Abstract
Candida dubliniensis is often associated with C. albicans in cultures. Easy-to-perform selective isolation procedures for these closely related species do not exist. Therefore, we evaluated previously described discriminatory phenotypic markers for C. dubliniensis. A total of 150 oral rinses from human immunodeficiency virus (HIV)-infected patients were cultured on CHROMagar Candida. Dark green colonies described as being indicative of C. dubliniensis and other green colonies, 170 in total, were isolated. Chlamydospore formation, intracellular β-d-glucosidase activity, ability to grow at 42°C, carbohydrate assimilation pattern obtained by the API ID 32C, and Fourier transform infrared (FT-IR) spectroscopy were used for phenotypic characterization. Sequencing of the 5′ end of the nuclear large-subunit (26S) ribosomal DNA gene was used for definitive species identification for C. dubliniensis. C. dubliniensis was found in 34% of yeast-colonized HIV-infected patients. The color of the colonies on CHROMagar Candida proved to be insufficient for selecting C. dubliniensis, since only 30 of 53 proven C. dubliniensis isolates showed a dark green color in primary cultures. The described typical chlamydospore formation can give only some indication of C. dubliniensis. The assimilation pattern proved to be insufficient to discriminate C. dubliniensis from C. albicans. All C. dubliniensis strains showed no or highly restricted growth at 42°C and a lack of β-d-glucosidase activity. Unfortunately, atypical C. albicans strains can also exhibit these phenotypic traits. FT-IR spectroscopy combined with hierarchical clustering proved to be as reliable as genotyping for discriminating the two species.
Various reports of the recently described yeast species Candida dubliniensis (32) indicate a worldwide occurrence (11, 26, 30, 31) of this fungus, which is phylogenetically closely related to C. albicans. The pathogenic potential of C. dubliniensis, so far recovered mainly from the oral cavity of human immunodeficiency virus (HIV)-infected patients (2, 23) and rarely from cases of candidemia in HIV-negative patients (16), is virtually unknown. While some C. dubliniensis isolates showed a higher level of adherence to human buccal epithelial cells when grown in glucose-rich media, the in vivo virulence of C. dubliniensis in a mouse model was found to be lower than that of C. albicans (4). Evidence for the inducibility of stable fluconazole resistance in vitro in C. dubliniensis strains (17, 18) may indicate an emerging pathogen for immunocompromised patients receiving long-term fluconazole prophylaxis.
Since C. dubliniensis is normally found in mixed cultures with C. albicans, several methods for the identification of C. dubliniensis and discrimination from C. albicans have been reported; these include the formation of dark green colonies on CHROMagar Candida (29), no or strictly reduced growth at 42°C (26), and lack of intracellular β-d-glucosidase activity (29). C. dubliniensis often shows characteristic chlamydospore formation (32) and may differ from C. albicans in its distinctive carbohydrate assimilation pattern (27). Furthermore, genetic differences between C. albicans and C. dubliniensis have been described (32). For a limited number of strains it has already been shown that Fourier transform infrared (FT-IR) spectroscopy can successfully discriminate C. albicans from C. dubliniensis (35).
The detection and identification of microorganisms strongly depend on the availability of easy-to-perform screening methods. In planning an epidemiological study, we were confronted with the problem of selective isolation of C. dubliniensis. Primary experiments clearly showed that the sole use of green color on CHROMagar Candida was insufficient. Therefore, we evaluated systematically the discriminatory power of various phenotypic markers, i.e., colony color on CHROMagar Candida, growth at 42°C, intracellular β-d-glucosidase activity, kind of chlamydospore formation, assimilation pattern, and FT-IR spectroscopy. For reference, definitive species identification was achieved by sequence analysis of the 5′ end of the nuclear large-subunit (26S) ribosomal DNA (rDNA) gene for suspected C. dubliniensis isolates or atypical C. albicans isolates (14).
MATERIALS AND METHODS
Clinical specimens.
Oral rinses from 150 consecutive HIV-infected patients attending an outpatient clinic for infectious diseases at Humboldt University in Berlin, Germany, between September and November 1997 were studied. HIV-infected patients (30 female and 120 male patients; 24 to 70 years old) with different stages of HIV infection (57 with AIDS-related complex and 66 with AIDS) were consequently receiving different treatments. T-cell (CD4+) counts were more than 500/μl in 13 patients, 200 to 500/μl in 78 patients, 100 to 200/μl in 42 patients, and less than 100/μl in 17 patients. Samples were taken regardless of clinical symptoms.
In addition to the patient isolates, the following reference strains were studied: C. dubliniensis CBS 7987T and CBS 7988 and C. albicans ATCC 10261, ATCC 48867, ATCC 76615, ATCC 90028, CBS 1905 (formerly the type strain of “C. stellatoidea”), A 1618, A 1626, and A 1634. The latter three, originating from vaginal swabs taken from women in Angola, had been described as morphologically and biochemically “atypical” C. albicans strains not capable of forming chlamydospores and of assimilating glucosamine and/or N-acetylglucosamine (34). Stock cultures of yeast isolates were kept on ceramic beads (Microbank; Mast Diagnostica, Reinfeld, Germany) at −70°C.
Media.
CHROMagar Candida (lot 332-c; CHROMagar Company, Paris, France; distributed by Mast Diagnostica), brain heart infusion (BHI) agar (Difco, Detroit, Mich.), and Sabouraud dextrose (SD) agar containing 2% glucose (E. Merck AG, Darmstadt, Germany) were prepared according to the manufacturers' instructions. Potato dextrose agar (PDA) was prepared using a filtrate of 100 g of boiled new potatoes, 20 g of dextrose, and 20 g of agar/liter, adjusted to pH 5.6. Rice-Tween 80 agar (RTagar) was prepared as described by Taschdjian (33).
Sample processing.
