Abstract
Cardiovascular disease (CVD) is recognized as a leading cause of death worldwide. Obesity, dyslipidemia, insulin resistance (IR), interconnected pathological conditions constitute risk factors that are closely associated with CVD. The aim of the present study was to highlight the association of IR with cardiovascular risk (CVR). The epidemiological, cross-sectional, non-interventional study was conducted over 12 months (2019-2020) within a research grant and included a sample of 400 subjects divided into 2 subgroups: group 1 (control) subjects did not have diabetes (n=200) and group 2 had type 2 diabetes (T2DM) (n=200). The Framingham risk score (FRS) was calculated according to the 2008 general CVD risk model from the Framingham Heart Study. Subsequent to a correlation of the value of homeostasis model assessment of insulin resistance (HOMA-IR) with the degree of CVR, the IR was higher in both groups, and CVR also increased. After being quantified by the Spearman correlation coefficient, the correlation in group 2 was higher at 0.625 compared to group 1 where this coefficient had a value of 0.440. A high FRS (FRS of 20%) was significantly associated with IR. The results therefore show that HOMA-IR is an independent risk factor for high FRS. New therapies focused on decreasing IR may contribute to decreased CVD.
Keywords: insulin resistance, type 2 diabetes mellitus, HOMA-IR, cardiovascular risk
Introduction
Cardiovascular disease (CVD) is recognized as a leading cause of death globally. Obesity, dyslipidemia, insulin resistance (IR), and interconnected pathological conditions are considered risk factors closely associated with CVD. IR is defined as a reduction in insulin sensitivity in peripheral tissues (1). It is manifested especially in the tissues that depend on the action of insulin for intracellular transport of glucose: liver, muscle and adipose tissue. The effects of IR are, however, multiple, as insulin exerts physiological actions on carbohydrate, lipid and protein metabolism, as well as on endothelial function (2).
Excess adipose tissue is not an inert tissue, it can directly produce and release inflammatory cytokines and other atherogenic molecules and it is associated with a low insulin response (3). Similarly, the liver takes up increased amounts of lipids from increased endogenous production and absorption of fatty acids from the blood, the consequence being the appearance of IR at this level. Insulin-resistant liver increases gluconeogenesis and decreases glycogen synthesis, perpetually increasing blood glucose levels (4). The combination of hepatic IR and high levels of circulating fatty acids also leads to increased very low-density lipoprotein (VLDL) production, lower high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) particles, all of which are associated with an increased risk of heart disease (5,6).
IR increases the release of free fatty acids from adipocytes, which increases circulating levels of free fatty acids, which stimulates the synthesis of triglyceride-rich VLDL particles in the liver, leading to increased HDL and triglyceride (TG)-rich LDL particles. The increase in triglycerides in lipid particles changes their metabolism. HDL particles are hydrolyzed more rapidly and HDL levels decrease. LDL particles are further subjected to lipolysis leading to the formation of small and dense LDL particles. The resulting dyslipidemia is extremely atherogenic and represents at least part of the increased risk of CVD in insulin-resistant individuals (7,8). Increased accumulation of lipids in the pancreas affects its response to increased plasma glucose levels and reduces insulin secretion (9).
At the level of the skeletal muscle, IR causes a decrease in peripheral glucose utilization, contributing to chronic hyperglycemia and/or hyperinsulinism (10). In addition to these organ-specific effects, hyperinsulinemia (itself a consequence of IR) increases blood pressure by activating the sympathetic nervous system (11). IR also decreases the synthesis and release of nitric oxide, which can directly affect vascular function thereby increasing cardiovascular risk (CVR) (12,13). In addition, hyperglycemia has been associated with the increased production of reactive oxygen species in several organ systems, which has been independently linked to CVD (14,15).
Taken together, these mechanisms suggested various pathways by which IR, hyperinsulinemia, and hyperglycemia directly contribute to the development and progression of atherosclerotic disease (16,17). The aim of the present study was to emphasize the association of IR with CVR.
Patients and methods
Study population
The epidemiological, cross-sectional, non-interventional study was conducted over 12 months (1 October, 2019-30 September, 2020) and included 400 subjects divided into 2 groups: group 1 (control) subjects did not have diabetes (n=200) and group 2 had type 2 diabetes (T2DM) (n=200). Subject characteristics are provided in Table I.
Table I.
