected at day 0. The adequacy of study blinding was assessed on day 0, after 28 days of supplementation but prior to virus challenge, by asking volunteers whether they believed they were receiving the active or placebo preparation or “don't know”.
Challenge virus: The challenge virus used for this study was RV-A39. The starting material for this pool was nasal lavage from a donor volunteer who was infected with RV-A39 as a participant in a previous challenge study. The challenge pool was produced under GMP conditions and safety tested according to protocols used for intranasal viral vaccines before use in this study under FDA IND #15241. All subjects were inoculated with 20-100 tissue culture infectious dose 50 (TCID50) of virus by intranasal drops.
Virus isolation and serology: Nasal lavage specimens collected on day 0, before virus challenge, were tested by multiplex PCR (Biofire Diagnostics, SLC, Utah) for the detection of unsuspected viral infections. Nasal lavage collected on study days 1-5 was cultured for RV by standard methods. Sera were tested for neutralizing antibody to RV-A39 by a standard microtiter assay. Volunteers who had RV-A39 isolated from at least one post-challenge specimen or had at least a 4-fold increase in serum neutralizing antibody to RV-A39 between the acute and convalescent specimens were considered infected with the study virus. Viral titers were determined in the original nasal wash specimens stored at -80C by culturing serial 10-fold dilutions in microtiter plates of MRC-5 cells as previously described [12].
Symptom assessment: Symptom scoring was done daily on each of the five days after virus challenge using the modified Jackson symptom score [12], [13], [14]. The symptoms of nasal obstruction, rhinorrhea, sore throat, cough, sneezing, headache, myalgia, and chilliness were scored as 0-4 corresponding to absent, mild, moderate, severe or very severe by an interactive interview with study staff. The total symptom score was the total score for all symptoms over the five days after virus challenge. Volunteers who had a total symptom score of at least 6 and either three days of rhinorrhea or the subjective impression they had experienced a common cold illness were defined as having a symptomatic illness for the data analysis. On study days 6-14, volunteers recorded in a study diary the presence and severity of the same symptoms using the same scoring scale.
Assessment of airway inflammation: Interleukin (IL)-8/ chemokine (C-X-C motif) ligand 8 (CXCL8) concentration was measured in nasal lavage using a commercially available ELISA assay (R&D Systems, Minneapolis, MN). Granulocyte colony-stimulating factor (G-CSF), IL-1β, IL-6, interferon gamma-induced protein (IP-10), monocyte chemoattractant protein-1 (MCP-1), interferon (IFN)-γ, and IL-10 cytokine concentrations from the nasal lavages were analyzed using multiplex ELISA (Aushon Biosystems, Billerica, MA, USA). Only data points within the standard range were accepted for statistical analysis.
Transcriptomics: Blood samples were collected into PAXgene RNA extractor tubes on study day -28 at baseline, day 0 before RV infection, and day 2 during RV infection. RNA was extracted for subsequent analysis of relative gene expression by templated oligonucleotide ligation followed by sequencing using an oligo panel targeted at inflammatory and antiviral genes (genes listed in Supplemental Material, Table 2). The effect of the probiotic and the infection on gene expression over time was assessed by comparing differential gene expression overall and at the different time points collected between the study groups, between infected and uninfected subjects, and between those with and without a rhinovirus-associated illness. A complete description of the methods is provided in the Supplemental Material.
Microbiota analyses: Nasal swabs were collected on study days -28, 0 and 3, throat swabs were collected on days -28, 0, 3, 5 and 14, and a fecal specimen was collected on day 0 for microbiota analysis. The microbiota composition from the per-protocol population samples was assessed by Illumina sequencing of the V4 variable region of the 16S rRNA gene and data were analyzed using QIIME2 (v. 2019.6) [15]. Alpha diversity was calculated for the pre-infection period as the change from day -28 to day 0 and post-infection as the change from day 0 using the unique amplicon sequence variants (ASVs). Differential taxa tests were conducted for the main effect of study group for each time point and overall (including adjustment for day, cohort, viral load, day*treatment and random effect of subject). The effect of day -28 or day 0 abundance of the predominant genus-level nasal taxa (Corynebacterium, Staphylococcus, Moraxella, Alloiococcus) on the clinical outcomes was investigated by including each taxa as a fixed effect into the statistical models used for the primary clinical endpoint analyses. Also, the two-way interaction of taxa and treatment was investigated. Similarly, the effect of the most abundant fecal bacterial genera (Blautia, Faecalibacterium, Coprococcus) on duration of illness and clinical symptom scores were investigated. A detailed description of the microbiomic methods is provided in the supplemental material.
In addition to the sequencing approach, a strain-specific PCR was used to detect the supplemented probiotic strain Bl-04 from the fecal (quantitative real-time PCR) and nasal/throat swab samples (droplet digital PCR). Throat swab samples were further analyzed for the presence of pathogenic bacterial DNA (real-time qPCR) which included: Haemophilus influenzae, Staphylococcus aureus, Neisseria meningitidis, Streptococcus pneumoniae, Streptococcus pyogenes group A.
