Abstract
Polyvinyl alcohol (PVA) containing the fixative mercuric chloride is considered the “gold standard” for the fixation of ova and parasites in the preparation of permanently stained smears of stool specimens. However, mercuric chloride is potentially hazardous to laboratory personnel and presents disposal problems. We compared three new alternative, nontoxic fixatives with PVA, analyzing ease of sample preparation and quality of smears. Sixty-eight fresh stool specimens were divided into aliquots and placed in each of four different fixatives: PARASAFE (PS) (Scientific Devices Laboratory, Inc., Des Plaines, Ill.), ECOFIX (EC) (Meridian Diagnostics, Inc., Cincinnati, Ohio), Proto-Fix (PF) (Alpha-Tec Systems, Inc., Vancouver, Wash.), and low-viscosity PVA fixative (PVA) (Meridian). Specimens were processed and stained according to each manufacturer's directions. Parasites were found in 31 of 68 slide preparations with PVA, 31 with PF, 30 with EC, and 30 with PS. Blastocystis hominis and Iodamoeba bütschlii were preserved in a readily identifiable state by all methods of fixation. However, some parasites were more easily identified with some of the fixatives because of differences in parasite distortion. For example, Entamoeba histolytica (Entamoeba dispar) was detected in 13 stools fixed with PF, 7 with PVA, and 6 with EC but none with PS. Likewise, Chilomastix mesnili was identified in 13 specimens fixed with PF, 8 with EC, and 5 with PVA but only 1 with PS, while Entamoeba coli was seen much less frequently with PS than with the other three fixatives. A dirty background was observed in 41% of specimens prepared with PS, whereas background quality was acceptable with other fixatives. Sample preparation was most rapid with PS, although the EC method involved the fewest steps. In conclusion, PVA and PF produced the least parasite distortion, while PS proved unsatisfactory for the identification of E. histolytica, E. coli, and C. mesnili. Both PF and EC appear to be acceptable, environmentally safe substitutes for PVA.
A permanently stained smear preparation is routinely made whenever an ovum and parasite examination is to be performed on a stool specimen (1, 2), since it allows the detection not only of protozoan cysts but also of trophozoites, which may be destroyed or lost during concentration procedures used for wet preparations, such as formalin-ethyl acetate concentration. The preparation of permanently stained slides for routine parasite examination is required by the College of American Pathologists in order for laboratories to be accredited for service in parasitology (1, 4). Fixation and staining of stool specimens should provide the technologist with a smear that renders the internal structures of the parasites clearly defined so as to permit their identification in a timely manner.
Certain chemicals used in the clinical laboratory are now recognized to present dangers to humans and the environment. To avoid these hazards without jeopardizing the quality of diagnostic testing, alternative methods which do not use these chemicals must be developed and implemented. In the United States, proficiency-testing samples and patient specimens are generally preserved with polyvinyl alcohol (PVA) fixative, which is recognized as the “gold standard” by many laboratories. The PVA component of this fixative serves as an adhesive, which glues the stool material to the slide, whereas the fixative properties are due to Schaudinn's fluid, which contains a saturated aqueous solution of mercuric chloride. However, mercury produces highly toxic vapors upon exposure to heat, and these vapors can be absorbed by the skin and mucous membranes, causing chronic mercury poisoning. Procedures for the disposal of waste containing mercury must comply with all local, state, and federal regulations, and the additional expense of contracting with an approved and licensed disposal agency for the removal of such material is often necessary. Modified PVA fixatives which substitute copper or zinc for mercury are now available, but the quality of parasite morphology achieved with these fixatives is generally not as good as that obtained with PVA fixative containing mercury. To circumvent these problems, several manufacturers recently have developed alternative fixatives which do not contain mercury and are potentially less hazardous, more environmentally safe, and not subject to governmental restrictions. However, to date, there have been few independent comparisons of the effectiveness of these new fixatives with that of PVA.
The objective of the present study was to compare the performance of three new environmentally safe fixatives with that of the current gold standard, PVA fixative. Permanently stained smears of stool, preserved in each of these four fixatives and stained according to the respective manufacturer's instructions, were examined for quality of background, clarity of parasite morphology, and number and species of parasites identified. In addition, the new fixatives and matched staining methods were compared with PVA-preserved specimens stained with trichrome (Wheatley's modification) for ease and time of preparation as well as cost.
