Abstract
Purpose
To describe the clinical course of COVID-19 in patients with cystic fibrosis (CF) and to identify risk factors for severe COVID-19.
Methods
We conducted a prospective study within the Italian CF Society. CF centers collected baseline and follow-up data of patients with virologically confirmed SARS-CoV-2 infection between March 2020 and June 2021. Odds ratios (ORs) for severe SARS-CoV-2 (as defined by hospital admission) were estimated by logistic regression models.
Results
The study included 236 patients with positive molecular test for SARS-CoV-2. Six patients died, 43 patients were admitted to hospital, 4 admitted to intensive care unit. Pancreatic insufficiency was associated with increased risk of severe COVID-19 (OR 4.04, 95% CI 1.52; 10.8). After adjusting for age and pancreatic insufficiency, forced expiratory volume in one second (FEVp) < 40% (OR 4.54, 95% CI 1.56; 13.2), oxygen therapy (OR 12.3, 95% CI 2.91–51.7), underweight (OR 2.92, 95% CI 1.12; 7.57), organ transplantation (OR 7.31, 95% CI 2.59; 20.7), diabetes (OR 2.67, 95% CI 1.23; 5.80) and liver disease (OR 3.67, 95% CI 1.77; 7.59) were associated with increased risk of severe COVID-19, while use of dornase alfa was associated with a reduced risk (OR 0.34, 95% CI 0.13–0.88). No significant changes were observed in FEVp from baseline to a median follow-up of 2 months (median difference: 0, interquartile range: − 4; 5, P = 0.62).
Conclusion
Clinical features indicative of severe form of CF are associated with increased risk of COVID-19 hospitalization. SARS-CoV-2 infected patients do not experience a deterioration of respiratory function.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-021-01737-z.
Keywords: Cystic fibrosis, SARS-CoV-2, COVID-19, Pandemic
Introduction
Cystic fibrosis (CF) is a genetic recessive disease caused by mutations of the CF Transmembrane Conductance Regulator (CFTR) gene. CFTR mutations affect the production and/or function of the CFTR ion channel altering chloride transport in many organs, most frequently the pancreas and the lungs. The main clinical features of CF are in fact fat malabsorption, corrected by pancreatic enzyme replacement therapy, and recurrent respiratory infections, which cause structural and functional lung deterioration since the first years of life.
Patients with respiratory diseases are considered at high risk of developing severe COVID-19 [1], and this is true also for CF patients for whom higher incidence and hospitalization rates were documented as compared to the age-matched general population [2].
However, most of the so far available data were collected during the first wave of the pandemic, when diagnostic capacity was suboptimal, stringent physical distance measures were in place and vaccines against SARS-CoV-2 were not available. More recently, with the implementation of the vaccine campaign, distancing measures were relaxed, and new variants of SARS-CoV-2 started to circulate. Some of the new variants showed increased transmissibility and uncertain consequences on the protection given by previous infections and vaccines [3, 4].
This study aimed at evaluating the clinical course and risk factors for severe COVID-19 among patients with CF who were infected by SARS-CoV-2 from the beginning of the pandemic up to June 2021 in Italy.
Methods
We carried out a prospective multicentre cohort study within the Italian Cystic Fibrosis Society involving 20 out of 32 CF centers and covering about 80% of the Italian CF population. All centers were compliant with the standard of care for CF which encompasses the diagnosis and treatment of this multisystemic disease [5].
Centers were asked to collect baseline and follow-up data of patients who had undergone nasopharyngeal swab for symptoms suggestive of COVID-19 or for having been in contact with a positive/suspected case between March 2020 and June 2021. The following symptoms were considered indicative of COVID-19: fever, cough, asthenia, anosmia, dysgeusia, shortness of breath, sore throat, headache, myalgia and diarrhea [6]. Swab samples were tested with polymerase chain reaction (PCR) analysis for SARS-CoV-2 detection. Patients with a positive PCR test were reported to the coordinating center for inclusion in this study.
A specifically designed report form was used to collect demographic, clinical, anthropometric, microbiological and respiratory function data as well as symptoms, CF maintenance and COVID-19 treatments. The clinical course of the disease was evaluated in terms of vital status, admission in intensive care unit (ICU), need for oxygen supplementation and non-invasive and invasive ventilation. Changes in forced expiratory volume in one second (FEV1) were also evaluated from baseline (pre-SARS-CoV-2 infection) to the first follow-up visit after the end of symptoms or to the first visit after testing positive for SARS-CoV-2 for asymptomatic patients. Baseline FEV1 value was obtained for each patient by averaging the last three measurements. The Global Lung Function Initiative 2012 reference equations were used to obtain predicted values [7].
