Abstract
By using ligands with various affinities for the T-cell receptor (TCR) and by altering the contribution of the CD45 tyrosine phosphatase, the effects of the potency of TCR-induced signals on the function of small GTPases Ras and Rap1 were studied. T cells expressing low-molecular-weight CD45 isoforms (e.g., CD45RO) exhibited the strongest activation of the Ras-dependent Elk-1 transcription factor and the highest sensitivity to the inhibitory action of dominant negative mutant Ras compared to T cells expressing high-molecular-weight CD45 isoforms (ABC). Moreover, stimulation of CD45RO+, but not CD45ABC+, T cells with a high-affinity TCR ligand induced suboptimal Elk-1 activation compared with the stimulation induced by an intermediate-affinity TCR-ligand interaction. This observation suggested that the Ras-dependent signaling pathway is safeguarded in CD45RO+ expressors by a negative regulatory mechanism(s) which prohibits maximal activation of the Ras-dependent signaling events following high-avidity TCR-ligand engagement. Interestingly, the biochemical activity of another small GTPase, the Ras-like protein Rap1, which has been implicated in the functional suppression of Ras signaling, was inversely correlated with the extent of Elk-1 activation induced by different-affinity TCR ligands. Consistently, overexpression of putative Rap dominant negative mutant RapN17 or the physiologic inhibitor of Rap1, the Rap GTPase-activating protein RapGAP, augmented the Elk-1 response in CD45RO+ T cells. This is in contrast to the suppressive effect of RapN17 and RapGAP on CD45ABC+ T cells, underscoring the possibility that Rap1 can act as either a repressor or a potentiator of Ras effector signals, depending on CD45 isoform expression. These observations suggest that cells expressing distinct isoforms of CD45 employ different signal transduction schemes to optimize Ras-mediated signal transduction in activated T lymphocytes.
The affinity of interaction between antigen and T-cell receptor (TCR) is essential in determining the level of TCR phosphorylation (17) and other early signaling parameters (4, 44). Such differential regulation of TCR signaling has important biological consequences during the immune response, including early T-cell maturation (13), peripheral Th1-Th2 helper subset differentiation (21), and memory T-cell generation (28). Furthermore, signaling output from the TCR is tightly regulated by a constitutively highly expressed CD45 membrane protein tyrosine phosphatase (19, 20). Whereas the role of the cytoplasmic domain of CD45 is thought to regulate the activities of tyrosine kinases Lck (31) and Fyn (29), the function of the heavily glycosylated CD45 ectodomain is not well established. Complexity of this domain is introduced by alternative splicing of four exons encoding the O-glycosylated N-terminal sequence of the CD45 ectodomain and generation of several isoforms with molecular masses of 170, 180, 190, 205, and 220 kDa (47). Although the heterogeneity of the CD45 ectodomain is correlated with different stages of T-cell activation, differentiation, and maturation (9, 34) and certain biochemical differences exist between T cells with differential expression of CD45 isoforms (27, 33, 39), the precise function of individual CD45 isoforms during acquisition of different functional profiles remains poorly understood.
Similarly, despite the knowledge that the character of the antigen binding by the TCR complex is a key factor influencing different cellular outcomes of the immune response, the mechanisms by which individual signaling pathways from the TCR contribute to the development of different cellular functions are not clear. Of interest in this regard are studies indicating that a classic Ras-induced signaling pathway influences the processes of thymocyte development (1, 45), cytokine gene expression (2, 32, 36), and Th2 helper cell differentiation (50), suggesting that Ras proteins provide an important signaling intermediate necessary for coupling of the TCR to distinct cellular phenotypes.
p21Ras and p21Rap1 are members of the Ras protein family which are prominently activated from the stimulated TCR by distinct but similarly organized signaling cascades (3, 10, 24, 37). These two closely related small GTPases share absolute identity within the core effector domain and can bind a similar spectrum of target molecules (6). However, the functional consequences of these interactions appear to be different, depending on whether Ras or Rap1 is involved. It has been proposed that because Rap1 is located in the endoplasmic reticulum and Golgi, it may sequester Ras effectors in a subcellular location that prevents their complete activation, thereby suppressing Ras effector signaling. Consistent with this model is the observation that Rap1 antagonizes the effects of oncogenic Ras (18). Also, it has been suggested that the cause of T-cell anergy lies in a block in the Ras–Raf-1–mitogen-activated protein (MAP) kinase cascade (11, 25) and that inhibition of this pathway in functionally unresponsive T cells correlates with active, GTP-bound Rap1 complexed with Raf-1 (3). These considerations raise the possibility that Ras and Rap1 maintain a close functional relationship which may effectively integrate and modify signal transduction events induced by the TCR.
Work from our laboratory (30) and by others (27) revealed that T cells carrying distinct isoforms of CD45 produce significantly different amounts of interleukin-2 (IL-2). Our motivation to analyze the role of Ras during this differential response was spurred by several reports demonstrating that Ras is critical for IL-2 transcription (2, 32, 36) and that the expression of CD45 has been correlated with distinct abilities of T cells to activate Ras (33). In this study, we simulated conditions which are required in primary T lymphocytes for the induction of different cellular outcomes by using differential TCR ligation and selective CD45 display. Under these conditions, we analyzed Ras and Rap1 signaling and potential cross talk between the pathways and linked the expression of single CD45 isoforms to distinct response patterns in T cells.
MATERIALS AND METHODS
Antibodies.
Anticlonotypic D10 TCR monoclonal antibodies (MAbs) 3D3 (immunoglobulin G1 [IgG1]) and 5A (IgG1) were used for stimulation (42). The antibodies were purified from culture supernatants on protein G columns and dialyzed against phosphate-buffered saline (PBS) before use. Fluorescein isothiocyanate-labeled 30-F11 (panspecific anti-mouse CD45, rat IgG2b) and phyroerythrin-labeled MAbs 16A (anti-mouse CD45RB, rat IgG2a), GK1.4 (anti-mouse CD4, rat IgG2b), and H57-597 (anti-mouse TCR β-chain, hamster IgG) were used to phenotype BW clones in flow cytometry analysis. MAb 30-F11 was also used in a Western blot analysis to verify the proper molecular weight of CD45 isoforms in individual BW clones (see Fig. 1C). All labeled MAbs were purchased from PharMingen. Anti-Rap1/Krev-1 rabbit polyclonal IgG was from Santa Cruz Biotech.
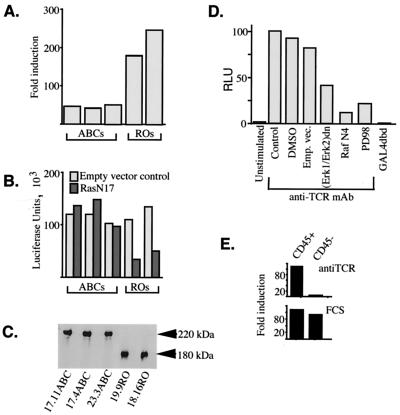
FIG. 1.