Assessment of safety: Information on adverse events (AEs) or severe adverse events (SAEs) was gathered by interactive interview with study staff at each study visit for all randomized subjects in the study and coded using the latest MedDRA dictionary. The study interventions in this study included the study procedures, the virus infection and the administration of the study product. The study investigator assessed the relatedness of all adverse events to the study interventions prior to the unblinding of the study groups.
2.1. Statistical analysis
The primary outcome variable for this study was the incidence of rhinovirus-associated illness compared between the active and placebo groups. A rhinovirus-associated illness was defined by the study protocol as an illness that met the study criteria for illness in a volunteer who also met the definition for RV infection as described above. The per-protocol analysis cohort was the volunteers who were susceptible to RV-A39 (serum neutralizing antibody titer ≤ 1:4), had no virus detected in the nasal lavage on day 0, were infected with RV-A39, and completed the study taking at least 80% of the intended treatment doses. Two subjects voluntarily withdrew from the study after the virus challenge. These volunteers were excluded from the per-protocol analysis. There were two missing symptom score data points of 1160 in the per-protocol analysis cohort. No correction or imputation was made for the missing data.
Sample size analysis: The sample size for this study was calculated on the primary endpoint of rhinovirus-induced illnesses. The challenge model results in an infection rate of approximately 90% in the control group. Approximately 60% of all infected volunteers are expected to develop a symptomatic illness and will meet the study definition of a rhinovirus-induced illness. A sample size of 95 completed subjects/arm would be expected to detect a reduction in rhinovirus-induced illness of 20% (from 60% to 40%) with 80% power with pα= 0.05. A reduction from 60% to 40% was established as a target effect based on results of previous successful studies in the rhinovirus challenge model [12,16]. To ensure that a sufficient number of volunteers would be evaluable by the protocol criteria, a target of 254 subjects challenged with the study virus was established.
Analysis of results: The relationship between treatment and incidence of rhinovirus-associated illness was assessed by a chi-square test. Duration of illness, incidence of viral infection, quantitative viral shedding, shedding of virus in nasal lavage, and total symptom score both during the acute illness period and during the total study period were analyzed as secondary endpoints. The relationship between treatment and incidence of infection and shedding of virus in nasal lavage were analyzed exactly the same way as the primary response. For duration of illness Wilcoxon-Mann-Whitney test was used to compare durations between treatments. Quantitative viral shedding was analyzed with a repeated measures analysis of variance model (RM-ANOVA). Total symptom scores were analyzed with Poisson regression analysis models using the generalized estimating equations (GEE) technique for repeated measures. The main interest in these analysis models was the over-time treatment effect. All p values for these analyses were two-sided tests. A hierarchical strategy was used to control the type I error in the secondary analyses. As exploratory endpoints, changes in CXCL8, IFN-γ, IL-10, G-CSF, MCP-1, IP-10, IL-6, and IL-1β concentrations both from day -28 until virus challenge and from day 0 onwards were analyzed with repeated measures analysis of covariance models (RM-ANCOVA). For the exploratory endpoints there was no correction for multiple comparisons. All p-values are reported as two-tailed tests. A complete description of the statistical methods for the study is provided as Supplemental Material and the Statistical Analysis Plan is provided at clinicaltrials.gov. The data in the study were captured in an electronic database (Viedoc™ Clinic; PCG Solutions AB, Uppsala, Sweden), all data were entered at the study site and monitored for accuracy. Inquiries about access to the original clinical data should be directed to the Corresponding Author.
Role of the funding source: This study was funded by Danisco Sweeteners Oy, Kantvik, Finland. The scientists at Danisco Sweeteners OY (IFF Health & Biosciences) were active participants in the development of the protocol and design of the study, completion of assays and analysis for cytokine, transcriptomic and microbiomic studies, and preparation of the manuscript. RBT, MJL, LL, SM, TH had access to all data. All authors agreed to submit the manuscript for publication.
3. Results
Subjects: Three-hundred eighty (380) subjects signed consent for participation in the trial. The flow of subjects through the study is shown in Figure 1. The study population was drawn primarily from the UVA student population. Age, gender, race, and ethnicity were comparable between the two study arms (Table 1). Three-hundred thirty-four (334) subjects were randomized to the study product and 318 volunteers (95%) completed the study as designed. All volunteers who received study product were included in the demographic, safety, and blinding analyses. After withdrawals and per protocol exclusions, 296 (143 active, 153 placebo), were challenged with RV-A39 (Figure 1). The pivotal analysis for the study was done on those subjects, 112 in the active group and 120 in the placebo group, who completed the study and met the per-protocol criteria detailed above.
Figure 1.
Flow chart of the enrollment and disposition of the study subjects over the course of the study.
Table 1.