(Portions of this work were presented at the 98th General Meeting of the American Society for Microbiology, Atlanta, Ga., 17 to 21 May 1998.)
MATERIALS AND METHODS
Fecal specimens from 38 patients who were being treated at the Atlanta VA Medical Center and 30 nonhuman primates housed at Yerkes Primate Center, Atlanta, Ga., were each divided into four aliquots and fixed with (i) low-viscosity PVA (Meridian Diagnostics, Inc., Cincinnati, Ohio), (ii) PARASAFE (PS) (Scientific Devices Laboratory, Inc., Des Plaines, Ill.), (iii) ECOFIX (EC) (Meridian), or (iv) Proto-Fix (PF) (Alpha-Tec Systems, Inc., Vancouver, Wash.). Specimens were processed and a smear was made from each preparation according to the respective manufacturer's directions. Fixatives were matched with the staining procedure suggested by the respective manufacturer to provide optimal morphologic results.
Specimens fixed by the PVA procedure were centrifuged for 10 min at 500 × g, the supernatant was decanted and, after the excess fluid was drained, a portion of the plug of fixed fecal material was used to prepare a permanent smear for staining. After drying was complete, the staining procedure with Wheatley's trichrome stain took 40 min. The PS method uses ethanol bis-carbonyl compounds as fixatives, and the reagent is claimed to contain no harmful chemicals. This method uses a centrifugation time of 3 min. The staining procedure recommended with this method, a modification of the standard Wheatley's trichrome stain protocol, eliminates the carbol-xylene step and reduces the time of the xylene step, for a total staining time of 23 min. The EC reagent contains zinc sulfate but is stated to contain no mercury. The centrifugation time for this method is 10 min, and the staining time for EcoStain is 15 min. The PF procedure uses a mixture of ethanol, methanol, isopropanol, and formaldehyde as fixatives, and the reagent contains no heavy metals. With the PF procedure, centrifugation takes 2 to 5 min, and staining with trichrome-plus takes 13 min.
All smears were reviewed by one of us (W.K.), a technologist with 15 years of experience in parasitology. In addition, representative smears prepared by each of the methods were examined by a second technologist (J.C.) to confirm the observations of the primary screener. Three hundred oil immersion fields on each slide were examined at a magnification of ×1,000. The reviewers were blinded as to specimen identification. Specimens were examined in random order, and there was no linkage of different preparations made from the same stool specimen. Organisms identified from each smear, quality of background, and clarity of internal structures necessary for parasite recognition were recorded. In addition, all methods were evaluated for the number of steps in the processing and staining procedures and the approximate technologist time required for processing and staining. Costs for reagents and stains were analyzed. Costs for technologist time and capital equipment were not included in this assessment, since these may vary from institution to institution.
Frequencies of detection of different parasites by the different methods of fixation and staining were compared using chi-square tests. In addition, the sensitivity of each fixative for detecting each of the parasites in all of the specimens was calculated.
RESULTS
Sixty-eight fresh stool specimens were examined by each of the four procedures under study. Of the 272 preparations (slides) examined, 122 were positive for at least one parasite (PVA, 31 slides; EC, 30 slides; PS, 30 slides; and PF, 31 slides). Parasites were detected in 3 of 38 stool specimens from human patients and 29 of 30 specimens from nonhuman primates. Table 1 compares the qualities of the microscopic slides prepared by the four fixation and staining methods. Slides prepared by the PF procedure had a clean, pale blue background. The morphologic features of the protozoa seen on slides prepared by this procedure were extremely well defined, even more so, in some instances, than those seen on slides made by the PVA procedure, simplifying parasite identification. Likewise, slides prepared by the EC method had a clean, blue-to-purple background and compared fairly well with those prepared by the PVA method with respect to quality of background and ease of parasite identification. Another advantage was that the staining of organisms and background produced by the EC method resembled the familiar coloration produced by the PVA method. As other investigators have found with fixatives which contain zinc (5), internal structures of parasites were not always as clearly defined with EC as with PVA; nonetheless, the parasites could usually be identified. In contrast, slides prepared with the PS procedure were less easy to read. The background on slides prepared by this procedure was considered to be dirty in 41% of cases. Furthermore, the dense blue-green color of the background made it difficult to distinguish internal protozoan structures for positive species identification. Also, this procedure resulted in a higher degree of distortion of parasitic architecture, which was responsible, in part, for lower rates of detection and identification of certain parasites.
