Abstract
Background: To date, evidence on whether sexualized drug use (SDU) and chemsex occur less frequently in rural compared to urban areas in Britain has been conflicting. This study aimed to better measure and understand whether attending urban versus rural sexual health clinics in the United Kingdom was associated with a difference in men who have sex with men’s (MSM) experience of SDU or their access to SDU support. Methods: Men from 29 sexual health services across England and Scotland were recruited by self-completing a waiting room survey. Results: A total of 2655 men (864 MSM) took part. There was no statistically significant difference in recent SDU or chemsex identified in MSM attending rural compared to urban clinics. Gamma-Hydroxybutyrate/Gamma-Butyrolactone (GHB/GBL) was the most commonly reported chemsex drug used in a sexual setting, with equal prevalence of use in urban and rural MSM attendees. Distance travelled for SDU was not significantly different for rural compared to urban MSM. Rural MSM reported a higher rate of unmet need for SDU specific services, although this difference was not statistically significant. Conclusion: Within this sample of MSM, there were no significant differences in sexualized drug use behaviours between those attending rural compared to urban sexual health settings.
Keywords: illicit drugs, drug users, sexual health, rural health services, urban health services
Introduction
Sexualized drug use (SDU), taking drugs during or immediately before sex, is reportedly increasing 1 and creates significant public health concern. 2 Chemsex, a subset of SDU, refers to particular drugs used in this context, by men who have sex with men (MSM). Chemsex drugs are usually defined as mephedrone, methamphetamine or Gamma-Hydroxybutyrate/Gamma-Butyrolactone (GHB/GBL), 3 although some UK and European studies have also included ketamine4,5 and cocaine 5 within the definition.
The effect of SDU and chemsex on individual and public health is well demonstrated but complex. By lowering inhibitions, individuals may take more risks during sexual activity when using drugs. Increased rates of condomless anal intercourse (CAI) have been demonstrated.6–13 SDU substances and chemsex drugs are sometimes injected (also known as ‘slamming’), with associated risks of infections with blood borne viruses. 3
Increased rates of sexually transmitted infections (STIs),8,12,14–16 blood borne viruses17,18 and sexually transmitted enteric pathogens (such as Shigella sp.) 19 have been linked to SDU and chemsex. SDU and chemsex have also been associated with increased use of HIV post-exposure prophylaxis (PEP) and pre-exposure prophylaxis (PrEP). 17 Chemsex has been demonstrated to have a negative effect on well-being, 12 mental health 20 and life satisfaction. 21
Responses to SDU and chemsex by service providers vary, by location and setting, from giving simple advice to provision of in-house ‘bespoke’ chemsex services. These latter services are only available in certain urban areas. 3 Sexual health clinics (SHC) are well positioned to identify those engaging in SDU and offer interventions. 3 SDU and chemsex have been demonstrated to be associated with recent SHC attendance and STI testing,13,21 and there is evidence that integrating services for both drug- and sexual-related harm reduction for MSM can be efficacious 22 and that most MSM attending these services had otherwise not had contact with drug support. 23
Estimates of the prevalence of SDU and chemsex vary greatly depending on the definition used, the recruitment setting and the subset of MSM studied. Most prevalence data available focus on MSM in large urban conurbations with a paucity of generalisable data available from rural areas. 2 However, a survey of sexual healthcare providers across the United Kingdom found that people reporting chemsex regularly attend SHCs throughout the United Kingdom including rural areas. 24 What also remains unclear is whether SDU is taking place near the individuals’ home address or whether people are travelling for SDU but accessing care more locally. This study aimed to better measure and understand the similarities and differences in chemsex and sexualized drug use in MSM in urban and rural Britain.