Demographics of the study participants randomized to study treatment.
| Active (N=165) | Placebo (N=169) | |
| Gender Male Female |
59 (36%) 106 (64%) |
75 (44%) 94 (56%) |
| Race Asian Black White Unknow/Other |
16 (10%) 10 (6%) 123 (75%) 16 (10%) |
16 (10%) 12 (7%) 120 (71%) 37 (12%) |
| Ethnicity Hispanic Non-hispanic Unknown |
6 (4%) 132 (80%) 27 (16%) |
11 (7%) 130 (77%) 28 (16%) |
| Age (yrs, median (Quartile 1, Quartile 3)) | 21 (20, 22) | 21 (20, 22) |
Compliance and blinding: Compliance with the study product was good. Two volunteers, one in the active group and one in the placebo group took less than 80% of intended doses between randomization and virus challenge based on counts of returned used sachets and were excluded from the per-protocol analysis. Bl-04 was detected in stool by PCR on day 0 before challenge in 83% of the volunteers in the active group (data not shown).
The majority of volunteers, 56% in the active group and 50% in the placebo group, reported they didn't know which supplementation they were taking (data not shown). Of those subjects randomized to the active group, 17% believed they were taking the active and 27% believed they were taking the placebo preparation. Of those randomized to placebo, 19% believed they were taking the active and 31% believed they were taking placebo (χ2=1·81, p=0·41; data not shown).
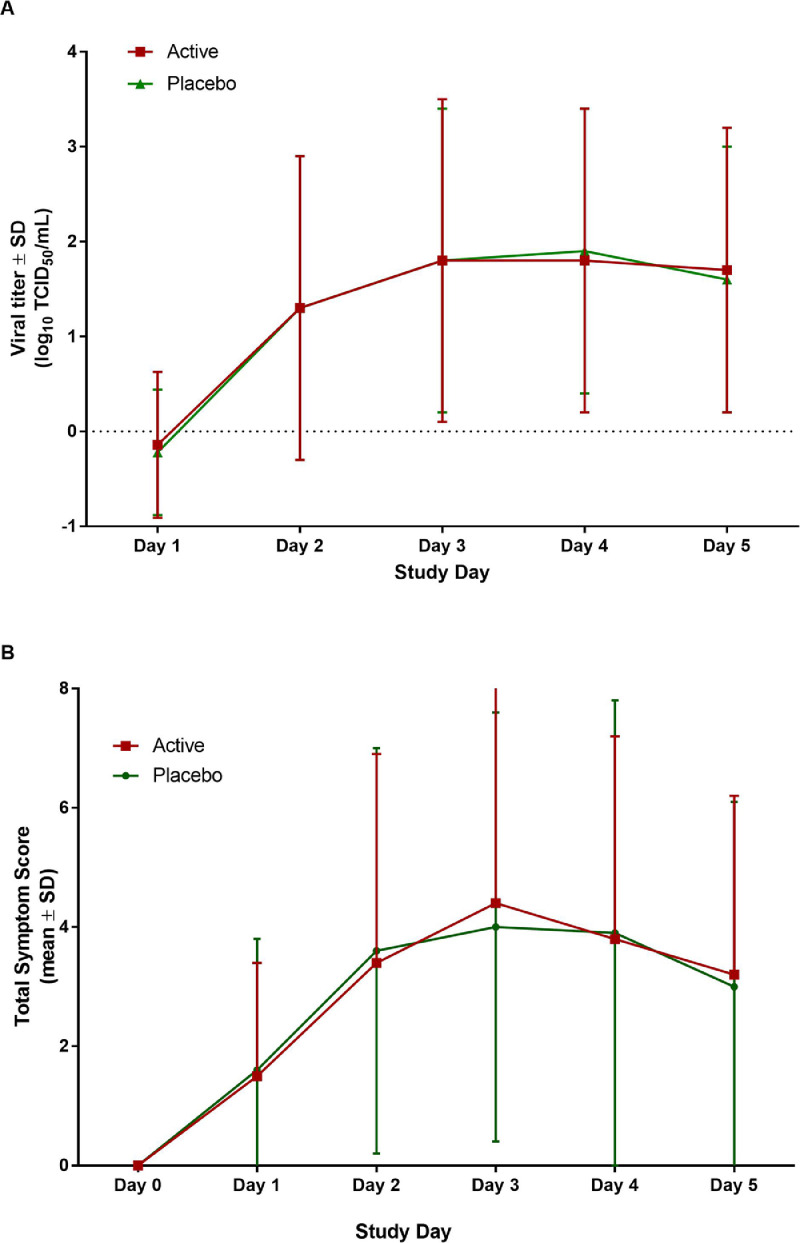
Effect of probiotic on RV infection and associated illness: There was no effect of administration of the probiotic Bl-04 on the occurrence of rhinovirus-associated illness. Sixty-three (56%, 95% CI [47%; 66%]) of the 112 subjects in the active group and 60 (50%,95% CI [41%; 59%]) of the 120 subjects in the placebo group had a protocol-defined rhinovirus-associated illness (χ2=0·91, p=0·34; Table 2). Ninety-six (86%, 95% CI [78%; 92%]) of 112 volunteers in the active group were infected with RV after challenge compared to 96 (80%, 95% CI [72%; 87%]) of 120 volunteers in the placebo group (χ2=1·33, p=0·25). There was no difference in the proportion of subjects who shed virus in nasal secretions (78%, 95% CI [69%; 85%] active, 69%, 95% CI [60%; 77%] placebo; Table 2) or seroconverted to the study virus (62%, 95% CI [52%; 71%] active, 60%, 95% CI [51%; 69%] placebo; data not shown). Quantitative titers of virus in nasal secretions on the five study days after challenge were similar in the two groups (Figure 2).