We evaluated a series of potential risk factors for severe COVID-19 (defined by hospital admission) collected at baseline visit, including sex, age, pancreatic insufficiency, severely impaired respiratory function (FEV1 as percent of predicted, FEVp < 40%), chronic P. aeruginosa infection, underweight, organ transplant, CF-related diabetes and liver disease, current medical treatment (oxygen therapy, CFTR modulators [8], inhaled steroids, dornase alfa and azithromycin).
Underweight was defined as BMI-for-age percentile < 5th for patients aged 2–19 and as BMI < 18.5 kg/m2 for older patients [9]. BMI percentiles were obtained using the Italian growth standards [10].
As a measure of association between each putative risk factor and severe COVID-19, we estimated the odds ratio (OR) of hospitalization for COVID-19 and corresponding 95% confidence intervals (CIs) through logistic regression models. Multivariable models were also fit to verify if each significant association found at the univariate analysis was independent from age and pancreatic insufficiency (considered as a proxy of severe genotype). Due to the limited number of patients requiring hospitalization, no further covariates were included in the models to avoid overfitting. The statistical significance of each risk factor was tested using the likelihood ratio test between nested models, namely the model with and without the risk factor.
Since CF is a progressive condition and usually adult patients have a more advanced lung disease than younger patients, we evaluated the risk factors for severe COVID-19 in this subgroup of patients by restricting the multiple variable regression analysis to patients aged ≥ 18 years.
FEVp data measured at baseline and at follow-up visit were compared using the Wilcoxon signed-rank test. Subgroup analyses were also performed according to baseline characteristics, between symptomatic and asymptomatic patients and between those who developed severe COVID-19 and those who did not. Comparisons between subgroups were made using the Wilcoxon sum-rank test. All tests were two-tailed with α = 0.05.
Results
Table 1 gives the demographic and clinical characteristics as well as the COVID-19 symptoms complained by the 236 patients reported by the Italian CF centers. Their median age was 25 years (Interquartile range, IQR 16–36, range 0–78), FEVp values (median 85, IQR 63–100) indicated on average a moderate impairment of lung function, 19 subjects were organ transplant recipients (18 lungs and one liver). The most common COVID-19 symptoms were: fever (reported in more than half of the patients), cough and asthenia (both reported in about one-third of the patients). Fifty-two (22%) patients were asymptomatic. The median duration of symptoms was 13 days, although 26 out of 178 (14.6%) symptomatic patients with available data on symptoms duration reported symptoms for more than 30 days and four patients had symptoms lasting 90 days or more. The characteristics of patients with symptom duration ≥ 90 days are shown in the Supplemental Table S1.
Table 1.
Characteristics and symptoms of patients with cystic fibrosis infected by SARS-CoV-2 between March 2020 and June 2021 in Italy
| No | % | |
|---|---|---|
| Total number of patients | 236 | 100 |
| Sex | ||
| Females | 130 | 55.1 |
| Males | 106 | 44.9 |
| Age in years | ||
| 0–19 | 81 | 34.3 |
| 20–39 | 110 | 46.6 |
| ≥ 40 | 45 | 19.1 |
| CFTR genotype | ||
| F508del homozygous | 53 | 22.5 |
| F508del heterozygous | 117 | 49.6 |
| Other mutations | 66 | 28.0 |
| Pancreatic insufficiency | 164 | 69.5 |
| P. aeruginosa infection | ||
| Chronic | 83 | 35.2 |
| Intermittent | 40 | 16.9 |
| First infection | 1 | 0.4 |
| B. cepacia complex infection | 5 | 2.1 |
| Diabetes | 51 | 21.6 |
| Liver disease | 65 | 27.5 |