Preferential activation of the Ras-MAP kinase signaling cascade in CD45RO+ T cells (part 1). (A) CD45ABC+ and CD45RO+ TCR+ CD3+ CD4+ BW5147 T-cell clones, in the order indicated in panel C, were transfected with an Elk-1–GAL4 transactivator and a 5×GAL4-luciferase reporter at a ratio of 1:10 and stimulated for 24 h with plate-bound anti-D10 TCR MAb 5A (10 μg/ml). The relative activity of the reporter is expressed as fold induction over reporter transactivation in unstimulated cells. (B) CD45ABC+ and CD45RO+ cells were transfected with the Elk-1–GAL4–5×GAL4–luciferase reporter, cotransfected with 50 ng of RasN17 or a matched empty vector, and stimulated with MAb 5A. Luciferase activities were normalized against an internal control (pRL-CMV). (C) Western blot analysis of whole-cell lysates of CD45ABC+ (clones 17.11, 17.4, and 23.3) and CD45RO+ (clones 19.9 and 18.16) cells stained with anti-CD45 MAb 30-F11. (D) CD3+ CD4+ CD45RO+ BW5147 cells were transfected with Elk-1–GAL4 (or the GAL4 DNA binding domain [GAL4dbd] alone) and a 5×GAL4-luciferase reporter and cotransfected with either the empty vector (Emp. vec.) or an expression plasmid(s) containing the dominant negative mutant forms of Erk or Raf (RafN4) under the control of a constitutive promoter. At 24 h posttransfection, cells were left unstimulated or were stimulated for another 24 h with an anti-TCR MAb. Luciferase activity (in relative luciferase units [RLU]) was normalized against the protein concentration of the cell lysates and expressed as a percentage of the control stimulation. DMSO, dimethyl sulfoxide. (E) Transactivation of the Elk-1–GAL4–5×GAL4–luciferase reporter system in CD45-positive and CD45-negative BW cells upon stimulation with an anti-TCR MAb (top) or 20% fetal calf serum (FCS) following overnight starvation (bottom). The data shown are representative of at least two independent experiments.
Cell culture and activation.
A CD45-negative variant of the AKR thymoma BW5147 reconstituted with the TCR from the D10.G4.1 (D10) Th2 cell clone, wild-type murine CD4, and single CD45 isoforms (ABC, RO, or Exon-1) has been described previously (30). BW cells were maintained in Eagle's high-amino-acid medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 10 mM HEPES, and antibiotics. Neomycin, puromycin, and hygromycin were added to maintain stable expression of the D10 TCR, CD4, and CD45, respectively. BW T-cell clones that express similar levels of CD45, CD4 coreceptor, and the D10 TCR were sorted and routinely immunophenotyped by immunofluorescence to ensure similar expression of these molecules. Stimulations were performed in 96-well flat-bottom microtiter plates precoated for 4 h with different dilutions of anticlonotypic TCR MAb 3D3 or 5A. BW cells (105) were added to each well and stimulated for 24 h. For stimulation with the antigen-presenting cells, 5 × 105 BW cells were cocultured with 2.5 × 106 mitomycin C-treated B10BR (H-2k) T-cell depleted splenocytes and the CA37 conalbumin peptide (100 μg/ml) and incubated for 48 h. Following stimulation, BW cells were harvested and analyzed for reporter gene expression.
Expression plasmids and reporter systems.
Cells were transfected by the standard DEAE-dextran method with the following constructs. pCGN-Raf-N4 encodes residues 23 to 284 of c-Raf-1 and was donated by C. J. Der (University of North Carolina at Chapel Hill). The dominant negative construct pcDNA3-RasN17 was a gift from K. L. Guan (University of Michigan). pZIPneoSV(X)1-RapN17, Rap63E, and RapGAP were kindly provided by L. A. Quilliam (Indiana University). The dominant negative mutant forms of Erk-1 and Erk-2 were gifts from M. H. Cobb (University of Texas).
The PathDetect trans-reporting system (Stratagene) consists of the plasmid that expresses a chimeric fusion consisting of the GAL4 DNA binding domain fused to the activation domain of Elk-1 and the reporter vector that contains luciferase downstream of a basic promoter element (TATA box) which is joined to five tandem repeats of the 17-bp GAL4 binding element. The chimeric IL-2–Luc reporter construct contains the luciferase gene under the control of the immediate upstream region (positions −7 to −293) of a mouse IL-2 gene and was provided by C. Dong (Yale University) with permission from E. Serfling (University of Würzburg) (43). The pRL-CMV plasmid containing the Renilla luciferase gene under the control of the cytomegalovirus immediate-early enhancer-promoter region and the dual-luciferase reporter assay system were purchased from Promega and used for normalization of the experimental firefly luciferase gene expression.
The pB4X-CAT reporter plasmid contains the bacterial gene for chloramphenicol acetyltransferase (CAT) driven by a minimal promoter that contains four tandem copies of the Ets/AP-1 Ras-responsive element from the polyomavirus enhancer (5). At 48 h posttransfection, cells were collected, washed with PBS, resuspended in 0.25 M Tris (pH 7.8), and lysed by three cycles of freezing-thawing. Portions of the cleared cell lysates were incubated with acetyl coenzyme A and [14C]chloramphenicol (DuPont, Boston, Mass.) for 45 min at 37°C. Following extraction in ethyl acetate, samples were dried under vacuum, resuspended in ethyl acetate, and chromatographed on silica gel 1B thin-layer chromatography plates (Baker, Phillipsburg, N.J.) in chloroform-methanol (95/5 ratio). Radioactivity in each spot was measured by the Molecular Imager System GS-525 (Bio-Rad Laboratories) and analyzed by the Molecular Analyst/Macintosh data analysis software. The amount of acetylated [14C]chloramphenicol was calculated as a percentage of the total [14C]chloramphenicol.
Rap1 activation assay.
Rap1 activity was measured by means of an activation-specific probe assay as previously described (12). Briefly, 5 × 106 rested BW cells were stimulated with plate-bound MAb 5A or 3D3 for 30 min, washed once with ice-cold PBS, and lysed in 1% NP-40–50 mM Tris (pH 7.4)–150 mM NaCl. Lysates were clarified by centrifugation, and supernatants were incubated with 5 μg of glutathione S-transferase (GST) bound to the 97-amino-acid sequence spanning the Rap1 binding domain of RalGDS precoupled to glutathione-agarose beads. After 1 h at 4°C, the beads were washed four times with lysis buffer and the materials collected were analyzed by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis, followed by transfer to a nitrocellulose membrane which was blocked for 1 h and probed with an anti-Rap1 polyclonal antibody.
Special reagents.
The conalbumin peptide CA37 (HRGAIEWEGIESG) was synthesized by the W. M. Keck Foundation Biotechnology Resource Laboratory and purified by high-pressure liquid chromatography prior to use. Phorbol myristate acetate (PMA; Sigma) was used at 50 ng/ml. PD98059 is a MEK1 inhibitor and was obtained from New England Biolabs Inc.
Western immunoblotting for CD45.