Table 2.
Results of Primary and Secondary Analyses
| Endpoint | Active | Placebo | Test statistic and p-value |
| Rhinovirus-associated illness# (%, 95% CI) | 63 (56, 47; 66) | 60 (50, 41; 59) | χ2=0•91, p=0•34 |
| Duration of illness* (days, Median) | 11•0 | 11•5 | Z value=0•05 p=0•934 |
| Number of subjects infected (%, 95% CI) | 96 (86, 78; 92) | 96 (80, 72; 87) | χ2=1•33 |
| Viral titer (log. (95% CI)) | 1.28 (1.15; 1.41) | 1.26 (1.13; 1.39) | F value= 0•28 |
| Number of subjects virus positive (%, 95% CI) | 87 (78, 69; 85) | 83 (69, 60; 77) | χ2=0.82 |
| Daily total symptom score, study days 1-5 (log. (95% CI)) | 1.12 (0.95; 1.28) | 1.12 (0.96; 1.29) | Z value= -0•069 |
| Daily total symptom score, study days 1-14 (log.) | 0.76 (0.60; 0.93) | 0.82 (0.63; 1.01) | Z value= -0•470 |
For endpoints analyzed with statistical tests, descriptive summaries are presented.
Primary endpoint
Duration of illness in subjects who met the protocol definition of a RV-associated illness
Figure 2.
Effect of probiotic administration on nasal lavage virus titer and total symptom score in rhinovirus-infected volunteers. The active group is shown in red the placebo group in green. A) Quantitative nasal wash viral titer in the active (n=112) and placebo (n=120) supplemented volunteers on study days 1 to 5 post-infection. B) Mean total symptom score by day in the active (n=112) and placebo (n=120) supplemented volunteers.
Evaluation of symptoms in infected volunteers revealed no effect of the administration of the probiotic. There was no difference in the mean daily total symptom scores in the two groups over the five days after virus challenge (Figure 2, estimated difference (log.) = -0·01, 95% CI [-0·24; 0·22], p=0·94, GEE Poisson regression analysis). For those subjects who developed illness, the median duration of illness was 11·0 (IQR=6·0) days in the active group and 11·5 (IQR=6·0) days in the placebo group (p=0·96, Wilcoxon Mann-Whitney test; Table 2).
Effect of probiotic on nasal cytokine/chemokine response to RVs: Cytokine/chemokine concentrations were compared for the subjects in the study groups who were infected (96 active and 96 placebo) by the RV challenge (Figure 3). IL-1β concentrations in the nasal mucosa showed statistically significant increases during probiotic ingestion from day -28 to day 0 (estimated difference (log.) 0·33, 95% CI [0·.12; 0·54], p=0·002, RM-ANCOVA) in the active group when compared with the placebo. There were no other statistically significant effects of probiotic supplementation either on baseline cytokine responses or on the cytokine response to infection (Figure 4).
Figure 3.
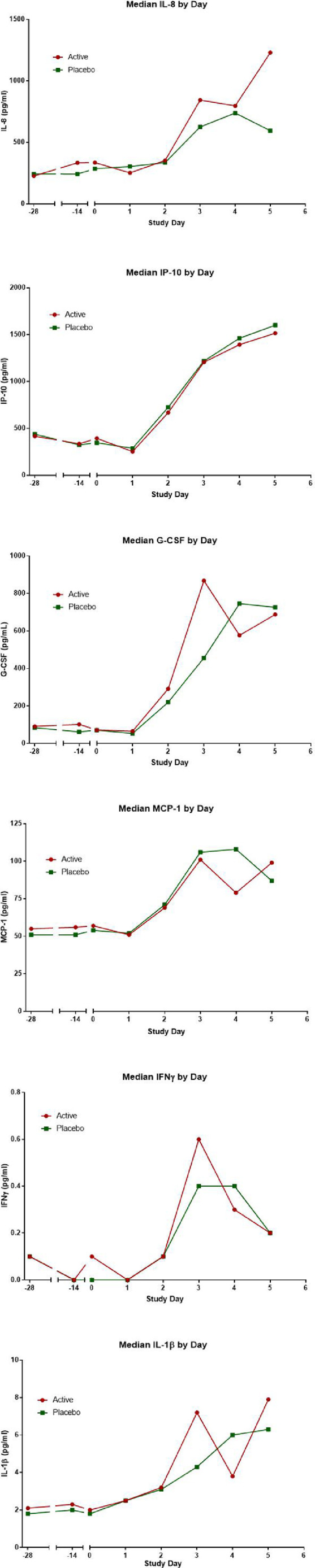
Cytokine and chemokine concentrations in nasal lavage fluid in relation to probiotic administration and rhinovirus infection. Probiotic or placebo was started on day -28 and virus challenge occurred on day 0. The active group is shown in red the placebo group in green.