Methods
Study design
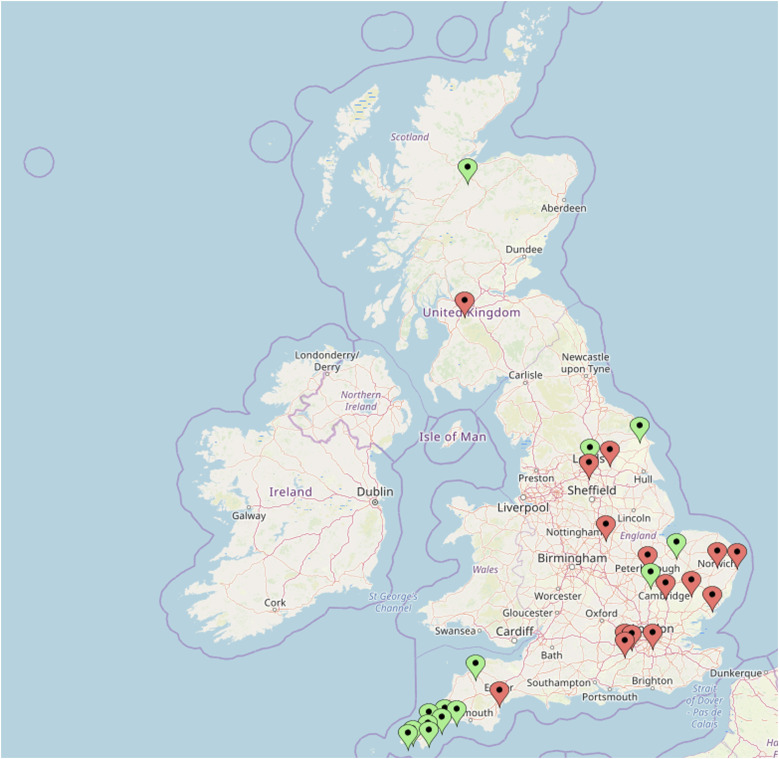
The Drugs and Sex Survey was a multi-site study of men attending SHCs in the United Kingdom between August and December 2018. Clinics were recruited through the British Association for Sexual Health and HIV (BASHH) Trainees Collaborative Audit, Research and Quality Improvement (TCARQ) network, professional networks or by the research team directly approaching clinics in representative areas. Twenty-nine clinics across England and Scotland agreed to take part (Supplementary File 1 and Figure 1). 25 Figure 1 and Supplementary Appendix 1 summarize site geography and contribution. SHCs were classified as urban or rural, based on clinic postcode according to Rural Urban Classification (2011) of Lower Layer Super Output Areas in England and Wales from the Office for National Statistics for clinics in England, 26 and Scottish Government Data Zone Urban Rural Classification 2013–2014 for clinics in Scotland. 27
Figure 1.
Map of participating urban (red) and rural (green) SHCs. SHC: sexual health clinics.
Participants
All men aged ≥18 years attending study clinics were invited to self-complete a paper or electronic questionnaire in the waiting room. A patient information leaflet explaining the purpose of the study and use of the data was provided to each participant prior to completion of the questionnaire. Participants were presumed to provide consent by choosing to participate in the survey.
Measures
The questionnaire had four sections collecting data on demographics, sexual health, SDU and chemsex and support needs for SDU and chemsex. Self-reported demographic data included age, gender, gender of partners and country of birth (UK or non-UK). For this survey, men and transmen with male sexual partners (whether exclusively or in addition to female and/or transfemale partners) were classified as MSM. Questions about sexual health, drug use and use of services asked about experiences in the previous 6 months. Sexual health questions asked about HIV status, PEP and PrEP use, diagnosis of an STI (gonorrhoea, chlamydia, syphilis, lymphogranuloma venereum (LGV) or shigella). Participants were asked about use of drugs before or during a sexual encounter including mephedrone, crystal methamphetamine, GHB/GBL, ketamine or other drugs (with a free text option). Recent SDU was defined as the use of any recreational drug before or during a sexual encounter in the past 6 months, and chemsex was defined as the use, by MSM, in a sexual context of mephedrone, crystal methamphetamine, GHB/GBL or ketamine. Participants were asked to report whether they had injected or ‘slammed’ drugs; whether they had shared injecting equipment; about concurrent use of alcohol and how far they had travelled from their home address the last time they had taken part in SDU. Distance travelled for most recent encounter was used as it was felt this would give a more accurate overall reflection of the variation in distances travelled as opposed asking about the furthest distance travelled. Participants were also asked whether a relative or friend, a doctor or healthcare worker had been concerned about their SDU and suggested they cut down. Support for SDU at SHC was assessed by asking participants to report the type of support they had received from a SHC in the past 6 months. Unmet need for SDU was defined as participants highlighting a service they needed and either could not get or did not try to get. The original questionnaire is available on request via the corresponding author.
Sample size
The sampling strategy aimed to maximize the number of MSM, chemsex user, respondents in the survey. We used published estimates of chemsex use in MSM of 13% in urban sites and 8% in rural sites. 24 The number of monthly attendances by MSM was estimated for clinics in England using the GUMCAD STI surveillance system data for 2017 and for Scottish clinics using national averages from NaSH (National Sexual Health System). These figures were used to assign a target number of questionnaires to be distributed to each clinic and to estimate the time needed to reach this target.
Data entry and statistical analysis
Data were double entered onto a secure database, with automated validation checks. Analysis and data cleansing was conducted in STATA 15.1. Missing data were assumed to be missing at random. Descriptive and comparative data analysis (using chi-squared or Fisher’s exact tests as appropriate) was completed.
Patient and public involvement
Patient peer groups from HIV services in both Leeds and Glasgow were consulted on the penultimate version of the questionnaire and provided input into the final questionnaire content.