| Chronic kidney disease | 4 | 1.7 |
| ABPA | 7 | 3.0 |
| Transplant recipients | 19 | 8.1 |
| CF maintenance treatment | ||
| Antibiotics | 104 | 44.1 |
| Azithromycin | 80 | 33.9 |
| Inhaled steroids | 98 | 41.5 |
| Oral steroids | 23 | 9.7 |
| Steroids (Administration route unknown) | 4 | 1.7 |
| Dornase alfa | 60 | 25.4 |
| Highly effective CFTR modulator therapya | 19 | 8.1 |
| Other CFTR modulator therapies | 35 | 14.8 |
| Oxygen therapy | 11 | 4.7 |
| Underweightb | 24 | 10.2 |
| FEV1 (% of predicted) < 40%c | 17 | 8.0 |
| COVID-19 symptoms | ||
| Fever | 131 | 55.5 |
| Headache | 54 | 22.9 |
| Dyspnea | 34 | 14.4 |
| Cough | 89 | 37.7 |
| Increased sputum | 23 | 9.7 |
| Chest pain | 8 | 3.4 |
| Rhinitis | 37 | 15.7 |
| Pharyngodynia | 37 | 15.7 |
| Anosmia | 15 | 6.4 |
| Dysgeusia | 21 | 8.9 |
| Nausea | 9 | 3.8 |
| Diarrhea | 29 | 12.3 |
| Asthenia | 83 | 35.2 |
| Myalgia | 14 | 5.9 |
| Joint pain | 50 | 21.2 |
| Asymptomatic | 52 | 22.0 |
Data are presented as numbers and percentages
ABPA allergic broncho-pulmonary aspergillosis, CFTR cystic fibrosis transmembrane conductance regulator, COVID-19 coronavirus disease 2019, IQR interquartile range, FEV1 forced expiratory volume in one second
aIvacaftor alone (4 patients) or in combination with tezacaftor and elexacaftor (15 patients) were considered highly effective CFTR modulator therapies
bUnderweight was defined as BMI-for-age percentile < 5th for patients aged 2–19 years and BMI < 18.5 kg/m2 for older patients. Percentage of underweight was computed on patients aged ≥ 2 years (N = 230) and excluding one patient with missing anthropometric values
cData not available for 24 patients, of whom 16 were too young (i.e., aged < 6 years) to perform a spirometry test
Figure 1 shows the number of patients infected by SARS-CoV-2 by month. Most cases (N = 217, 91.9%) were registered during the second and the third wave of the pandemic in Italy (September 2020–June 2021), with a peak during the months of October and November 2020 when 40 (16.9%) and 55 (23.3%) cases were observed, respectively. With the start of the vaccination campaign for all patients aged ≥ 16 years in mid-March 2021, the number of reported cases decreased progressively and only one case was reported in June 2021.
Fig. 1.
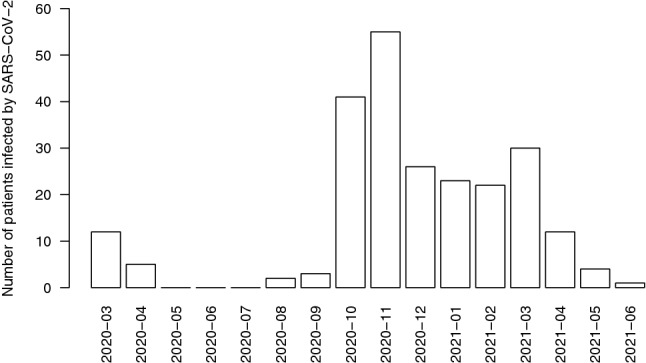
Number of patients reported by the Italian cystic fibrosis centers between March 2020 and June 2021 with documented SARS-CoV-2 infection. The x-axis indicates the month of the positive test for SARS-CoV-2
Table 2 shows the treatment received and the outcome of the disease. More than 70% of patients received azithromycin or other antibiotics, around 1/3 were treated with steroids, a minority received antiviral (7.6%) or anti-thrombotic (8.9%) drugs and only four patients were treated with monoclonal antibodies. Forty-three patients (10 children and 33 adults) (18.2%) required hospital admission, 4 patients (one child and 3 adults) (1.7%) were admitted to ICU and six patients (2.5%) died at the age of 14, 15, 33, 39, 48 and 78. All deceased patients were pancreatic insufficient with chronic P. aeruginosa infection; two patients were lung transplanted, underweight in oxygen therapy and with baseline FEVp < 40%. COVID-19 was a contributing cause of death in four patients, while death was not related to COVID-19 for the other two patients: one patient who fully recovered from COVID-19 died after 2 months due to a respiratory exacerbation while waiting for lung transplant; the other patient who had been included in the transplant list before SARS-CoV-2 infection, died two weeks after receiving lung-liver-pancreas transplantation due to complications. All the other patients (N = 230, 97.5%) fully recovered without important sequelae.
Table 2.