Fresh oral rinse (10 ml) was centrifuged at 3,000 × g for 10 min, and the resulting sediment was resuspended in 0.5 ml of sterile saline. This suspension was microscopically examined and, if the yeast cell count was >104 ml−1, diluted to about 103 cells ml−1 with sterile saline. A 100-μl quantity of this suspension was plated onto CHROMagar Candida and cultivated for 48 h at 37°C in ambient air. The occurrence of colonies in various shades of green (Fig. 1) was determined, and one colony of each green shade was randomly isolated and further characterized phenotypically.
FIG. 1.
Primary culture of an oral rinse on CHROMagar Candida after 48 h at 37°C showing colonies with three different shades of green (arrowheads), all finally identified as C. dubliniensis.
Chlamydospore formation.
According to the description given by Sullivan et al. (32), chlamydospore formation on RTagar was studied after prior subculturing on PDA for 48 to 72 h at 37°C. Chlamydospore formation was scored on days 2, 3, and 7 of incubation at 26°C as (i) suspected C. dubliniensis, i.e., abundant chlamydospores, often occurring in clusters or contiguous pairs on short branching pseudomycelia, or (ii) suspected C. albicans, i.e., chlamydospores occurring mostly singly on elongated pseudomycelia. Scoring was done by inspection of 20 fields (magnification, ×200) independently by two trained persons.
Intracellular β-d-glucosidase activity.
Intracellular β-d-glucosidase activity was assessed twice as described elsewhere in detail (1, 29). Isolates grown on BHI agar were subcultured overnight in BHI broth in Erlenmeyer flasks on an orbital shaker at 120 rpm and 35°C. The following steps were performed at room temperature. One milliliter of the broth was centrifuged at 15,000 × g for 2 min. Pellets were resuspended in 1 ml of sodium acetate buffer (pH 5.5) containing 1 mg of 4-methylumbelliferyl-β-d-glucoside (Sigma, Deisenhofen, Germany) per ml. Cells were disrupted by vigorous vortexing four times for 30 s each time after the addition of 0.4 g of glass beads (0.5-mm diameter). After centrifugation at 15,000 × g for 2 min, 0.1 ml of supernatant was transferred into a well of a microtiter plate. The occurrence of bright fluorescence indicative of intracellular β-d-glucosidase activity was judged visually after 15, 30, and 60 min using a UV transilluminator (Spectroline TR 302; Spectronics Corp., Westbury, N.Y.) with an excitation of 302 nm.
Growth at 42°C.
Each isolate was subcultured on PDA at 37 and 42°C (30) for 48 h using a multipoint inoculator (Denley Corp., Sussex, United Kingdom). The growth of isolates at 42°C was scored as (i) none or poor and (ii) not reduced in comparison to that at 37°C.
Extended phenotypic characterization.
Yeast isolates suspected of being C. dubliniensis, i.e., having at least two of the aforementioned phenotypic markers indicative of this species (e.g., abundant chlamydospore formation, lack of intracellular β-d-glucosidase activity, and/or significant growth reduction at 42°C; color on CHROMagar Candida not included), were further characterized by the assimilation pattern and FT-IR spectroscopy.
Carbohydrate assimilation.
The assimilation pattern was studied using the API ID 32C (bioMérieux, Nürtingen, Germany). Strains were precultured on SD agar at 37°C for 48 h. Reactions were examined after 48 h of incubation at 30°C. Reading was done visually in strict accordance with the manufacturer's instructions; i.e., any cupule more turbid than the control was judged positive. Evaluation of the assimilation pattern was done with the ID 32C database (A-Software 2.6.8, version 2.0, 1999).
FT-IR spectroscopy.
For FT-IR spectroscopy, yeast cells were subcultured on SD agar for 24 h at 30°C. Cells were harvested and prepared for FT-IR measurements as already described for bacteria (9, 10, 20). Briefly, 25 μl of a yeast cell suspension in distilled water was transferred to a ZnSe optical plate and dried to a transparent film under mild vacuum (2.5 to 7.5 kPa). All spectra between 500 and 4,000 cm−1 were recorded on an IFS 28/B spectrometer (Bruker Analytik, Karlsruhe, Germany) equipped with a deuterated triglycerine sulfate detector. Nominal physical resolution was set to 6 cm−1, Blackman-Harris apodization was used for Fourier transformation, and a zero filling factor of 4 was applied to yield an encoding interval of approximately one datum point per wave number. Spectral data were collected and evaluated with OPUS 3.0 software. Data processing included calculation of second derivatives to minimize baseline problems and to enhance apparent resolution. For hierarchical cluster analysis, an unsupervised classification technique (10), Ward's algorithm, was used to construct dendrograms. As a distance measure, Pearson's product-moment correlation coefficient was used as defined previously (9, 10).
For artificial neural network (ANN) analysis, the Stuttgart Neuronal Net Simulator was used. Analysis was performed as described previously (28). Automatic feature selection was based on variance analysis (28). This strategy generally improved the quality of the classifier and reduced the complexity and dimensionality of the classification model. Prior to feature selection, data reduction was performed by averaging three datum points, resulting in 1,206 new datum points out of 3,620. The spectral range used for ANN analysis was 750 to 1,500 cm−1. Only vector-normalized spectra were used. A three-layer feed-forward network with 150 input neurons, 75 hidden layers, and 2 output units allowing shortcut connections within the net was established. As the learning function, resilient back propagation was used. A total of 150 training cycles were performed, with a relative training error (i.e., error divided by the variance of the outputs) of 0%.
Sequencing for genetic characterization.
Partial sequence analysis of the nuclear rDNA was performed for all isolates presumed to be C. dubliniensis (groups VI and VII in Table 1; n = 53) and for doubtful C. albicans isolates (groups III, IV, and V in Table 1; n = 18).
TABLE 1.