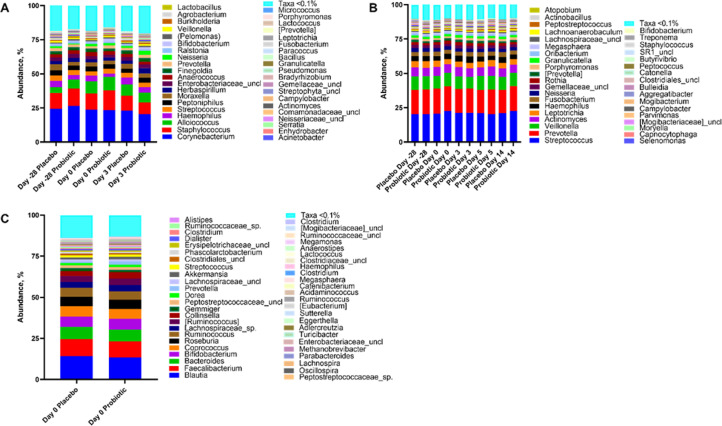
Figure 4.
Effect of probiotic administration and rhinovirus infection on the host microbiome. Genus-level taxa abundance in the nose (A), throat (B), and stool (C) in relation to study day and probiotic supplementation.
Effect of probiotic on gene expression following RV infection: Expression analysis by transcriptomics revealed a large cohort bias likely due to methodological variation among the study cohorts. Principal component analysis at day -28 showed each cohort clustering, with a PC1 of ∼50% and a PC2 of ∼20% (Supplemental Material, Figure 1). This variation was consistant through day 0 and was only changed after RV challenge resulting in clustering into two distinct groups (Supplemental Material, Figure 2). No genes were differentially expressed between the active and placebo groups on study days -28, 0, or 2 (data not shown).
As expected, viral effects were shown for the subjects on day 2 after infection with 298 genes shown to be upregulated and 303 down regulated when comparing samples that were infected with virus and those that were not infected (Supplemental material, Figure 2). When performing pathway analysis most genes classifying into pathways involved in immune response, cell signalling, cell repair, and inflammatory response (Supplemental Material, Table 3). No genes were differentially expressed in the samples from day -28 or day 0 when volunteers who became infected or ill were compared with those who remained uninfected or infected but not ill (data not shown).
Effect of probiotic on nasal, throat, and fecal microbiota and association with the response to infection: Supplementation with Bl-04 had little effect on the nasal, throat or fecal microbiota. In the nose, there was no difference between the study groups either in alpha-or beta-diversity (data not shown). The most abundant organisms were from the genera Corynebacterium, Staphylococcus, Alloiococcus, Haemophilus, Streptococcus, Peptoniphilus and Moraxella (Figure 5A) and the taxonomic composition was similar between the study groups over the course of the study. Four genus level nasal taxa were previously found relevant by Lehtinen et al [11]. (Corynebacterium, Staphylococcus, Alloiococcus and Moraxella) and the effect of day -28 and day 0 abundance levels of these taxa on clinical endpoints was further analyzed. We identified potential associations between at least one of these genera and a variety of study parameters (Supplemental Material, Table 6).
Evaluation of throat microbiota revealed alpha-diversity (observed ASVs) decreased in the probiotic group whereas in the placebo group the diversity increased between day -28 and day 0 (estimated difference (log.) = -0·06, 95% CI [-0·126; -0·003] p=0·039, ANCOVA; Supplemental material, Figure 10). Furthermore, there were potential associations between day 0 alpha-diversity and clinical endpoints unrelated to the study group. The most abundant organisms in the throat samples were from the genera Streptococcus, Prevotella, Veillonella, and Actinomyces. Their abundance, on average, remained generally similar throughout the course of the infection between the study groups (Figure 5B).
The fecal microbiota analysis revealed no detectable differences between the study groups in terms of alpha-and beta-diversity at day 0. The most abundant genera were Blautia, Faecalibacterium, and Coprococcus, which were similar between the study groups (Figure 5C). Higher Blautia abundance on day 0 was statistically significantly associated with a decrease in total symptom score (estimate (log.) for one unit increase in Blautia = -2·13 95% CI [-3·87; -0·38], p=0·022, GEE Poisson regression analysis), and higher Faecalibacterium abundance statistically significantly associated with a longer duration of illness (estimate (log.) for one unit change in Faecalibacterium = 1·48 95% CI [0·35; 2·62], p=0·010, Poisson regression analysis; Supplemental Material, Figure 11).
Adverse events: AEs were recorded for all subjects who were randomized to study product (165 active, 169 placebo). Three (2%) subjects in the active group and seven (4%) subjects in the placebo group reported AEs that were judged as definitely or probably related to the study interventions (χ2=1·55, p=0·21). There were no significant differences in the occurrence of AEs between the study groups (Table 3). The AES that were judged as definitely and probably related to the study interventions were related to study procedures or the virus infection. There were no AEs that were judged to be definitely or probably related to the administration of the study product.