Treatment and clinical course of COVID-19 in patients with cystic fibrosis infected by SARS-CoV-2 between March 2020 and June 2021 in Italy
| No | % | |
|---|---|---|
| COVID-19 treatment | ||
| Antiviral drugs | 18 | 7.6 |
| Hydroxychloroquine | 7 | 3.0 |
| Azithromycin | 67 | 28.4 |
| Other antibiotics | 99 | 41.9 |
| Steroids | 73 | 30.9 |
| Monoclonal antibodies | 4 | 1.7 |
| Anti-thrombotic drugs | 21 | 8.9 |
| Clinical course | ||
| Hospitalization | 43 | 18.2 |
| Intensive care unit | 4 | 1.7 |
| Additional oxygen therapy | 17 | 7.2 |
| Intensive ventilation | 2 | 0.8 |
| CPAP | 11 | 4.7 |
| ECMO | 1 | 0.4 |
| Change in FEVp at follow-up, median IQRa | 0 (− 4; 5) | |
| Clinically recovered | 230 | 97.5 |
| Died | 6 | 2.5 |
Data are presented as numbers and percentages, unless otherwise specified
CPAP continuous positive airway pressure therapy, ECMO extracorporeal membrane oxygenation, IQR Interquartile Range, FEVp forced expiratory volume in one second expressed as percentage of the predicted value
aData were not available for 43 patients: for 26 patients spirometry data at follow-up were not collected, 13 patients were too young (i.e., aged < 6 years) to perform a spirometry, 4 patients died with COVID-19 as cause of death
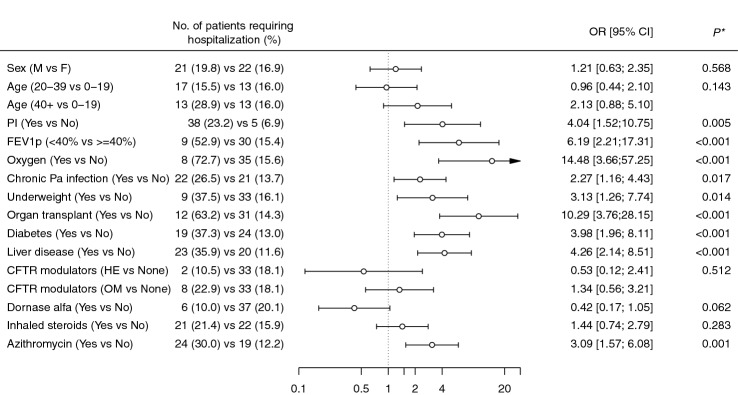
Figure 2 shows the OR for severe COVID-19 according to some selected demographic and clinical variables. Sex and age were not significantly associated with severe COVID-19, although the OR for age ≥ 40 years (2.13, 95% CI 0.88–5.10) indicated an increased risk as compared to younger patients (10–19 years). All the other clinical variables were associated with an increased risk of severe COVID-19, with organ transplant and oxygen therapy being the risk factors showing the highest ORs. No significant associations were found for CFTR modulator use and inhaled steroids. The OR indicated a reduced risk among patients receiving dornase alfa (although not significant, P = 0.062) and an increased risk among those receiving azithromycin.
Fig. 2.
Odds ratios (ORs) and corresponding 95% confidence intervals for severe COVID-19 in patients with cystic fibrosis infected by SARS-CoV-2 between March 2020 and June 2021 in Italy, according to selected demographic and clinical characteristics. HE highly effective CFTR modulators; OM moderately effective. *P values were obtained using the likelihood ratio test between two nested models (i.e., the null model and the model including the variable of interest)
In the multivariable analysis, adjusted for age and pancreatic insufficiency, most associations remained significant, with the only exception of chronic P. aeruginosa infection (Table 3). The adjusted OR indicated a significant reduced risk of severe COVID for patients receiving dornase alfa. When we restricted the analysis to adult patients, most of the associations found in the main analysis resulted attenuated, and were no longer significant for diabetes and underweight (Supplemental table S2).
Table 3.
Odds ratios (ORs) for severe COVID-19 obtained from logistic regression models adjusted for age and pancreatic insufficiency in patients with cystic fibrosis infected by SARS-CoV-2 between March 2020 and June 2021 in Italy, according to selected clinical characteristics
| OR [95% CI] | P* | |
|---|---|---|
| FEV1p (< 40% vs ≥ 40%) | 4.54 [1.56–13.19] | 0.005 |
| Oxygen (Yes vs No) | 12.27 [2.91–51.68] | < 0.001 |
| Chronic Pa infection (Yes vs No) | 1.77 [0.84–3.73] | 0.135 |
| Underweight (Yes vs No) | 2.92 [1.12–7.57] | 0.028 |
| Organ transplant (Yes vs No) | 7.31 [2.59–20.65] | < 0.001 |
| Diabetes (Yes vs No) | 2.67 [1.23–5.80] | 0.013 |
| Liver disease (Yes vs No) | 3.67 [1.77–7.59] | < 0.001 |
| CFTR modulators (HE vs None) | 0.42 [0.09–2.02] | 0.501 |
| CFTR modulators (OM vs None) | 0.97 [0.39–2.41] | |
| Inhaled steroids (Yes vs No) | 1.20 [0.59–2.43] | 0.621 |
| Dornase alfa (Yes vs No) | 0.34 [0.13–0.88] | 0.026 |
| Azithromycin (Yes vs No) | 2.58 [1.27–5.25] | 0.009 |
HE highly effective modulators, OM Other CFTR modulators
*P values were obtained using the likelihood ratio test between two nested models (i.e., the null model and the model including the variable of interest)
FEVp data collected after a median number of 61 days (IQR 32–96 days) from the end of COVID-19-related symptoms or from the day asymptomatic patients tested positive for SARS-CoV-2 indicated no significant differences from baseline measures. Median FEVp at baseline was 85% (IQR63; 100) and 83% (IQR 62; 100) at recovery and the median difference between the two measurement was: 0 (IQR − 4; 5), P = 0.619.