Ethics and funding
The work was undertaken as a BASHH/Public Health England (PHE) fellowship and received support from the Sandyford Research Endowment Fund for data entry and survey logistics. The funder had no role in the design, analysis or interpretation of the study. Ethical approval was reviewed and granted by the West of Scotland Research Ethics Committee (ref 18/WS/0071). Health Research Authority approval was achieved for sites in England (IRAS 237310), and individual centres were given local Research and Innovation confirmation of capacity and capability.
Results
Demographics of survey participants
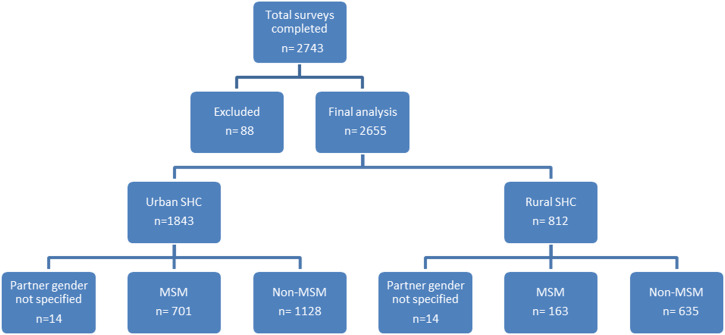
A total of 2743 questionnaires were received. Participants were excluded from the analysis if they were under 18 or did not identify as a man or transman when completing the survey. For final analysis, 2655 surveys were included of which 864 (32.5%) where completed by MSM (Figure 2).
Figure 2.
Exclusions and categories of survey participants.
MSM responses are detailed in Table 1. The majority were born in the United Kingdom (73.0%) with a mean age of 35.7 years (SD = 11.36, range 18–75 years). MSM participating in an urban SHC were younger than those participating in rural SHCs and more likely to be born outside the United Kingdom. A higher proportion of MSM in rural clinics reported being HIV-positive, but PEPSE and PrEP use were reported more frequently among MSM in urban clinics.
Table 1.
Demographics and SDU responses in MSM attending urban vs rural SHC settings.
| MSM urban SHC (n = 701) | MSM rural SHC (n = 163) | Total MSM (n = 864) | p value (urban vs rural) | |
| n (%) | n (%) | n (%) | ||
| Country of birth | ||||
| United Kingdom | 486 (69.3%) | 145 (89.0%) | 631 (73.0%) | <.001 |
| Non-UK | 213 (30.4%) | 18 (11.0%) | 231 (26.7%) | |
| Missing data | 2 (0.3%) | 0 (0%) | 2 (0.2%) | |
| Age group | ||||
| 18–24 | 114 (16.3%) | 29 (17.8%) | 143 (16.6%) | .002 |
| 25–34 | 272 (38.8%) | 59 (36.2%) | 331 (38.3%) | |
| 35–44 | 164 (23.4%) | 23 (14.1%) | 187 (21.6%) | |
| 45–54 | 92 (13.1%) | 27 (16.6%) | 119 (13.8%) | |
| 55+ | 50 (7.1%) | 25 (15.3%) | 75 (8.7%) | |
| Missing data | 9 (1.3%) | 0 (0%) | 9 (1.0%) | |
| HIV status | ||||
| Negative | 557 (79.5%) | 114 (69.9%) | 671 (77.7%) | .02 |
| Positive | 63 (9.0%) | 24 (14.7%) | 87 (10.1%) | |
| I don’t know/never had a test | 39 (5.6%) | 14 (8.6%) | 53 (6.1%) | |
| Missing data | 42 (6.0%) | 11 (6.7%) | 53 (6.1%) | |
| Taken PEPSE in last 6 months | ||||
| Yes | 63 (9.0%) | 6 (3.7%) | 69 (8.0%) | .009 |
| No | 590 (84.2%) | 141 (86.5%) | 731 (84.6%) | |
| I don’t know | 11 (1.6%) | 7 (4.3%) | 18 (2.1%) | |
| Missing data | 37 (5.3%) | 9 (5.5%) | 46 (5.3%) | |
| Taken PrEP in the last 6 months | ||||
| Yes | 152 (21.7%) | 27 (16.6%) | 179 (20.7%) | .03 |
| No | 503 (71.8%) | 122 (74.8%) | 625 (72.3%) | |
| I don’t know | 8 (1.1%) | 6 (3.7%) | 14 (1.6%) | |
| Missing data | 38 (5.4%) | 8 (4.9%) | 46 (5.3%) | |
| SDU in last 6 months | ||||
| Yes | 116 (16.5%) | 29 (17.8%) | 145 (16.8%) | .77 |
| No | 560 (79.9%) | 131 (80.4%) | 691 (80%) | |
| Missing data | 25 (3.6%) | 3 (1.8%) | 28 (3.2%) | |
| Chemsex in last 6 months | ||||
| Yes | 66 (9.4%) | 17 (10.4%) | 83 (9.6%) | .74 |
| No | 610 (87.0%) | 143 (87.7%) | 753 (87.2%) | |
| Missing data | 25 (3.6%) | 3 (1.8%) | 28 (3.2%) | |
SDU practices in MSM
SDU in the last 6 months was reported by 16.8% of MSM participants and chemsex by 9.6% (Table 1). GBL/GHB was the most commonly used chemsex drug. Stimulants were the most commonly reported non-chemsex SDU substance. In those reporting recent SDU, 11.0% reported injecting drugs during or prior to sex (‘slamming’). Sharing injecting equipment was reported by 25% of this group. These patterns were similar between urban and rural participants (Table 2).