General characteristics of the study participantsa.
| Variables | Total (n=400) | Group 1 (n=200) | Group 2 (n=200) | P-value |
|---|---|---|---|---|
| Age (years) | 62±10.261 | 61.83±10.231 | 62.16±10.313 | 0.728 |
| Sex (%) | ||||
| Women | 50 | 50 | 50 | |
| Men | 50 | 50 | 50 | |
| SBP (mmHg) | 139.16±22.09 | 139.88±20.09 | 138.44±23.95 | 0.515 |
| The presence of IR (%) | 35.7 | 79 | ||
| HOMA-IR index | 3.259±2.586 | 2.116±1.725 | 4.402±2.794 | <0.001 |
| FRS (%) | 19.75±9.48 | 16.23±9.6 | 23.20±8 | <0.001 |
| Smoker (%) | 12 | 17 | 7 | |
| HTN (%) | 69.5 | 59 | 80 | |
| DM (%) | 50 | - | 100 | |
| HDL-C (mg/dl) | 52.71±16.10 | 56.19±18.44 | 49.23±12.47 | <0.001 |
| TC (mg/dl) | 207.54±49.70 | 221.04±54.00 | 194.04±40.86 | <0.001 |
aClinical characteristics are expressed as the mean ± SD for continuous variables and n (%) for categorical variables. P-values were derived from a one-way analysis of variance (ANOVA) for continuous variables and Chi-square tests for categorical variables. SBP, systolic blood pressure; IR, insulin resistance; FRS, Framingham risk score; HOMA-IR, homeostasis model assessment of insulin resistance; HTN, hypertension; DM, diabetes mellitus; HDL-C, high-density lipoprotein-cholesterol; TC, total cholesterol. Group 1, control subjects without DM; group 2, subjects with type 2 diabetes.
Caucasian subjects without a history of carbohydrate metabolism and patients diagnosed with T2DM at the beginning of the study were included, according to the criteria of the American Diabetes Association (ADA) with minor modifications (18). Subjects with acute metabolic imbalance, acute or chronic diseases, drug treatments that potentially experience a secondary disruption of carbohydrate metabolism were excluded.
Informed consent was signed, in full knowledge of the facts, by each participant in the study, after all the necessary aspects were communicated to take a decision for or against participation in the study. This study was approved by the Academic and Scientific Ethics and Deontology Committee of the University of Medicine and Pharmacy in Craiova (registration no. 18/2019) in accordance with the European Union Guidelines (Declaration of Helsinki), in accordance with good clinical practice, respecting the right to integrity, confidentiality, or the option of the subject to withdraw from the study at any time.
Demographic data (age, sex), personal pathological history (diabetes, treated/untreated hypertension), lifestyle data aimed at identifying smoker status were collected. In this regard, the patients completed a questionnaire, the answers being subsequently grouped into the categories: current smoker, former smoker and non-smoker. Respondents admitting to consuming ≥100 cigarettes during their lifetime and who, at the time of the survey, smoked daily, or several days or weeks were defined as current smokers. Respondents who reported smoking ≥100 cigarettes during their lifetime and who, at the time of the survey, no longer smoked were regarded as former smokers. Respondents who stated that they smoked <100 cigarettes during their lifetime were interpreted as non-smokers (19). Blood pressure was measured using an automated sphygmomanometer after the subjects were relaxed and seated for >10 min.
Laboratory tests
Subjects were required to fast for ≥12 h and avoid a high-fat diet or alcohol consumption prior to blood sampling. The blood samples obtained were stored in a refrigerator at 4˚C and subsequently sent to the hospital laboratory. Clinical biochemical tests measured were represented by the fasting plasma glucose (FPG); fasting insulin; fasting lipid profiles, especially HDL cholesterol and total cholesterol (TC).
Assessment of IR
IR was assessed via HOMA-IR (20), which was expressed as:
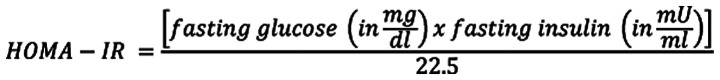 |
Subjects were considered to have IR if the value of HOMA-IR index was ≥1.9.
CVR assessments
The 2008 Framingham risk score (FRS) assessments were employed to determine the risk of CVD (21). CVR stratification was performed in the 3 categories: low risk (<10%), moderate risk (10-20%), and high risk (>20%).
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences software, version 26.0 (IBM Corp.). Patient characteristics and clinical data are expressed as means ± standard deviations for continuous variables and countable with percentages for discrete variables. Descriptive statistics were provided. Differences among groups with varying HOMA-IR levels were compared via one-way ANOVA and Post Hoc Multiple Comparisons for continuous data and the Chi-square test was used for categorical data. Pearson's analysis was used to evaluate the correlation of CVD risk factors and HOMA-IR levels. Multiple logistic regression analysis was used to adjust for covariates. Receiver operating characteristic (ROC) curve was used to assess the utility of the HOMA-IR to predict a high FRS. In addition, a favorable cut-off point and the corresponding sensitivity, specificity, and area undercurve (AUC) were determined. P<0.05 (two-sided) was regarded as statistically significant.