TABLE 1.
Quality of microscopic slides prepared with different fixatives
| Fixation method | % of slides with the following:
|
|||
|---|---|---|---|---|
| Quality of background
|
Ease of parasite identification
|
|||
| Good | Dirty | Well defined | Distorted | |
| PVA | 100 | 0 | 97 | 3 |
| PS | 59 | 41 | 35 | 65 |
| EC | 96 | 4 | 80 | 20 |
| PF | 100 | 0 | 100 | 0 |
Five species of parasites (Entamoeba histolytica or Entamoeba dispar, Iodamoeba bütschlii, Blastocystis hominis, Chilomastix mesnili, and Entamoeba coli) were frequently identified in the stool specimens examined in this study. The morphologic appearances of representative examples of four of these organisms (E. histolytica [or E. coli], I. bütschlii, B. hominis, and C. mesnili) on slides prepared by each of the four fixation and staining procedures are shown in Fig. 1 and 2. Table 2 lists the numbers of cases in which various parasites were found on smears prepared by each of the four paired fixation and staining procedures, while Table 3 provides an estimate of the number of parasites on each slide according to fixation procedure. B. hominis was the parasite most frequently detected, regardless of the method of preparation. This organism was found in slightly more samples prepared by the PVA and PF methods than by the EC and PS methods but was generally preserved in a recognizable state by all methods. The next most frequently detected parasite, I. bütschlii, was found in about 20 samples prepared by each of the fixation and staining procedures, and the features of this organism were generally recognizable, regardless of the fixation or staining procedure. On the other hand, the remaining species of parasites detected, E. histolytica or E. dispar, E. coli, and C. mesnili, showed substantial differences in the degree of preservation of parasite morphology and in rates of detection, depending on the method. E. coli was detected in about equal numbers of smears (n = 14 to 16) prepared by the PVA, PF, and EC methods but in only one-third as many smears prepared by the PS method. More significantly, E. histolytica or E. dispar was found in the largest number of specimens (n = 13) by the PF method and substantially fewer by the PVA (7) or EC (6) method. Importantly, no definitive identifications of this parasite were made with PS. Likewise, C. mesnili was found in 13 samples prepared with PF, 8 with EC, and 5 with PVA but in only a single sample prepared by the PS method. Overall, the largest number of parasites was detected by the PF method, somewhat fewer parasites were detected by the PVA or EC method, and the fewest were detected by the PS method. Of particular concern, the PS method failed to preserve E. histolytica or E. dispar, C. mesnili and, in many cases, E. coli in an easily recognizable form.
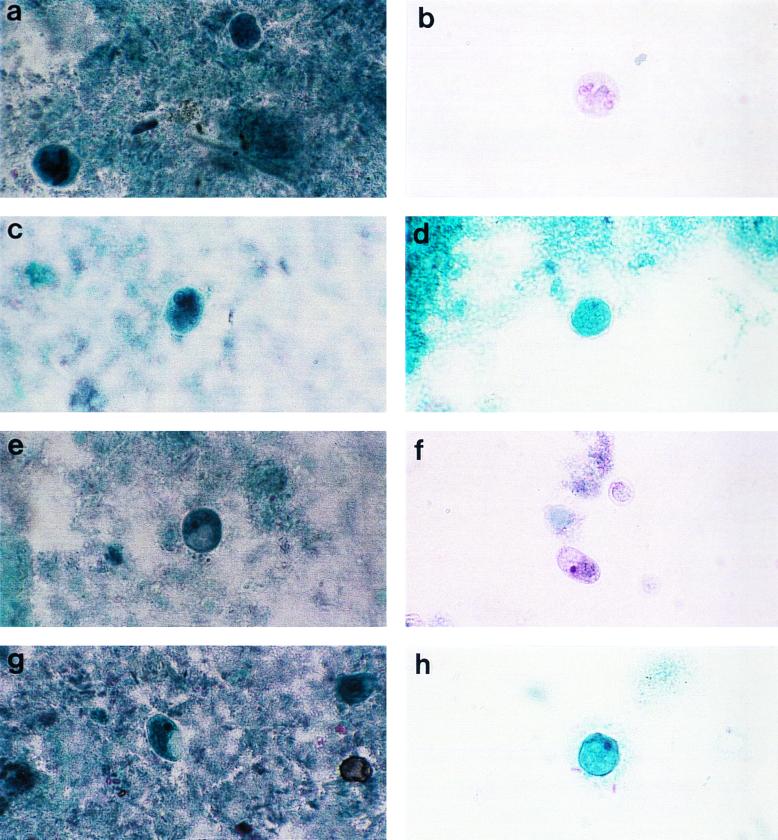
FIG. 1.
