Figure 1.
Experimental measurement of cell volume and of the temporal evolution of cell height
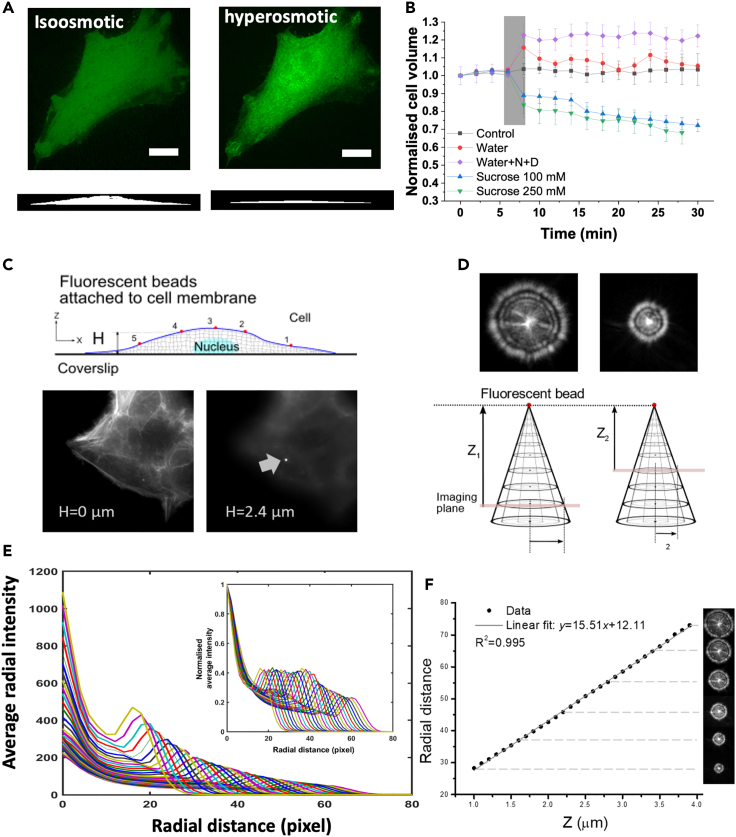
(A) The volume of HeLa cells was measured by acquiring xyzt confocal stacks. Top images show the cytoplasmic GFP cells under isosmotic and hyperosmotic conditions and the bottom images are orthogonal views of the 3D reconstruction of the cell volume. Scale bars = 10μm.
(B) Cell volume change over time in response to changes in extracellular osmolarity. Error bars indicate the standard deviation from n = 3–5 independent experiments. The shadow area indicates the time of addition of osmolytes. The water + N + D and sucrose 100mM curves are extracted from our previous work (Moeendarbary et al., 2013). Error bars indicate the standard deviation.
(C) Schematic of fluorescent nanobeads covalently linked to the cell membrane. The height of a bead attached to the cell is estimated by first finding the z position of the bottom of the dish (shown in the left focused image) where stress fibers are in focus, and then the position for which the bead attached to the cell membrane is in focus (shown in the right focused image).
(D) Keeping the focal plane fixed, the change in vertical position of each bead after swelling/shrinkage was determined from the radius of the outer ring formed in the defocused image.
(E) Moving the imaging plane (red line in panel d) in 100 nm increments away from the bead focal plane creates a series of curves showing the averaged radial fluorescence intensity as a function of distance from the center of the nanobeads. Inset shows the averaged radial intensity curves normalized to the maximum intensity in their center.
(F) Corresponding calibration curve demonstrating the linear relationship between the radial distance of outer ring and the height (z-position). The radial position of the ring (peak intensity) is linearly related to the distance between the imaging plane and the nanobead focal plane and therefore the z-position of the nanobeads can be tracked with very high accuracy (∼10 nm).