Table 2.
Responses by MSM who report recent SDU.
| MSM urban SHC reporting SDU (n = 116) | MSM rural SHC reporting SDU (n = 29) | Total MSM reporting SDU (n = 145) | p value (urban vs rural) | |
| n (%) | n (%) | n (%) | ||
| Distance travelled from home address for last episode of SDU | ||||
| Less than 10 miles | 68 (58.6%) | 17 (58.6%) | 85 (58.6%) | .87 |
| 10–49 miles | 16 (13.8%) | 3 (10.3%) | 19 (13.1%) | |
| 50–99 miles | 6 (5.2%) | 2 (6.9%) | 8 (5.5%) | |
| 100 miles+ | 11 (9.5%) | 4 (13.8%) | 15 (10.3%) | |
| Missing data | 15 (12.9%) | 3 (10.3%) | 18 (12.4%) | |
| More than one drug (excluding alcohol) at a time for recent SDU | ||||
| Yes | 63 (54.3%) | 17 (58.6%) | 80 (55.2%) | .71 |
| No | 48 (41.4%) | 11 (37.9%) | 59 (40.7%) | |
| Missing data | 5 (4.3%) | 1 (3.4%) | 6 (4.1%) | |
| Self-, healthcare provider- or friend/family-identified concerns | ||||
| Yes | 18 (15.5%) | 6 (20.7%) | 24 (16.6%) | .44 |
| No | 95 (81.9%) | 21 (72.4%) | 116 (80.0%) | |
| Missing data | 3 (2.6%) | 2 (6.9%) | 5 (3.4%) | |
| An identified need for support from SHC for SDU but could not get it | ||||
| Yes | 4 (3.4%) | 3 (10.3%) | 7 (4.8%) | .14 |
| No | 112 (96.6%) | 26 (89.7%) | 138 (95.2%) | |
| Drugs and alcohol together for a sexual encounter in the last 6 months | ||||
| Never/occasionally | 97 (83.6%) | 20 (69.0%) | 117 (80.7%) | .045 |
| Mostly/always | 12 (10.3%) | 7 (24.1%) | 19 (13.1%) | |
| Missing data | 7 (6.0%) | 2 (6.9%) | 9 (6.2%) | |
| Chemsex drugs during/immed. before sex (any) | 66 (56.9%) | 17 (58.6%) | 83 (57.2%) | .74 |
| GBL/GHB (for sex) | 44 (37.9%) | 11 (37.9%) | 55 (37.9%) | 1.00 |
| Mephedrone (for sex) | 36 (31%) | 6 (20.7%) | 42 (29.0%) | .27 |
| Crystal Meth (for sex) | 27 (23.3%) | 11 (37.9%) | 38 (26.2%) | .11 |
| Ketamine (for sex) | 26 (22.4%) | 7 (24.1%) | 33 (22.8%) | .84 |
| Non-chemsex drug during/immed. before sex (any) | 67 (57.8%) | 19 (65.5%) | 86 (59.3%) | .45 |
| Stimulants (inc. Cocaine) (for sex) | 38 (32.8%) | 9 (31.0%) | 47 (32.4%) | .86 |
| Cannabinoids (for sex) | 18 (15.5%) | 5 (17.2%) | 23 (15.9%) | .82 |
| Nitrates (‘poppers’) (for sex) | 14 (12.1%) | 2 (6.9%) | 16 (11.0%) | .74 |
| PDE5 inhibitors (e.g. Viagra) (for sex) | 2 (1.7%) | 2 (6.9%) | 4 (2.8%) | .18 |
| Hallucinogenics (for sex) | 2 (1.7%) | 1 (3.4%) | 3 (2.1%) | .49 |
| Opioids (for sex) | 0 (0%) | 1 (3.4%) | 1 (0.7%) | .20 |
| Chemsex drugs outside a sexual setting (any) | 37 (31.9%) | 6 (20.7%) | 43 (29.7%) | .24 |
| GBL/GHB (out-with sex) | 15 (12.9%) | 4 (13.8%) | 19 (13.1%) | 1.00 |
| Mephedrone (out-with sex) | 16 (13.8%) | 1 (3.5%) | 17 (11.7%) | .20 |
| Crystal meth (out-with sex) | 6 (5.2%) | 3 (10.3%) | 9 (6.2%) | .38 |
| Ketamine (out-with sex) | 22 (19.0%) | 3 (10.3%) | 25 (17.2%) | .41 |
| Non-chemsex drug outside a sexual setting (any) | 50 (43.1%) | 10 (34.5%) | 60 (41.4%) | .40 |
| Slamming for SDU in last 6 months | ||||
| Yes | 10 (8.6%) | 6 (20.7%) | 16 (11%) | .07 |
| No | 101 (87.0%) | 22 (75.9%) | 123 (84.8%) | |
| Missing data | 5 (4.3%) | 1 (3.4%) | 6 (4.1%) | |
| If slammed in last 6 months, have you ever shared injecting equipment? | ||||
| Yes | 4 (40.0%) | 0 (0.0%) | 4 (25%) | .10 |
| No | 5 (50.0%) | 6 (100.0%) | 11 (68.85) | |
| Missing data | 1 (10.0%) | 0 (0.0%) | 0 (0.0%) | |
Using more than one drug at a time (excluding alcohol) for sexual purposes was common and reported by 55.2% which was similar by urban/rural classification. The use of alcohol in conjunction with SDU was rare. However, those reporting mostly or always using alcohol in combination with SDU were significantly more likely to be attending a rural clinic.