Seventeen out of 186 patients (9.1%) with available data at baseline and at follow-up had a FEVp reduction > 10%. Their baseline characteristics are shown in the Supplemental table S3. Median age was 25 years (range 6–50 years), most were pancreatic insufficient (13, 76.5%), one was lung transplanted, none had severely impaired lung function or was in oxygen therapy at baseline, none of them died.
Figure 3 shows the subgroup analysis of changes in FEVp after COVID-19. Median changes were all close to zero and no significant differences were detected between all the groups considered, with the only exception of a slight increase in FEVp among patients with advanced lung disease at baseline.
Fig. 3.
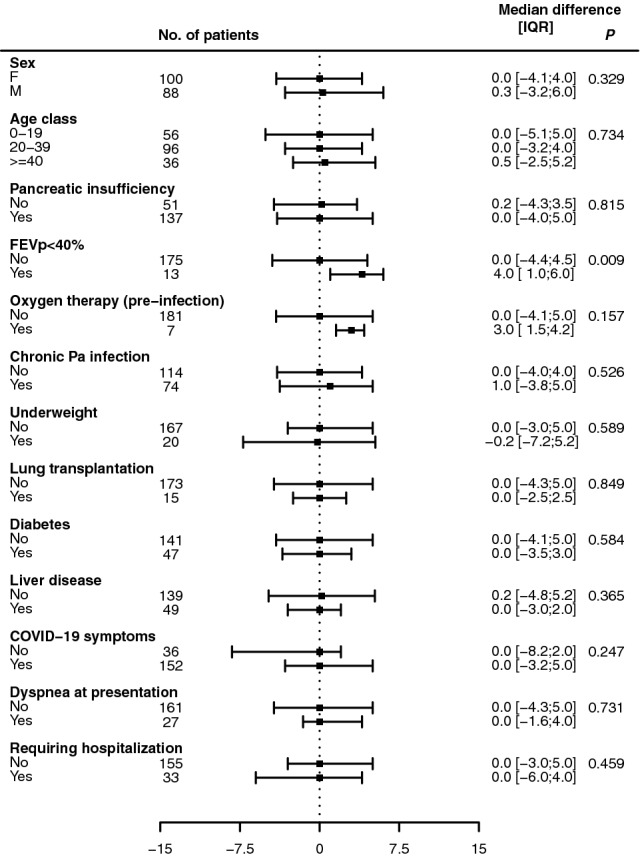
Changes in forced expiratory volume in one second (expressed as percentage of predicted) from baseline to follow-up visit (first visit after the end of symptoms or after testing positive for SARS-CoV-2 for asymptomatic patients) in patients with cystic fibrosis infected by SARS-CoV-2 between March 2020 and June 2021 in Italy, according to baseline characteristics and clinical course of the disease. Symbols indicate the median value, bars show the 25th and the 75th percentile. The Wilcoxon sum-rank test was used to compare FEVp changes between groups. Pa Pseudomonas aeruginosa.
Discussion
Our study indicates that about 20% of the patients with CF infected by SARS-CoV-2 in Italy developed severe COVID-19. Organ transplantation was identified as a major risk factor of severe COVID-19, and some clinical characteristics of CF, including pancreatic insufficiency, severe impairment of respiratory function, chronic infection by P. aeruginosa, underweight, and presence of the two major comorbidities of CF, namely CF-related diabetes and liver disease were also associated to an increased risk.
Use of dornase alfa was found to be associated with reduced risk of severe COVID-19. Our study also indicates that most patients fully recovered, although around 10% of them had a significant reduction in FEVp after recovery.
These findings agree with what was documented during the first wave of the pandemic in multinational reports based on Registry data of patients with CF infected by SARS-CoV-2 showing higher hospitalization rates among patients with severe lung function impairment and among transplant recipients [2, 11].
Increasing concern has been raised on the severity of SARS-CoV-2 infection in non-CF organ transplant recipients due to chronic immunosuppressive treatment [12, 13]. In a case–control study [14] on non-CF lung transplant recipient patients (median age 60 years) based on 24 cases with COVID-19 and 48 controls free of SARS-CoV-2 infection, COVID-19 was associated with increased risk of secondary bacterial infection, need of bronchoscopy and readmission for respiratory exacerbation. Ninety-day mortality was not significantly different between cases and controls but the comparison was based on only 2 deaths among cases and one death among controls. In a French study [15] including 35 lung transplant recipients infected by SARS-CoV-2 (median age 50 years, including 11 patients with CF), most patients (~ 90%) required hospitalization, 13 were admitted to ICU, and among them seven received intensive mechanical ventilation. Over a follow-up of 50 days, five patients died. In our cohort of patients with CF, probably due to the younger age, SARS-CoV-2 infection was less severe with 12 out of 19 transplant recipients (63%) requiring hospitalization; only one patient was admitted to ICU, none received intensive ventilation, however 2 patients died.