Results
Patient characteristics
The general characteristics of the participants are summarized in Table I. The groups were constructed without significant differences in terms of sex, age, mean of systolic blood pressure (SBP), personal history of hypertension, HDL-C and TC values. Evaluating the average value of HOMA-IR in the two groups, an average value of 2.116±1.725 was obtained for the group without DM. For the group with T2DM, an average value of 4.402±2.794 was obtained, with a statistically significant difference (P<0.001) between the two groups. This confirms the close association of IR with T2DM.
Following evaluation of CVR for the two groups, an average value of 16.23±9.6 was obtained for the group without diabetes and 23.20±8 for the group with T2DM (Table I). The results indicated a statistically significant difference (P<0.001), confirming diabetes as an important factor in increasing CVR. Analyzing the groups regarding the presence of IR, the following data were obtained: in group 1 IR was registered in 35.7% of patients, while in group 2 it was 79% (Table I). The results indicated a statistically significant difference (P<0.001) in favor of patients with diabetes.
Stratifying CVR by groups, in group 1 there were no significant differences among the CVR categories, unlike group 2 where most patients had a moderate or increased CVR (24 or 68%, respectively), with a statistically significant difference (P<0.001) in favor of the T2DM (Table II).
Table II.
Stratification of cardiovascular risk in the two groups.
| Framingham risk score | Group 1 (%) | Group 2 (%) | P-value |
|---|---|---|---|
| Low | 34.67 | 8 | P<0.001 |
| Moderate | 29.6 | 24 | P<0.005 |
| High | 35.7 | 68 | P<0.001 |
Group 1, control subjects without DM; group 2, subjects with type 2 diabetes.
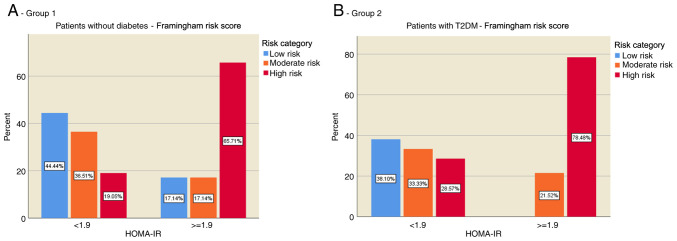
The distribution of CVR in the two groups according to the presence of IR was evaluated. The results showed that most of the individuals without diabetes (group 1) and without IR were in the low CVR category (44.44%), while individuals with IR were predominantly in the high CVR category (65.71%) (Fig. 1A). In patients with T2DM (group 2), the risk was evenly distributed in those without IR, but for those with IR the present risk was predominantly increased (78.48%) (Fig. 1B).
Figure 1.
Distribution of cardiovascular risk according to the presence of IR. (A) Patients without diabetes (group 1), and (B) with T2DM (group 2). IR, insulin resistance; HOMA-IR, homeostasis model assessment of insulin resistance; T2DM, type 2 diabetes mellitus.
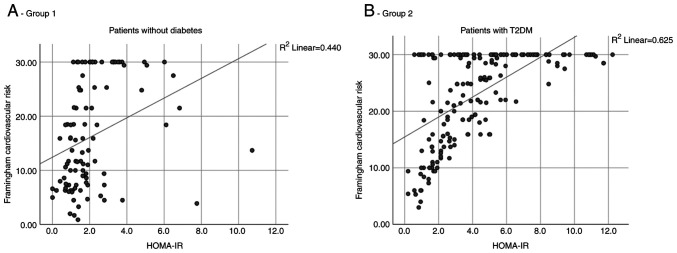
After correlating the value of HOMA-IR with the degree of CVR it was observed in both groups that as the IR increased, the CVR also increased. However, in group 2, the correlation was higher, quantified by a value of the Spearman correlation coefficient of 0.625, compared to group 1 where this coefficient had a value of 0.440 (Fig. 2A and B).
Figure 2.
Correlations between the value of HOMA-IR and FRS. (A) Patients without diabetes (group 1), and (B) with T2DM (group 2). HOMA-IR, homeostasis model assessment of insulin resistance; T2DM, type 2 diabetes mellitus; FRS, Framingham risk score.