Table 3.
Table of adverse events judged possibly, probably or definitely related to the study treatment or procedures
| ADVERSE EVENT | ACTIVE | PLACEBO |
| Total | 17 | 28 |
| Ear Discomfort | 2 | |
| Decreased Hearing | 1 | |
| Abdominal Distension | 2 | |
| Abdominal Pain | 1 | 2 |
| Constipation | 2 | |
| Diarrhea | 3 | 2 |
| Dyspepsia | 2 | |
| Flatulence | 2 | |
| Nausea | 6 | |
| Pain | 1 | 3 |
| Laryngitis | 1 | |
| Sinusitis | 1 | 1 |
| Decreased appetite | 1 | |
| Muscle spasm | 1 | 1 |
| Presyncope | 1 | |
| Dysphonia | 1 | |
| Epistaxis | 2 | 6 |
4. Discussion
This randomized, double-blind, placebo-controlled study using the RV challenge model found no effect of oral administration of Bl-04 on RV infection or illness. These results are similar to the outcome of a previous study using the challenge model.[10] However, in contrast to the results of our previous study, we also found no substantial evidence of an effect of probiotic administration on the innate inflammatory response in the nose or on virus shedding. Similarly, an exploratory analysis in our previous study found evidence of an interaction between the nasal microbiome and clinical, virologic and immunologic responses to the virus infection that were not fully replicated in the current study [11].
The challenge model has been used for decades for the study of pathogenesis and treatment of viral respiratory infection in general and RV infection in particular and has proven to be a sensitive tool for detecting the effect of various treatments and a reliable predictor of the results in subsequent field trials [17]. The challenge model is limited by the fact that the route of inoculation is artificial and only a single serotype of rhinovirus is tested. Although a previous field trial found a modest effect of Bl-04 on the incidence of viral respiratory illness, that study did not characterize the infection status of the ill subjects [18]. Previous experience with the challenge model suggests that it is unlikely that an effect would be found in a field trial directed at rhinovirus infections and illness.
Many clinical trials have explored the effect of probiotics on URTI. However, most clinical trials have been conducted in children and the low number of studies in adults have made systematic comparison challenging [2,19]. Although some of these trials have reported beneficial effects, the results have been inconsistent. Many of the studies have not included viral diagnostics and the reported effects, whether frequency of illness, duration of illness, or severity of illness vary from study-to-study. Furthermore, there is little information about probiotic strain-specific effects, optimal dose, and potential mechanisms of action. Reviews of these studies have concluded that the usefulness of probiotics in viral upper respiratory disease is uncertain and more studies are needed to clarify the effectiveness of probiotics in healthy adults [2,7]. Our study was designed to address some of the deficiencies in the earlier studies by using a well-defined and well-controlled model involving documented infection with single pathogen and an adequate sample size to detect a meaningful effect. The study also included extensive analysis by virologic, immunologic, transcriptomic, and microbiomic assays to assess the clinical and biologic effects of probiotic administration and interaction with viral infection.
One potential mechanism of action of the probiotics is alteration of the gastrointestinal or nasopharyngeal microbiota in a way that influences the innate immune response [20]. The ability to conduct longitudinal assessments of individual volunteers with collection of specimens in a fixed relation to the time of consumption of the probiotic and the initiation of the viral infection provides a powerful tool for the study of both microbiota and immunological responses to the probiotic. The administration of Bl-04 in the present study had no clinically relevant effect on cytokine responses or expression of innate immune or proinflammatory genes. Similarly, the oral administration of the probiotic did not produce a detectable change in either the fecal or the nasopharyngeal microbiota, supporting the idea that the microbiota of healthy subjects is relatively resistant to change by probiotic supplementation [21]. Future studies of probiotics that have purported clinical effects should include similar assessment of both immunological and microbiota parameters.
A number of studies, including an exploratory analysis of data from our previous challenge study, have suggested an association between the composition of the nasopharyngeal microbiota and the incidence or severity of viral respiratory infection [11,[22], [23], [24], [25], [26], [27], [28], [29], [30]]. Many of these studies have been done in young infants and have reported a positive correlation between the abundance of Haemophilus species and severity of bronchiolitis. Interestingly, the influence of the microbiota on disease severity appears to vary by viral pathogen [27]. There is little longitudinal information about the interaction of the nasopharyngeal microbiota and viral respiratory infection in adults. Allen, et al. [31], reported that bacterial detection from nasal lavage fluid was not statistically significantly influenced during a RV infection although Haemophilus species were less abundant in specimens from infected subjects. In our exploratory analysis and in the current study we also found that the composition of the nasal microbiota was not altered by the RV infection. In contrast to the findings of the current study, our previous study found evidence that different patterns of species dominance in the nasal cavity were associated with effects on viral replication, cytokine elaboration and symptom severity. The failure to detect these patterns in this study may be due to the fact that the species were differentiated at a more granular level (ASVs rather than OTUs) that would potentially obscure this finding. Additional work will be required to resolve this difference.