Characterization of 170 isolates derived from green colonies on CHROMagar Candida upon primary culturing
| Group (no. of isolates) | Unrestricted growth at 42°Ca | Type of chlamydospore formation | β-d-Glucosidase activity | Presumed species |
|---|---|---|---|---|
| I (76) | + | Mostly single on elongated hyphae | + | C. albicans |
| II (23) | + | Abundant, in clusters or contiguous pairs | + | C. albicans |
| III (3) | + | None | + | C. albicansb,c |
| IV (9) | + | Mostly single on elongated hyphae | − | C. albicansb,c |
| Vd (6) | + | Abundant, in clusters or contiguous pairs | − | C. dubliniensis or C. albicansb,c |
| VId (46) | − | Abundant, in clusters or contiguous pairs | − | C. dubliniensisb |
| VIId (7) | − | Mostly single on elongated hyphae | − | C. dubliniensisb |
In comparison to growth at 37°C. +, positive; −, negative.
Further characterized by sequencing.
All confirmed as C. albicans.
Isolates were further characterized by the assimilation pattern determined with the API ID 32C gallery and FT-IR spectroscopy.
Yeast cells were harvested after 2 days of incubation at 30°C on SD agar and suspended in sterile saline. DNA was prepared according to the procedure described by Goodwin and Lee (7). With primers NS7 and ITS2 (39), the 3′ end of the nuclear 18S rRNA gene, along with the adjacent internal transcribed spacer 1 (ITS1) region, was amplified by PCR, whereas the 5′ end of the nuclear large-subunit (26S) rDNA gene was amplified by use of a procedure described recently (13).
Direct sequencing of the resulting products was done using a PRISM Ready Reaction DyeDeoxye Terminator Cycle Sequencing Kit and a 373 A DNA Sequencer (both from Applied Biosystems, Weiterstadt, Germany) as well as amplification primers for sequencing. Details of this procedure have been given recently (8). Concatenation of the resulting sequences and alignment using the respective sequences of C. albicans (EMBL accession no. AB013586 and AB032172) as a reference were done with the help of the DCSE software package (3). The ITS1 sequences of C. dubliniensis CBS 7987T and of C. albicans CBS 1905 have been deposited under EMBL accession no. AJ010332 and AJ010333, respectively.
Fluconazole susceptibility testing.
A microdilution assay was performed in duplicate according to National Committee for Clinical Laboratory Standards performance standard M 27A (18a), with the modification that a final glucose concentration of 2% was used in RPMI medium. Final fluconazole concentrations ranged from 0.1 to 128 μg/ml. After 24 h of incubation at 35°C, each isolate showed good growth in the control well. Growth was then determined spectrophotometrically, and MICs were calculated as the MICs at which 80% of isolates were inhibited.
RESULTS
Yeasts could be cultured from 117 (78%) of 150 oral rinses.
Colony color on CHROMagar Candida and characterization of yeast isolates.
All samples showing growth of yeasts on CHROMagar Candida also yielded green colonies. As one specimen provided colonies with up to three different shades of green, 170 different colonies in total (light green, 49; green, 55; dark green, 66) were isolated randomly and further characterized phenotypically (Table 2). FT-IR spectroscopic measurements were obtained for 59 isolates after judgment of their phenotypic characteristics, i.e., abundant chlamydospore formation, no or highly restricted growth at 42°C, and/or no intracellular β-d-glucosidase activity (Table 1, groups V, VI, and VII). Analysis of the sequence of the 5′ end of the nuclear large-subunit (26S) rDNA gene allowed unequivocal species assignment with the homolog signature nucleotides A (278), G (288), T (492), C (494), and G (496) for C. albicans (positions from GenBank sequence X70659) and G (176), A (186), A (392), T (394), and A (396) for C. dubliniensis (positions from GenBank sequence U57685). Therefore, all isolates of groups III, IV, and V (Table 1) proved to be C. albicans, whereas isolates of groups VI and VII proved to be C. dubliniensis. Together with definitive phenotypic identification for groups I and II, all green colonies were finally identified as either C. albicans (n = 117) or C. dubliniensis (n = 53).
TABLE 2.
Phenotypic characteristics of 170 isolates derived from green colonies on CHROMagar Candida
| Characteristic | Resulta for:
|
|
|---|---|---|
| C. albicans(n = 117) | C. dubliniensis(n = 53) | |
| Colony color on CHROMagar Candida | ||
| Light green | 34 (29) | 15 (28) |
| Green | 47 (40) | 8 (15) |
| Dark green | 36 (31) | 30 (57) |
| Growth at 42°Cb | ||
| Unrestricted | 117 (100) | — |
| None or highly restricted | — | 53 (100) |
| Chlamydospore formation in clusters or contiguous pairs | 29 (25) | 46 (87) |
| MIC80 of fluconazole | ND | |
| ≤0.5 μg/ml | 50 (94) | |
| 2 to 8 μg/ml | 3 (6) | |
| Intracellular β-d-glucosidase activity | 102 (87) | — |
| Assimilation (including weak) ofc: | ||
| d-Xylose | 5/6d | 31 (58) |
| dl-Lactate | 6/6d | 46 (87) |
| MDG | 6/6d | 29 (55) |
Reported as number (percent) of strains, unless indicated otherwise. ND, not done; —, not found.
In comparison to that at 37°C.
API ID 32C results were read after 48 h.
For isolates belonging to group V (Table 1).
Only 30 of the 53 C. dubliniensis isolates showed the dark green pigmentation described as typical of this species in primary cultures. Fifteen isolates were clearly light green, a color regarded as indicative of C. albicans. Eight of the C. dubliniensis isolates showed an intermediate intensity of green. Furthermore, C. albicans colonies also displayed every shade of green noted on CHROMagar Candida (Table 2).
Chlamydospore formation.
All but 3 of 170 isolates tested produced chlamydospores. ITS1 sequencing revealed that the three non-chlamydospore-forming isolates were C. albicans. “Typical” chlamydospore formation of C. dubliniensis, i.e., abundant chlamydospores frequently arranged in triplets or contiguous pairs on short branching pseudomycelia (30, 32), was found in 46 isolates. Of 117 C. albicans strains, 85 showed single terminal chlamydospores, regarded as typical of C. albicans, whereas 29 showed chlamydospores typical of C. dubliniensis.