We also found that the clinical features of classic CF, including pancreatic insufficiency, poor respiratory function and inadequate nutritional status were associated with increasing risk of hospitalization. Patients with pancreatic insufficiency have a more severe form of CF and may have suboptimal nutritional status and worse respiratory function, both conditions being important prognostic factors in CF [16, 17]. Thus, our data suggest that these patients are also more vulnerable to SARS-CoV-2 infection and require special attention.
Similar to what has been found in the general population, the presence of comorbidities, such as diabetes and liver disease, increases the risk of severe COVID-19 also in the CF population [18, 19].
As previously reported for the first pandemic wave [20], CF patients infected by SARS-CoV-2 presented unspecific symptoms, which could have been easily mistaken for a typical pulmonary exacerbation. However, for a non-negligible share of the symptomatic population (∼15%), they lasted more than one month. In addition, during the first wave of the pandemic, most COVID-19 cases were reported in Northern Italy [20], while during the subsequent waves, all the Italian centers involved in this study documented COVID-19 cases.
With regard to CF maintenance therapy, we did not find a significant association between CFTR modulator therapy and reduced risk of severe COVID-19. CFTR modulators are oral drugs which improve CFTR function (potentiators) or facilitate its trafficking toward the cell membrane (correctors). Some of them proved to be highly effective in improving the clinical manifestations of the disease, while others showed only slight/moderate effects [21]. The potential role of CFTR modulators in preventing hospitalization in CF patients with COVID-19 was also evaluated in a study based on a research network of Health Care Organizations in the USA, which found a reduced risk, although not statistically significant (OR 0.57, 95% CI 0.30–1.08), among 68 patients with CF treated with CFTR modulators as compared with 353 untreated patients [22].
Of interest, patients receiving dornase alfa showed a reduced risk of severe COVID-19. Dornase alfa is a recombinant human deoxyribonuclease (DNase), which cleaves extracellular DNA released by the breakdown of neutrophils in the infected lungs of CF patients, thus reducing the viscosity of the respiratory secretions and improving ciliary clearance [23]. Interestingly, neutrophil extracellular trap formation was also identified as one of the pathogenetic mechanisms of COVID-19 [24].
Azithromycin, a commonly used antibiotic in CF, was initially proposed as a potential treatment for COVID-19 due to its immunomodulatory properties and mild antiviral activity, but its efficacy has not been proven [25]. We found that azithromycin treatment was associated with an increased risk of severe COVID-19. However, this association may reflect the severity of lung disease (P. aeruginosa and other Gram-negative bacterial infections and recurrent pulmonary exacerbations) among patients receiving azithromycin rather than a real effect of the treatment.
Monoclonal antibodies have been shown to reduce SARS-CoV-2 viral load and to prevent severe COVID-19 [26]; however, in the present study, we could not evaluate their effect on the course of the infection in this high-risk population. Indeed, at the time of the study, this therapeutic strategy was not available in many of the participating centers and it was administered only to four of our patients (none of whom died and two were hospitalized).
To our knowledge, this is the first study showing that respiratory function of patients with CF, as measured by FEVp, does not deteriorate after SARS-CoV-2 infection. We did not observe a significant reduction in FEVp even among the more vulnerable patients, such as transplant recipients, those with poor respiratory function and malnutrition as well as among those who experienced severe COVID-19.
Sequelae of COVID-19 on respiratory function in non-CF patients have not been clearly described and mainly involve residuals symptoms up to 6 months from hospital discharge [27, 28]. The clinical significance of this condition, which was defined as long COVID, is not fully understood; however, advanced age, severe symptoms in the acute phase, pneumonia, acute respiratory distress syndrome and need of mechanical ventilation were identified as main risk factors [29–31]. In addition, post-infection data on non-CF patients showed altered respiratory function [27, 28], with impaired carbon monoxide diffusion capacity of the lungs (DLCO) in ~ 40% of the patients, restrictive patterns in ~ 15% and broncho-obstructive syndrome in ~ 7% [32]. However, these abnormalities could not be directly related to COVID-19 since it is not possible to exclude a pre-existing respiratory involvement, as pre-infection respiratory assessment tests were not available. According to the British Thoracic Society (BTS), the evaluation of pulmonary function test must be performed at three months post-discharge, especially in patients suspected of having pulmonary sequelae [33]. In those studies, most respiratory assessment tests were performed one month after the onset of COVID-19 or one month after discharge [31, 34, 35], probably too early to discriminate how much of these changes reflect long-term respiratory complications and how much are due to the inflammation resulting from the acute event.