BW cells (107) cultured in normal media were lysed in buffer containing 1% NP-40–20 mM Tris (pH 7.5)–150 mM NaCl–1 mM MgCl2–1 mM EGTA–leupeptin at 10 μg/ml–1 mM phenylmethylsulfonyl fluoride–1 mM sodium vanadate. Equal amounts of protein from precleared cell lysates were fractioned under nonreducing conditions on a sodium dodecyl sulfate–6% polyacrylamide gel. After electrophoresis, proteins were electroblotted onto nitrocellulose membranes (Bio-Rad Laboratories), blocked with 5% nonfat dry milk, probed for 1 h with biotin-conjugated MAb 30-F11, and then incubated with avidin-coupled horseradish peroxidase. The immunoblots were developed with the ECL chemiluminescence detection system (Amersham Pharmacia Biotech).
RESULTS
Expression of low-molecular-weight isoform of CD45 increases transactivation from Ras-dependent molecular reporter constructs.
To study the effects of distinct CD45 isoforms on lymphocyte function, our laboratory has developed a system in which CD45-negative mutant murine thymoma BW5147 T cells were reconstituted with single isoforms of CD45. Using the BW transfectants, it was demonstrated that clones with the lowest-molecular-weight (RO) isoforms of CD45 produce significantly more IL-2 following antigen stimulation compared with the high-molecular-weight (ABC) CD45 expressors (23, 30) and that this preferential cytokine response was strongly correlated with the increased physical interaction of CD45RO isoforms with the TCR-CD4 complex (22, 23). We now wished to understand the mechanism(s) that couples differential organization of the TCR-CD4-CD45 interacting complex to different IL-2 cytokine outcomes. In this regard, we have concentrated our efforts on the Ras-MAP kinase cascade, a signaling pathway which has been repeatedly implicated in the regulation of the IL-2 response (2, 32, 36). However, since activation of the IL-2 gene is a complex process depending on input from various signaling pathways, we have used Ras-dependent plasmid reporter constructs rather than full-length or minimal-length IL-2 promoter constructs. We hoped that in this way we could separate the effects of CD45 isoforms on Ras-MAP kinase signaling from the effects that these CD45 isoforms exhibit toward other signaling processes. In the first system, the activity of a chimeric GAL4–Elk-1 reporter containing the Ras-dependent C-terminal domain of Elk-1 fused to the DNA binding domain of GAL4 was tested. In this experiment, TCR-induced transcriptional activity of GAL4–Elk-1 was potently inhibited by RafN4, Mek inhibitor PD098059, and interfering forms of Erks (extracellular signal-regulated kinases) (Fig. 1D). These data indicate that TCR stimulation activates Elk-1 via the Ras-Erk signaling pathway. A CD45-negative control cell clone did not activate Elk-1 via the TCR, despite the ability of these cells to respond to serum (Fig. 1E). Subsequently, all of the CD45ABC+ and CD45RO+ clones were transfected with the reporter stimulated with anti-TCR clonotypic MAb 5A. As shown in Fig. 1A, CD45RO expressors demonstrated the strongest relative transactivation upon treatment with the antibody, suggesting that Ras is most efficiently activated via the TCR in these cells.
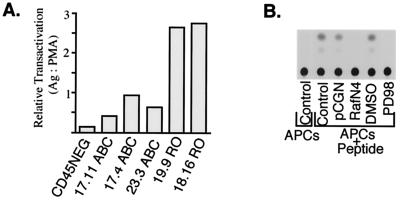
Similar data were obtained with a second construct which contains four ets–Ap-1 promoter elements from the polyomavirus enhancer and can be transactivated by the cytoplasmic oncogenes that encode v-Ras and v-Raf (5). First, we confirmed that the Ras–Raf-1 cascade elicits similar responses from pB4X-CAT during TCR stimulation of the BW cells used in these studies, since both the dominant negative Raf mutant (RafN4) and PD098059 strongly inhibited reporter activity following stimulation of cells with the antigen (Fig. 2B). Importantly, CD45RO expressors, upon stimulation with the peptide-pulsed antigen-presenting cells, transactivated the CAT reporter much better than CD45ABC+ cells, again suggesting that Ras is more efficiently upregulated via the TCR in cells carrying low-molecular-weight CD45 isoforms (Fig. 2A).
FIG. 2.
Preferential activation of the Ras-Raf-Mek signaling cascade in CD45RO+ T cells (part 2). Transactivation of the reporter construct pB4X-CAT in CD45-negative (CD45NEG), CD45ABC+ (clones 17.11, 17.4, and 23.3), and CD45RO+ (clones 19.9 and 18.16) TCR+ CD3+ CD4+ BW5147 T cells. Cells in the indicated order were transfected with the CAT reporter and stimulated for 48 h with the antigen (Ag; conalbumin peptide CA37 [100 μg/ml] or PMA [50 ng/ml]). The relative activity of the reporter is expressed as the ratio of stimulation with the peptide to stimulation with PMA. (B) Autoradiogram of thin-layer chromatography and conversion of acetylated [14C]chloramphenicol in a CAT assay of TCR+ CD3+ CD4+ CD45+ T cells transiently transfected with pB4X-CAT alone (control, dimethyl sulfoxide [DMSO], and PD98059) or transfected with pB4X-CAT and cotransfected with either an empty vector control (pCGN) or an expression plasmid containing dominant negative mutant Raf (RafN4). Posttransfection, cells were nonstimulated, stimulated for 48 h with CA37 without additional treatment, or stimulated and simultaneously treated with dimethyl sulfoxide or 10 μM Mek1 inhibitor PD98058. APCs, antigen-presenting cells.
Next, we measured the sensitivity of TCR-induced CD45ABC+ and CD45RO+ BW cells to the dominant negative mutant form of Ras. Interestingly, CD45RO+ clones, which in previous experiments showed the highest relative reporter transactivation, here also were the most efficiently inhibited by RasN17 (Fig. 1B). These two observations together strongly suggest that the preferential transactivation of the Ras-responsive promoters in CD45RO+ cells is due to the greater proportional contribution of Ras during TCR-induced responses in these cells. Importantly, the data obtained using luciferase and CAT assays are consistent with previous work done in this laboratory showing that BW thymoma RO but not ABC CD45 expressors are good IL-2 producers. The Ras-MAP kinase pathway may therefore, at least in part, be responsible for the phenomenon of this preferential cytokine responsiveness.
CD45RO expression prohibits maximal T-cell activation following stimulation with high-affinity TCR ligands.