In contrast to the C. albicans American Type Culture Collection reference strains, which produced single terminal chlamydospores, strains A 1618, A 1626, and A 1634 produced no chlamydospores (34). Incubation for more than 72 h did not alter these results.
Intracellular β-d-glucosidase activity.
Reproducible determination of β-d-glucosidase activity was achievable only after 30 min of incubation with the substrate. For all 53 proven C. dubliniensis isolates, no fluorescence could be detected. Bright fluorescence was seen for 102 (87%) of the 117 confirmed C. albicans isolates as well as the reference strains. Fifteen (13%) of 117 C. albicans isolates and strains A 1618, A 1626, and A 1634 showed no fluorescence, indicating a lack of intracellular β-d-glucosidase activity.
Growth at 42°C.
No or highly restricted growth at 42°C was found for all C. dubliniensis isolates (Table 2), whereas all studied C. albicans strains, except for strains CBS 1905, A 1618, A 1626, and A 1634, grew well at this temperature.
Carbohydrate assimilation.
No unique assimilation pattern for C. dubliniensis isolates was obtained with α-methyl-d-glucoside (MDG), dl-lactate, and d-xylose. Seven of 53 C. dubliniensis isolates failed to assimilate dl-lactate, and 24 were unable to assimilate MDG after 48 h. Twenty-two of 53 isolates failed to assimilate d-xylose. Only one of the 31 isolates able to assimilate d-xylose showed a strong reaction. Of note, the two C. dubliniensis reference strains also showed very weak assimilation of the above-mentioned three carbohydrates, but after a further 5 days of incubation, the assimilation reactions were clear-cut.
When the 53 C. dubliniensis isolates were identified using the assimilation pattern and the API 32C database, only for 2 isolates was C. dubliniensis quoted first (percentage of identification [%id], 81.9; T = 0.50). For the other isolates, an identification of “C. albicans” was given (%id, 68.0 to 99.9; T, 0.40 to 1.00). These results were due to the frequencies of assimilation of d-xylose, MDG, and dl-lactate being given as 0, 0, and 10%, respectively, in the database. Interestingly, one C. dubliniensis isolate was unable to assimilate N-acetylglucosamine.
FT-IR spectroscopy. (i) Hierarchical cluster analysis.
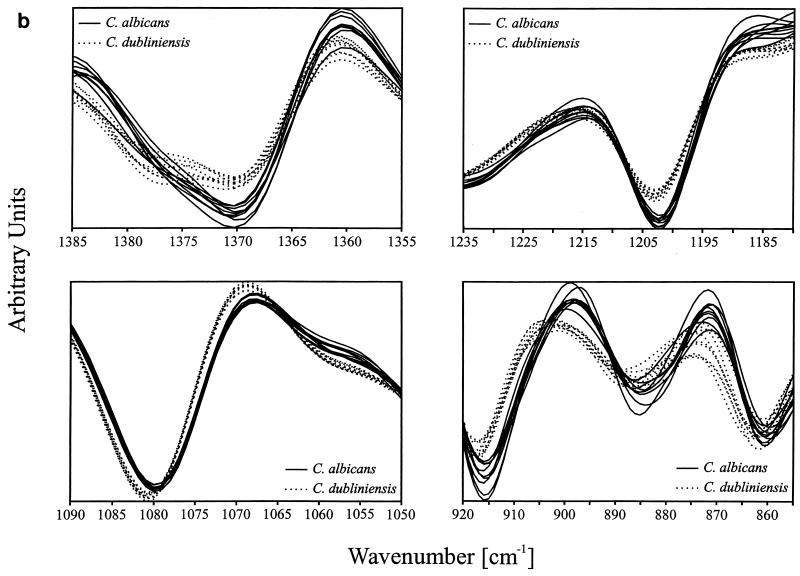
Grouping of the spectra into distinct clusters was achieved by varying the number and combination of the spectral ranges contributing maximally to the desired classification (9). By use of the information encoded in five spectral ranges (822 to 799, 920 to 855, 1,090 to 1,050, 1,235 to 1,180, and 1,385 to 1,355 cm−1) (equally weighted) as input data for the calculation of spectral distances and cluster analysis, a dendrogram was obtained (Fig. 2a). This dendrogram, comprising more than 350 spectra, exhibited two main clusters clearly separating the C. albicans and C. dubliniensis strains into two groups. All replicate measurements on the strains gave very similar distances, yielding extremely dense subgroupings within the two main clusters. Atypical C. albicans strains A 1618, A 1626, A 1634, and CBS 1905 clustered as a monophyletic group together with the other C. albicans strains studied.
FIG. 2.
(a) Dendrogram of a hierarchical cluster analysis performed on 421 spectra measured from 58 strains of C. albicans and 53 strains of C. dubliniensis. The discriminating information used for clustering is based on five spectral ranges: 822 to 799, 920 to 855, 1,090 to 1,050, 1,235 to 1,180, and 1,385 to 1,355 cm−1. (b) Representative second-derivative spectra of C. albicans and C. dubliniensis in the four most discriminatory spectral windows.
Figure 2b shows typical center-of-class spectra extracted from the C. albicans and C. dubliniensis clusters. The spectra are plotted for the spectral ranges used to calculate spectral distances. Figure 2b evidences a number of phenotypic differences responsible for the separation of the two Candida species into two spectral classes.
(ii) ANN analysis.