The detailed information collected on baseline characteristics, treatment and outcomes and the completeness of the information are certainly among the main strengths of the study. They provide new insights in the clinical consequences of SARS-CoV-2 infection on CF patients as well as on risk factors for severe COVID-19.
Our study has some limitations. First, the follow-up was relatively short and only the short-term clinical consequences of SARS-CoV-2 infection could be described. Second, we measured only FEVp to evaluate the effects of SARS-CoV-2 infection on respiratory function; other more sensitive measures might provide different results, especially in younger patients who usually show FEVp values within the normal range [36]. Third, risk factors for severe COVID-19 were evaluated mostly in a population of cases presenting with symptomatic infection. Since at the time of the study large-scale antibody testing among the CF population was not in place, the study could not include all infected patients with asymptomatic infection. Fourth, we could not evaluate the different impact of SARS-CoV-2 variants as virus genome was not sequenced. However, the alfa variant (B.1.1.7) was the main variant circulating in Italy during the study period, while new variants started to circulate only in May 2021 [37]. Finally, it should be noted that since the beginning of the pandemic patients with CF have been considered a vulnerable population and were more likely to be hospitalized even if they had a less severe form of COVID-19.
In conclusion, clinical features indicative of severe form of CF are associated with increased risk of hospital admission due to COVID-19. However, most patients infected by SARS-CoV-2 do not experience a relevant deterioration of respiratory function. The reduced risk of severe COVID-19 among patients receiving dornase alfa warrants further investigation.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization: CC, GA; methodology: CC, GA; formal analysis GA; writing—original draft preparation: CC, GA, VD; writing—review and editing: all authors. All authors read and approved the final manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of the Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico and by all the Ethics Committees of the participating centers.
Informed consent
Written informed consent was obtained from each patient or their legal representative.
References
- 1.Beltramo G, Cottenet J, Mariet A-S, Georges M, Piroth L, Tubert-Bitter P, et al. Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: a nationwide study. Eur Respir J. 2021 doi: 10.1183/13993003.04474-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naehrlich L, Orenti A, Dunlevy F, Kasmi I, Harutyunyan S, Pfleger A, et al. Incidence of SARS-CoV-2 in people with cystic fibrosis in Europe between February and June 2020. J Cyst Fibros. 2021 doi: 10.1016/j.jcf.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stowe J, Andrews N, Gower C, Gallagher E, Utsi L, Simmons R, et al. Effectiveness of COVID-19 vaccines against hospital admission with the Delta (B.1.617.2) variant. Public Heal Engl 2021;37. Available at: https://khub.net/web/phe-national/public-library.
- 4.Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, Konings F, Batra N, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance. 2021 doi: 10.2807/1560-7917.es.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellani C, Duff AJA, Bell SC, Heijerman HGM, Munck A, Ratjen F, et al. ECFS best practice guidelines: the 2018 revision. J Cyst Fibros. 2018;17:153–178. doi: 10.1016/j.jcf.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard n.d. https://covid19.who.int. Accessed May 20, 2021.
- 7.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramos KJ, Pilewski JM, Taylor-Cousar JL. Challenges in the use of highly effective modulator treatment for cystic fibrosis. J Cyst Fibros. 2021;20:381–387. doi: 10.1016/j.jcf.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Use and Interpretation of the WHO and CDC Growth Charts for Children from Birth to 20 Years in the United States. https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/growthchart.pdf. Accessed 9 Aug 2021
- 10.Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr) J Endocrinol Invest. 2006;29:581–593. doi: 10.1007/BF03344156. [DOI] [PubMed] [Google Scholar]
- 11.McClenaghan E, Cosgriff R, Brownlee K, Ahern S, Burgel PR, Byrnes CA, et al. The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J Cyst Fibros. 2020 doi: 10.1016/j.jcf.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E. COVID-19 and solid organ transplantation: a review article. Transplantation. 2021 doi: 10.1097/TP.0000000000003523. [DOI] [PubMed] [Google Scholar]
- 13.Mathew HR, Choi MY, Parkins MD, Fritzler MJ. Systematic review: cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic. BMC Pulm Med. 2021 doi: 10.1186/s12890-021-01528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Permpalung N, Bazemore K, Chiang T, Mathew J, Barker L, Nematollahi S, et al. Impact of COVID-19 on lung allograft and clinical outcomes in lung transplant recipients. Transplantation. 2021 doi: 10.1097/TP.0000000000003839. [DOI] [PubMed] [Google Scholar]
- 15.Messika J, Eloy P, Roux A, Hirschi S, Nieves A, Le Pavec J, et al. COVID-19 in lung transplant recipients. Transplantation. 2021 doi: 10.1097/TP.0000000000003508. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson AL, Tom M, Berthiaume Y, Singer LG, Aaron SD, Whitmore GA, et al. A contemporary survival analysis of individuals with cystic fibrosis: a cohort study. Eur Respir J. 2015;45:670–679. doi: 10.1183/09031936.00119714. [DOI] [PubMed] [Google Scholar]
- 17.Breuer O, Caudri D, Stick S, Turkovic L. Predicting disease progression in cystic fibrosis. Expert Rev Respir Med. 2018;12:905–917. doi: 10.1080/17476348.2018.1519400. [DOI] [PubMed] [Google Scholar]
- 18.Pugliese G, Vitale M, Resi V, Orsi E. Is diabetes mellitus a risk factor for COronaVIrus Disease 19 (COVID-19)? Acta Diabetol. 2020;57:1275–1285. doi: 10.1007/s00592-020-01586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Istituto Superiore di Sanità. Characteristics of SARS-CoV-2 patients dying in Italy Report. Istitvto Svperiore Di Sanita 2020:4–8.