Composite photograph showing the appearances of Entomeba species and I. bütschlii in slides prepared by each of the four fixation and staining methods under study. (a) E. histolytica, PVA. (b) E. histolytica, PF. (c) E. histolytica, EC. (d) E. coli, PS. (e) I. bütschlii, PVA. (f) I. bütschlii, PF. (g) I. bütschlii, EC. (h) I. bütschlii, PS. Magnification, ×750.
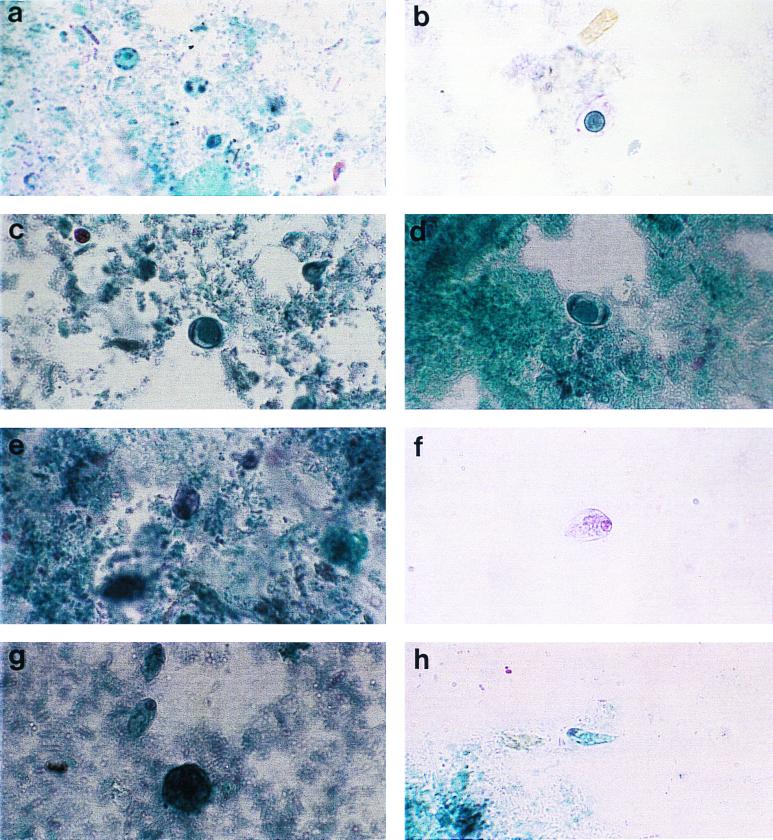
FIG. 2.
Composite photograph showing the appearances of B. hominis and C. mesnili in slides prepared by each of the four fixation and staining methods under study. (a) B. hominis, PVA. (b) B. hominis, PF. (c) B. hominis, EC. (d) B. hominis, PS. (e) C. mesnili, PVA. (f) C. mesnili, PF. (g) C. mesnili, EC. (h) C. mesnili, PS. Magnification, ×750.
TABLE 2.
Number of samples in which parasites were identified after fixation by various procedures
| Fixation method | No. of samples with the following parasite:
|
||||
|---|---|---|---|---|---|
| E. histolytica or E. dispar | E. coli | I. bütschlii | B. hominis | C. mesnili | |
| PVA | 7 | 16 | 19 | 30 | 5 |
| EC | 6 | 14 | 19 | 27 | 8 |
| PS | 0a | 5 | 21 | 24 | 1 |
| PF | 13 | 15 | 22 | 30 | 13 |
One possible E. histolytica parasite was noted, but the species could not be definitively identified.
TABLE 3.