A second approach, ANN analysis, a supervised classification method known to be superior to hierarchical clustering (5, 6, 35), was used to discriminate between C. albicans and C. dubliniensis and to establish a reference database suitable for the rapid identification of the two Candida species. The whole set of 270 FT-IR spectra collected from 40 strains of C. albicans and 41 isolates of C. dubliniensis was divided into two subsets, comprising 135 spectra of C. albicans and 135 spectra of C. dubliniensis for training and validation of the ANN. Prior to training and validation, the data set was subjected to an automatic wavelength selection process to extract class-specific spectral traits from the spectra and to obtain data reduction. The selected wavelengths (data not shown) were used as input data for ANN analysis. All spectra (100%) of the training and validation data sets were correctly assigned to their respective classes, with the activation value of the output neurons being either 0 or 1. In a last step of analysis, the ANN was challenged with an independent test set of 87 FT-IR spectra obtained from 13 C. albicans strains and 17 C. dubliniensis strains not included in the training and validation. Spectra of this test data set were assigned correctly to their respective classes, indicating that the ANN is a stable reference for identifying unknown isolates of C. albicans and C. dubliniensis.
ITS1 sequencing.
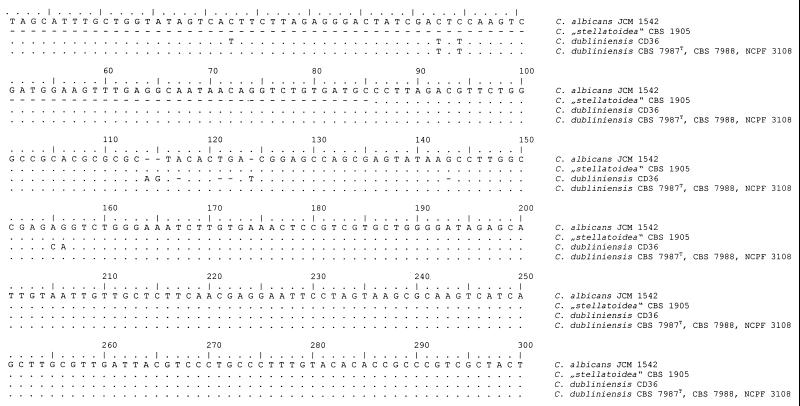
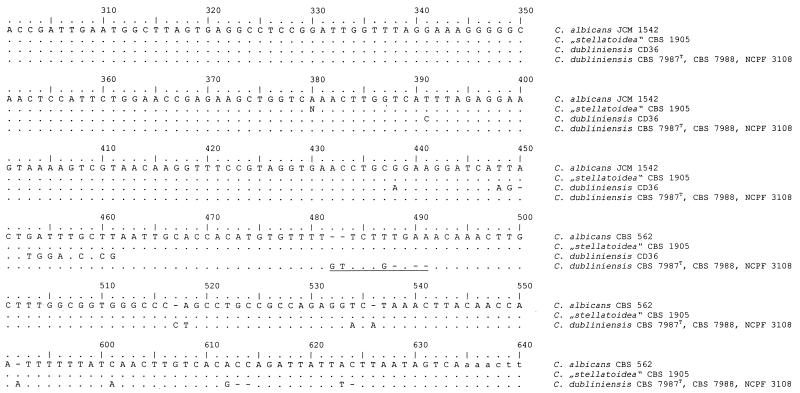
Analyses of the alignment of all ITS1 sequences obtained revealed two clusters (Fig. 3). All sequenced C. albicans strains (groups III, IV, and V in Table 1; n = 18) showed a sequence identical to that of CBS 1905. The type strain of C. dubliniensis and the other C. dubliniensis isolates showed identical sequences, thus representing a second cluster. The alignment (Fig. 3) showed that one region (positions 482 to 491) is very distinctive for both species and thus is capable of being used as a signature sequence.
FIG. 3.
Alignment of the 3′ end of the small-subunit (18S) rDNA sequences of C. albicans JCM 1542 (EMBL accession no. AB013586), C. dubliniensis CD36 (GenBank accession no. X99399), and C. dubliniensis CBS 7987T, CBS 7988, and NCPF 3108 and of ITS1 sequences of C. albicans CBS 562 (EMBL accession no. AB032172), “C. stellatoidea” CBS 1905, and C. dubliniensis CBS 7987T, CBS 7988, and NCPF 3108. Identical nucleotides are indicated by dots, and gaps are indicated by dashes. The ITS1 region begins at position 451 and ends at position 634. Underlining indicates the species-specific signature sequence that could be used to assign a strain unequivocally to C. albicans or C. dubliniensis.
Additional sequencing and alignment of the 3′ end of the nuclear small-subunit (18S) rDNA for the three reference strains of C. dubliniensis (CBS 7987T, CBS 7988, and NCPF 3108) showed no difference from the results for C. albicans (Fig. 3), a result which is in contrast to the deposited sequence of the type strain of C. dubliniensis (X99399).
Fluconazole susceptibility.
For all 53 C. dubliniensis isolates, the MIC was ≤8 μg/ml, indicating fluconazole susceptibility for all isolates, according to recent interpretation guidelines (25). Fifty isolates were highly susceptible (MIC, ≤0.5 μg/ml). Three isolates for which the MIC was between 2 and 8 μg/ml were isolated from a single sample.
DISCUSSION
Twenty-seven percent of HIV-infected patients studied were colonized with C. dubliniensis, a rate comparable to the rate reported from Ireland (2), whereas colonization is much lower in HIV-negative individuals (2, 23). The presence of C. dubliniensis may be underestimated when phenotypic markers used for selective isolation prove to be insufficient. Colonization with C. dubliniensis was not found to be correlated with the stage of HIV infection. Induction of fluconazole resistance in C. dubliniensis strains due to treatment with this drug, as seen in in vitro studies (18), seemed not to have occurred in our patients.
C. dubliniensis as the sole identified yeast species cultivated in 11 oral rinses cannot be definitively interpreted because isolation of different green colonies was done randomly, with possible C. albicans not being recognized.