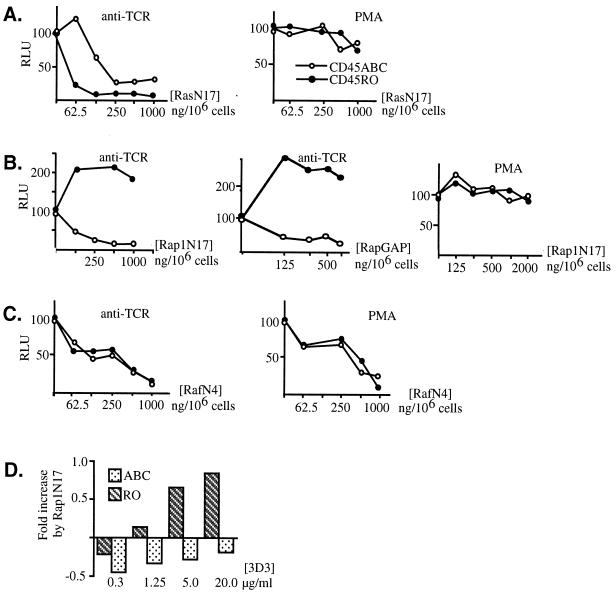
BW transfectants are advantageous because, in addition to CD45, they express the CD4 accessory molecule, as well as the CD3 complex and the functional TCR derived from the D10 Th2 cell clone (30). A panel of MAbs was previously raised against this TCR and characterized extensively with respect to the epitopes recognized in the D10 TCR, as well as affinity for the D10 TCR (40, 42). Importantly, some of these D10 TCR-specific antibodies potently recruit CD4 upon TCR binding, thereby mimicking physiologic stimulation of T cells with the antigen-myosin heavy chain complex. Since the relative affinity of ligands is known in this system, mutant BW cells bearing the D10 TCR are ideal for experiments aimed at determining differential effects of high- and low-affinity ligand-TCR interactions on signal transduction events. Interestingly, the above-described increased activation of the Ras signaling pathway in a CD45RO+ BW clone (Fig. 1A) was observed in the context of stimulation with anti-D10 TCR clonotypic MAb 5A but not 3D3. Hence, we wondered whether MAb 5A- and 3D3-induced changes in D10 TCR+ CD4+ BW T cells are differentially regulated by distinct CD45 isoforms. Dose-response experiments were conducted in which maximal activation of Elk-1 was compared in cells stimulated with MAbs 5A and 3D3. These experiments revealed significantly higher transactivation of Elk-1 upon treatment of CD45RO+ cells with MAb 5A, as opposed to the stimulation of these cells with MAb 3D3 (Fig. 3A). Since MAb 3D3 exhibits higher affinity for the D10 TCR than does MAb 5A (51), as well as greater potency as defined by the ability to cocap CD4 with the TCR (40), it was unexpected to find more prominent absolute Elk-1 activation in BW cells treated with MAb 5A. We also investigated the generality of this phenomenon by testing other BW clones carrying single isoforms of CD45. From these experiments, it became clear that the MAb 5A-induced augmentation of Elk-1 transactivation, compared to that induced by MAb 3D3, was seen primarily in CD45RO+ cells but not in CD45ABC+ cells (Fig. 3C).
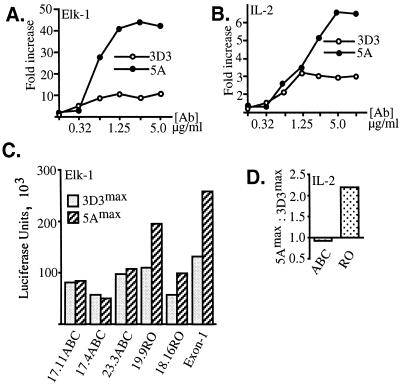
FIG. 3.
Intermediate- but not high-avidity TCR-CD4 ligands induce maximal Elk-1 transactivation and IL-2 responses in CD45RO+ T cells. CD45ABC+ and CD45RO+ BW cells were transfected with the Elk-1–GAL4–5×GAL4–luciferase construct (A and C) or with the IL-2–luciferase construct (B and D). At 24 h posttransfection, 105-cell aliquots were incubated in 96-well plates precoated with MAb 3D3 or 5A. After stimulation, cells were collected and lysed and the luciferase activity was measured and normalized against the protein concentration. (A and B) Transcriptional functional assay monitoring activity of Elk-1 (A) or the IL-2 minimal promoter (B) in CD45RO+ 19.9 cells stimulated with increasing concentrations of anti-TCR clonotypic MAb 3D3 (open) or 5A (closed). (C) Comparison of maximal Elk-1 activations during stimulation with MAbs 3D3 (3D3max) and 5A (5Amax) in three independent CD45ABC+ clones (17.11, 17.4, and 23.3) clones, two CD45RO+ clones, (19.9 and 18.16), and one CD45 Exon-1 BW clone. (D) Similar analysis of the maximal responses from the IL-2 promoter conducted with one representative CD45ABC+ clone and one CD45RO+ clone. The data in panel D are the ratios between the maximal responses obtained with the two antibodies for each cell clone. The data shown are representative of two to five independent experiments. Ab, antibody.
Next, we studied whether MAb 5A-induced potentiation of T-cell activation leads to enhanced activation of cytokine genes. We therefore transiently transfected BW cells with the IL-2–luciferase reporter construct and monitored its transactivation following TCR ligation. Again, in CD45RO+ but not in CD45ABC+ cells, MAb 5A-induced stimulation caused a twofold stronger response from the IL-2 reporter over analogous treatment with MAb 3D3 (Fig. 3B and D).
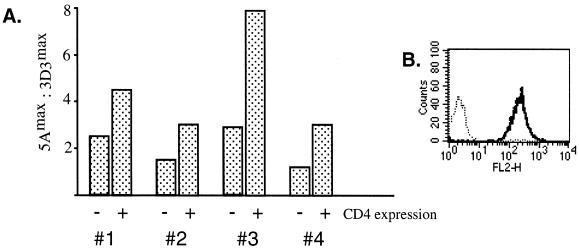
Since the activities of anti-D10 TCR clonotypic MAbs are believed to involve a physical association of CD4 with the TCR (40, 42), we decided to next determine whether CD4 expression contributes to the MAb 5A-induced increase in Elk-1 activation. To answer this question, TCR+ CD45RO+ CD4-negative BW cells were sorted (Fig. 4B) and compared to CD4-positive counterparts in the Elk-1 transcriptional assay. In these experiments, CD4-negative cells, like CD4-positive cells, were more strongly stimulated with MAb 5A than with MAb 3D3. However, this effect appeared to be weaker compared with that in CD4+ derivatives (Fig. 4A). Together, these data suggest that both CD4-dependent and CD4-independent mechanisms operate in the process of inhibition of the Ras-MAP kinase signaling pathway in CD45RO+ cells following stimulation of these cells with the high-affinity TCR ligand.
FIG. 4.
Augmentation of Elk transactivation by the intermediate-affinity TCR ligand is partially dependent on CD4. (A) CD4+ or CD4− BW5147 TCR+ CD3+ CD45RO+ cells (clone 18.16) were transfected with the Elk-1–GAL4–5×GAL4–luciferase reporter system and the pRL-CMV internal vector control and stimulated with different concentrations of plate-bound MAb 3D3 or 5A. Luciferase activity was normalized against the internal reporter, and the ratio between the maximal responses obtained with the two MAbs from each cell type was determined. Numbers 1, 2, 3, and 4 indicate separate experiments. (B) Flow cytometric analysis of CD45RO+ CD4+ (solid) and CD45RO+ CD4− (dots) BW cells stained with phycoerythrin-labeled anti-CD4 MAb GK1.5. The cell surface expression of CD45 and the TCR-CD3 complex was also measured and was the same in both cell types (data not shown).