Enumeration of parasites on each slide according to fixation method
| Specimena | No. of the following parasites revealed by the indicated fixation methodb:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
E. histolytica
|
E. coli
|
I. bütschlii
|
B. hominis
|
C. mesnili
|
||||||||||||||||
| PVA | EC | PS | PF | PVA | EC | PS | PF | PVA | EC | PS | PF | PVA | EC | PS | PF | PVA | EC | PS | PF | |
| 1 | + | + | 0 | 0 | ++ | + | + | ++ | ++ | ++ | + | ++ | +++ | +++ | 0 | + | 0 | 0 | 0 | + |
| 2 | 0 | + | 0 | 0 | ++ | + | 0 | + | ++ | + | + | + | +++ | +++ | 0 | + | 0 | 0 | 0 | + |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ++ | ++ | 0 | + | 0 | 0 | 0 | 0 |
| 4 | + | 0 | 0 | 0 | 0 | + | 0 | 0 | + | 0 | + | 0 | ++ | ++ | + | + | 0 | 0 | 0 | + |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | +++ | ++ | ++ | +++ | 0 | 0 | 0 | 0 |
| 6 | + | 0 | 0 | 0 | 0 | 0 | + | 0 | +++ | 0 | + | ++ | ++ | 0 | 0 | +++ | 0 | 0 | 0 | ++ |
| 7 | 0 | 0 | 0 | + | + | 0 | + | 0 | 0 | ++ | 0 | + | +++ | 0 | +++ | ++ | 0 | 0 | 0 | + |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | + | +++ | + | ++ | + | 0 | 0 | 0 | 0 |
| 9 | + | 0 | 0 | + | + | + | 0 | + | ++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | 0 | 0 | 0 | + |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | +++ | ++ | +++ | ++ | +++ | ++ | ++ | ++ | 0 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ++ | + | 0 | +++ | +++ | +++ | +++ | 0 | 0 | 0 | 0 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | + | ++ | ++ | +++ | + | 0 | 0 | 0 | 0 |
| 13 | 0 | 0 | 0 | + | + | 0 | 0 | + | +++ | ++ | ++ | + | +++ | +++ | 0 | +++ | 0 | 0 | 0 | 0 |
| 14 | 0 | + | 0 | + | 0 | 0 | 0 | 0 | + | + | + | ++ | +++ | ++ | + | + | 0 | 0 | 0 | 0 |
| 15 | 0 | + | 0 | ++ | ++ | 0 | + | ++ | + | ++ | ++ | ++ | +++ | ++ | ++ | ++ | 0 | 0 | 0 | 0 |
| 16 | 0 | 0 | 0 | + | + | + | 0 | + | +++ | +++ | +++ | +++ | ++ | 0 | +++ | + | 0 | 0 | 0 | 0 |
| 17 | 0 | 0 | 0 | + | + | 0 | 0 | + | 0 | 0 | 0 | ++ | +++ | +++ | 0 | +++ | 0 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | + | ++ | 0 | 0 | 0 | 0 | + | +++ | 0 | +++ | +++ | ++ | +++ | 0 | 0 | 0 | 0 |
| 19 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | + | + | + | 0 | + | + | + | 0 | ++ | +++ | +++ | + | +++ | 0 | + | 0 | ++ |
| 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | + | ++ | + | + | +++ | ++ | + | +++ | +++ | 0 | 0 | + |
| 22 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | ++ | + | 0 | ++ | +++ | +++ | + | ++ | +++ | ++ | + | ++ |
| 23 | 0 | 0 | 0 | 0 | 0 | + | 0 | + | + | + | ++ | + | ++ | +++ | ++ | +++ | 0 | + | 0 | 0 |
| 24 | ++ | 0 | 0 | + | + | + | 0 | 0 | + | + | + | + | ++ | ++ | + | ++ | 0 | 0 | 0 | 0 |
| 25 | 0 | 0 | 0 | 0 | + | + | 0 | 0 | + | 0 | ++ | + | +++ | ++ | ++ | ++ | 0 | 0 | 0 | 0 |
| 26 | + | + | 0 | + | 0 | 0 | 0 | + | + | + | + | + | ++ | + | +++ | ++ | 0 | 0 | 0 | 0 |
| 27 | 0 | 0 | 0 | 0 | + | ++ | 0 | + | 0 | + | + | + | ++ | ++ | ++ | ++ | ++ | + | 0 | + |
| 28 | 0 | 0 | 0 | 0 | + | + | 0 | + | + | + | 0 | + | +++ | +++ | ++ | ++ | 0 | + | 0 | + |
| 29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | + | 0 | 0 | 0 | ++ | ++ | ++ | ++ | + | + | 0 | + |
| 30 | 0 | 0 | 0 | + | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ++ | ++ | + | + | + | + | 0 | 0 |
| 31 | 0 | 0 | 0 | 0 | + | + | 0 | + | 0 | 0 | 0 | 0 | +++ | +++ | + | +++ | 0 | + | 0 | + |
| 32 | + | + | 0 | + | 0 | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Specimens 3, 19, and 32 were from humans; other specimens were from nonhuman primates.