Respondents who reported SDU were also asked about the use of drugs outside of a sexual context. Ketamine was the most common chemsex drug used with 17.9% (26/145) reporting use in the previous 6 months in this context. This was followed by GBL/GHB at 13.1% (19/145), mephedrone at 12.4% (18/145) and crystal meth at 6.2% (9/145). Almost half (44.8%) reported taking another (non-chemsex) drug in this context. Rates of drug use outside a sexual setting were not significantly different between urban and rural SHC attendees (Table 2).
The majority of MSM reported travelling less than 10 miles the last time they engaged in SDU. Distance travelled was similar for both urban and rural MSM (Table 2).
Support for MSM SDU
Table 2 details responses by MSM about levels of support and concern for their SDU; 16.6% of respondents reported that they had concerns about their SDU or that someone else had raised concerns about their SDU, and there was no statistical difference between urban or rural participants. Overall, even fewer reported needing support but being unable to get it when attending their local SHC in the previous 6 months. This result was three times as high in rural MSM (3.4% urban vs 10.3% rural, p=.14) although did not meet statistical significance.
Factors associated with SDU in MSM
MSM reporting recent SDU were more likely to have had a bacterial STI diagnosed in the previous 6 months compared to those who did not. Recent SDU was not associated with HIV status. The use of recent HIV post-exposure prophylaxis (PEP) and PrEP use was significantly associated with SDU (Table 3).
Table 3.
Analyses of factors associated with MSM SDU in past 6 months.
| MSM not engaged in SDU (n = 691) | MSM engaged in SDU (n = 145) | ||
| n (%) | n (%) | p value | |
| Diagnosed bacterial STI in last 6 months | |||
| Yes | 176 (25.5%) | 62 (42.8%) | <.001 |
| No | 515 (74.5%) | 83 (57.2%) | |
| HIV status | |||
| Positive | 69 (10.0%) | 18 (12.4%) | .63 |
| Negative | 556 (80.5%) | 114 (78.6%) | |
| Unknown: I don’t know/never had a test | 45 (5.5%) | 8 (5.5%) | |
| Missing data | 21 (3%) | 5 (3.4%) | |
| PEPSE use in last 6 months | |||
| Yes | 45 (6.5%) | 23 (15.9%) | <.001 |
| No | 618 (89.4%) | 113 (77.9%) | |
| I don’t know | 17 (2.5%) | 1 (0.7%) | |
| Missing data | 11 (1.6%) | 8 (5.5%) | |
| PrEP use in last 6 months | |||
| Yes | 129 (18.7%) | 50 (34.5%) | <.001 |
| No | 538 (77.9%) | 87 (60%) | |
| I don’t know | 11 (1.6%) | 3 (2.1%) | |
| Missing data | 13 (1.9%) | 5 (3.4%) | |
SDU in men who exclusively had sex with women and transwomen (MSW)
In total, 661 of 1763 MSW answered questions on SDU, of whom 16.4% reported SDU in the previous 6 months. This is a similar proportion to SDU use in MSM. SDU was reported more frequently among MSW attending a rural clinic (19%) compared to those attending an urban clinic (14%) (p=.01). Chemsex was reported by 3.8% MSW overall, and more commonly in rural settings, although not statistically significant (3.2% urban; 4.8% rural; p=.10).
When comparing MSM SDU with MSW SDU, the use of stimulants (for example cocaine) (p=.04) and nitrates (poppers) (p<.01) was significantly more likely in MSM than MSW.