Rap1 mediates negative signaling in CD45RO+ T cells stimulated with the high-affinity TCR ligand.
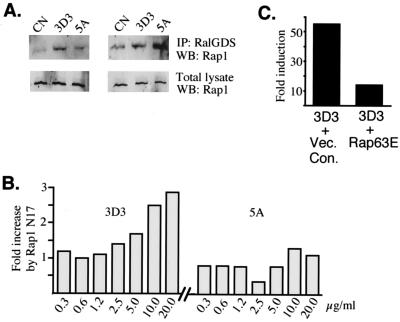
One possible explanation of the finding that strong stimulation of the TCR-CD4 complex results in suboptimal transcriptional activation could be that under this condition a negative regulatory mechanism is activated to prohibit full triggering of Ras-MAP kinase pathway activation. Since Rap1 has been implicated in the suppression of Ras-mediated effector signaling in T cells (3), we deemed it important to compare the levels of activated Rap1 in BW cells treated with MAbs 5A and 3D3. Pulldown experiments employing the GST-Rap1 binding domain of RalGDS as a probe for the active form of Rap1 (12) showed that stimulation of CD45RO+ BW cells with MAb 5A induces less accumulation of Rap1-GTP than does similar treatment with MAb 3D3 (Fig. 5A, left). In contrast, no such reduction was seen in BW cells expressing CD45ABC (Fig. 5A, right). This observation supports the view that the MAb 5A-dependent increase in Elk-1 activation can be attributed to the reduced intracellular concentration of Rap-GTP and diminished negative signaling in CD45RO+ cells.
FIG. 5.
Rap1 suppresses T-cell stimulation in CD45RO+ cells stimulated with high-affinity anti-TCR ligand. (A) Pulldown experiments assessing Rap1 activity (12). Equal numbers of TCR+ CD4+ CD45RO+ (left) or TCR+ CD4+ CD45ABC+ (right) BW5147 cells were unstimulated or treated with plate-bound MAb 3D3 or 5A, lysed, and incubated with the GST-Rap binding domain of RalGDS precoupled to glutathione-Sepharose 4B. The washed, pulled down materials (top) or total lysates (bottom) were run on the gel and stained with an anti-Rap1 antibody. IP, immunoprecipitate; CN, unstimulated control; WB, Western blotting. (B) Effect of dominant negative mutant RapN17 on Elk-1–GAL4–5×GAL4–luciferase transactivation in CD45RO+ cells stimulated with different-affinity TCR ligands. CD45RO+ BW thymona cells were transiently transfected with the Elk-1 reporter and the pRL-CMV internal control and cotransfected with either the empty vector control or the expression plasmid encoding RapN17. Cells were than stimulated with different concentrations of MAb 3D3 (left) or 5A (right), and the effect of the mutant was determined for each antibody concentration as the ratio of stimulation in cells transfected with RapN17 to the stimulation in cells transfected with the empty vector. Luciferase activities were normalized to the pRL-CMV internal control. Similar results were obtained in three independent experiments. (C) Effect of constitutively active mutant Rap63E on Elk-1–GAL4–5×GAL4–luciferase in BW cells stimulated via the TCR. TCR+ CD4+ CD45RO+ cells (clone 18.16) were transfected with the reporter, the internal control (pRL-CMV), and 0.5 μg of the Rap63E expression plasmid (right) or the empty vector (left) and stimulated with MAb 3D3. Luciferase activities were normalized and expressed as fold induction over the reporter transactivation in unstimulated cells. Vec. Con., vector control.
Next, utilizing the putative dominant negative mutant form of Rap1, we reexamined the function of this small GTPase. Transient expression of RapN17 augmented by severalfold the activation of the Elk-1 reporter during stimulation with MAb 3D3 (Fig. 5B, left; see also Fig. 6B, left), mimicking previously seen effects of the treatment of CD45RO+ BW cells with MAb 5A (Fig. 3A). Treatment of CD45RO+ cells with MAb 3D3 plus RapN17 produced a similar or higher absolute transactivation level of the Elk-1–luciferase reporter than the parallel MAb 5A-induced stimulation of the control cells transfected with the empty vector. Moreover, this prominent potentiation by RapN17 was particularly strong during stimulation with high antibody concentrations, suggesting that the negative function of Rap1 is accentuated with increasing TCR-ligand interaction engagement (Fig. 5B, left). Interestingly, stimulation of the TCR with MAb 5A was less sensitive to this enhancing effect of mutant Rap1 (Fig. 5B, right). In a control experiment with constitutively active Rap63E, we found that this mutant protein potently suppressed TCR-induced Elk-1 in CD45RO+ cells (Fig. 5C). Together, the biochemical and functional studies strongly implicate Rap1 as an inhibitor of the Ras signaling pathway in T cells which carry the low-molecular-weight CD45 isoform and whose TCR is highly engaged by the ligand.
FIG. 6.
Differential requirements for Ras and Rap1 in transactivation of Elk-1 in CD45ABC+ and CD45RO+ T cells. CD45ABC+ (open) and CD45RO+ (closed) BW cells were transfected with the Elk-1–GAL4–5×GAL4 reporter; cotransfected with 0, 62.5, 125, 250, 500, or 1,000 ng of dominant negative mutant RasN17 (A) or RafN4 (C), with 125, 250, 500, or 1,000 ng of RapGAP (B middle), or with 125, 250, 500, 1,000, or 2,000 ng of RapN17 (B, left and right); and stimulated with an anti-TCR MAb (A, B, and C, left; B, middle) or PMA (A, B, and C, right). The amount of total DNA used for each transfection was adjusted with the matched empty vector control to 1.0 or 2.0 μg, as required. (D) Comparison of the effects of RapN17 on CD45ABC+ and CD45RO+ cells stimulated with different concentrations of MAb 3D3. Cells were transfected with the Elk-1–GAL4–4×GAL4–luciferase reporter and cotransfected with a single dose of RapN17 or the empty vector control and stimulated with different concentrations of MAb 3D3. The data are expressed as the ratio of the stimulation in cell transfected with RapN17 to the stimulation in cells transfected with the empty vector control. Luciferase activities were normalized against the internal control (pRL-CMV). RLU, relative luciferase units.
Transcriptional activation of Elk-1 requires involvement of distinct members of the Ras protein family in CD45RO+ and CD45ABC+ cells.