0, none; +, 1 or 2 parasites/oil immersion field (OIF); ++, 3 to 9 parasites/OIF; +++, >10 parasites/OIF.
Both trophozoites and cysts were detected by all of the fixation and staining procedures. No significant difference in the ratio of cysts to trophozoites detected was seen for any of the fixation methods.
Statistical analysis was conducted to assess the significance of differences in the numbers of parasites detected with the various fixatives. A series of chi-square tests was used to evaluate the homogeneity of distribution of parasite identification for type of organism and type of fixative. The overall matrix showed a lack of homogeneity (χ2, 22.31; df, 12; P, 0.034), indicating differences in test results justifying further evaluation. Using PVA as the standard, we found that the distribution of identification for organism type was homogeneous for all fixatives except PS (χ2, 11.53; df, 4; P, 0.021). These results indicated that recoveries obtained with PS were different from those obtained with the other three fixatives. When PS data were eliminated from the overall matrix, the resulting chi-square analysis revealed homogeneity (χ2, 4.98; df, 8; P, 0.760), indicating that parasite recoveries obtained with PF and EC were not statistically different from the recovery obtained with PVA.
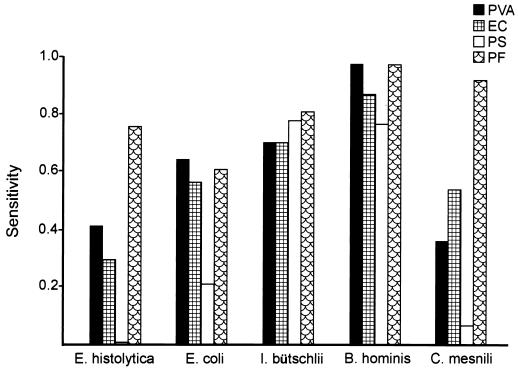
The sensitivity of each of the fixatives for each of the parasites (the number of samples in which a specific parasite was identified with a specific fixative divided by the number of samples in which that parasite was identified with any of the fixatives) was computed. The resulting sensitivities are shown in Fig. 3. I. bütschlii and B. hominis were detected with approximately equal sensitivities by all fixatives, whereas E. coli, E. histolytica, and C. mesnili were detected less frequently by the PS method than by the other methods. PF showed a higher sensitivity than the other fixatives for the identification of E. histolytica and C. mesnili.
FIG. 3.
Bar graphs showing the sensitivities of PVA, EC, PS, and PF for the identification of E. histolytica, E. coli, I. bütschlii, B. hominis, and C. mesnili.
Table 4 summarizes a number of technical aspects regarding each of the four fixation and staining methods and provides the cost of materials per slide required to perform each procedure. None of the procedures is difficult to perform, although the numbers of processing and staining steps differ significantly between them. The EC and PVA methods involve fewer steps (15 or 16 steps) than the PF and PS methods (21 or 22 steps). However, overall processing and staining time for the technologist (not including centrifugation time) is about 20 min for each of the newer techniques, compared with 45 min for the PVA procedure. All of the procedures are adaptable to either batch tests or single tests.
TABLE 4.
Technical aspects and costs per slide for each fixation and staining procedure
| Procedure | Fixative(s) used | No. of steps | Centrifugation time (min) | Slide preparation time (min)a | Staining method | Staining time (min) | Fixation and staining costs ($) (reagents only) |
|---|---|---|---|---|---|---|---|
| PVA | Mercuric chloride | 16 | 10 | 15 | Wheatley's trichrome | 40 | 3.36 |
| EC | Zinc sulfate | 15 | 10 | 14 | EcoStain | 15 | 3.92 |
| PS | Ethanol bis-carbonyl compounds | 22 | 3 | 8 | Modified Wheatley's trichrome | 23 | 2.76 |
| PF | Ethanol, methanol, isopropanol, CH2O | 21 | 2 | 11 | Trichrome plus | 13 | 3.28 |
Including cenrifugation time.