Discussion
This large survey is the first, of which we are aware, that directly compares SDU in MSM in both urban and rural settings in multiple sites across the United Kingdom. It supports previous literature suggesting that chemsex users attend both urban and rural SHCs, 24 and demonstrates comparable SDU and chemsex prevalence to other UK studies. 2 Furthermore, it suggests that the prevalence of SDU in rural settings is not significantly different from urban settings, which should be taken into account when planning and commissioning services.
The European MSM Internet Survey (EMIS) 4 identified higher rates of sexualized drug use occurring in London-based respondents compared to the rest of the United Kingdom (13.1% vs 4.1%) but SHC attendee data collected from more diverse areas of the United Kingdom, which provides a more reliable estimate for those involved in commissioning and service planning for SHCs, has not previously been examined.
HIV PrEP and PEP were significantly associated with both SDU and with attending an urban SHC. It is not possible to ascertain if this was due to differences in access to/awareness of these interventions or if this is due to differences in eligibility between the two groups. MSM attending rural SHCs who identified as being involved in SDU were as likely to have experienced/participated in this within their local area as those attending urban SHCs. This is particularly relevant for rural services, which are less likely to have bespoke SDU services. MSM reporting bacterial STI in the previous 6 months was significantly associated with SDU, in keeping with previous findings.8,12,14–16
Examining chemsex, specifically, previous data have suggested that men aged 36–45 years were more likely to engage in chemsex as compared to other age groups. 13 Although our data showed an increased use of chemsex in those over 35 years, the difference was not statistically significant. A recent review article looking at chemsex use in developed countries identified a wide prevalence range from 3 to 29%, with a prevalence of 17–27% in MSM recruited from sexual health clinics. 17 These are higher rates than observed in this study. In this cohort, GHB/GBL was the most commonly used chemsex substance, consistent with most other studies that have examined this. 17 Overall ketamine use was 3.8% in MSM in this sample, which is in keeping with review article findings of 1–4%. 17 Similarly, ketamine was used more commonly than mephedrone in this sample as with previous review article findings. 17 Injecting prevalence in this cohort was 1.9%, within the range of 1–9% in previous large MSM samples. 17 Thirteen percent of MSM who identified recent SDU said they used alcohol alongside SDU most or all of the time; a quarter of rural MSM reported concurrent alcohol which was significantly higher than in urban areas. Living with HIV was not significantly associated with SDU or chemsex in this sample. There have been conflicting data regarding this previously, with no association found in some studies,14,21 but significant association demonstrated in others.13,14,19,28 The lack of a significant association in this study might be explained by this survey being conducted in sexual health clinics, which often care for clients living with HIV or provide HIV services, but not in HIV clinics specifically. Therefore, clients living with HIV in studied geographical areas may have been under-represented.
Reported unmet need for specialist SDU services in rural MSM was proportionately higher compared to urban clinic responses. Although numbers were small and differences did not meet statistical significance, it does highlight a potential question over whether there is currently adequate access to these services across all areas of the United Kingdom and would benefit from further study. Concerns over SDU were raised by either the individual, a healthcare professional or a friend or relative in 16.6% of MSM reporting SDU. This may reflect a perceived lower risk, but may also be a consequence of limited assessment or understanding of the risks of SDU. MSM should be counselled appropriately regarding safety measures with SDU alongside alcohol consumption, especially in rural areas where specialist SDU services are less available. Online services could also be used to provide useful information and sign-posting to MSM requesting sexual health information and STI testing.
This is the first survey of which we are aware that also included data on MSW. However, this group was not the primary focus of the study and therefore underwent limited data analysis. Overall, 16.4% MSW reported drug use during or immediately prior to sexual intercourse in the last 6 months, which is a similar proportion to that in MSM, and 3.8% identified as this being with one or more chemsex drug. Those caring for individuals in a sexual health setting should be aware that this is a problem that affects a wider population than the MSM community.
Recent review articles2,17 have identified that most previous prevalence studies have not specifically separated the use of specific substances into use during or out-with a sexual setting. This study therefore gives us a better understanding of how people use certain drugs, including chems, both for sex events and in other situations.
It is important to acknowledge that as a limitation to this analysis we found less reported unmet need for chemsex support services than expected in both urban and rural settings. We hypothesized that there would be a greater unmet need in rural settings and the relatively small number of rural chemsex users in our sample may have been too small to have detected a significant difference. Alternately, this may be because individuals involved in SDU perhaps do not view this as an area of their health that needs addressed, and this may be incongruent to a healthcare provider’s view on that individual’s drug use. It is acknowledged that someone attending an urban clinic may live in a rural area, and vice versa. It is also acknowledged that there is potential for reporting bias of those involved in SDU, where confidentiality concerns may have led to under-reporting of SDU; this may be more of a concern in rural settings where people may have been more anxious regarding anonymity and stigma.