The main obstacle to clarification of the epidemiological role of C. dubliniensis is the lack of an easy-to-perform screening method capable of selective identification of this yeast, which occurs mostly in mixed cultures. Such a “positive” phenotypic marker is still missing. CHROMagar Candida is a medium with chromogenic substances allowing presumptive identification of several clinically important Candida species (22). A dark green color on CHROMagar Candida was described as a potential phenotypic marker for C. dubliniensis in primary cultures (2, 29). Since this phenomenon was found not to be reproducible after subculturing and storage of isolates (29, 30), use of this marker may be limited to primary cultures. In a large-scale retrospective study by Odds et al. (23), the shade of green proved to be unreliable for discrimination between C. albicans and C. dubliniensis. The results obtained in our study are in accordance with this observation. Therefore, an approach that uses color on CHROMagar Candida in primary or secondary cultures (11) as a sole selective criterion may underestimate the frequency of C. dubliniensis.
The use of reduction of 2,3,5-triphenyltetrazolium chloride (TTC) by C. dubliniensis and not by C. albicans as a positive screening parameter (38) is also of limited value, since TTC reduction is also seen regularly in C. tropicalis, a species frequently encountered in human specimens.
The type of chlamydospore formation was found to be variable in both C. dubliniensis and C. albicans strains and therefore is also not reliable for differentiation of these two species (29, 30). Since 57% of C. dubliniensis isolates (compared to 15% of C. albicans isolates) showed abundant chlamydospores frequently arranged in clusters of contiguous pairs, the predictive value of this characteristic is low.
No or highly restricted growth at 42°C proved to be an easily obtainable and reliable phenotypic marker for the presence of C. dubliniensis; this trait is rarely encountered in atypical C. albicans strains. Use of an incubation temperature of 45°C, as recently proposed (24), in order to obtain more clear-cut discrimination seems not to be mandatory, since the highly restricted growth of some C. dubliniensis isolates at 42°C did not negatively affect the discriminatory power, because of unrestricted growth of C. albicans isolates. This difference could best be seen after incubation for 48 h, since the growth of C. albicans was less evident after 24 h at 42°C (data not shown). Of note, the maximum growth temperature of C. albicans varies from 43 to 46°C (37). Nevertheless, one should be aware that rare atypical C. albicans strains, e.g., the former “C. stellatoidea” type I and strains A 1618, A 1626, and A 1634 (34), are not able to grow at 42°C.
When the recommended procedure was used (1), none of the C. dubliniensis isolates showed intracellular β-d-glucosidase activity. Nevertheless, the predictive value of this trait is lower than that of the inability to grow at 42°C, since 13% of C. albicans isolates showed no β-d-glucosidase activity. This result is in accordance with the results reported by Odds et al. (23), who found nearly the same percentage of C. albicans isolates without β-d-glucosidase activity.
According to Schoofs et al. (29) but contrary to the reported uniformly negative assimilation of dl-lactate, d-xylose, and MDG in C. dubliniensis strains (27, 30–32), we found considerable variability in the assimilation of dl-lactate, d-xylose, and MDG when using the API ID 32C gallery and reading the test visually. This result may have been due to very weak reactions after 48 h of incubation.
FT-IR spectroscopy monitors the composition and particular structures of all cell compounds within an intact cell environment. Thus, the spectral signatures observed are the complex superposition of the infrared active vibrational spectroscopic features of all cell components present in a cell (9, 10, 19, 21). To extract the relevant discriminatory information from large spectral data sets, data reduction and application of pattern recognition techniques are mandatory.
FT-IR spectroscopy is undoubtedly capable of discriminating between clinical isolates of C. albicans and C. dubliniensis. This discrimination is, however, achievable only when multivariate statistical techniques are applied to the analysis of complex spectral data. These spectral differences cannot yet be interpreted in terms of biochemical and/or chemical structures, however. The two classification models elaborated in this study prove that the information contained in the microbial FT-IR spectra is sufficient to differentiate phenotypically between the two closely related species of Candida. The potency of FT-IR spectroscopy for differentiating these two Candida species has already been shown by Timmins et al. (35). The present study, however, is the first example of a successful application of FT-IR spectroscopy to previously unidentified clinical isolates of C. albicans and C. dubliniensis. Subgrouping within the two species evidences the presence of strain-specific substructures within the classification scheme and demonstrates the clear ability to use the FT-IR spectroscopy technique as a fingerprinting-like method for further studies. The ANN approach elaborated in this paper proved to be extremely efficient, since once a validated ANN had been generated, unknown isolates could be identified within seconds without again performing the whole elaboration procedure. Thus, FT-IR spectroscopy can be used as a very rapid and versatile technique for screening large numbers of isolates in the context of epidemiology and outbreaks.
Species assignment based on analysis of the 5′ end of the nuclear large-subunit (26S) rDNA proved to be a reliable procedure, especially for yeasts (12, 13), including medically important species (14). The variability found in the ITS1 region of the rDNA gene is also often used for the discrimination of phylogenetically closely related fungi (36). Upon comparison, both regions of the nuclear rRNA gene showed identical clustering. The ITS1 sequence contained one species-specific signature sequence that could be used to assign a strain unequivocally to C. albicans or C. dubliniensis. The same was true for phenotypically atypical strains of C. albicans, such as the former type strain of “C. stellatoidea” or C. albicans strains not producing chlamydospores. Therefore, this signature sequence may be used for the design of a species-specific gene probe, allowing a colony blot to be performed in order to recognize C. dubliniensis in mixed cultures. Since there are several different adjacent nucleotides, the design and application of a gene probe might be easier than the use of single different nucleotides, as in the case of the 5′ end of the nuclear large-subunit (26S) rDNA (15). Such an approach might help to overcome the difficulties encountered when phenotypic markers are used for this purpose, as revealed in our study. However, sequence differences in the region of the 3′ end of the nuclear 18S rRNA gene in one strain of C. dubliniensis relative to C. albicans (32) could not be revealed in the reference strains (CBS 7987T, CBS 7988, and NCPF 3108) studied by us. This finding may provide an explanation for the unsuccessful application of a gene probe (data not shown) designed on the basis of the sequence differences in positions 114 to 124 in the alignment (Fig. 3) when we started our study.