PS was the most economical system from the standpoint of reagent costs. Reagents and other disposable items required for fixation by this method cost $2.00 per sample, compared with $2.60 for PVA, $2.63 for EC, and $2.47 for PF. The EC stain was the most expensive at $1.29 per slide. The costs for the PVA, PS, and PF stains were $0.76, $0.76, and $0.81 per slide, respectively.
DISCUSSION
PVA, the fixative most commonly used for parasite examination, presents safety and disposal problems to laboratories, because of its mercuric chloride content. Relevant to this situation, a recent Memorandum of Understanding concluded between the American Hospital Association and the U.S. Environmental Protection Agency calls for a virtual elimination of mercury pollution by 2005 (see http://www.ada.org/memofunder.html). To eliminate this hazard, several manufacturers have recently developed alternative fixatives to replace PVA. Since it is likely that these newer fixatives will be used by many laboratories to avoid the toxicity problems associated with PVA, it is essential that the performance of these alternative fixatives be evaluated and compared with that of PVA, which remains, to this point, the gold standard for parasite fixation.
Our comparison of three new fixatives with PVA showed significant differences in performance between the four fixation and staining procedures. The background quality of smears prepared by the PVA, EC, and PF methods was clean in almost all instances, allowing relatively easy identification of organisms, whereas 41% of smears prepared from specimens fixed with PS had a dirty background. Although the PS procedure was simple and rapid, slides prepared by this method had a dense blue-green background, which made it difficult to resolve the internal structures of parasites, rendering their identification difficult. (It should be noted that although our reviews were blinded as to specimen, slides prepared by each method were so characteristic as to the amount and color of background that blinding for fixation and staining procedures was not possible.)
For identification of the five species of parasite most frequently observed in our specimens, the PF and EC methods were not statistically different from the PVA method, as determined by chi-square testing with PVA as the gold standard. Comparison of sensitivities of the four methods for detecting each of the five parasites showed that PF (which uses a mixture of alcohols and formaldehyde for fixation) performed better than PVA for the identification of E. histolytica and C. mesnili and was comparable to PVA for the other organisms detected. The zinc sulfate-based fixative, EC, showed a sensitivity similar to that of PVA with respect to identification of all five types of parasites. Other investigators have noted that specimens fixed with zinc sulfate do not always show the internal structures of organisms as clearly as do specimens fixed with PVA (6), and our qualitative observations were in agreement with these results. However, this limitation did not appear to adversely affect our ability to identify organisms with this fixative in most cases.
On the other hand, chi-square analysis revealed that the data obtained with the PS procedure were significantly different from those obtained with the other procedures. The sensitivities of this method for the identification of E. histolytica, E. coli, and C. mesnili were all lower than those obtained with the other methods. Examination of the individual slides suggested that the lower sensitivities seen with PS were due, at least in part, to distortion of the internal structures of the organisms upon which diagnoses were based, perhaps because of inadequate fixation. Interference by the dirty green background seen on many of the slides fixed with PS also may have contributed. It is particularly important to be able to distinguish E. histolytica from E. coli, as the former parasite can cause severe gastroenteritis with mucosal ulceration leading, in some cases, to fulminant parasitemia and resulting in abscesses in other internal organs, such as the liver, lungs, and brain (7). Persons infected with E. histolytica also serve as reservoirs of infection for other individuals.
Processing and staining times for all the newer fixatives and stains were very similar, and all of these procedures were more rapid than the PVA procedure. An additional advantage of the PF method was that the bright red reagents used with this procedure stained gloves and laboratory apparel, showing very clearly when any splashing had occurred. A spray bottle of 10% bleach solution removed the droplets readily.
Although costs for reagents, materials, and stains were fairly similar for all four methods, the PS method cost the least, whereas the EC method was the most expensive. Parenthetically, it is noteworthy that the larger filter funnels with a filter diameter of about 1 in., now sold by many manufacturers, are much easier to use with stool specimens than were gauze and a paper funnel or a funnel with a filter diameter of only 0.5 in.