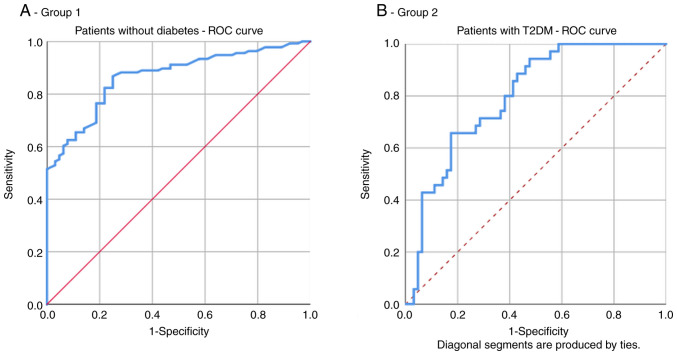
In group 1, the area under the ROC curve for HOMA-IR as a predictor of increased CVR (≥20%) was 0.797. The optimal cut-off value of HOMA-IR for high CVR prediction was 1.965 with a sensitivity of 65.7% and specificity of 82.5% (Fig. 3A and Table III).
Figure 3.
Receiver operating characteristic (ROC) curve for the HOMA-IR index as a predictor of the FRS. (A) Patients without diabetes (group 1), and (B) with T2DM (group 2). HOMA-IR, homeostasis model assessment of insulin resistance; FRS, Framingham risk score; T2DM, type 2 diabetes mellitus.
Table III.
AUC, sensitivity, and specificity of the optimized cut-off points for the HOMA-IR index in predicting high Framingham risk score (FRS >20%).
| Variable | Group | AUC (95% CI) | P-value | Cut-off point | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| HOMA-IR | 1 | 0.797 | 0.001 | 1.965 | 65.7 | 82.5 |
| 2 | 0.867 | 0.001 | 2.926 | 82.4 | 75 |
AUC, area under the ROC curve; HOMA-IR, homeostasis model assessment of insulin resistance. Group 1, control subjects without DM; group 2, subjects with type 2 diabetes.
In group 2, the area under the ROC curve for HOMA-IR as a predictor of increased CVR (≥20%) was 0.867. The optimal cut-off value of HOMA-IR for high CVR prediction was 2.926 with a sensitivity of 82.4% and specificity of 75% (Fig. 3B and Table III).
Discussion
As expected, in the present study, IR was found at a higher percentage among patients with diabetes when compared with individuals without diabetes. In addition, the CVR was statistically significantly higher among patients with diabetes vs. individuals without diabetes. CVR was significantly higher among people with IR with or without DM providing that IR is a pathological condition with adverse cardiovascular influences.
Early identification of IR, lifestyle and dietary interventions could decrease the development and progression of IR and its detrimental consequences on the liver, kidney, blood pressure, heart and endothelium (22-27). Lifestyle interventions include body weight loss of >5% of initial weight, physical activity for 150 min/week or more, or a regular exercise program. Dietary interventions include daily calorie restriction by reducing 500 Kcal from regular food intake, fructose restriction and choosing a Mediterranean diet.
Supplementation with antioxidants, amino acids, vitamins, n-3 polyunsaturated fatty acids and prebiotics/probiotics may have benefits; however, further studies are needed to confirm these benefits (22). Nevertheless, the present study has some limitations. First, the study was cross-sectional; therefore, a cause-and-effect relationship could not be established between the FRS and IR. Second, we did not have a direct measure outcome of CVD. Finally, the size of our sample was relatively small; thus, further studies are required.
In conclusion, a high FRS (FRS >20%) was significantly associated with IR. The homeostasis model assessment of insulin resistance (HOMA-IR) is an independent risk factor for high FRS. New therapies focusing on decreasing IR may contribute to a decreased CVD.
Acknowledgements
Not applicable.