In conclusion, the main problem in the selection and identification of C. dubliniensis, which is present in more than one-third of yeast-colonized HIV-infected patients, is still the lack of a reliable discriminative phenotypic marker. Use of dark green colonies on CHROMagar Candida proved to be insufficient. One indication of the presence of C. dubliniensis in chlamydospore-forming isolates is the combination of no or highly restricted growth at 42°C and the absence of intracellular β-d-glucosidase activity. Both ITS1 sequencing and FT-IR spectroscopy with multivariant data analysis allow reliable identification of C. dubliniensis.
The flow scheme proposed in Fig. 4 is based on the results obtained in our study and might help in identifying C. dubliniensis.
FIG. 4.
Proposed scheme for identification of C. dubliniensis. Some authors claim that a clearer distinction between C. albicans and C. dubliniensis is obtained by subculturing at 45°C (24).
ACKNOWLEDGMENTS
We thank Mareike Kurz and Beate Melzer-Krick for excellent work and H.-J. Tietz for providing us with three atypical C. albicans strains. The help and suggestions offered by J. Schmitt in applying ANN analysis to the FT-IR spectroscopy data are particularly acknowledged.
Part of this work was supported by EC Biomed II project BMH4-97-2054.
K.T. and G.H. contributed equally to this work.
REFERENCES
- 1.Boerlin P, Boerlin-Petzold F, Durussel C, Addo M, Pagani J L, Chave J P, Bille J. Cluster of oral atypical Candida albicans isolates in a group of human immunodeficiency virus-positive drug users. J Clin Microbiol. 1995;33:1129–1135. doi: 10.1128/jcm.33.5.1129-1135.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman D C, Sullivan D J, Bennett D E, Moran G P, Barry H J, Shanley D B. Candidiasis: the emergence of a novel species, Candida dubliniensis. AIDS. 1997;11:557–567. doi: 10.1097/00002030-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 3.De Rijk P, De Wachter R. DCSE, an interactive tool for sequence alignment and secondary structure research. Comput Applic Biosci. 1993;9:735–740. doi: 10.1093/bioinformatics/9.6.735. [DOI] [PubMed] [Google Scholar]
- 4.Gilfillan G D, Sullivan D J, Haynes K, Parkinson T, Coleman D C, Gow N A. Candida dubliniensis: phylogeny and putative virulence factors. Microbiology. 1998;144:829–838. doi: 10.1099/00221287-144-4-829. [DOI] [PubMed] [Google Scholar]
- 5.Goodacre R, Rooney P J, Kell D B. Rapid analysis of microbial systems using vibrational spectroscopy and supervised learning methods: application to the discrimination between methicillin-resistant and methicillin-susceptible Staphylococcus aureus. In H. H. Mantsch and M. Jackson (ed.), Infrared spectroscopy: new tool in medicine. Proceedings of SPIE. Vol. 3257. 1998. pp. 220–229. , Bellingham, Wash. [Google Scholar]
- 6.Goodacre R, Timmins E M, Rooney P J, Rowland J J, Kell D B. Rapid identification of Streptococcus and Enterococcus species using diffuse reflectance-absorbance Fourier transform infrared spectroscopy and artificial neural networks. FEMS Microbiol Lett. 1996;140:233–239. doi: 10.1016/0378-1097(96)00186-3. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin D C, Lee S B. Microwave miniprep of total genomic DNA from fungi, plants, protists and animals for PCR. BioFeedback. 1993;15:438–444. [PubMed] [Google Scholar]
- 8.Haase G, Sonntag L, van de Peer Y, Uijthof J M J, Podbielski A, Melzer-Krick B. Phylogenetic analysis of ten black yeast species using nuclear small subunit rRNA gene sequences. Antonie Leeuwenhoek. 1995;68:19–33. doi: 10.1007/BF00873289. [DOI] [PubMed] [Google Scholar]
- 9.Helm D, Labischinski H, Naumann D. Elaboration of a procedure for identification of bacteria using Fourier-transform infrared spectral libraries: a stepwise correlation approach. J Microbiol Methods. 1991;14:127–142. [Google Scholar]
- 10.Helm D, Labischinski H, Schallehn G, Naumann D. Classification and identification of bacteria by Fourier-transform infrared spectroscopy. J Gen Microbiol. 1991;137:69–79. doi: 10.1099/00221287-137-1-69. [DOI] [PubMed] [Google Scholar]
- 11.Jabra-Rizk M A, Baqui A A, Kelley J I, Falkler W A, Jr, Merz W G, Meiller T F. Identification of Candida dubliniensis in a prospective study of patients in the United States. J Clin Microbiol. 1999;37:321–326. doi: 10.1128/jcm.37.2.321-326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurtzman C P. Molecular taxonomy of the yeasts. Yeast. 1994;10:1727–1740. doi: 10.1002/yea.320101306. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzman C P, Robnett C J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek. 1998;73:331–371. doi: 10.1023/a:1001761008817. [DOI] [PubMed] [Google Scholar]
- 14.Kurtzman C P, Robnett C J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol. 1997;35:1216–1223. doi: 10.1128/jcm.35.5.1216-1223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannarelli B M, Kurtzman C P. Rapid identification of Candida albicans and other human pathogenic yeasts by using short oligonucleotides in a PCR. J Clin Microbiol. 1998;36:1634–1641. doi: 10.1128/jcm.36.6.1634-1641.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meis J F, Ruhnke M, De Pauw B E, Odds F C, Siegert W, Verweij P E. Candida dubliniensis candidemia in patients with chemotherapy-induced neutropenia and bone marrow transplantation. Emerg Infect Dis. 1999;5:1–4. doi: 10.3201/eid0501.990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran G P, Sanglard D, Donnelly S M, Shanley D B, Sullivan D J, Coleman D C. Identification and expression of multidrug transporters responsible for fluconazole resistance in Candida dubliniensis. Antimicrob Agents Chemother. 1998;42:1819–1830. doi: 10.1128/aac.42.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moran G P, Sullivan D J, Henman M C, McCreary C E, Harrington B J, Shanley D B, Coleman D C. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob Agents Chemother. 1997;41:617–623. doi: 10.1128/aac.41.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. M-27A. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 19.Naumann D. Infrared and NIR Raman spectroscopy in medical microbiology. In: Mantsch H H, Jackson M, editors. Infrared spectroscopy: new tool in medicine. Proceedings of SPIE. Vol. 3257. 1998. pp. 245–257. , Bellingham, Wash. [Google Scholar]