Rap1 appears to negatively influence the activation of CD45RO+ T cells. We next wished to determine the primary function of Rap1 in T lymphocytes expressing the high-molecular-weight CD45 isoforms. To answer this question and better understand the regulation of Ras proteins in T cells, experiments were performed with the dominant negative mutant forms of Ras and Rap and the physiologic Rap1 inhibitor RapGAP (41). CD45RO+ and CD45ABC+ T cells were transiently transfected with the Elk-1–GAL4–5×GAL4–luciferase constructs and cotransfected with increasing doses of RasN17, RapN17, or RapGAP. As before (Fig. 1B), the inhibitory capacity of RasN17 prevailed in CD45RO+ cells (Fig. 6A left), and this finding is consistent with earlier data showing that Ras-dependent elements are better transactivated in CD45RO+ T cells (Fig. 1A and 2A). In contrast, RapN17 (Fig. 6B, left, and D) and RapGAP (Fig. 6B, middle) significantly increased Elk-1 transactivation in CD45RO+ T cells. This was acutely distinct from an inhibitory effect of these constructs on the Elk-1 reporter in CD45ABC+ expressors. This differential ability of transdominant negative RasN17, and RapGAP or RapN17, to inhibit TCR-induced activation of Ras proteins in cells expressing CD45RO or CD45ABC suggests the possibility that distinct CD45 isoforms influence TCR coupling to downstream signaling events via different Ras proteins. In addition, because RapN17 and RapGAP augmented or suppressed Elk-1 transactivation in CD45RO+ and CD45ABC+ cells, respectively, an argument can be made that Rap1 is activated in CD45ABC+, as well as in CD45RO+, cells. However, its primary function toward the MAP kinase signaling pathway is distinctly determined by these different CD45 isoforms.
Several controls were included in this set of experiments. In the first one, employing treatment of cells with phorbol ester, activation of the Elk-1 reporter was only mildly inhibited by mutant RasN17 and with no essential difference between CD45ABC+ and CD45RO+ cells (Fig. 6A, right). This observation is consistent with the fact that PMA inhibits Ras GTPase-activating protein (10) and increases the level of Ras-GTP independently of the nucleotide exchange factor activities affected by the mutant RasN17 used in this experiment. Also in the second control experiment, no major difference in the effects of RapN17 on PMA-induced Elk-1 activation was seen between CD45RO+ and CDABC+ cells (Fig. 6B, right). In the third control, mutant RafN4, which binds to the Ras and Rap1 effector domains, was able to inhibit TCR-induced transactivation of Elk-1 in both cell types with similar degrees of efficiency (Fig. 6C, left). This finding suggests that the differential effects of distinct CD45 isoforms on the MAP kinase signaling pathway involve primarily proximal signal transduction events which control distinct small G proteins and that these differences merge at the level of the effector signals positioned downstream of Ras and Rap1. Finally, in contrast to mutant RasN17, RafN4 also inhibited the PMA-induced responses from the Elk-1 reporter (Fig. 6C, right). This result is in agreement with the notion that PMA-induced active GTP-bound Ras requires a free effector domain in order to exercise its biological function on Elk-1.
DISCUSSION
The aim of this study was to define the role of different-size isoforms of CD45 in the regulation of small-GTPase-mediated signaling in T lymphocytes. Three observations emerged from our work. First, we provided evidence that the expression of small CD45 isoforms (p170 and p180) improves the ability of T cells to activate the Ras-MAP kinase signaling pathway. In this regard, we showed that two distinct Ras-regulated reporter systems are preferentially transactivated in T cells expressing CD45RO and in addition that these strong responses are highly sensitive to RasN17. Although it is unclear why CD45RO+ cells preferentially transactivate Ras-dependent signals rather than CD45ABC+ expressors, previous work in this laboratory demonstrated that low- rather than high-molecular-weight isoforms of CD45 interact with the TCR-CD3 complex (22, 23), suggesting an early mechanism by which the extracellular portion of CD45 may regulate the extent of proximal signaling events. According to this model, close interaction between the TCR and CD45RO tyrosine phosphatase may cause higher activity of the TCR-recruited proximal protein tyrosine kinases, leading to more efficient recruitment of the adapter proteins and exchange factor Sos, and ultimately result in improved Ras activation. Since Ras signaling has been implicated in the upregulation of cytokine genes (2, 32, 36), such augmented function of Ras and its effectors in activated CD45RO+ memory T cells might facilitate synthesis and/or secretion of the effector cytokines in these cells.
Second, we have demonstrated that in CD45RO+ cells, the clonotypic MAb (3D3) with high affinity for the TCR and high CD4 cocapping potency produced a suboptimal transcriptional outcome of the Ras-MAP kinase pathway and that Rap1 activation is likely responsible for the negative regulation of Ras-induced effector signals during this stimulation. One interpretation of this finding is that strong stimulation of the TCR complex, together with expression of low-molecular-weight CD45 isoforms, may actually be harmful rather than beneficial in obtaining maximal transcriptional responses. It is possible that not only positive but also inhibitory signals operate at a higher rate in CD45RO+ than in CD45ABC+ cells. Such negative regulation of the Ras-MAP kinase pathway in primary T lymphocytes may be important in the prevention of uncontrolled and cytokine-independent cell proliferation, it may protect T cells from overstimulation and antigen-induced death, or alternatively, through downregulation of the antiapoptotic Ras effector signals (15), it might decrease the survival potential of activated CD45RO+ memory T cells.
Finally, experiments with the dominant negative mutant form of Rap1 suggested that, in contrast to CD45RO+ cells in which p21Ras appears to be a major inducer of MAP kinase–Elk-1 responses, in CD45ABC+ cells this role is also played by p21Rap1. We therefore propose that in T cells, Rap1 does not act as a pure functional antagonist of Ras but rather that under certain conditions it can also mimic Ras effects. This conclusion is consistent with information available in the literature. Signaling pathways consisting of Cbl and Crk adapter proteins and the C3G exchange factor upregulate Rap1 in anergic T cells and implicate Rap1 in these cells in the suppression of Ras effector signals (3). However, it has also been demonstrated that Rap1 can cause positive effects independently of Ras while using similar or identical Ras effector pathways (52). For example, in neuronal PC12 cells, Rap has been connected with the cyclic AMP (cAMP)-induced activation of B-Raf and subsequently the activation of Mek and Erk and the transcription of Elk-1 (49). Thus, in contrast to Ras, for which the predominant mechanism of activation is association of guanine nucleotide exchange factors with the cell membrane, Rap1 can be activated by highly motile second messengers. The recently discovered protein Epac, an exchange protein activated by cAMP, provides a potential mechanism by which cAMP may directly activate Rap1 (8). Moreover, discoveries of other Rap1-specific exchangers have revealed additional complexity of Rap1 regulation (7, 16). Although we do not fully understand the proximal routes which couple TCR-CD45RO+ and TCR-CD45ABC+ to distinct functions of Rap1, the existence of multiple exchange factors for this small GTPase may provide an effective way to polarize Rap1 functions in a single cell type.
It is noteworthy that in addition to Ras antagonistic and, under certain circumstances, Ras synergistic effects on the MAP kinase signaling pathway, Rap has also been indicated to have other unique functions. Cell adhesion (14, 38, 48), cytoskeleton organization (46), and events controlling the mitochondrial oxidative burst (26, 35) are examples of the processes in which Rap is thought to be involved. In particular, two recent reports have strongly implicated Rap as an important immunological modulator during T-lymphocyte intercellular adhesion molecule and vascular adhesion molecule binding (14, 38). Therefore, it is plausible that in our studies examining the outcomes of Rap inhibition, RapN17 and RapGAP do not necessarily work through the block of influences on Raf kinases but rather might interfere with other processes controlled by this small GTPase.