- 20.Colombo C, Alicandro G, Dacco V, Gagliano V, Morlacchi LC, Casciaro R, et al. SARS-CoV-2 infection in cystic fibrosis: a multicentre prospective study with a control group, Italy, February-July 2020. PLoS ONE. 2021 doi: 10.1371/journal.pone.0251527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dave K, Dobra R, Scott S, Saunders C, Matthews J, Simmonds NJ, et al. Entering the era of highly effective modulator therapies. Pediatr Pulmonol. 2021;56:S79–89. doi: 10.1002/ppul.24968. [DOI] [PubMed] [Google Scholar]
- 22.Hadi Y, Lakhani D, Naqvi S, Fatima N, Sarwari A. Outcomes of SARS-CoV-2 infection in patients with cystic fibrosis: a multicenter retrospective research network study. Respir Med. 2021 doi: 10.1016/j.rmed.2021.106606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konstan MW, Ratjen F. Effect of dornase alfa on inflammation and lung function: potential role in the early treatment of cystic fibrosis. J Cyst Fibros. 2012;11:78–83. doi: 10.1016/j.jcf.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler CC, Dorward J, Yu LM, Gbinigie O, Hayward G, Saville BR, et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397:1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. J Am Med Assoc. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munker D, Veit T, Barton J, Mertsch P, Mümmler C, Osterman A, et al. Pulmonary function impairment of asymptomatic and persistently symptomatic patients 4 months after COVID-19 according to disease severity. Infection. 2021 doi: 10.1007/s15010-021-01669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortini A, Torrigiani A, Sbaragli S, Lo Forte A, Crociani A, Cecchini P, et al. COVID-19: persistence of symptoms and lung alterations after 3–6 months from hospital discharge. Infection. 2021 doi: 10.1007/s15010-021-01638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tosato M, Carfì A, Martis I, Pais C, Ciciarello F, Rota E, et al. Prevalence and predictors of persistence of COVID-19 symptoms in older adults: a single-center study. J Am Med Dir Assoc. 2021 doi: 10.1016/j.jamda.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anastasio F, Barbuto S, Scarnecchia E, Cosma P, Fugagnoli A, Rossi G, et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021 doi: 10.1183/13993003.04015-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y, Tan C, Wu J, Chen M, Wang Z, Luo L, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020 doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres-Castro R, Vasconcello-Castillo L, Alsina-Restoy X, Solis-Navarro L, Burgos F, Puppo H, et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2020 doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.British Thoracic Society. British Thoracic Society Guidance on Respiratory Follow Up of Patients with a Clinico-Radiological Diagnosis of COVID-19 Pneumonia. 2020. https://www.brit-thoracic.org.uk/document-library/quality-improvement/covid-19/resp-follow-up-guidance-post-covid-pneumonia. Accessed 9 Aug 2020.
- 34.Frija-Masson J, Debray MP, Gilbert M, Lescure FX, Travert F, Borie R, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020 doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You J, Zhang L, Zhang J, Hu F, Chen L, et al. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J Infect. 2020 doi: 10.1016/j.jinf.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mondéjar-López P, Horsley A, Ratjen F, Bertolo S, de la Vicente H, de Cruz AÒ. A multimodal approach to detect and monitor early lung disease in cystic fibrosis. Expert Rev Respir Med. 2021;15:761–772. doi: 10.1080/17476348.2021.1908131. [DOI] [PubMed] [Google Scholar]
- 37.Istituto Superiore di Sanità. Speciale COVID-19 Varianti del virus 2021. https://www.iss.it/cov19-cosa-fa-iss-varianti. Accessed December 10, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.