We found no effect of the probiotic supplementation on blood transcriptome in healthy subjects relative to supplementation or during the infection. In the statistical analyses, we found a statistically significant and unexplained cohort effect, however, within cohort results also showed no effect by the probiotic. These findings are consistent with a previous clinical study where Bl-04 or placebo supplementation to an active healthy adult cohort resulted in no difference in blood cytokine profiles, regulatory T cells or clinical chemistry [32], [33], [34], indicating that in healthy subjects with normal immune function and physiology probiotics have no measurable effect on peripheral immune markers. On the other hand, modest cytokine level changes were observed in the nasal washes of subject supplemented with Bl-04 in this and the previous challenge study [10], indicating that the effect of probiotics on mucosal immune function should be investigated further.
This large study in the RV challenge model provided an opportunity for a carefully-controlled assessment of Bl-04 effects on the fecal, throat and nasal microbiome, nasal cytokine profiles and blood transcriptomics. No clinically relevant effects were found. The study did find limited evidence of the effect of the baseline microbiomic profile on clinical, virologic and cytokine responses to the viral infection consistent with but not identical to our previous study [11]. These interactions will continue to be evaluated in future studies.
Funding
This work was supported by Danisco Sweeteners Oy, Kantvik, Finland (now IFF Health & Biosciences).
Data Sharing
Inquiries about access to the original clinical data should be directed to the Corresponding Author.
Author contributions
RBT, MJL, LL designed the study; RBT oversaw the conduct of the study; FRB, BZ designed and conducted the analysis of the transcriptomic studies; AH, BZ, designed and conducted the microbiota analyses; NY conducted the qPCR analysis: SM, TH designed and conducted the statistical analysis; RBT, MJL, LL, SM, TH had access to all data; RBT, MJL, LL, AH, BZ, SM wrote the manuscript and all authors reviewed and contributed to the final manuscript.
Declaration of Competing Interest
LL, AH, BZ, FB, NY, and MJL report other from IFF Health & Biosciences, outside the submitted work. LL, AH, and MJL report a patent pending based on the results. Dr. Turner reports grants from Danisco Sweeteners OY, during the conduct of the study; other from PrEP BioPharm, Inc, other from Henkel Corporation, other from G.ST Antivirals GmbH, other from American Cleaning Institute, other from Avrio, other from Bayer Consumer Health, outside the submitted work. All the other authors have nothing to disclose.
Acknowledgements
Jaana Larsson-Leskelä, Henri Ahokoski, Kirsi Stenström, and Minna Eskola are acknowledged from DuPont in assisting with the laboratory analyses. Cheree Denby-Taylor was the study coordinator for the human volunteer aspects of the study at the University of Virginia.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101224.
Appendix. Supplementary materials
References
- 1.Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 2.Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;2 doi: 10.1002/14651858.CD006895.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Guo Q, Goldenberg JZ, Humphrey C, El Dib R, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4 doi: 10.1002/14651858.CD004827.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70(11):1356–1371. doi: 10.1111/all.12700. [DOI] [PubMed] [Google Scholar]
- 5.Sniffen JC, McFarland LV, Evans CT, Goldstein EJC. Choosing an appropriate probiotic product for your patient: An evidence-based practical guide. PLoS One. 2018;13(12) doi: 10.1371/journal.pone.0209205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su GL, Ko CW, Bercik P, et al. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology. 2020;159(2):697–705. doi: 10.1053/j.gastro.2020.05.059. [DOI] [PubMed] [Google Scholar]
- 7.Lehtoranta L, Pitkaranta A, Korpela R. Probiotics in respiratory virus infections. Eur J Clin Microbiol Infect Dis. 2014;33(8):1289–1302. doi: 10.1007/s10096-014-2086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehtoranta L, Latvala S, Lehtinen MJ. Role of Probiotics in Stimulating the Immune System in Viral Respiratory Tract Infections: A Narrative Review. Nutrients. 2020;12(10) doi: 10.3390/nu12103163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahbazi R, Yasavoli-Sharahi H, Alsadi N, Ismail N, Matar C. Probiotics in Treatment of Viral Respiratory Infections and Neuroinflammatory Disorders. Molecules. 2020;25(21) doi: 10.3390/molecules25214891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner RB, Woodfolk JA, Borish L, et al. Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection - a randomised controlled trial. Benef Microbes. 2017;8(2):207–215. doi: 10.3920/BM2016.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehtinen MJ, Hibberd AA, Mannikko S, et al. Nasal microbiota clusters associate with inflammatory response, viral load, and symptom severity in experimental rhinovirus challenge. Sci Rep. 2018;8(1):11411. doi: 10.1038/s41598-018-29793-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner RB, Wecker MT, Pohl G, et al. Efficacy of tremacamra, a soluble intercellular adhesion molecule 1, for experimental rhinovirus infection. J Am Med Assoc. 1999;281:1797–1804. doi: 10.1001/jama.281.19.1797. [DOI] [PubMed] [Google Scholar]