Due to the relatively small number of patient specimens received at the Atlanta VA Medical Center and containing parasites, we decided to include specimens from nonhuman primates, so that more substantial numbers of the various parasites could be studied by the four fixation and staining procedures. Unfortunately, no Giardia lamblia cysts or trophozoites were recovered by any of the methods from either humans or nonhuman primates during the course of our study. A few other parasites or parasite ova (e.g., Balantidium coli trophozoites and eggs of Trichiuris trichiura) were identified in single slides prepared by one or more of the methods, but these were not included in our analysis because of their rare occurrence and because the staining procedures under study are not designed for the detection of helminths.
Our data suggesting that PF has a higher sensitivity for the detection of E. histolytica and C. mesnili than the other methods of fixation tested should be confirmed by larger studies. Furthermore, it should be noted that the PF procedure is less “forgiving” of small variations in reagent quality than the other methods being evaluated. The pH of the distilled water used in this procedure must be between 4 and 5, and the water should be changed daily. Likewise, the alcohols and clearing agent should be prepared freshly each day for optimal results. It should also be mentioned that PF contains 0.75% formaldehyde. The current permissible level of formaldehyde authorized by the U.S. Occupational Safety and Health Administration is 0.75 ppm (measured as a time-weighted average for an 8-h exposure) or 2 ppm (measured as a 15-min short-term exposure). It would appear that as long as appropriate monitoring is performed, the small amount of formaldehyde present in PF should not present a significant safety hazard.
It has been shown that a permanently stained smear increases the recovery of parasites compared with the examination of concentrated sediment alone (3). In this era of managed care and cost containment, one group of investigators has suggested that a single specimen per patient is sufficient for outpatients and for inpatients hospitalized for 3 days or less (8). We disagree with this viewpoint, since organisms such as Giardia can be present in the stool one day and absent the next. Nonetheless, regardless of the number of specimens to be examined, a reliable fixation and staining procedure for preparing permanently stained smears and providing definitive, recognizable characteristics for optimal recovery and identification of parasites is a prerequisite for any analysis. In our experiments, the PF procedure with trichrome-plus staining best provides this quality while avoiding the use of mercury.
ACKNOWLEDGMENTS
We thank the staff of the VAMC Microbiology Laboratory, Decatur, Ga., for skillful technical assistance and Ken Lowery for reviewing the manuscript.
REFERENCES
- 1.Commission on Laboratory Accreditation, College of American Pathologists. Checklist in microbiology, section 4. Northfield, Ill: College of American Pathologists; 1997. [Google Scholar]
- 2.Committee on Education, American Society of Parasitologists. Procedures suggested for use in examination of clinical specimens for parasitic infection. J Parasitol. 1977;63:959–960. [Google Scholar]
- 3.Garcia L S, Brewer T C, Bruckner D A. A comparison of the formalin-ether concentration and trichrome stained smear methods for the recovery and identification of intestinal parasites. Am J Med Technol. 1979;45:932–935. [PubMed] [Google Scholar]
- 4.Garcia L S, Bruckner D A. Diagnostic medical parasitology. Washington, D.C.: ASM Press; 1997. pp. 623–624. [Google Scholar]
- 5.Garcia L S, Shimizu R Y. Evaluation of intestinal protozoan morphology in human fecal specimens preserved in EcoFix: comparison of Wheatley's trichrome stain and EcoStain. J Clin Microbiol. 1998;36:1974–1976. doi: 10.1128/jcm.36.7.1974-1976.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia L S, Shimizu R Y, Brewer T C, Bruckner D A. Evaluation of intestinal morphology in polyvinyl alcohol preservative: comparison of copper sulfate and mercuric chloride base for use in Schaudinn's fixative. J Clin Microbiol. 1983;17:1092–1095. doi: 10.1128/jcm.17.6.1092-1095.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Healy G R, Garcia L S. Intestinal and urogenital protozoa. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: ASM Press; 1995. pp. 1204–1228. [Google Scholar]
- 8.Morris A J, Wilson M L, Reller J B. Application of rejection criteria for stool ovum and parasite examinations. J Clin Microbiol. 1992;30:3213–3216. doi: 10.1128/jcm.30.12.3213-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]