Funding Statement
Funding: This work was supported by the by dates obtained from the grant (research contract no. 49/09.09.2019) with the research topic: Cardiovascular risk assessment in patients with type 2 diabetes conducted by Ionela Mihaela Vladu, as project director, in collaboration with the ‘The Holy Apostles Medical Center’ and the University of Medicine and Pharmacy Craiova.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
IMV, MF, DC, MCF, RP, LR, ȘTȚ, PMR and VP contributed equally to acquisition, analysis and systematization of data, manuscript writing and critical revision of it for important intellectual content. The authenticity of all raw data was assessed to ensure their legitimacy by IMV and MF. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was approved by the Scientific Ethics and Deontology Committee of the Craiova Municipal Hospital (registration no. 17283/2019) in accordance with the European Union Guidelines (Declaration of Helsinki). Written informed consent was obtained for patient participation.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–3325. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 2.Grigorescu ED, Lăcătușu CM, Crețu I, Floria M, Onofriescu A, Ceasovschih A, Mihai BM, Șorodoc L. Self-reported satisfaction to treatment, quality of life and general health of type 2 diabetes patients with inadequate glycemic control from north-eastern Romania. Int J Environ Res Public Health. 2021;18(3249) doi: 10.3390/ijerph18063249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 4.Adiels M, Olofsson SO, Taskinen MR, Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 5.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 6.Borén J, Taskinen M-R, Olofsson S-O, Levin M. Ectopic lipid storage and insulin resistance: A harmful relationship. J Intern Med. 2013;274:25–40. doi: 10.1111/joim.12071. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reaven GM. Insulin resistance/compensatory hyperinsulinemia, essential hypertension, and cardiovascular disease. J Clin Endocrinol Metab. 2003;88:2399–2403. doi: 10.1210/jc.2003-030087. [DOI] [PubMed] [Google Scholar]
- 9.Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: The last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32 (Suppl):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrannini E, Cushman WC. Diabetes and hypertension: The bad companions. Hypertension. 2012;380:601–610. doi: 10.1016/S0140-6736(12)60987-8. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clenciu D, TeneaCojan TS, Dijmarescu A, Ene CG, Davitoiu DV, Baleanu VD, Ciora CA, Socea B, Voiculescu D, NedelcuŢă RM, et al. Diabetic retinopathy in relation with eGDR value in patients with type 1 diabetes mellitus. Rev Chim (Bucharest) 2019;70:1434–1438. [Google Scholar]
- 14.Laakso M. Heart in diabetes: A microvascular disease. Diabetes Care. 2011;34 (Suppl):S145–S149. doi: 10.2337/dc11-s209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. The International Expert Committee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Socea B, Radu L, Clenciu D, TeneaCojan TS, Baleanu VD, Ene CG, Girgavu SR, Vladu IM. The utility of visceral adiposity index in prediction of metabolic syndrome and hypercholesterolemia. RevChim (Bucharest) 2018;69:3112–3114. [Google Scholar]
- 17.Vladu IM, Radu L, Girgavu SR, Baleanu V, Clenciu D, Ene CG, Socea B, Mazen E, Cristea OM, Mota M, et al. An easy way to detect cardiovascular risk. Rev Chim (Bucharest) 2018;69:3229–3232. [Google Scholar]
- 18.Standards of medical care in diabetes-2021. Diabetes Care. 2021;44 (Suppl):S15–S33. doi: 10.2337/dc21-S002. American Diabetes Association: 2. Classification and diagnosis of diabetes. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro J, Kannel WB. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Rodas MC, Valenzuela R, Videla LA. Relevant aspects of nutritional and dietary interventions in non-alcoholic fatty liver disease. Int J Mol Sci. 2015;16:25168–25198. doi: 10.3390/ijms161025168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forțofoiu MC, Popescu DM, Pădureanu V, Dobrinescu AC, Dobrinescu AG, Mită A, Foarfă MC, Bălă VS, Muşetescu AE, Ionovici N, ForŢofoiu M. Difficulty in positive diagnosis of ascites and in differential diagnosis of a pulmonary tumor. Rom J Morphol Embryol. 2017;58:1057–1064. [PubMed] [Google Scholar]
- 23.Valenzuela R, Videla LA. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011;2:644–648. doi: 10.1039/c1fo10133a. [DOI] [PubMed] [Google Scholar]
- 24.Grigorescu ED, Sorodoc V, Floria M, Anisie E, Popa AD, Onofriescu A, Ceasovschih A, Sorodoc L. The inflammatory marker hsCRP as a predictor of increased insulin resistance in type 2 diabetics without atherosclerotic manifestations. Rev Chim (Bucharest) 2019;70:1791–1794. [Google Scholar]
- 25.Forţofoiu M, Forţofoiu MC, Comănescu V, Dobrinescu AC, Pădureanu V, Vere CC, Streba CT, Ciurea PL. Hepatocellular carcinoma and metabolic diseases-histological perspectives from a series of 14 cases. Rom J Morphol Embryol. 2015;56:1461–1465. [PubMed] [Google Scholar]
- 26.Mihailovici AR, Padureanu V, Albu CV, Dinescu VC, Pirlog MC, Dinescu SN, Malin RD, Calborean V. Myocardial Noncompaction. Rev Chim (Bucharest) 2018;69:2209–2212. [Google Scholar]
- 27.Vladu M, Clenciu D, Efrem IC, Forţofoiu MC, Amzolini A, Micu ST, Mota M, Fortofoiu M. Insulin resistance and chronic kidney disease in patients with type 1 diabetes mellitus. J Nutr Metab. 2017;2017(6425359) doi: 10.1155/2017/6425359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.