- 13.Barrett B, Brown R, Voland R, Maberry R, Turner R. Relations among questionnaire and laboratory measures of rhinovirus infection. Eur Respir J. 2006;28(2):358–363. doi: 10.1183/09031936.06.00002606. [DOI] [PubMed] [Google Scholar]
- 14.Gwaltney JM, Jr., Moskalski PB, Hendley JO. Interruption of experimental rhinovirus transmission. J Infect Dis. 1980;142(6):811–815. doi: 10.1093/infdis/142.6.811. [DOI] [PubMed] [Google Scholar]
- 15.Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayden FG, Gwaltney JM., Jr. Intranasal interferon alpha 2 for prevention of rhinovirus infection and illness. J Infect Dis. 1983;148(3):543–550. doi: 10.1093/infdis/148.3.543. [DOI] [PubMed] [Google Scholar]
- 17.Lambkin-Williams R, Noulin N, Mann A, Catchpole A, Gilbert AS. The human viral challenge model: accelerating the evaluation of respiratory antivirals, vaccines and novel diagnostics. Respir Res. 2018;19(1):123. doi: 10.1186/s12931-018-0784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West NP, Horn PL, Pyne DB, et al. Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin Nutr. 2014;33(4):581–587. doi: 10.1016/j.clnu.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 19.King S, Glanville J, Sanders ME, Fitzgerald A, Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112(1):41–54. doi: 10.1017/S0007114514000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 21.Maukonen J, Saarela M. Human gut microbiota: does diet matter? Proc Nutr Soc. 2015;74(1):23–36. doi: 10.1017/S0029665114000688. [DOI] [PubMed] [Google Scholar]
- 22.Chonmaitree T, Jennings K, Golovko G, et al. Nasopharyngeal microbiota in infants and changes during viral upper respiratory tract infection and acute otitis media. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Steenhuijsen Piters WA, Heinonen S, Hasrat R, et al. Nasopharyngeal Microbiota, Host Transcriptome, and Disease Severity in Children with Respiratory Syncytial Virus Infection. American journal of respiratory and critical care medicine. 2016;194(9):1104–1115. doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ederveen THA, Ferwerda G, Ahout IM, et al. Haemophilus is overrepresented in the nasopharynx of infants hospitalized with RSV infection and associated with increased viral load and enhanced mucosal CXCL8 responses. Microbiome. 2018;6(1):10. doi: 10.1186/s40168-017-0395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa K, Linnemann RW, Mansbach JM, et al. The Fecal Microbiota Profile and Bronchiolitis in Infants. Pediatrics. 2016;138(1) doi: 10.1542/peds.2016-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasegawa K, Mansbach JM, Ajami NJ, et al. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur Respir J. 2016;48(5):1329–1339. doi: 10.1183/13993003.00152-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mansbach JM, Hasegawa K, Henke DM, et al. Respiratory syncytial virus and rhinovirus severe bronchiolitis are associated with distinct nasopharyngeal microbiota. J Allergy Clin Immunol. 2016;137(6) doi: 10.1016/j.jaci.2016.01.036. 1909-13 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mansbach JM, Hasegawa K, Piedra PA, et al. Haemophilus-Dominant Nasopharyngeal Microbiota Is Associated With Delayed Clearance of Respiratory Syncytial Virus in Infants Hospitalized for Bronchiolitis. J Infect Dis. 2019;219(11):1804–1808. doi: 10.1093/infdis/jiy741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosas-Salazar C, Shilts MH, Tovchigrechko A, et al. Differences in the Nasopharyngeal Microbiome During Acute Respiratory Tract Infection With Human Rhinovirus and Respiratory Syncytial Virus in Infancy. J Infect Dis. 2016;214(12):1924–1928. doi: 10.1093/infdis/jiw456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toivonen L, Camargo CA, Jr., Gern JE, et al. Association between rhinovirus species and nasopharyngeal microbiota in infants with severe bronchiolitis. J Allergy Clin Immunol. 2019;143(5) doi: 10.1016/j.jaci.2018.12.1004. 1925-8 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen EK, Koeppel AF, Hendley JO, Turner SD, Winther B, Sale MM. Characterization of the nasopharyngeal microbiota in health and during rhinovirus challenge. Microbiome. 2014;2:22. doi: 10.1186/2049-2618-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox AJ, West NP, Horn PL, et al. Effects of probiotic supplementation over 5 months on routine haematology and clinical chemistry measures in healthy active adults. European Journal of Clinical Nutrition. 2014;68(11):1255–1257. doi: 10.1038/ejcn.2014.137. [DOI] [PubMed] [Google Scholar]
- 33.West NP, Horn PL, Barrett S, et al. Supplementation wiht a single and double strain probiotic on the innate immune system for respiratory illness. e-SPEN. 2014;9 e178-e84. [Google Scholar]
- 34.West NP, Horn PL, Pyne DB, et al. Probiotic supplementation has little effect on peripheral blood regulatory T cells. J Allergy Clin Immunol. 2016;138(6) doi: 10.1016/j.jaci.2016.06.055. 1749-52 e7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.