Conclusions
In this large survey across England and Scotland, we identified no significant difference between the prevalence of self-reported recent sexualized drug use, chemsex use or distance travelled for SDU for MSM attending rural compared to urban sexual health clinics. While the limited number of responses who identify an unmet need for SDU services make it difficult to draw strong conclusions, there does seem to be a disparity in this sample between access for rural attendees versus urban attendees.
Supplemental Material
Supplemental Material, sj-pdf-1-std-10.1177_09564624211041456 for Sexualized drug use and specialist service experience among MSM attending urban and rural sexual health clinics in England and Scotland by Richard Kennedy, Jennifer Murira, Kirsty Foster, Ellen Heinsbroek, Frances Keane, Nisha Pal, Lynn Chalmers and Katy Sinka in International Journal of STD & AIDS
Acknowledgements
With thanks to the survey participants; to Sandyford Endowment Fund who supplied funding for this project; to Professor Claudia Estcourt and Helen Wiggins for their support; to Dana Ogaz and Stuart Wrigglesworth for their support with data collection. Principle investigators: Lynn Chalmers; Sophia Davies; Michael Ewans; Frances Keane; Caroline Oswald; Nisha Pal; Manjula Pammi; Durba Raha and Nina Vora.
Author Contributions: R Kennedy: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing - original draft, visualization, supervision, project administration and funding acquisition; J Murira: conceptualization, methodology, formal analysis, investigation, data curation, writing - original draft, visualization, supervision and project administration; K Foster: conceptualization, methodology, writing - review and editing and supervision; E Heinsbroek: conceptualization, methodology, software, formal analysis and writing - review and editing; F Keane: investigation and writing - review and editing; N Pal: investigation and writing - review and editing; L Chalmers: investigation and writing - review and editing; K Sinka: conceptualization, methodology, writing - review and editing and supervision.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Sandyford Endowment Fund.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Richard Kennedy https://orcid.org/0000-0002-3844-9642
References
- 1.Sewell J, Cambiano V, Speakman A, et al. Changes in chemsex and sexual behaviour over time, among a cohort of MSM in London and Brighton: findings from the AURAH2 study. Int J Drug Pol 2019; 68: 54–61. [DOI] [PubMed] [Google Scholar]
- 2.Edmundson C, Heinsbroek E, Glass R, et al. Sexualised drug use in the United Kingdom (UK): a review of the literature. Int J Drug Pol 2018; 55: 131–148. [DOI] [PubMed] [Google Scholar]
- 3.Public Health England . Substance Misuse Services for Men Who Have Sex with Men Involved in Chemsex. London: PHE Publications, 2015. [Google Scholar]
- 4.Schmidt AJ, Bourne A, Weatherburn P, et al. Illicit drug use among gay and bisexual men in 44 cities: findings from the European MSM Internet Survey (EMIS). Int J Drug Pol 2016; 38: 4–12. [DOI] [PubMed] [Google Scholar]
- 5.Bourne A, Reid D, Hickson F, et al. The Chemsex Study: Drug Use in Sexual Settings Among Gay and Bisexual Men in Lambeth, Southwark and Lewisham. London: Sigma Research, London School of Hygiene & Tropical Medicine. [Google Scholar]
- 6.Ahmed AK, Weatherburn P, Reid D, et al. Social norms related to combining drugs and sex (“chemsex”) among gay men in South London. Int J Drug Pol 2016; 38: 29–35. [DOI] [PubMed] [Google Scholar]
- 7.Melendez-Torres GJ, Hickson F, Reid D, et al. Nested event-level case-control study of drug use and sexual outcomes in multipartner encounters reported by men who have sex with men. AIDS Behav 2016; 20(3): 646–654. [DOI] [PubMed] [Google Scholar]
- 8.Ottaway Z, Finnerty F, Amlani A, et al. Men who have sex with men diagnosed with a sexually transmitted infection are significantly more likely to engage in sexualised drug use. Int J STD AIDS 2017; 28(1): 91–93. [DOI] [PubMed] [Google Scholar]
- 9.Melendez-Torres GJ, Hickson F, Reid D, et al. Findings from within-subjects comparisons of drug use and sexual risk behaviour in men who have sex with men in England. Int J STD AIDS 2017; 28(3): 250–258. [DOI] [PubMed] [Google Scholar]
- 10.Gilbart VL, Simms I, Jenkins C, et al. Sex, drugs and smart phone applications: findings from semistructured interviews with men who have sex with men diagnosed with Shigella flexneri 3a in England and Wales. Sex Transm Infect 2015; 91(8): 598–602. [DOI] [PubMed] [Google Scholar]