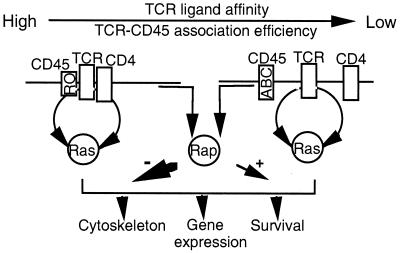
In conclusion, we propose that two factors, the size of the CD45 isoform expressed on the T-cell surface and the interaction affinity between TCR-CD4 and antigen-major histocompatibility complex are critical determinants of the signaling contributions of Ras and Rap1. Accordingly, the stimulation of T cells with low-affinity ligands in the presence of the high-molecular-weight isoform of CD45 results primarily in functional synergism between Ras and Rap1. In contrast, stimulation of T cells with high-affinity TCR ligands in the presence of low-molecular-weight CD45 isoforms induces Rap activation, which antagonizes Ras pathway signaling (Fig. 7). It will be interesting to know whether the different functional relationships between Ras and Rap1 contribute to remote lymphocyte behaviors such as Th1-Th2 helper differentiation, thymocyte maturation, or memory cell acquisition.
FIG. 7.
Model of the putative functional organization of Ras and Rap1 by the TCR-CD4-CD45 interacting complex. Low-molecular-weight isoforms of CD45 (RO) efficiently interact with the TCR, thereby increasing the amplitude of the TCR effector signals. Under these conditions, stimulation of the TCR-CD4 complex with the high-affinity ligand (e.g., MAb 3D3) delivers a strong signal which involves Rap1 primarily in the negative regulation of Ras effector functions. In contrast, stimulation of the TCR with intermediate-to-low-affinity ligands (e.g., MAb 5A) fails to recruit CD4 and, in the presence of the high-molecular-weight isoform of CD45 (ABC), generates a signal that is below the threshold required for the negative regulation of Ras by Rap1 yet is sufficient to positively engage Rap1 toward the Ras-MAP kinase signaling pathway.
ACKNOWLEDGMENTS
We thank C. A. Janeway and G. M. Losyev for MAbs 5A and 3D3, C. J. Der for helpful suggestions and RafN4, L. A. Quilliam for Rap constructs and RapGAP, Teresa Brtva and K. L. Guan for RasN17, M. H. Cobb for dominant negative Erk-1 and Erk-2, J. L. Bos for GST-RBD of RalGDS, and C. Dong and E. Serfling for the IL-2–luciferase reporter construct. We also thank Teresa Brtva for critical reading of the manuscript.
REFERENCES
- 1.Alberola-Ila J, Forbush K A, Seger R, Krebs E G, Perlmutter R M. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 2.Baldari C T, Macchia G, Telford J L. Interleukin-2 promoter activation in T-cells expressing activated Ha-ras. J Biol Chem. 1992;267:4289–4291. [PubMed] [Google Scholar]
- 3.Boussiotis V A, Freeman G J, Berezovskaya A, Barber D L, Nadler L M. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science. 1997;278:124–128. doi: 10.1126/science.278.5335.124. [DOI] [PubMed] [Google Scholar]
- 4.Boutin Y, Leitenberg D, Tao X, Bottomly K. Distinct biochemical signals characterize agonist- and altered peptide ligand-induced differentiation of naïve CD4+ T cells into Th1 and Th2 subsets. J Immunol. 1997;159:5802–5809. [PubMed] [Google Scholar]
- 5.Bruder J T, Heidecker G, Rapp U R. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992;6:545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- 6.Campbell S L, Khosravi-Far R, Rossman K L, Clark G J, Der C J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 7.de Rooij J, Boenink N M, van Triest M, Cool R H, Wittinghofer A, Bos J L. PDZ-GEF1, a guanine nucleotide exchange factor specific for rap1 and rap2. J Biol Chem. 1999;274:38125–38130. doi: 10.1074/jbc.274.53.38125. [DOI] [PubMed] [Google Scholar]
- 8.de Rooij J, Zwartkruis F J, Verheijen M H, Cool R H, Nijman S M, Wittinghofer A, Bos J L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 9.Dianzani U, Luqman M, Rojo J, Yagi J, Baron J L, Woods A, Janeway C A, Bottomly K. Molecular association on the T cell surface correlate with immunological memory. Eur J Immunol. 1990;20:2249–2257. doi: 10.1002/eji.1830201014. [DOI] [PubMed] [Google Scholar]
- 10.Downward J, Graves J D, Warne P H, Rayter S, Cantrell D A. Stimulation of p21ras upon T-cell activation. Nature. 1990;346:719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- 11.Fields P E, Gajewski T F, Fitch F W. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 12.Franke B, Akkerman J W, Bos J L. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 1997;16:252–259. doi: 10.1093/emboj/16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogquist K A, Jameson S C, Heath W R, Howard J L, Bevan M J, Carbone F R. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 14.Katagari K, Hattori M, Minato N, Irie S-K, Takatsu K, Kinashi T. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol Cell Biol. 2000;20:1956–1969. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature. 1997;385:544–548. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki H, Springett G M, Toki S, Canales J J, Harlan P, Blumenstiel J P, Chen E J, Bany I A, Mochizuki N, Ashbacher A, Matsuda M, Housman D E, Graybiel A M. A Rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proc Natl Acad Sci USA. 1998;95:13278–13283. doi: 10.1073/pnas.95.22.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kersh E N, Shaw A S, Allen P M. Fidelity of T cell activation through multistep T cell receptor zeta phosphorylation. Science. 1998;281:572–575. doi: 10.1126/science.281.5376.572. [DOI] [PubMed] [Google Scholar]
- 18.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 19.Koretzky G A, Picus J, Schultz T, Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci USA. 1991;88:2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koretzky G A, Picus J, Thomas M L, Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature. 1990;346:66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- 21.Leitenberg D, Bottomly K. Regulation of naïve T cell differentiation by varying the potency of TCR signal transduction. Sem Immunol. 1999;11:283–292. doi: 10.1006/smim.1999.0184. [DOI] [PubMed] [Google Scholar]
- 22.Leitenberg D, Boutin Y, Lu D D, Bottomly K. Biochemical association of CD45 with the T cell receptor complex: regulation by CD45 isoform and during T cell activation. Immunity. 1999;10:701–711. doi: 10.1016/s1074-7613(00)80069-2. [DOI] [PubMed] [Google Scholar]