- 20.Naumann D, Helm D, Labischinski H. Microbiological characterizations by FT-IR spectroscopy. Nature. 1991;351:81–82. doi: 10.1038/351081a0. [DOI] [PubMed] [Google Scholar]
- 21.Naumann D, Labischinski H, Giesbrecht P. The characterization of microorganisms by Fourier-transform infrared spectroscopy (FT-IR) In: Nelson W H, editor. Modern techniques for rapid microbiological analysis. New York, N.Y: VCH Publishers; 1991. pp. 43–96. [Google Scholar]
- 22.Odds F C, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol. 1994;32:1923–1929. doi: 10.1128/jcm.32.8.1923-1929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odds F C, Van Nuffel L, Dams G. Prevalence of Candida dubliniensis isolates in a yeast stock collection. J Clin Microbiol. 1998;36:2869–2873. doi: 10.1128/jcm.36.10.2869-2873.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinjon E, Sullivan D, Salkin I, Shanley D, Coleman D. Simple, inexpensive, reliable method for differentiation of Candida dubliniensis from Candida albicans. J Clin Microbiol. 1998;36:2093–2095. doi: 10.1128/jcm.36.7.2093-2095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rex J H, Pfaller M A, Galgiani J N, Barlett M S, Espinel-Ingroff A, Ghannoum M A. Development of interpretative breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 26.Ruhnke M, Grosch-Woerner I, Steinmüller A, Neubauer A. Molekulare Epidemiologie von Candida-Infektionen bei HIV-infizierten Müttern und ihren Kindern. Wien Med Wochenschr. 1997;147:446–449. [PubMed] [Google Scholar]
- 27.Salkin I F, Pruitt W R, Padhye A A, Sullivan D, Coleman D, Pincus D H. Distinctive carbohydrate assimilation profiles used to identify the first clinical isolates of Candida dubliniensis recovered in the United States. J Clin Microbiol. 1998;36:1467. doi: 10.1128/jcm.36.5.1467-1467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitt J, Udelhoven T, Naumann D, Flemming H C. Stacked spectral data processing and artifical neural networks applied to FT-IR and FT-Raman spectra in biomedical applications. In: Mantsch H H, Jackson M, editors. Infrared spectroscopy: new tool in medicine. Proceedings of SPIE. Vol. 3257. 1998. pp. 236–244. , Bellingham, Wash. [Google Scholar]
- 29.Schoofs A, Odds F C, Colebunders R, Ieven M, Goossens H. Use of specialised isolation media for regonition and identification of Candida dubliniensis isolates from HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1997;16:296–300. doi: 10.1007/BF01695634. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan D, Coleman D. Candida dubliniensis: characteristics and identification. J Clin Microbiol. 1998;36:329–334. doi: 10.1128/jcm.36.2.329-334.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan D, Haynes K, Bille J, Boerlin P, Rodero L, Lloyd S, Henman M, Coleman D. Widespread geographic distribution of oral Candida dubliniensis strains in human immunodeficiency virus-infected individuals. J Clin Microbiol. 1997;35:960–964. doi: 10.1128/jcm.35.4.960-964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan D J, Westerneng T J, Haynes K A, Bennett D E, Coleman D C. Candida dubliniensis sp. nov.: phenotypic and molecular characterization of a novel species associated with oral candidosis in HIV-infected individuals. Microbiology. 1995;141:1507–1521. doi: 10.1099/13500872-141-7-1507. [DOI] [PubMed] [Google Scholar]
- 33.Taschdjian C L. A simply prepared identification medium for Candida albicans. Mycologia. 1953;45:474–475. [Google Scholar]
- 34.Tietz H J, Kussner A, Thanos M, De Andrade M P, Presber W, Schonian G. Phenotypic and genotypic characterization of unusual vaginal isolates of Candida albicans from Africa. J Clin Microbiol. 1995;33:2462–2465. doi: 10.1128/jcm.33.9.2462-2465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timmins E M, Howell S A, Alsberg B K, Noble W C, Goodacre R. Rapid differentiation of closely related Candida species and strains by pyrolysis-mass spectrometry and Fourier transform-infrared spectroscopy. J Clin Microbiol. 1998;36:367–374. doi: 10.1128/jcm.36.2.367-374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uijthof J M J, van Belkum A, de Hoog G S, Haase G. Exophiala dermatitidis and Sarcinomyces phaeomuriformis: ITS1-sequencing and nutritional physiology. Med Mycol. 1998;36:143–152. [PubMed] [Google Scholar]
- 37.van Uden N, Buckley H. Genus 2. Candida Berkhout. In: Lodder J, editor. The yeasts. Ataxonomic study. 2nd ed. Amsterdam, The Netherlands: North-Holland Publishing Company; 1971. pp. 914–1087. [Google Scholar]
- 38.Velegraki A, Logotheti M. Presumptive identification of an emerging yeast pathogen: Candida dubliniensis (sp. nov.) reduces 2,3,5-triphenyltetrazolium chloride. FEMS Immunol Med Microbiol. 1998;20:239–241. doi: 10.1111/j.1574-695X.1998.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 39.White T J, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for genetics. In: Innis M A, Gelfand D H, Sninsky J S, White T J, editors. PCR protocols: a guide to methods and applications. New York, N.Y: Academic Press, Inc.; 1990. pp. 129–141. [Google Scholar]