- 11.Bourne A, Reid D, Hickson F, et al. Illicit drug use in sexual settings ('chemsex') and HIV/STI transmission risk behaviour among gay men in South London: findings from a qualitative study. Sex Transm Infect 2015; 91(8): 564–568. [DOI] [PubMed] [Google Scholar]
- 12.Glynn RW, Byrne N, O'Dea S, et al. Chemsex, risk behaviours and sexually transmitted infections among men who have sex with men in Dublin, Ireland. Int J Drug Pol 2018; 52: 9–15. [DOI] [PubMed] [Google Scholar]
- 13.Frankis J, Flowers P, McDaid L, et al. Low levels of chemsex among men who have sex with men, but high levels of risk among men who engage in chemsex: analysis of a cross-sectional online survey across four countries. Sex Health 2018; 15(2): 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegazi A, Lee MJ, Whittaker W, et al. Chemsex and the city: sexualised substance use in gay bisexual and other men who have sex with men attending sexual health clinics. Int J STD AIDS 2017; 28(4): 362–366. [DOI] [PubMed] [Google Scholar]
- 15.Rosinska M, Gios L, Nostlinger C, et al. Prevalence of drug use during sex amongst MSM in Europe: results from a multi-site bio-behavioural survey. Int J Drug Pol 2018; 55: 231–241. [DOI] [PubMed] [Google Scholar]
- 16.Druckler S, van Rooijen MS, de Vries HJC. Chemsex among men who have sex with men: a sexualized drug use survey among clients of the sexually transmitted infection outpatient clinic and users of a gay dating app in Amsterdam, the The Netherlands. Sex Transm Dis 2018; 45(5): 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Pol 2019; 63: 74–89. [DOI] [PubMed] [Google Scholar]
- 18.Page EE, Nelson M. Hepatitis C and sex. Clin Med (Lond) 2016; 16(2): 189–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbart VL, Simms I, Jenkins C, et al. Sex, drugs and smart phone applications: findings from semistructured interviews with men who have sex with men diagnosed with Shigella flexneri 3a in England and Wales. Sex Transm Infect 2015; 91(8): 598–602. [DOI] [PubMed] [Google Scholar]
- 20.Pakianathan M, Whittaker W, Lee MJ, et al. Chemsex and new HIV diagnosis in gay, bisexual and other men who have sex with men attending sexual health clinics. HIV Med 2018; 19: 485–490. [DOI] [PubMed] [Google Scholar]
- 21.Hibbert MP, Brett CE, Porcellato LA, et al. Psychosocial and sexual characteristics associated with sexualised drug use and chemsex among men who have sex with men (MSM) in the UK. Sex Transm Infect 2019; 95(5): 342–350. [DOI] [PubMed] [Google Scholar]
- 22.Knight R, Karamouzian M, Carson A, et al. Interventions to address substance use and sexual risk among gay, bisexual and other men who have sex with men who use methamphetamine: a systematic review. Drug Alcohol Depend 2019; 194: 410–429. [DOI] [PubMed] [Google Scholar]
- 23.Stevens O, Moncrieff M, Gafos M. Chemsex-related drug use and its association with health outcomes in men who have sex with men: a cross-sectional analysis of Antidote clinic service data. Sex Transm Infect 2020; 96(2): 124–130. [DOI] [PubMed] [Google Scholar]
- 24.Wiggins H, Ogaz D, Mebrahtu H, et al. Demand for and availability of specialist chemsex services in the UK: a cross-sectional survey of sexual health clinics. Int J Drug Pol 2018; 55: 155–158. [DOI] [PubMed] [Google Scholar]
- 25.Google Earth . Participating Urban and Rural Clinics. 2019. [Google Scholar]
- 26.Office for National Statistics. Rural Urban Classification (2011) of Lower Layer Super Output Areas in England and Wales. Available at: https://data.gov.uk/dataset/b1165cea-2655-4cf7-bf22-dfbd3cdeb242/rural-urban-classification-2011-of-lower-layer-super-output-areas-in-england-and-wales (accessed 01 October 2018). [Google Scholar]
- 27.Scottish Government . Scottish Government Urban Rural Classification 2013-2014. Available at: https://www2.gov.scot/Publications/2014/11/2763 (Accessed 01 October 2018). [Google Scholar]
- 28.Curtis TJ, Rodger AJ, Burns F, et al. Patterns of sexualised recreational drug use and its association with risk behaviours and sexual health outcomes in men who have sex with men in London, UK: a comparison of cross-sectional studies conducted in 2013 and 2016. Sex Transm Infect 2020; 96: 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-std-10.1177_09564624211041456 for Sexualized drug use and specialist service experience among MSM attending urban and rural sexual health clinics in England and Scotland by Richard Kennedy, Jennifer Murira, Kirsty Foster, Ellen Heinsbroek, Frances Keane, Nisha Pal, Lynn Chalmers and Katy Sinka in International Journal of STD & AIDS