- 23.Leitenberg D, Novak T J, Farber D, Smith B R, Bottomly K. The extracellular domain of CD45 controls association with the CD4-T cell receptor complex and the response to antigen-specific stimulation. J Exp Med. 1996;183:249–259. doi: 10.1084/jem.183.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B, Subleski M, Fusaki N, Yamamoto T, Copeland T, Princler G L, Kung H, Kamata T. Catalytic activity of the mouse guanine nucleotide exchanger mSOS is activated by Fyn tyrosine protein kinase and the T-cell antigen receptor in T cells. Proc Natl Acad Sci USA. 1996;93:1001–1005. doi: 10.1073/pnas.93.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Whaley C D, Mondino A, Mueller D L. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 26.Maly F-E, Quilliam L A, Dorseuil O, Der C J, Bokoch G M. Activated or dominant inhibitory mutants of Rap1A decrease the oxidative burst of Epstein-Barr virus-transformed human B lymphocytes. J Biol Chem. 1994;269:18743–18746. [PubMed] [Google Scholar]
- 27.McKenney D W, Onodera H, Gorman L, Mimura T, Rothstein D M J. Distinct isoforms of the CD45 protein-tyrosine phosphatase differentially regulate interleukin 2 secretion and activation signal pathways involving Vav in T cells. J Biol Chem. 1995;270:24949–24954. doi: 10.1074/jbc.270.42.24949. [DOI] [PubMed] [Google Scholar]
- 28.Metz D P, Bottomly K. Function and regulation of memory T cells. Immunol Res. 1999;19:127–141. doi: 10.1007/BF02786482. [DOI] [PubMed] [Google Scholar]
- 29.Mustelin T, Pessa-Morikawa T, Autero M, Gassmann M, Andersson L C, Gahmberg C G, Burn P. Regulation of the p59fyn protein tyrosine kinase by the CD45 phosphotyrosine phosphatase. Eur J Immunol. 1992;22:1173–1178. doi: 10.1002/eji.1830220510. [DOI] [PubMed] [Google Scholar]
- 30.Novak T J, Farber D, Leitenberg D, Hong S C, Johnson P, Bottomly K. Isoforms of the transmembrane tyrosine phosphatase CD45 differentially affect T cell recognition. Immunity. 1994;1:109–119. doi: 10.1016/1074-7613(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 31.Ostergaard H L, Shackelford D A, Hurley T R, Johnson P, Hyman R, Sefton B M, Trowbridge I S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci USA. 1989;86:8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owaki H, Vartma R, Gillis B, Bruder J T, Rapp U R, Davis L S, Geppert T D. Raf-1 is required for T cell IL2 production. EMBO J. 1993;12:4367–4373. doi: 10.1002/j.1460-2075.1993.tb06121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel H R, Renz H, Terada N, Gelfand E W. Differential activation of p21ras in CD45RA+ and CD45RO+ human T lymphocytes. J Immunol. 1994;152:2830–2836. [PubMed] [Google Scholar]
- 34.Pilarski L M, Gillitzer R, Zola H, Shortman K, Scollay R. Definition of the thymic generative lineage by selective expression of high molecular weight isoforms of CD45 (T200) Eur J Immunol. 1989;19:589–597. doi: 10.1002/eji.1830190403. [DOI] [PubMed] [Google Scholar]
- 35.Quinn M T, Parkos C A, Walker L, Orkin S H, Dinauer M C, Jesaitis A J. Association of a Ras-related protein with cytochrome b of human neutrophils. Nature. 1989;342:198–200. doi: 10.1038/342198a0. [DOI] [PubMed] [Google Scholar]
- 36.Rayter S I, Woodrow M, Lucas S C, Cantrell D A, Downward J. p21ras mediates control of IL-2 gene promoter function in T cell activation. EMBO J. 1992;11:4549–4556. doi: 10.1002/j.1460-2075.1992.tb05556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reedquist K A, Bos J L. Costimulation through CD28 suppresses T cell receptor-dependent activation of the Ras-like small GTPase Rap1 in human T lymphocytes. J Biol Chem. 1998;273:4944–4949. doi: 10.1074/jbc.273.9.4944. [DOI] [PubMed] [Google Scholar]
- 38.Reedquist K A, Ross E, Koop E A, Wolthuis R M F, Zwartkruis F J T, van Kooyk Y, Salmon M, Buckley C D, Bos J L. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson A T, Miller N, Alexander D R. CD3 antigen-mediated calcium signals and protein kinase C activation are higher in CD45RO+ than in CD45RA+ human T lymphocyte subsets. Eur J Immunol. 1993;23:61–68. doi: 10.1002/eji.1830230111. [DOI] [PubMed] [Google Scholar]
- 40.Rojo J M, Saizawa K, Janeway C A. Physical association of CD4 and the T-cell receptor can be induced by anti-T-cell receptor antibodies. Proc Natl Acad Sci USA. 1989;86:3311–3315. doi: 10.1073/pnas.86.9.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubinfeld B, Munemitsu S, Clark R, Conroy L, Watt K, Crosier W J, McCormick F, Polakis P. Molecular cloning of a GTPase activating protein specific for the Krev-1 protein p21rap1. Cell. 1991;65:1033–1042. doi: 10.1016/0092-8674(91)90555-d. [DOI] [PubMed] [Google Scholar]
- 42.Saizawa K, Rojo J, Janeway C A. Evidence for a physical association of CD4 and CD3:alpha:beta T-cell receptor. Nature. 1987;328:260–263. doi: 10.1038/328260a0. [DOI] [PubMed] [Google Scholar]
- 43.Serfling E, Barthelmas R, Pfeuffer I, Schenk B, Zarius S, Swoboda R, Mercurio F, Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocyte. EMBO J. 1989;8:465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sloan-Lancaster J, Shaw A S, Rothbard J B, Allen P M. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 45.Swat W, Shinkai Y, Cheng H L, Davidson L, Alt F W. Activated Ras signals differentiation and expansion of CD4+8+ thymocytes. Proc Natl Acad Sci USA. 1996;93:4683–4687. doi: 10.1073/pnas.93.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Torti M, Bertoni A, Canobbio I, Sinigaglia F, Lapetina E G, Balduini C. Rap1B and Rap2B translocation to the cytoskeleton by von Willebrand factor involves FcγII receptor-mediated protein tyrosine phosphorylation. J Biol Chem. 1999;274:13690–13697. doi: 10.1074/jbc.274.19.13690. [DOI] [PubMed] [Google Scholar]
- 47.Trowbridge I S, Thomas M L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 48.Tsukamoto N, Hattori M, Yang H, Bos J L, Minato N. Rap1 GTPase-activating protein SPA-1 negatively regulates cell adhesion. J Biol Chem. 1999;274:18463–18469. doi: 10.1074/jbc.274.26.18463. [DOI] [PubMed] [Google Scholar]
- 49.Vossler M R, Yao H, York R D, Pan M G, Rim C S, Stork P J. cAMP activated MAP kinase and Elk-1 through a B-Raf and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita M, Kimura M, Kuba M, Shimizu C, Tada T, Perlmutter R M, Nakayama T. T cell antigen receptor-mediated activation of the Ras/mitogen-activated protein kinase pathway controls interleukin 4 receptor function and type-2 helper T cell differentiation. Proc Natl Acad Sci USA. 1999;96:1024–1029. doi: 10.1073/pnas.96.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoon S T, Dianzani U, Bottomly K, Janeway C A. Both high and low avidity antibodies to the T cell receptor can have agonist or antagonist activity. Immunity. 1994;1:563–569. doi: 10.1016/1074-7613(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 52.York R D, Yao H, Dillon T, Ellig C L, Eckert S P, McCleskey E W, Stork P J. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]